Hepatic Hilar Block as an Adjunct to Transarterial Embolization of Neuroendocrine Tumors: A Retrospective Review of Safety and Efficacy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Demographics and Data Collection
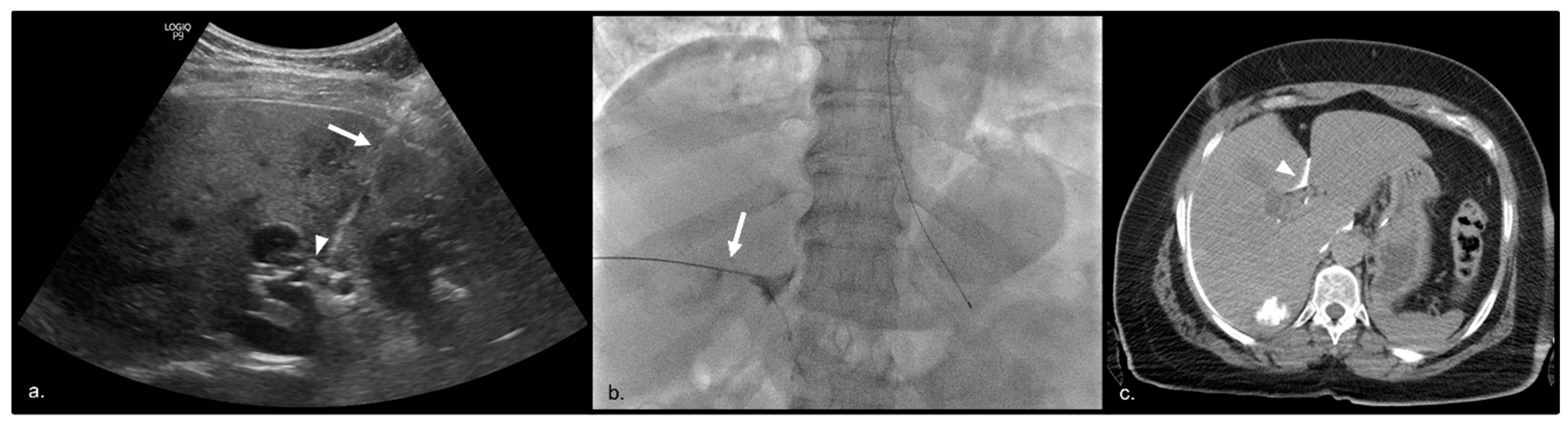
2.2. Treatment Technique
2.3. Statistical Analysis
3. Results
3.1. Pre-Procedural Findings
3.2. Post-Procedural Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.M.; Phan, A.T.; Dagohoy, C.G.; Leary, C.C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Pericleous, M.; Caplin, M.E.; Tsochatzis, E.; Yu, D.; Morgan-Rowe, L.; Toumpanakis, C. Hepatic artery embolization in advanced neuroendocrine tumors: Efficacy and long-term outcomes. Asia Pac. J. Clin. Oncol. 2016, 12, 61–69. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Choi, J.; Cantor, A.B.; Kvols, L.K. Selective Hepatic Artery Embolization for Treatment of Patients with Metastatic Carcinoid and Pancreatic Endocrine Tumors. Cancer Control 2006, 13, 72–78. [Google Scholar] [CrossRef]
- Barbier, C.E.; Garske-Román, U.; Sandström, M.; Nyman, R.; Granberg, D. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur. J. Nucl. Med. 2016, 43, 1425–1431. [Google Scholar] [CrossRef]
- Da Dong, X.; Carr, B.I. Hepatic artery chemoembolization for the treatment of liver metastases from neuroendocrine tumors: A long-term follow-up in 123 patients. Med. Oncol. 2011, 28, 286–290. [Google Scholar] [CrossRef]
- Fiore, F.; Del Prete, M.; Franco, R.; Marotta, V.; Ramundo, V.; Marciello, F.; Di Sarno, A.; Carratù, A.C.; Roseto, C.d.L.d.; Colao, A.; et al. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine 2014, 47, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.A.; Goin, J.E.; Sickles, C.; Raskay, B.J.; Soulen, M.C. Determinants of Postembolization Syndrome after Hepatic Chemoembolization. J. Vasc. Interv. Radiol. 2001, 12, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, H.; West, S. Management of Postembolization Syndrome Following Hepatic Transarterial Chemoembolization for Primary or Metastatic Liver Cancer. Cancer Nurs. 2016, 39, E1–E18. [Google Scholar] [CrossRef]
- Fiorentini, G.; Aliberti, C.; Tilli, M.; Mulazzani, L.; Graziano, F.; Giordani, P.; Mambrini, A.; Montagnani, F.; Alessandroni, P.; Catalano, V.; et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: Final results of a phase III study. Anticancer Res. 2012, 32, 1387–1395. [Google Scholar] [PubMed]
- Gregorian, R.S.; Gasik, A.; Kwong, W.J.; Voeller, S.; Kavanagh, S. Importance of Side Effects in Opioid Treatment: A Trade-Off Analysis with Patients and Physicians. J. Pain 2010, 11, 1095–1108. [Google Scholar] [CrossRef]
- Tessier, L.; Guilcher, S.J.; Bai, Y.Q.; Ng, R.; Wodchis, W.P. The impact of hospital harm on length of stay, costs of care and length of person-centred episodes of care: A retrospective cohort study. Can. Med. Assoc. J. 2019, 191, E879–E885. [Google Scholar] [CrossRef] [PubMed]
- Siramolpiwat, S.; Punjachaipornpon, T.; Pornthisarn, B.; Vilaichone, R.-K.; Chonprasertsuk, S.; Tangaroonsanti, A.; Bhanthumkomol, P.; Phumyen, A.; Yasiri, A.; Kaewmanee, M. N-Acetylcysteine Prevents Post-embolization Syndrome in Patients with Hepatocellular Carcinoma Following Transarterial Chemoembolization. Dig. Dis. Sci. 2019, 64, 3337–3345. [Google Scholar] [CrossRef]
- Ogasawara, S.; Chiba, T.; Ooka, Y.; Kanogawa, N.; Motoyama, T.; Suzuki, E.; Tawada, A.; Nagai, K.; Nakagawa, T.; Sugawara, T.; et al. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology 2018, 67, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Seon, J.; Sung, P.S.; Oh, J.S.; Lee, H.L.; Jang, B.; Chun, H.J.; Jang, J.W.; Bae, S.H.; Choi, J.Y.; et al. Dexamethasone Prophylaxis to Alleviate Postembolization Syndrome after Transarterial Chemoembolization for Hepatocellular Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Study. J. Vasc. Interv. Radiol. 2017, 28, 1503–1511.e2. [Google Scholar] [CrossRef]
- Agrawal, R. Identifying predictors and evaluating the role of steroids in the prevention of post-embolization syndrome after transarterial chemoembolization and bland embolization. Ann. Gastroenterol. 2020, 34, 241–246. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, C.; Liang, B.; Xiong, B. Efficacy and safety analysis of dexamethasone-lipiodol emulsion in prevention of post-embolization syndrome after TACE: A retrospective analysis. BMC Gastroenterol. 2021, 21, 256. [Google Scholar] [CrossRef]
- Bessar, A.A.; Nada, M.G.; Wadea, F.M.; Elsayed, A.E.; Farag, A.; Bessar, M.A. Hepatic Hilar and Celiac Plexus Nerve Blocks as Analgesia for Doxorubicin-Eluting Microsphere Chemoembolization Procedures for Hepatocellular Carcinoma: A Nonblinded Randomized Clinical Trial. J. Vasc. Interv. Radiol. 2021, 32, 1179–1185. [Google Scholar] [CrossRef]
- Lee, S.H.; Hahn, S.T.; Park, S.H. Intraarterial Lidocaine Administration for Relief of Pain Resulting from Transarterial Chemoembolization of Hepatocellular Carcinoma: Its Effectiveness and Optimal Timing of Administration. Cardiovasc. Interv. Radiol. 2001, 24, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Bingham, A.E.; Fu, R.; Horn, J.-L.; Abrahams, M.S. Continuous Peripheral Nerve Block Compared with Single-Injection Peripheral Nerve Block. Reg. Anesthesia Pain Med. 2012, 37, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Coldwell, D.M.; Loper, K.A. Regional Anesthesia for Hepatic Arterial Embolization. Radiology 1989, 172, 1039–1040. [Google Scholar] [CrossRef]
- Aguirre, J.; Del Moral, A.; Cobo, I.; Borgeat, A.; Blumenthal, S. The Role of Continuous Peripheral Nerve Blocks. Anesthesiol. Res. Pract. 2012, 2012, 560879. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; McClenny, T.E.; Cardella, J.F.; Lewis, C.A. Society of Interventional Radiology Clinical Practice Guidelines. J. Vasc. Interv. Radiol. 2003, 14, S199–S202. [Google Scholar] [CrossRef]
- Zener, R.; Yoon, H.; Ziv, E.; Covey, A.; Brown, K.T.; Sofocleous, C.T.; Thornton, R.H.; Boas, F.E. Outcomes After Transarterial Embolization of Neuroendocrine Tumor Liver Metastases Using Spherical Particles of Different Sizes. Cardiovasc. Interv. Radiol. 2019, 42, 569–576. [Google Scholar] [CrossRef]
- Parhar, D.; Baum, R.A.; Spouge, R.; Yan, T.; Ho, S.; Hadjivassiliou, A.; Machan, L.; Legiehn, G.; Klass, D.; Dhatt, R.; et al. Hepatic Hilar Nerve Block for Adjunctive Analgesia during Percutaneous Thermal Ablation of Hepatic Tumors: A Retrospective Analysis. J. Vasc. Interv. Radiol. 2023, 34, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Hassan, S.; Lewandowski, R.J.; Grace, K.; Martin, R.C.; Sichlau, M.J.; Fung, J.; Kim, E.; Chao, S.; Rosner, B.I. Quality of Life after Radioembolization for Hepatocellular Carcinoma Using a Digital Patient-Reported Outcome Tool. J. Vasc. Interv. Radiol. 2020, 31, 311–314.e1. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.H.; Carrasco, C.H.; Hohn, D.C.; Curley, S.A. Hepatic artery chemoembolization or embolization for primary and metastatic liver tumors: Post-treatment management and complications. J. Surg. Oncol. 1995, 60, 116–121. [Google Scholar] [CrossRef]
- Mason, M.C.; Massarweh, N.N.; Salami, A.; Sultenfuss, M.A.; Anaya, D.A. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB 2015, 17, 1137–1144. [Google Scholar] [CrossRef]
- Ubillus, W.J.R.; Munoz, J.; Vekaria, M.; Wollner, I.S.; Getzen, T. Hematology—Oncology division, Interventional Radiology department Post-embolization syndrome: Outcomes regarding the type of embolization. J. Clin. Oncol. 2011, 29, e14582. [Google Scholar] [CrossRef]
- Yang, A.; Brown, J.; Mak, E. Persistent Diarrhea after Celiac Plexus Block in a Pancreatic Cancer Patient: Case Report and Literature Review. J. Palliat. Med. 2016, 19, 83–86. [Google Scholar] [CrossRef] [PubMed]
- He, K.S.; Fernando, R.; Cabrera, T.; Valenti, D.; Algharras, A.; Martínez, N.; Liu, D.M.; Noel, G.; Muchantef, K.; Bessissow, A.; et al. Hepatic Hilar Nerve Block for Hepatic Interventions: Anatomy, Technique, and Initial Clinical Experience in Thermal Ablation of Liver Tumors. Radiology 2021, 301, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Pryor, M.E.; Mather, L.E.; Veering, B.T. Cardiovascular and Central Nervous System Effects of Intravenous Levobupivacaine and Bupivacaine in Sheep. Obstet. Anesth. Dig. 1998, 86, 797–804. [Google Scholar] [CrossRef]
| Overall (n = 165) | Embo + Nerve Block (n = 12) | Embo (n = 153) | p-Value | |
|---|---|---|---|---|
| Age (mean, 95% CI) | 62 (59–64) | 62 (51–74) | 62 (59–64) | 0.934 |
| Prior liver interventions (ex. Y90, ablation, embolization) | 0.315 | |||
| Yes | 87 (52.73%) | 8 (66.67%) | 79 (51.63%) | |
| No | 78 (47.27%) | 4 (33.33%) | 74 (48.37%) | |
| Liver treatment data | ||||
| Size of largest lesion in treated region (mean cm, SD) | 5.20 (3.37) | 5.15 (0.27) | 5.88 (1.18) | 0.472 |
| Bilobar disease present | 0.834 | |||
| Yes | 155 (93.88%) | 12 (100%) | 143 (93.46%) | |
| No | 10 (6.12%) | 0 (0%) | 10 (6.54%) | |
| Number of hepatic lesions | 0.810 | |||
| 1 | 2 (1.21%) | 0 (0%) | 2 (1.31%) | |
| 2–5 | 27 (16.36%) | 3 (25%) | 24 (15.69%) | |
| >5 | 136 (82.43%) | 9 (75%) | 127 (83.0%) | |
| Percent of liver involved | 0.020 | |||
| >50% | 28 (16.97%) | 5 (41.67%) | 23 (15.03%) | |
| <50% | 137 (83.03) | 7 (58.33%) | 130 (74.97%) | |
| Extrahepatic liver metastases | ||||
| Yes | 133 (80.61%) | 11 (91.67%) | 122 (79.74%) | 0.314 |
| No | 32 (19.39%) | 1 (8.33%) | 31 (20.26%) | |
| Pre-procedure pain medication | ||||
| Yes | 82 | 8 (66.67%) | 74 (48.37%) | 0.222 |
| No | 83 | 4 (33.33%) | 79 (51.63%) | |
| Opioids | 55 | 4 (50%) | 51 (68.92%) | 0.279 |
| Non-opioids | 27 | 4 (50%) | 23 (31.08%) |
| Overall (n = 165) | Embo + Nerve Block (n = 12) | Embo (n = 153) | p-Value | |
|---|---|---|---|---|
| Post-procedure nausea | 0.347 | |||
| Yes | 128 | 8 (66.67%) | 120 (78.43%) | |
| No | 37 | 4 (33.33%) | 33 (21.57%) | |
| Post-procedure antiemetic use | 0.258 | |||
| Yes | 131 | 8 (66.67%) | 123 (80.39%) | |
| No | 34 | 4 (33.33%) | 30 (19.61%) | |
| 0.856 | ||||
| 1 antiemetic drug | 106 | 8 (66.67%) | 98 (64.05%) | |
| >1 drug | 59 | 4 (33.33%) | 55 (35.95%) | |
| Post-procedure pain | 0.185 | |||
| Yes | 144 | 9 (75.0%) | 135 (88.24%) | |
| No | 21 | 3 (25.0%) | 18 (11.76%) | |
| Number of analgesic drugs (mean, 95%CI) | 2.85 (2.55–3.16) | 2.83 (1.23–4.43) | 2.86 (2.54–3.17) | 0.8783 |
| Post-procedure oral NSAID | 0.293 | |||
| Yes | 13 | 0 (0%) | 13 (8.5%) | |
| No | 152 | 12 (100%) | 140 (91.5%) | |
| Post-procedure IV NSAID | 0.594 | |||
| Yes | 67 | 4 (33.33%) | 63 (41.8%) | |
| No | 98 | 8 (66.67%) | 90 (58.82%) | |
| Number of IV NSAID doses (mean, 95% CI) | 3.04 | 2.5 (1.29–6.29) | 3.08 (2.34–3.82) | 0.319 * |
| Post-procedure oral acetaminophen | 0.462 | |||
| Yes | 53 (32.12%) | 5 (41.67%) | 48 (31.37%) | |
| No | 112 (67.88%) | 7 (58.83%) | 105 (68.63%) | |
| Number of oral acetaminophen doses (mean, 95% CI) | 2.98 (2.19–3.78) | 1.8 (0.18–3.42) | 3.10 (2.23–3.97) | 0.274 * |
| Post-procedure IV acetaminophen | 0.927 | |||
| Yes | 57 (34.55%) | 4 (33.33%) | 53 (34.64%) | |
| No | 108 (65.45%) | 8 (66.67%) | 100 (65.36%) | |
| Number of IV acetaminophen doses (mean, 95% CI) | 1.68 | 1 (1–1) | 1.74 (1.16–2.31) | 0.485 * |
| Post-procedure opioids oral | 0.263 | |||
| Yes | 107 (64.85%) | 6 (50%) | 101 (66.01%) | |
| No | 58 (35.15%) | 6 (50%) | 52 (33.99%) | |
| Number of opiates oral doses (mean, 95% CI) | 4.47 (3.76–5.13) | 5.67 (1.87–9.46) | 4.40 (3.67–5.13) | 0.230 * |
| Post-procedure opiates IV | 0.455 | |||
| Yes | 93 (56.36%) | 8 (66.67%) | 85 (55.56%) | |
| No | 72 (43.64%) | 4 (33.33%) | 68 (44.44%) | |
| Number of opiates IV doses (mean, 95% CI) | 4.34 (3.32–5.37) | 4.25 (0.60–7.90) | 4.35 (3.26–5.45) | 1.00 * |
| Post-procedure PCA | 0.506 | |||
| Yes | 18 (10.91%) | 2 (16.67%) | 16 (10.46%) | |
| No | 147 (89.09%) | 10 (83.33%) | 137 (89.54%) | |
| Number of PCA doses (mean, 95% CI) | 7.44 | 4 (21.41–29.41) | 7.88 (1.41–14.33) | 0.723 * |
| Analgesic patch | 0.014 | |||
| Yes | 12 (7.27%) | 3 (25.0%) | 9 (5.88%) | |
| No | 153 (92.73%) | 9 (75.0%) | 144 (94.12%) |
| Overall (n = 165) | Embo + Nerve Block (n = 12) | Embo (n = 153) | p-Value | |
|---|---|---|---|---|
| Length of hospital stay (days, mean, 95% CI) | 2.2 (0.81–2.17) | 2.8 (1.43–4.26) | 2.2 (1.74–2.56) | 0.174 * |
| Length of hospital stay—pre-existing medical issues excluded (days, mean, 95% CI) | 1.73 (1.52–1.93) | 1.75 (0.59–2.91) | 1.72 (1.51–1.94) | 0.947 * |
| Rehospitalization within 30 days | 0.839 | |||
| For planned repeat | 8 (4.85%) | 1 (8.33%) | 7 (4.58%) | |
| For complications | 13 (7.88%) | 1 (8.33%) | 12 (7.84%) | |
| No hospitalization | 144 (87.27%) | 10 (83.33%) | 134 (87.58%) | |
| SIR-grade complications | 0.895 | |||
| A | 128 (77.85%) | 9 (75%) | 119 (77.78%) | |
| B | 9 (5.45%) | 1 (8.33%) | 8 (5.23%) | |
| C | 5 (3.03%) | 0 | 5 (3.27%) | |
| D | 6 (3.64%) | 1 (8.33%) | 5 (3.27%) | |
| F | 1 (0.61%) | 0 | 1 (0.65%) | |
| None | 16 (9.7%) | 1 (8.33%) | 15 (9.8%) |
| Factor | OR | 95% CI: | p-Value |
|---|---|---|---|
| Presence of extrahepatic metastases | 1.813 | 0.643–5.124 | 0.260 |
| Size of largest lesion in treated region | 1.147 | 0.962–1.365 | 0.125 |
| Bilobar disease | 0.751 | 0.090–6.240 | 0.790 |
| Liver involvement ≥50% | 5.044 | 0.649–39.147 | 0.122 |
| Treated region (≥ 4 segments) | 1.672 | 0.669–4.209 | 0.269 |
| Treatment of phrenic artery | 1.491 | 0.181–12.294 | 0.710 |
| Number of lesions >5 | 0.452 | 0.100–2.077 | 0.268 |
| 40–120 Embospheres | 0.550 | 0.219–1.385 | 0.205 |
| 100–300 Embospheres | 2.755 | 0.996–7.585 | 0.051 |
| 300–500 Embospheres | 1 | NA | NA |
| 500–700 Embospheres | 1 | NA | NA |
| 100 PVA | 1.813 | 0.394–8.343 | 0.444 |
| 300 PVA | 1 | NA | NA |
| Addition of block to embolization | 0.427 | 0.104–1.705 | 0.226 |
| Addition of biopsy to embolization | 1 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, S.; Blume, H.; Rodriguez, L.; Petre, E.; Moussa, A.; Zhao, K.; Sotirchos, V.; Raj, N.; Reidy, D.; Ziv, E.; et al. Hepatic Hilar Block as an Adjunct to Transarterial Embolization of Neuroendocrine Tumors: A Retrospective Review of Safety and Efficacy. Cancers 2023, 15, 5202. https://doi.org/10.3390/cancers15215202
Jain S, Blume H, Rodriguez L, Petre E, Moussa A, Zhao K, Sotirchos V, Raj N, Reidy D, Ziv E, et al. Hepatic Hilar Block as an Adjunct to Transarterial Embolization of Neuroendocrine Tumors: A Retrospective Review of Safety and Efficacy. Cancers. 2023; 15(21):5202. https://doi.org/10.3390/cancers15215202
Chicago/Turabian StyleJain, Samagra, Harrison Blume, Lee Rodriguez, Elena Petre, Amgad Moussa, Ken Zhao, Vlasios Sotirchos, Nitya Raj, Diane Reidy, Etay Ziv, and et al. 2023. "Hepatic Hilar Block as an Adjunct to Transarterial Embolization of Neuroendocrine Tumors: A Retrospective Review of Safety and Efficacy" Cancers 15, no. 21: 5202. https://doi.org/10.3390/cancers15215202
APA StyleJain, S., Blume, H., Rodriguez, L., Petre, E., Moussa, A., Zhao, K., Sotirchos, V., Raj, N., Reidy, D., Ziv, E., & Alexander, E. (2023). Hepatic Hilar Block as an Adjunct to Transarterial Embolization of Neuroendocrine Tumors: A Retrospective Review of Safety and Efficacy. Cancers, 15(21), 5202. https://doi.org/10.3390/cancers15215202






