Access to Care and Healthcare Quality Metrics for Patients with Advanced Genitourinary Cancers in Urban versus Rural Areas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Selection
2.2. Data Collection
2.3. Statistical Examination
3. Results
3.1. Demographics
3.2. Accessibility to Genomic Testing and Clinical Trials
3.3. Number of Treatment Lines Received
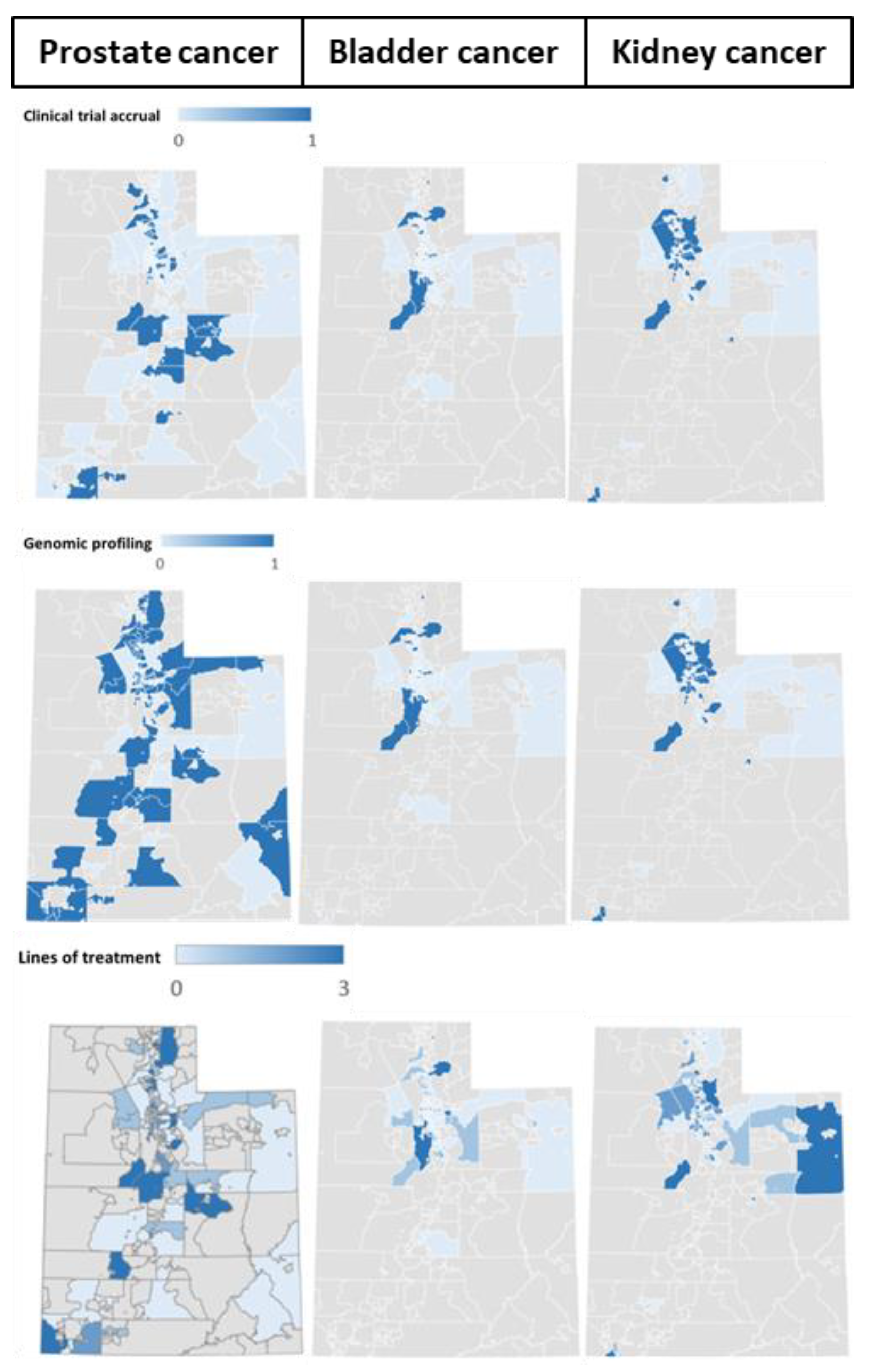
3.4. Geographic Distribution of Patients
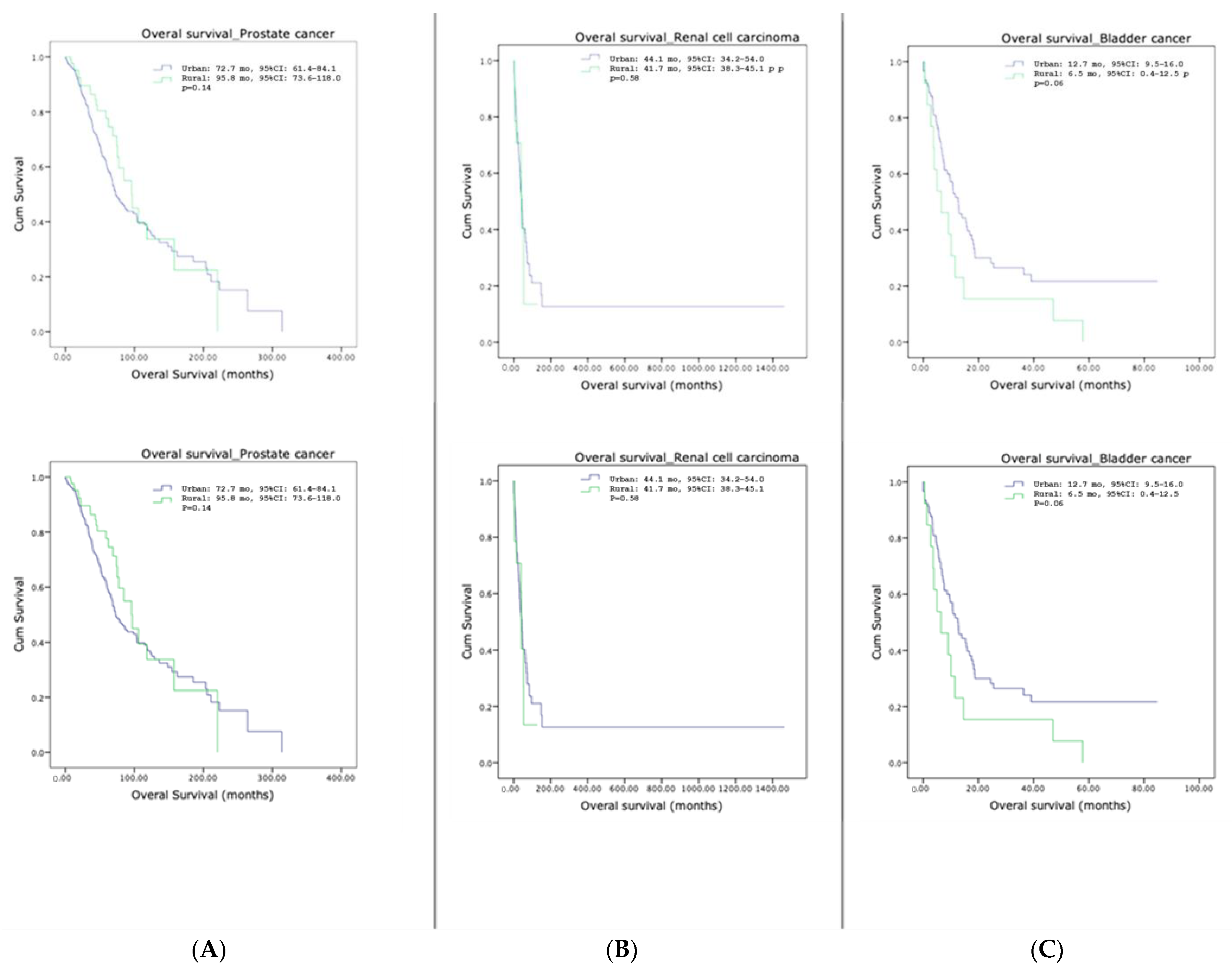
3.5. Survival Outcomes
4. Discussion
5. Limitation of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henley, S.J.; Anderson, R.N.; Thomas, C.C.; Massetti, G.M.; Peaker, B.; Richardson, L.C. Invasive Cancer Incidence, 2004–2013, and Deaths, 2006–2015, in Nonmetropolitan and Metropolitan Counties—United States. MMWR Surveill. Summ. 2017, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Schlichting, J.; Chioreso, C.; Ward, M.; Vikas, P. Challenges of Rural Cancer Care in the United States. Oncology 2015, 29, 633–640. [Google Scholar] [PubMed]
- Levit, L.A.; Byatt, L.; Lyss, A.P.; Paskett, E.D.; Levit, K.; Kirkwood, K.; Schenkel, C.; Schilsky, R.L. Closing the Rural Cancer Care Gap: Three Institutional Approaches. JCO Oncol. Pract. 2020, 16, 422–430. [Google Scholar] [CrossRef]
- Hashibe, M.; Kirchhoff, A.C.; Kepka, D.; Kim, J.; Millar, M.; Sweeney, C.; Herget, K.; Monroe, M.; Henry, N.L.; Lopez, A.M.; et al. Disparities in cancer survival and incidence by metropolitan versus rural residence in Utah. Cancer Med. 2018, 7, 1490–1497. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Han, X.; Zhao, J.; Nogueira, L.; Jemal, A. Rural Cancer Disparities in the United States: A Multilevel Framework to Improve Access to Care and Patient Outcomes. JCO Oncol. Pract. 2020, 16, 409–413. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Wi, C.I.; Srinivasan, S.G.; Choi, H.; Wheeler, P.H.; Stavlund, J.R.; Keller, D.A.; Bailey, K.R.; Juhn, Y.J. Participation of rural patients in clinical trials at a multi-site academic medical center. J. Clin. Transl. Sci. 2021, 5, e190. [Google Scholar] [CrossRef]
- Tanner, A.; Kim, S.H.; Friedman, D.B.; Foster, C.; Bergeron, C.D. Barriers to medical research participation as perceived by clinical trial investigators: Communicating with rural and African American communities. J. Health Commun. 2015, 20, 88–96. [Google Scholar] [CrossRef]
- Unger, J.M.; Moseley, A.; Symington, B.; Chavez-MacGregor, M.; Ramsey, S.D.; Hershman, D.L. Geographic Distribution and Survival Outcomes for Rural Patients With Cancer Treated in Clinical Trials. JAMA Netw. Open 2018, 1, e181235. [Google Scholar] [CrossRef]
- Hart, L.G.; Larson, E.H.; Lishner, D.M. Rural definitions for health policy and research. Am. J. Public Health 2005, 95, 1149–1155. [Google Scholar] [CrossRef]
- Sarfati, D. Why social inequalities matter in the cancer continuum. In Reducing Social Inequalities in Cancer: Evidence and Priorities for Research; Vaccarella, S., Lortet-Tieulent, J., Saracci, R., Conway, D.I., Straif, K., Wild, C.P., Eds.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Scanlon, B.; Brough, M.; Wyld, D.; Durham, J. Equity across the cancer care continuum for culturally and linguistically diverse migrants living in Australia: A scoping review. Glob. Health 2021, 17, 87. [Google Scholar] [CrossRef]
- The state of cancer care in America, 2014: A report by the American Society of Clinical Oncology. J. Oncol. Pract. 2014, 10, 119–142. [CrossRef]
- Montiel Ishino, F.A.; Odame, E.A.; Villalobos, K.; Rowan, C.; Whiteside, M.; Mamudu, H.; Williams, F. Sociodemographic and Geographic Disparities of Prostate Cancer Treatment Delay in Tennessee: A Population-Based Study. Am. J. Mens. Health 2021, 15, 15579883211057990. [Google Scholar] [CrossRef]
- Paskett, E.D.; Fisher, J.L.; Lengerich, E.J.; Schoenberg, N.E.; Kennedy, S.K.; Conn, M.E.; Roberto, K.A.; Dwyer, S.K.; Fickle, D.; Dignan, M. Disparities in underserved white populations: The case of cancer-related disparities in Appalachia. Oncologist 2011, 16, 1072–1081. [Google Scholar] [CrossRef]
- Foley, G.R.; Blizzard, C.L.; Stokes, B.; Skala, M.; Redwig, F.; Dickinson, J.L.; FitzGerald, L.M. Urban-rural prostate cancer disparities in a regional state of Australia. Sci. Rep. 2022, 12, 3022. [Google Scholar] [CrossRef]
- Maganty, A.; Sabik, L.M.; Sun, Z.; Eom, K.Y.; Li, J.; Davies, B.J.; Jacobs, B.L. Under Treatment of Prostate Cancer in Rural Locations. J. Urol. 2020, 203, 108–114. [Google Scholar] [CrossRef]
- Joshi, M.; Polimera, H.; Krupski, T.; Necchi, A. Geography Should Not Be an “Oncologic Destiny” for Urothelial Cancer: Improving Access to Care by Removing Local, Regional, and International Barriers. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 327–340. [Google Scholar] [CrossRef]
- Ward, M.M.; Ullrich, F.; Matthews, K.; Rushton, G.; Tracy, R.; Bajorin, D.F.; Goldstein, M.A.; Kosty, M.P.; Bruinooge, S.S.; Hanley, A.; et al. Access to Chemotherapy Services by Availability of Local and Visiting Oncologists. J. Oncol. Pract. 2014, 10, 26–31. [Google Scholar] [CrossRef]
- Baquet, C.R.; Commiskey, P.; Mullins, C.D.; Mishra, S.I. Recruitment and participation in clinical trials: Socio-demographic, rural/urban, and health care access predictors. Cancer Detect. Prev. 2006, 30, 24–33. [Google Scholar] [CrossRef]
- Sateren, W.B.; Trimble, E.L.; Abrams, J.; Brawley, O.; Breen, N.; Ford, L.; McCabe, M.; Kaplan, R.; Smith, M.; Ungerleider, R.; et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J. Clin. Oncol. 2002, 20, 2109–2117. [Google Scholar] [CrossRef]
- Salloum, R.G.; George, T.J.; Silver, N.; Markham, M.J.; Hall, J.M.; Guo, Y.; Bian, J.; Shenkman, E.A. Rural-urban and racial-ethnic differences in awareness of direct-to-consumer genetic testing. BMC Public Health 2018, 18, 277. [Google Scholar] [CrossRef]
- Wan, N.; Zhan, F.B.; Cai, Z. Socioeconomic disparities in prostate cancer mortality and the impact of geographic scale. South Med. J. 2011, 104, 553–559. [Google Scholar] [CrossRef] [PubMed]
| Urban | Rural | p-Value | ||
|---|---|---|---|---|
| Median age at diagnosis, years (range) | mPCa | 65 (44–88) | 65 (43–87) | 0.84 |
| mBCa | 66 (36–88) | 69 (41–86) | 0.22 | |
| mRCC | 60 (43–80) | 59 (37–74) | 0.56 | |
| Sex (male), n (%) | mBCa | 114 (72.2) | 17 (65.4) | 0.64 |
| mRCC | 99 (70.7) | 15 (68.2) | 0.81 | |
| Race (White), n (%) | mPCa | 524 (92.4) | 106 (94.6) | 0.87 |
| mBCa | 151 (95.6) | 26 (100.0) | 0.88 | |
| mRCC | 117 (83.6) | 18 (81.8) | 0.84 | |
| De novo metastatic disease at diagnosis, n (%) | mPCa | 227 (43.0) | 43 (40.6) | 0.72 |
| mBCa | 32 (20.3) | 6 (23.0) | 0.94 | |
| mRCC | 59 (42.0) | 8 (36.4) | 0.64 | |
| Pathology subtypes, n (%) | mPCa, adenocarcinoma subtype | 550 (97.0) | 111 (99.1) | 0.34 |
| mBCa, urothelial subtype | 85 (53.8) | 17 (65.4) | 0.37 | |
| mRCC, clear cell subtype | 105 (75.0) | 14 (63.6) | 0.25 | |
| Urban, (n = 860) | Rural, (n = 165) | p-Value | |
|---|---|---|---|
| Insurance plan, n (%) | 0.40 | ||
| No insurance | 106 (12.3) | 18 (11.0) | |
| Medicare | 386 (44.9) | 84 (50.9) | |
| Medicaid | 20 (2.3) | 5 (3.0) | |
| Commercial | 348 (40.4) | 58 (35.1) |
| Urban, n (%) | Rural, n (%) | p-Value | ||
|---|---|---|---|---|
| Clinical trial accrual | mPCa | 155 (27.3) | 37 (33.0) | 0.22 |
| mBCa | 32 (20.3) | 5 (19.2) | 0.90 | |
| mRCC | 69 (49.3) | 9 (40.9) | 0.46 | |
| Genomic profiling | mPCa | 376 (66.3) | 68 (60.7) | 0.25 |
| mBCa | 113 (71.5) | 18 (69.2) | 0.72 | |
| mRCC | 70 (50.0) | 11 (50.0) | 1.00 | |
| Patients who received more than one line of systemic treatment | mPCa | 243 (42.8) | 43 (38.4) | 0.44 |
| mBCa | 52 (32.9) | 8 (30.8) | 0.99 | |
| mRCC | 75 (53.6) | 9 (40.9) | 0.27 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Sahu, K.K.; Kumar, S.A.; Tripathi, N.; Sayegh, N.; Nordblad, B.; Chigarira, B.; Gupta, S.; Maughan, B.L.; Agarwal, N.; et al. Access to Care and Healthcare Quality Metrics for Patients with Advanced Genitourinary Cancers in Urban versus Rural Areas. Cancers 2023, 15, 5171. https://doi.org/10.3390/cancers15215171
Li H, Sahu KK, Kumar SA, Tripathi N, Sayegh N, Nordblad B, Chigarira B, Gupta S, Maughan BL, Agarwal N, et al. Access to Care and Healthcare Quality Metrics for Patients with Advanced Genitourinary Cancers in Urban versus Rural Areas. Cancers. 2023; 15(21):5171. https://doi.org/10.3390/cancers15215171
Chicago/Turabian StyleLi, Haoran, Kamal Kant Sahu, Shruti Adidam Kumar, Nishita Tripathi, Nicolas Sayegh, Blake Nordblad, Beverly Chigarira, Sumati Gupta, Benjamin L. Maughan, Neeraj Agarwal, and et al. 2023. "Access to Care and Healthcare Quality Metrics for Patients with Advanced Genitourinary Cancers in Urban versus Rural Areas" Cancers 15, no. 21: 5171. https://doi.org/10.3390/cancers15215171
APA StyleLi, H., Sahu, K. K., Kumar, S. A., Tripathi, N., Sayegh, N., Nordblad, B., Chigarira, B., Gupta, S., Maughan, B. L., Agarwal, N., & Swami, U. (2023). Access to Care and Healthcare Quality Metrics for Patients with Advanced Genitourinary Cancers in Urban versus Rural Areas. Cancers, 15(21), 5171. https://doi.org/10.3390/cancers15215171






