Heat Shock Factor 1 Inhibition: A Novel Anti-Cancer Strategy with Promise for Precision Oncology
Abstract
:Simple Summary
Abstract
1. Introduction
2. HSF1 Biology
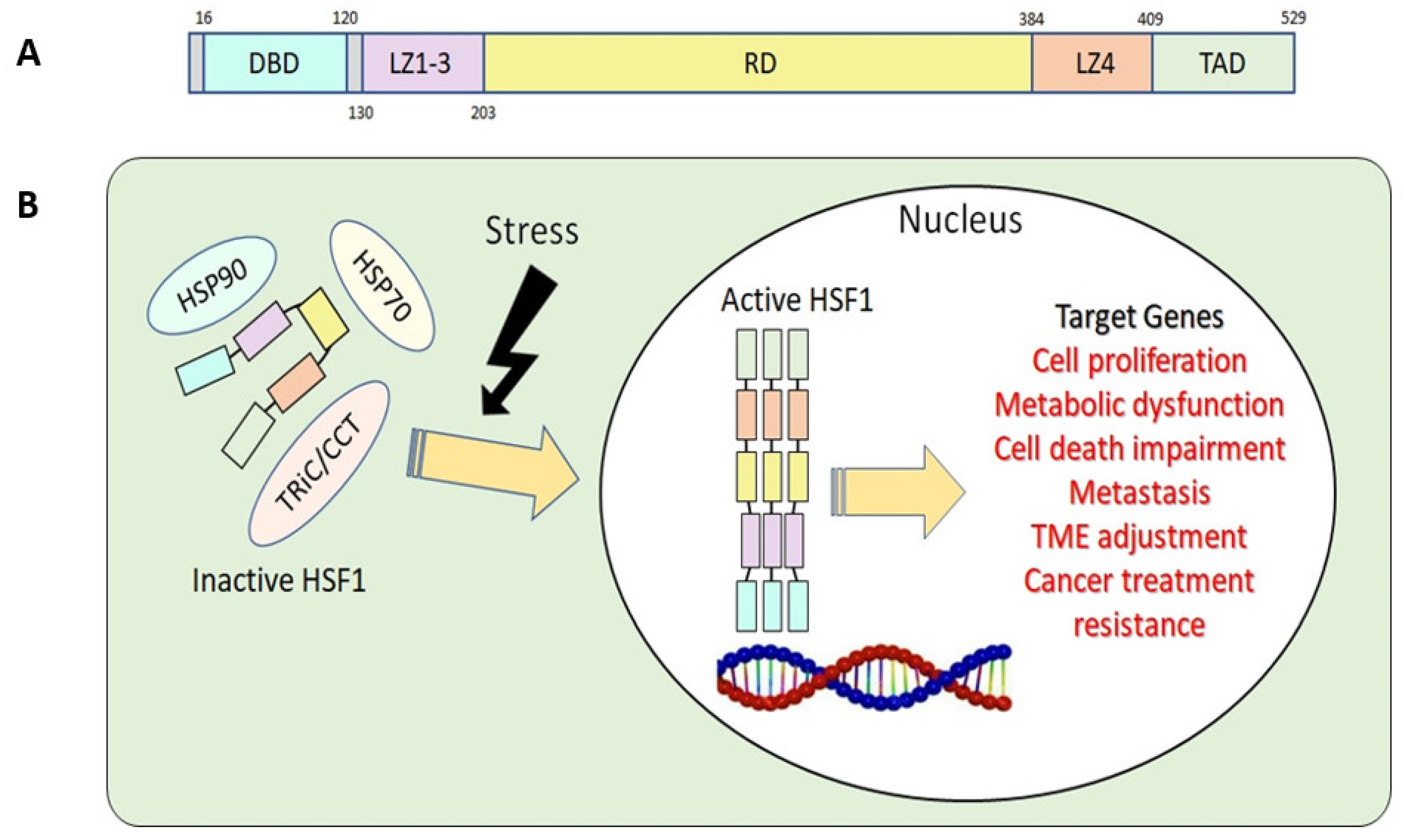
2.1. HSF1 Structure and Function
2.2. HSF1 Regulatory Mechanisms in Normal and Cancer Cells
3. HSF1 Involvement in Various Cancer Types
3.1. Breast Cancer
3.2. Lung Cancer
3.3. Ovarian Cancer
3.4. Endometrial Cancer
3.5. Prostate Cancer
| Cancer Type | Effect on Tumorigenesis | Reference |
|---|---|---|
| Liver cancer | Promotes cell proliferation, growth, migration, invasion, and survival, as well as kinase function, lipid metabolism, and glycolysis | [63,64,65,66,67] |
| Breast Cancer | Promotes cell motility, metastasis, and survival as well as receptor and kinase maturation, stemness, drug resistance, DNA repair, and EMT | [35,36,37,38,68,69] |
| Prostate Cancer | Promotes development of polyploidy, high Gleason score, and cancer re-occurrence. Decreases patient survival. | [31,59] |
| Lung Cancer | Promotes angiogenesis and Metastasis | [41,70,71] |
| ESCC | Promotes cell survival and expression of HSPs. | [10] |
| Colorectal Cancer | Promotes expression of anti-apoptotic proteins, cell growth, and glutaminolysis | [71,72] |
| Endometrial Cancer | Tumor progression | [32] |
| Ovarian Cancer | Proliferation | [46,47] |
| Tumor progression, cell spreading, ECM remodeling, and cancer invasion | [49,73,74] |
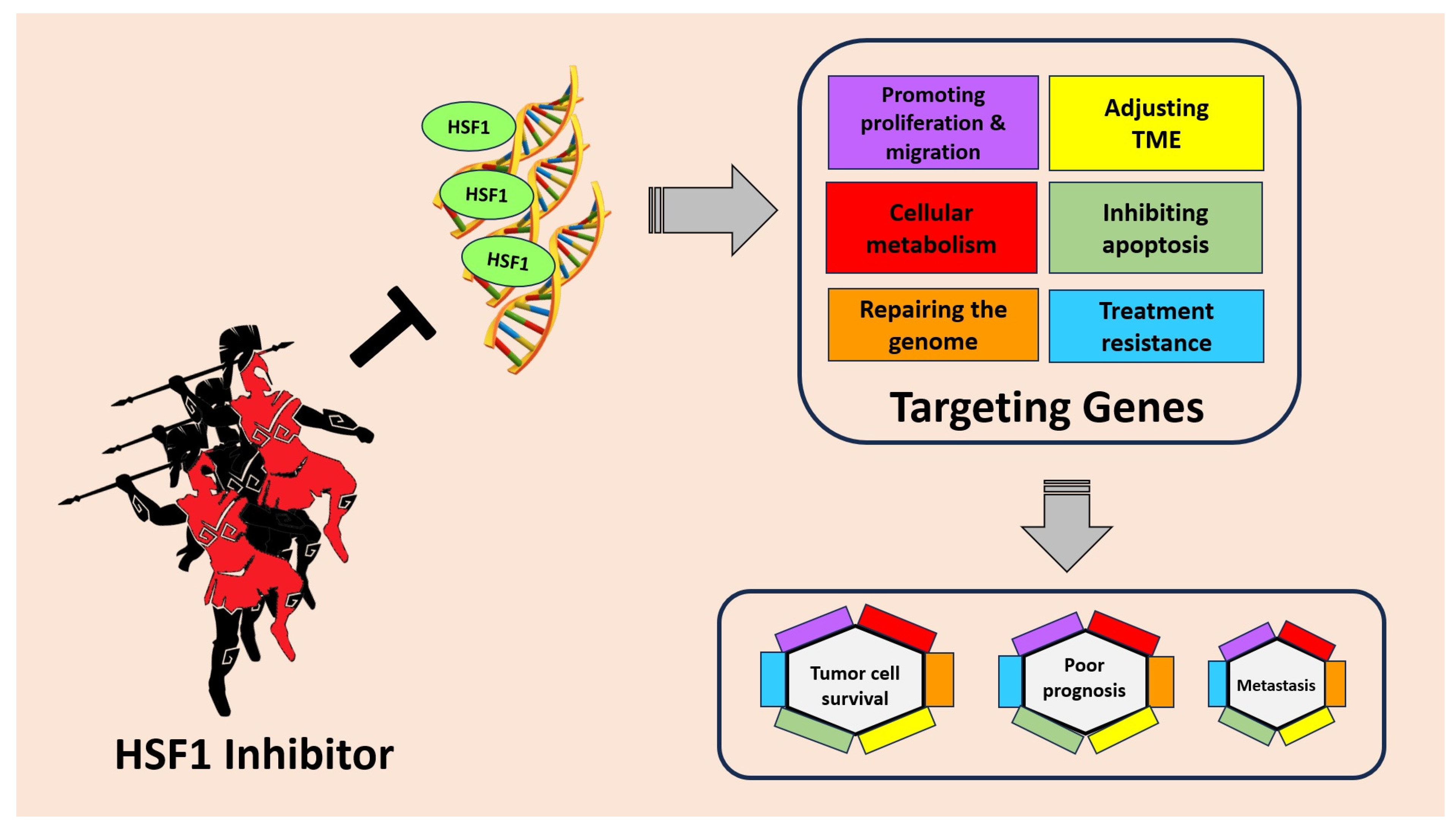
4. HSF1 in Therapeutic Resistance
5. Targeting HSF1 for Cancer Treatment
| Agents | Source | Cancer Type | Refs. |
|---|---|---|---|
| Cantharidin | Blister beetles (Meloidae spp.) | colon cancer; lung cancer; prostate cancer; breast cancer | [114] |
| CCT251236 | Bisamide | ovarian cancer | [112] |
| Dorsomorphin | colon cancer; prostate cancer | [115] | |
| IHSF1115 | Thiazole acrylamide | multiple myeloma; breast cancer | [116,117] |
| KNK437 | Benzylidene lactam | colon cancer; squamous cell carcinoma; breast cancer | [117,118,119] |
| KRIBB11 | Pyridinediamine | multiple myeloma; lung cancer | [41,117,120,121,122] |
| NZ28 | Emetine | myeloma; prostate cancer; lung cancer; breast cancer | [97,123] |
| NXP800 (CCT361814) | Bisamide | multiple myeloma; solid tumor (under clinical trial) | [109,110,111] |
| NZ28 | Emetine | myeloma; prostate cancer; lung cancer; breast cancer | [97,123] |
| PW3405 | Anthraquinone | HeLa cancer cells | [113,117] |
| Quercetin | plant pigment (flavonoid) | liver and breast cancer | [124] |
| Rohinitib (rocaglamide/rocaglates) | Flavaglines; | leukemia | [79,125] |
| SISU-102 (DTHIB) | prostate cancer; leukemia | [31,108] | |
| SNS-032 | Sulfur compounds | leukemia | [126,127,128] |
| Triptolide | Tripterygium wilfordii | chronic lymphocytic leukemia; pancreatic cancer; liver cancer multiple myeloma | [129] [130] [131] |
| 2,4-Bis (4-hydroxy benzyl) phenol | Gastrodia elata | lung cancer | [131] |
| 4,6-disubstituted pyrimidines | Aromatic heterocyclic organic compound | osteosarcoma | [128] |
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Morimoto, R.I. The heat shock response: Systems biology of proteotoxic stress in aging and disease. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tu, K.; Fu, Q.; Schmitt, D.C.; Zhou, L.; Lu, N.; Zhao, Y. Multifaceted roles of HSF1 in cancer. Tumour Biol. 2015, 36, 4923–4931. [Google Scholar] [CrossRef]
- Dai, C.; Sampson, S.B. HSF1: Guardian of Proteostasis in Cancer. Trends Cell Biol. 2016, 26, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Labbadia, J.; Morimoto, R.I. Rethinking HSF1 in Stress, Development, and Organismal Health. Trends Cell Biol. 2017, 27, 895–905. [Google Scholar] [CrossRef]
- Chen, F.; Fan, Y.; Cao, P.; Liu, B.; Hou, J.; Zhang, B.; Tan, K. Pan-Cancer Analysis of the Prognostic and Immunological Role of HSF1: A Potential Target for Survival and Immunotherapy. Oxid. Med. Cell. Longev. 2021, 2021, 5551036. [Google Scholar] [CrossRef]
- Wang, G.; Cao, P.; Fan, Y.; Tan, K. Emerging roles of HSF1 in cancer: Cellular and molecular episodes. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188390. [Google Scholar] [CrossRef]
- Mendillo, M.L.; Santagata, S.; Koeva, M.; Bell, G.W.; Hu, R.; Tamimi, R.M.; Fraenkel, E.; Ince, T.A.; Whitesell, L.; Lindquist, S. HSF1 Drives a Transcriptional Program Distinct from Heat Shock to Support Highly Malignant Human Cancers. Cell 2012, 150, 549–562. [Google Scholar] [CrossRef]
- Wan, T.; Shao, J.; Hu, B.; Liu, G.; Luo, P.; Zhou, Y. Prognostic role of HSF1 overexpression in solid tumors: A pooled analysis of 3,159 patients. Onco Targets Ther. 2018, 11, 383–393. [Google Scholar] [CrossRef]
- Dong, B.; Jaeger, A.M.; Thiele, D.J. Inhibiting Heat Shock Factor 1 in Cancer: A Unique Therapeutic Opportunity. Trends Pharmacol. Sci. 2019, 40, 986–1005. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. Regulation of the mammalian heat shock factor 1. FEBS J. 2017, 284, 1606–1627. [Google Scholar] [CrossRef] [PubMed]
- Neudegger, T.; Verghese, J.; Hayer-Hartl, M.; Hartl, F.U.; Bracher, A. Structure of human heat-shock transcription factor 1 in complex with DNA. Nat. Struct. Mol. Biol. 2016, 23, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef]
- Kmiecik, S.W.; Mayer, M.P. Molecular mechanisms of heat shock factor 1 regulation. Trends Biochem. Sci. 2022, 47, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Ravarani, C.N.; Erkina, T.Y.; De Baets, G.; Dudman, D.C.; Erkine, A.M.; Babu, M.M. High-throughput discovery of functional disordered regions: Investigation of transactivation domains. Mol. Syst. Biol. 2018, 14, e8190. [Google Scholar] [CrossRef]
- Pincus, D. Regulation of Hsf1 and the Heat Shock Response. Adv. Exp. Med. Biol. 2020, 1243, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kryczek, I.; Nam, J.; Li, X.; Li, S.; Li, J.; Wei, S.; Grove, S.; Vatan, L.; Zhou, J.; et al. LIMIT is an immunogenic lncRNA in cancer immunity and immunotherapy. Nat. Cell Biol. 2021, 23, 526–537. [Google Scholar] [CrossRef]
- Masser, A.E.; Ciccarelli, M.; Andreasson, C. Hsf1 on a leash—Controlling the heat shock response by chaperone titration. Exp. Cell Res. 2020, 396, 112246. [Google Scholar] [CrossRef]
- Guettouche, T.; Boellmann, F.; Lane, W.S.; Voellmy, R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005, 6, 4. [Google Scholar] [CrossRef]
- Holmes, B.; Benavides-Serrato, A.; Freeman, R.S.; Landon, K.A.; Bashir, T.; Nishimura, R.N.; Gera, J. mTORC2/AKT/HSF1/HuR constitute a feed-forward loop regulating Rictor expression and tumor growth in glioblastoma. Oncogene 2018, 37, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Takii, R.; Okada, M.; Fujimoto, M.; Nakai, A. MED12 interacts with the heat-shock transcription factor HSF1 and recruits CDK8 to promote the heat-shock response in mammalian cells. FEBS Lett. 2021, 595, 1933–1948. [Google Scholar] [CrossRef]
- Chou, S.D.; Prince, T.; Gong, J.; Calderwood, S.K. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS ONE 2012, 7, e39679. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lee, F.L.; Peng, S.F.; Lin, K.H.; Chen, R.J.; Ho, T.J.; Tsai, F.J.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. HSF1 phosphorylation by ERK/GSK3 suppresses RNF126 to sustain IGF-IIR expression for hypertension-induced cardiomyocyte hypertrophy. J. Cell. Physiol. 2018, 233, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Loew, C.; Korner, R.; Pinkert, S.; Theis, M.; Hayer-Hartl, M.; Buchholz, F.; Hartl, F.U. Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell 2014, 156, 975–985. [Google Scholar] [CrossRef]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M., Jr.; Sistonen, L.; Morimoto, R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, S.W.; Le Breton, L.; Mayer, M.P. Feedback regulation of heat shock factor 1 (Hsf1) activity by Hsp70-mediated trimer unzipping and dissociation from DNA. EMBO J. 2020, 39, e104096. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhou, M.; Liu, H.; Ding, Y.; Khong, H.T.; Yu, D.; Fodstad, O.; Tan, M. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene 2009, 28, 3689–3701. [Google Scholar] [CrossRef]
- Desai, S.; Liu, Z.; Yao, J.; Patel, N.; Chen, J.; Wu, Y.; Ahn, E.E.; Fodstad, O.; Tan, M. Heat shock factor 1 (HSF1) controls chemoresistance and autophagy through transcriptional regulation of autophagy-related protein 7 (ATG7). J. Biol. Chem. 2013, 288, 9165–9176. [Google Scholar] [CrossRef]
- Dong, B.; Jaeger, A.M.; Hughes, P.F.; Loiselle, D.R.; Hauck, J.S.; Fu, Y.; Haystead, T.A.; Huang, J.; Thiele, D.J. Targeting therapy-resistant prostate cancer via a direct inhibitor of the human heat shock transcription factor 1. Sci. Transl. Med. 2020, 12, eabb5647. [Google Scholar] [CrossRef]
- Engerud, H.; Tangen, I.L.; Berg, A.; Kusonmano, K.; Halle, M.K.; Oyan, A.M.; Kalland, K.H.; Stefansson, I.; Trovik, J.; Salvesen, H.B.; et al. High level of HSF1 associates with aggressive endometrial carcinoma and suggests potential for HSP90 inhibitors. Br. J. Cancer 2014, 111, 78–84. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Society, A.C. Cancer Facts & Figures 2021; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- He, L.; Lv, S.; Ma, X.; Jiang, S.; Zhou, F.; Zhang, Y.; Yu, R.; Zhao, Y. ErbB2 promotes breast cancer metastatic potential via HSF1/LDHA axis-mediated glycolysis. Med. Oncol. 2022, 39, 45. [Google Scholar] [CrossRef]
- Vydra, N.; Janus, P.; Kus, P.; Stokowy, T.; Mrowiec, K.; Toma-Jonik, A.; Krzywon, A.; Cortez, A.J.; Wojtas, B.; Gielniewski, B.; et al. Heat shock factor 1 (HSF1) cooperates with estrogen receptor α (ERα) in the regulation of estrogen action in breast cancer cells. eLife 2021, 10, e69843. [Google Scholar] [CrossRef] [PubMed]
- Vydra, N.; Janus, P.; Toma-Jonik, A.; Stokowy, T.; Mrowiec, K.; Korfanty, J.; Długajczyk, A.; Wojtaś, B.; Gielniewski, B.; Widłak, W. 17β-Estradiol Activates HSF1 via MAPK Signaling in ERα-Positive Breast Cancer Cells. Cancers 2019, 11, 1533. [Google Scholar] [CrossRef]
- Liu, K.; Ma, R. MicroRNA-615-5p regulates the proliferation and apoptosis of breast cancer cells by targeting HSF1. Exp. Ther. Med. 2021, 21, 192. [Google Scholar] [CrossRef]
- Yang, W.; Feng, B.; Meng, Y.; Wang, J.; Geng, B.; Cui, Q.; Zhang, H.; Yang, Y.; Yang, J. FAM3C-YY1 axis is essential for TGFbeta-promoted proliferation and migration of human breast cancer MDA-MB-231 cells via the activation of HSF1. J. Cell. Mol. Med. 2019, 23, 3464–3475. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ortega, M.; Carrera, A.C.; Garrido, A. Role of NRF2 in Lung Cancer. Cells 2021, 10, 1879. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, J.; Lee, Y.J.; Kim, S.K.; Kim, J.A.; Kim, B.K.; Park, K.C.; Kwon, B.M.; Han, D.C. Targeting HSF1 as a Therapeutic Strategy for Multiple Mechanisms of EGFR Inhibitor Resistance in EGFR Mutant Non-Small-Cell Lung Cancer. Cancers 2021, 13, 2987. [Google Scholar] [CrossRef]
- Dai, C.; Whitesell, L.; Rogers, A.B.; Lindquist, S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 2007, 130, 1005–1018. [Google Scholar] [CrossRef]
- Hoj, J.P.; Mayro, B.; Pendergast, A.M. The ABL2 kinase regulates an HSF1-dependent transcriptional program required for lung adenocarcinoma brain metastasis. Proc. Natl. Acad. Sci. USA 2020, 117, 33486–33495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, E.Y.; Kim, O.; Schilder, J.M.; Coffey, D.M.; Cho, C.H.; Bast, R.C., Jr. Cell Origins of High-Grade Serous Ovarian Cancer. Cancers 2018, 10, 433. [Google Scholar] [CrossRef]
- Wilson, A.L.; Moffitt, L.R.; Duffield, N.; Rainczuk, A.; Jobling, T.W.; Plebanski, M.; Stephens, A.N. Autoantibodies against HSF1 and CCDC155 as Biomarkers of Early-Stage, High-Grade Serous Ovarian Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 183–192. [Google Scholar] [CrossRef]
- Moody, R.; Wilson, K.; Kampan, N.C.; McNally, O.M.; Jobling, T.W.; Jaworowski, A.; Stephens, A.N.; Plebanski, M. Mapping Epitopes Recognised by Autoantibodies Shows Potential for the Diagnosis of High-Grade Serous Ovarian Cancer and Monitoring Response to Therapy for This Malignancy. Cancers 2021, 13, 4201. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, B.; Tang, D.; Bowden, N.A. Biomarkers of platinum resistance in ovarian cancer: What can we use to improve treatment. Endocr. Relat. Cancer 2018, 25, R303–R318. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, N.; Ranftl, R.; Chicherova, I.; Slaven, N.D.; Moeendarbary, E.; Farrugia, A.J.; Lam, M.; Semiannikova, M.; Westergaard, M.C.W.; Tchou, J.; et al. Dickkopf-3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer-associated fibroblasts. Nat. Commun. 2019, 10, 130. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Polycystic Ovary Syndrome. ACOG Pract. Bull. 2018, 131, 157–171. [Google Scholar]
- Hu, G.; Zhang, J.; Zhou, X.; Liu, J.; Wang, Q.; Zhang, B. Roles of estrogen receptor α and β in the regulation of proliferation in endometrial carcinoma. Pathol. Res. Pract. 2020, 216, 153149. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef]
- Guha, P.; Sen, K.; Chowdhury, P.; Mukherjee, D. Estrogen receptors as potential therapeutic target in endometrial cancer. J. Recept. Signal Transduct. 2023, 43, 19–26. [Google Scholar] [CrossRef]
- Ranhotra, H.S. The estrogen-related receptors in metabolism and cancer: Newer insights. J. Recept. Signal Transduct. Res. 2018, 38, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.; Tav, C.; Berube-Simard, F.A.; Cuppens, T.; Leclercq, M.; Fournier, E.; Cote, M.C.; Droit, A.; Bilodeau, S. Modulating HSF1 levels impacts expression of the estrogen receptor alpha and antiestrogen response. Life Sci. Alliance 2021, 4, e202000811. [Google Scholar] [CrossRef] [PubMed]
- Schatten, H. Brief Overview of Prostate Cancer Statistics, Grading, Diagnosis and Treatment Strategies; Part of the Advances in Experimental Medicine and Biology book series (AEMB, volume 1095). In Cell & Molecular Biology of Prostate Cancer; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–14. [Google Scholar] [CrossRef]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef]
- Björk, J.K.; Ahonen, I.; Mirtti, T.; Erickson, A.; Rannikko, A.; Bützow, A.; Nordling, S.; Lundin, J.; Lundin, M.; Sistonen, L.; et al. Increased HSF1 expression predicts shorter disease-specific survival of prostate cancer patients following radical prostatectomy. Oncotarget 2018, 9, 31200–31213. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, M.; Beraldi, E.; Cong, M.; Zoubeidi, A.; Gleave, M.; Peng, L. A Novel Triazole Nucleoside Suppresses Prostate Cancer Cell Growth by Inhibiting Heat Shock Factor 1 and Androgen Receptor. Anti-Cancer Agents Med. Chem. 2015, 15, 1333–1340. [Google Scholar] [CrossRef]
- Wyatt, A.W.; Gleave, M.E. Targeting the adaptive molecular landscape of castration-resistant prostate cancer. EMBO Mol. Med. 2015, 7, 878–894. [Google Scholar] [CrossRef]
- Moses, M.A.; Kim, Y.S.; Rivera-Marquez, G.M.; Oshima, N.; Watson, M.J.; Beebe, K.E.; Wells, C.; Lee, S.; Zuehlke, A.D.; Shao, H.; et al. Targeting the Hsp40/Hsp70 Chaperone Axis as a Novel Strategy to Treat Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 4022–4035. [Google Scholar] [CrossRef]
- Fang, F.; Chang, R.; Yang, L. Heat shock factor 1 promotes invasion and metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer 2012, 118, 1782–1794. [Google Scholar] [CrossRef]
- Shen, Z.; Yin, L.; Zhou, H.; Ji, X.; Jiang, C.; Zhu, X.; He, X. Combined inhibition of AURKA and HSF1 suppresses proliferation and promotes apoptosis in hepatocellular carcinoma by activating endoplasmic reticulum stress. Cell. Oncol. 2021, 44, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hu, J.; Jin, R.; Cheng, H.; Chen, H.; Li, L.; Guo, K. Effects of LRP1B Regulated by HSF1 on Lipid Metabolism in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2020, 7, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Huang, D.A.; Li, M.M.; Liu, H.D.; Guo, K. HSF1: A mediator in metabolic alteration of hepatocellular carcinoma cells in cross-talking with tumor-associated macrophages. Am. J. Transl. Res. 2019, 11, 5054–5064. [Google Scholar]
- Zhang, N.; Wu, Y.; Lyu, X.; Li, B.; Yan, X.; Xiong, H.; Li, X.; Huang, G.; Zeng, Y.; Zhang, Y.; et al. HSF1 upregulates ATG4B expression and enhances epirubicin-induced protective autophagy in hepatocellular carcinoma cells. Cancer Lett. 2017, 409, 81–90. [Google Scholar] [CrossRef]
- Santagata, S.; Hu, R.; Lin, N.U.; Mendillo, M.L.; Collins, L.C.; Hankinson, S.E.; Schnitt, S.J.; Whitesell, L.; Tamimi, R.M.; Lindquist, S.; et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 18378–18383. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ren, C.; Lu, C.; Qiao, P.; Han, X.; Wang, L.; Wang, D.; Lv, S.; Sun, Y.; Yu, Z. Phosphorylation of HSF1 by PIM2 Induces PD-L1 Expression and Promotes Tumor Growth in Breast Cancer. Cancer Res. 2019, 79, 5233–5244. [Google Scholar] [CrossRef]
- Yun, H.H.; Baek, J.Y.; Seo, G.; Kim, Y.S.; Ko, J.H.; Lee, J.H. Effect of BIS depletion on HSF1-dependent transcriptional activation in A549 non-small cell lung cancer cells. Korean J. Physiol. Pharmacol. 2018, 22, 457–465. [Google Scholar] [CrossRef]
- Song, P.; Feng, L.; Li, J.; Dai, D.; Zhu, L.; Wang, C.; Li, J.; Li, L.; Zhou, Q.; Shi, R.; et al. β-catenin represses miR455-3p to stimulate m6A modification of HSF1 mRNA and promote its translation in colorectal cancer. Mol. Cancer 2020, 19, 129. [Google Scholar] [CrossRef]
- Cen, H.; Zheng, S.; Fang, Y.M.; Tang, X.P.; Dong, Q. Induction of HSF1 expression is associated with sporadic colorectal cancer. World J. Gastroenterol. 2004, 10, 3122–3126. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Santagata, S.; Mendillo, M.L.; Sholl, L.M.; Ben-Aharon, I.; Beck, A.H.; Dias-Santagata, D.; Koeva, M.; Stemmer, S.M.; Whitesell, L.; et al. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell 2014, 158, 564–578. [Google Scholar] [CrossRef]
- Heng, B.C.; Zhang, X.; Aubel, D.; Bai, Y.; Li, X.; Wei, Y.; Fussenegger, M.; Deng, X. An overview of signaling pathways regulating YAP/TAZ activity. Cell. Mol. Life Sci. 2021, 78, 497–512. [Google Scholar] [CrossRef]
- Kijima, T.; Prince, T.L.; Tigue, M.L.; Yim, K.H.; Schwartz, H.; Beebe, K.; Lee, S.; Budzynski, M.A.; Williams, H.; Trepel, J.B.; et al. HSP90 inhibitors disrupt a transient HSP90-HSF1 interaction and identify a noncanonical model of HSP90-mediated HSF1 regulation. Sci. Rep. 2018, 8, 6976. [Google Scholar] [CrossRef] [PubMed]
- Workman, P. Reflections and Outlook on Targeting HSP90, HSP70 and HSF1 in Cancer: A Personal Perspective. Adv. Exp. Med. Biol. 2020, 1243, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Cyran, A.M.; Zhitkovich, A. Heat Shock Proteins and HSF1 in Cancer. Front. Oncol. 2022, 12, 860320. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.; Gumilar, K.E.; Li, X.G.; Tjokroprawiro, B.A.; Lu, C.H.; Lu, J.; Zhou, M.; Sobol, W.; Tan, M. Targeting HSF1 for cancer treatment: Mechanisms and inhibitor development. Theranostic 2023, 13, 2281. [Google Scholar] [CrossRef]
- Santagata, S.; Mendillo, M.L.; Tang, Y.C.; Subramanian, A.; Perley, C.C.; Roche, S.P.; Wong, B.; Narayan, R.; Kwon, H.; Koeva, M.; et al. Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science 2013, 341, 1238303. [Google Scholar] [CrossRef]
- Chang, Z.; Lu, M.; Park, S.M.; Park, H.K.; Kang, H.S.; Pak, Y.; Park, J.S. Functional HSF1 requires aromatic-participant interactions in protecting mouse embryonic fibroblasts against apoptosis via G2 cell cycle arrest. Mol. Cells 2012, 33, 465–470. [Google Scholar] [CrossRef]
- Luft, J.C.; Benjamin, I.J.; Mestril, R.; Dix, D.J. Heat shock factor 1-mediated thermotolerance prevents cell death and results in G2/M cell cycle arrest. Cell Stress Chaperones 2001, 6, 326–336. [Google Scholar] [CrossRef]
- Bruce, J.L.; Chen, C.; Xie, Y.; Zhong, R.; Wang, Y.Q.; Stevenson, M.A.; Calderwood, S.K. Activation of heat shock transcription factor 1 to a DNA binding form during the G(1)phase of the cell cycle. Cell Stress Chaperones 1999, 4, 36–45. [Google Scholar] [CrossRef]
- Fujimoto, M.; Takii, R.; Takaki, E.; Katiyar, A.; Nakato, R.; Shirahige, K.; Nakai, A. The HSF1-PARP13-PARP1 complex facilitates DNA repair and promotes mammary tumorigenesis. Nat. Commun. 2017, 8, 1638. [Google Scholar] [CrossRef]
- Shi, X.; Deng, Z.; Wang, S.; Zhao, S.; Xiao, L.; Zou, J.; Li, T.; Tan, S.; Tan, S.; Xiao, X. Increased HSF1 Promotes Infiltration and Metastasis in Cervical Cancer via Enhancing MTDH-VEGF-C Expression. Onco Targets Ther. 2021, 14, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhou, N.; Yuan, J.; Lu, L.; Zhang, Q.; Wei, M.; Zou, Y.; Yuan, L. Heat shock transcription factor 1 regulates exercise-induced myocardial angiogenesis after pressure overload via HIF-1α/VEGF pathway. J. Cell. Mol. Med. 2020, 24, 2178–2188. [Google Scholar] [CrossRef]
- Gabai, V.L.; Meng, L.; Kim, G.; Mills, T.A.; Benjamin, I.J.; Sherman, M.Y. Heat shock transcription factor Hsf1 is involved in tumor progression via regulation of hypoxia-inducible factor 1 and RNA-binding protein HuR. Mol. Cell. Biol. 2012, 32, 929–940. [Google Scholar] [CrossRef] [PubMed]
- McConnell, J.R.; Buckton, L.K.; McAlpine, S.R. Regulating the master regulator: Controlling heat shock factor 1 as a chemotherapy approach. Bioorg. Med. Chem. Lett. 2015, 25, 3409–3414. [Google Scholar] [CrossRef] [PubMed]
- Mun, G.I.; Choi, E.; Lee, Y.; Lee, Y.S. Decreased expression of FBXW7 by ERK1/2 activation in drug-resistant cancer cells confers transcriptional activation of MDR1 by suppression of ubiquitin degradation of HSF1. Cell Death Dis. 2020, 11, 395. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Vydra, N.; Toma, A.; Glowala-Kosinska, M.; Gogler-Piglowska, A.; Widlak, W. Overexpression of heat shock transcription factor 1 enhances the resistance of melanoma cells to doxorubicin and paclitaxe. BMC Cancer 2013, 13, 504. [Google Scholar] [CrossRef]
- Kourtis, N.; Moubarak, R.S.; Aranda-Orgilles, B.; Lui, K.; Aydin, I.T.; Trimarchi, T.; Darvishian, F.; Salvaggio, C.; Zhong, J.; Bhatt, K.; et al. FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat. Cell Biol. 2015, 17, 322–332. [Google Scholar] [CrossRef]
- Zheng, T. Long noncoding RNA NBAT1 inhibits autophagy via suppression of ATG7 in non-small cell lung cancer. Am. J. Cancer Res. 2018, 8, 1801–1811. [Google Scholar]
- Gabai, V.L.; Budagova, K.R.; Sherman, M.Y. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene 2005, 24, 3328–3338. [Google Scholar] [CrossRef]
- Schilling, D.; Bayer, C.; Li, W.; Molls, M.; Vaupel, P.; Multhoff, G. Radiosensitization of normoxic and hypoxic h1339 lung tumor cells by heat shock protein 90 inhibition is independent of hypoxia inducible factor-1α. PLoS ONE 2012, 7, e31110. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; McLaughlin, M.; Bhide, S.A.; Eccles, S.A.; Workman, P.; Nutting, C.M.; Huddart, R.A.; Harrington, K.J. The HSP90 inhibitor NVP-AUY922 radiosensitizes by abrogation of homologous recombination resulting in mitotic entry with unresolved DNA damage. PLoS ONE 2012, 7, e35436. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Martinez, J.D. Loss of HSF1 results in defective radiation-induced G(2) arrest and DNA repair. Radiat. Res. 2011, 176, 17–24. [Google Scholar] [CrossRef]
- Schilling, D.; Kuhnel, A.; Konrad, S.; Tetzlaff, F.; Bayer, C.; Yaglom, J.; Multhoff, G. Sensitizing tumor cells to radiation by targeting the heat shock response. Cancer Lett. 2015, 360, 294–301. [Google Scholar] [CrossRef]
- Jacobs, A.T.; Marnett, L.J. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J. Biol. Chem. 2009, 284, 9176–9183. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, Y.-S.; Yun, H.H.; Im, C.-N.; Ko, J.-H.; Lee, J.-H. ERK-mediated phosphorylation of BIS regulates nuclear translocation of HSF1 under oxidative stress. Exp. Mol. Med. 2016, 48, e260. [Google Scholar] [CrossRef] [PubMed]
- Janus, P.; Pakuła-Cis, M.; Kalinowska-Herok, M.; Kashchak, N.; Szołtysek, K.; Pigłowski, W.; Widlak, W.; Kimmel, M.; Widlak, P. NF-κB signaling pathway is inhibited by heat shock independently of active transcription factor HSF1 and increased levels of inducible heat shock proteins. Genes Cells 2011, 16, 1168–1175. [Google Scholar] [CrossRef]
- Li, W.; Hu, C.; Zhong, X.; Wu, J.; Li, G. Melatonin Induces AGS Gastric Cancer Cell Apoptosis via Regulating PERK/eIF2α and HSF1/NF-κB Signaling Pathway. Ann. Clin. Lab. Sci. 2022, 52, 40–47. [Google Scholar]
- Li, J.; Liu, Y.; Duan, P.; Yu, R.; Gu, Z.; Li, L.; Liu, Z.; Su, L. NF-κB regulates HSF1 and c-Jun activation in heat stress-induced intestinal epithelial cell apoptosis. Mol. Med. Rep. 2018, 17, 3388–3396. [Google Scholar] [CrossRef]
- Kühnel, A.; Schilling, D.; Combs, S.E.; Haller, B.; Schwab, M.; Multhoff, G. Radiosensitization of HSF-1 Knockdown Lung Cancer Cells by Low Concentrations of Hsp90 Inhibitor NVP-AUY922. Cells 2019, 8, 1166. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Gokmen-Polar, Y. HSF1 as a Cancer Biomarker and Therapeutic Target. Curr. Cancer Drug Targets 2019, 19, 515–524. [Google Scholar] [CrossRef]
- Nytko, K.J.; Thumser-Henner, P.; Russo, G.; Weyland, M.S.; Rohrer Bley, C. Role of HSP70 in response to (thermo)radiotherapy: Analysis of gene expression in canine osteosarcoma cells by RNA-seq. Sci. Rep. 2020, 10, 12779. [Google Scholar] [CrossRef]
- Multhoff, G.; Pockley, A.G.; Schmid, T.E.; Schilling, D. The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett. 2015, 368, 179–184. [Google Scholar] [CrossRef]
- Velayutham, M.; Cardounel, A.J.; Liu, Z.; Ilangovan, G. Discovering a Reliable Heat-Shock Factor-1 Inhibitor to Treat Human Cancers: Potential Opportunity for Phytochemists. Front. Oncol. 2018, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Xiu, Y.; Wang, Y.; Hodgson, C.; Borcherding, N.; Jordan, C.; Buchanan, J.; Taylor, E.; Wagner, B.; Leidinger, M.; et al. HSF1 is a driver of leukemia stem cell self-renewal in acute myeloid leukemia. Nat. Commun. 2022, 13, 6107. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Clarke, P.A.; Te Poele, R.; Powers, M.; Box, G.; De Billy, E.; De Haven Brandon, A.; Hallsworth, A.; Hayes, A.; McCann, H.; et al. Discovery and validation of biomarkers to support clinical development of NXP800: A first-in-class orally active, small-molecule HSF1 pathway inhibitor. Eur. J. Cancer 2022, 174, S35. [Google Scholar] [CrossRef]
- Menezes, K.; Aram, G.; Mirabella, F.; Johnson, D.C.; Sherborne, A.L.; Houlston, R.S.; Cheeseman, M.D.; Pasqua, E.; Clarke, P.; Workman, P.; et al. The Novel Protein HSF1 Stress Pathway Inhibitor Bisamide CCT361814 Demonstrates Pre-Clinical Anti-Tumor Activity in Myeloma. Blood 2017, 130, 3072. [Google Scholar] [CrossRef]
- Diane Marsolini, S.S. A Phase 1 Clinical Study of NXP800 in Subjects With Advanced Cancers. Available online: https://clinicaltrials.gov/ct2/show/NCT05226507?term=HSF1&draw=2&rank=3#contacts (accessed on 2 September 2023).
- Cheeseman, M.D.; Chessum, N.E.; Rye, C.S.; Pasqua, A.E.; Tucker, M.J.; Wilding, B.; Evans, L.E.; Lepri, S.; Richards, M.; Sharp, S.Y.; et al. Discovery of a Chemical Probe Bisamide (CCT251236): An Orally Bioavailable Efficacious Pirin Ligand from a Heat Shock Transcription Factor 1 (HSF1) Phenotypic Screen. J. Med. Chem. 2017, 60, 180–201. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, B. Selective killing of cancer cells by small molecules targeting heat shock stress response. Biochem. Biophys. Res. Commun. 2016, 478, 1509–1514. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, Y.; Kwon, B.M.; Han, D.C. The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J. Biol. Chem. 2013, 288, 28713–28726. [Google Scholar] [CrossRef]
- Li, N.; Wang, T.; Li, Z.; Ye, X.; Deng, B.; Zhuo, S.; Yao, P.; Yang, M.; Mei, H.; Chen, X.; et al. Dorsomorphin induces cancer cell apoptosis and sensitizes cancer cells to HSP90 and proteasome inhibitors by reducing nuclear heat shock factor 1 levels. Cancer Biol. Med. 2019, 16, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Vilaboa, N.; Bore, A.; Martin-Saavedra, F.; Bayford, M.; Winfield, N.; Firth-Clark, S.; Kirton, S.B.; Voellmy, R. New inhibitor targeting human transcription factor HSF1: Effects on the heat shock response and tumor cell survival. Nucleic Acids Res. 2017, 45, 5797–5817. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Seo, Y.H. Small Molecule Inhibitors of HSF1-Activated Pathways as Potential Next-Generation Anticancer Therapeutics. Molecules 2018, 23, 2757. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin. Ther. Targets 2009, 13, 469–478. [Google Scholar] [CrossRef]
- Oommen, D.; Prise, K.M. KNK437, abrogates hypoxia-induced radioresistance by dual targeting of the AKT and HIF-1α survival pathways. Biochem. Biophys. Res. Commun. 2012, 421, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Kim, J.A.; Shin, K.D.; Shin, D.S.; Han, Y.M.; Lee, Y.J.; Lee, J.S.; Kwon, B.M.; Han, D.C. KRIBB11 inhibits HSP70 synthesis through inhibition of heat shock factor 1 function by impairing the recruitment of positive transcription elongation factor b to the hsp70 promoter. J. Biol. Chem. 2011, 286, 1737–1747. [Google Scholar] [CrossRef]
- Kang, M.J.; Yun, H.H.; Lee, J.H. KRIBB11 accelerates Mcl-1 degradation through an HSF1-independent, Mule-dependent pathway in A549 non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2017, 492, 304–309. [Google Scholar] [CrossRef]
- Fok, J.H.L.; Hedayat, S.; Zhang, L.; Aronson, L.I.; Mirabella, F.; Pawlyn, C.; Bright, M.D.; Wardell, C.P.; Keats, J.J.; De Billy, E.; et al. HSF1 Is Essential for Myeloma Cell Survival and A Promising Therapeutic Target. Clin. Cancer Res. 2018, 24, 2395–2407. [Google Scholar] [CrossRef]
- Zaarur, N.; Gabai, V.L.; Porco, J.A., Jr.; Calderwood, S.; Sherman, M.Y. Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res. 2006, 66, 1783–1791. [Google Scholar] [CrossRef]
- Yang, W.; Cui, M.; Lee, J.; Gong, W.; Wang, S.; Fu, J.; Wu, G.; Yan, K. Heat shock protein inhibitor, quercetin, as a novel adjuvant agent to improve radiofrequency ablation-induced tumor destruction and its molecular mechanism. Chin. J. Cancer Res. 2016, 28, 19–28. [Google Scholar] [CrossRef]
- Iwasaki, S.; Floor, S.N.; Ingolia, N.T. Rocaglates convert DEAD-box protein eIF4A into a sequence-selective translational repressor. Nature 2016, 534, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wierda, W.G.; Chubb, S.; Hawtin, R.E.; Fox, J.A.; Keating, M.J.; Gandhi, V.; Plunkett, W. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood 2009, 113, 4637–4645. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, C.; Sun, X.; Shi, X.; Jin, B.; Ding, K.; Yeung, S.C.; Pan, J. Cyclin-dependent kinase 7/9 inhibitor SNS-032 abrogates FIP1-like-1 platelet-derived growth factor receptor alpha and bcr-abl oncogene addiction in malignant hematologic cells. Clin. Cancer Res. 2012, 18, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Rye, C.S.; Chessum, N.E.; Lamont, S.; Pike, K.G.; Faulder, P.; Demeritt, J.; Kemmitt, P.; Tucker, J.; Zani, L.; Cheeseman, M.D.; et al. Discovery of 4,6-disubstituted pyrimidines as potent inhibitors of the heat shock factor 1 (HSF1) stress pathway and CDK9. Medchemcomm 2016, 7, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Home, T.; McGuirk, J.; Rao, R.; Kambhampati, A.Y.; Shi, H.; Dandawate, P.; Saluja, S.P.; McGuirk, J.; Rao, R. Targeting HSF1 disrupts HSP90 chaperone function in chronic lymphocytic leukemia. Oncotarget 2015, 6, 31767. [Google Scholar] [CrossRef]
- Sangwan, V.; Banerjee, S.; Jensen, K.M.; Chen, Z.; Chugh, R.; Dudeja, V.; Vickers, S.M.; Saluja, A.K. Primary and liver metastasis-derived cell lines from KrasG12D; Trp53R172H; Pdx-1 Cre animals undergo apopto-sis in response to triptolide. Pancreas 2015, 44, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Heimberger, T.; Andrulis, M.; Riedel, S.; Stuhmer, T.; Schraud, H.; Beilhack, A.; Bumm, T.; Bogen, B.; Einsele, H.; Bargou, R.C.; et al. The heat shock transcription factor 1 as a potential new therapeutic target in multiple myeloma. Br. J. Haematol. 2013, 160, 465–476. [Google Scholar] [CrossRef]
- Yoon, T.; Kang, G.Y.; Han, A.R.; Seo, E.K.; Lee, Y.S. 2,4-Bis(4-hydroxybenzyl)phenol inhibits heat shock transcription factor 1 and sensitizes lung cancer cells to conventional anticancer modalities. J. Nat. Prod. 2014, 77, 1123–1129. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumilar, K.E.; Chin, Y.; Ibrahim, I.H.; Tjokroprawiro, B.A.; Yang, J.-Y.; Zhou, M.; Gassman, N.R.; Tan, M. Heat Shock Factor 1 Inhibition: A Novel Anti-Cancer Strategy with Promise for Precision Oncology. Cancers 2023, 15, 5167. https://doi.org/10.3390/cancers15215167
Gumilar KE, Chin Y, Ibrahim IH, Tjokroprawiro BA, Yang J-Y, Zhou M, Gassman NR, Tan M. Heat Shock Factor 1 Inhibition: A Novel Anti-Cancer Strategy with Promise for Precision Oncology. Cancers. 2023; 15(21):5167. https://doi.org/10.3390/cancers15215167
Chicago/Turabian StyleGumilar, Khanisyah Erza, Yeh Chin, Ibrahim Haruna Ibrahim, Brahmana A. Tjokroprawiro, Jer-Yen Yang, Ming Zhou, Natalie R. Gassman, and Ming Tan. 2023. "Heat Shock Factor 1 Inhibition: A Novel Anti-Cancer Strategy with Promise for Precision Oncology" Cancers 15, no. 21: 5167. https://doi.org/10.3390/cancers15215167
APA StyleGumilar, K. E., Chin, Y., Ibrahim, I. H., Tjokroprawiro, B. A., Yang, J.-Y., Zhou, M., Gassman, N. R., & Tan, M. (2023). Heat Shock Factor 1 Inhibition: A Novel Anti-Cancer Strategy with Promise for Precision Oncology. Cancers, 15(21), 5167. https://doi.org/10.3390/cancers15215167








