Unveiling the Association between HPV and Pan-Cancers: A Bidirectional Two-Sample Mendelian Randomization Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. HPV-Related Indicators Exposure Data Source
2.3. The Selection Criteria of Instrumental Variables for MR Analysis
2.4. Cancer Outcome Data Source
2.5. Bidirectional Two-Sample MR Analysis
2.6. MR Analyses in Validation Datasets
2.7. Immune Infiltration Analysis in HPV Associated Cancers
2.8. Statistical Analyses
3. Results
3.1. Detailed Information of Screened IVs
3.2. Causal Effect between Plasma Levels of HPV E7 Protein and Cancers
3.3. Validation of MR Analysis Results
3.4. Sensitivity Analysis of the MR Results
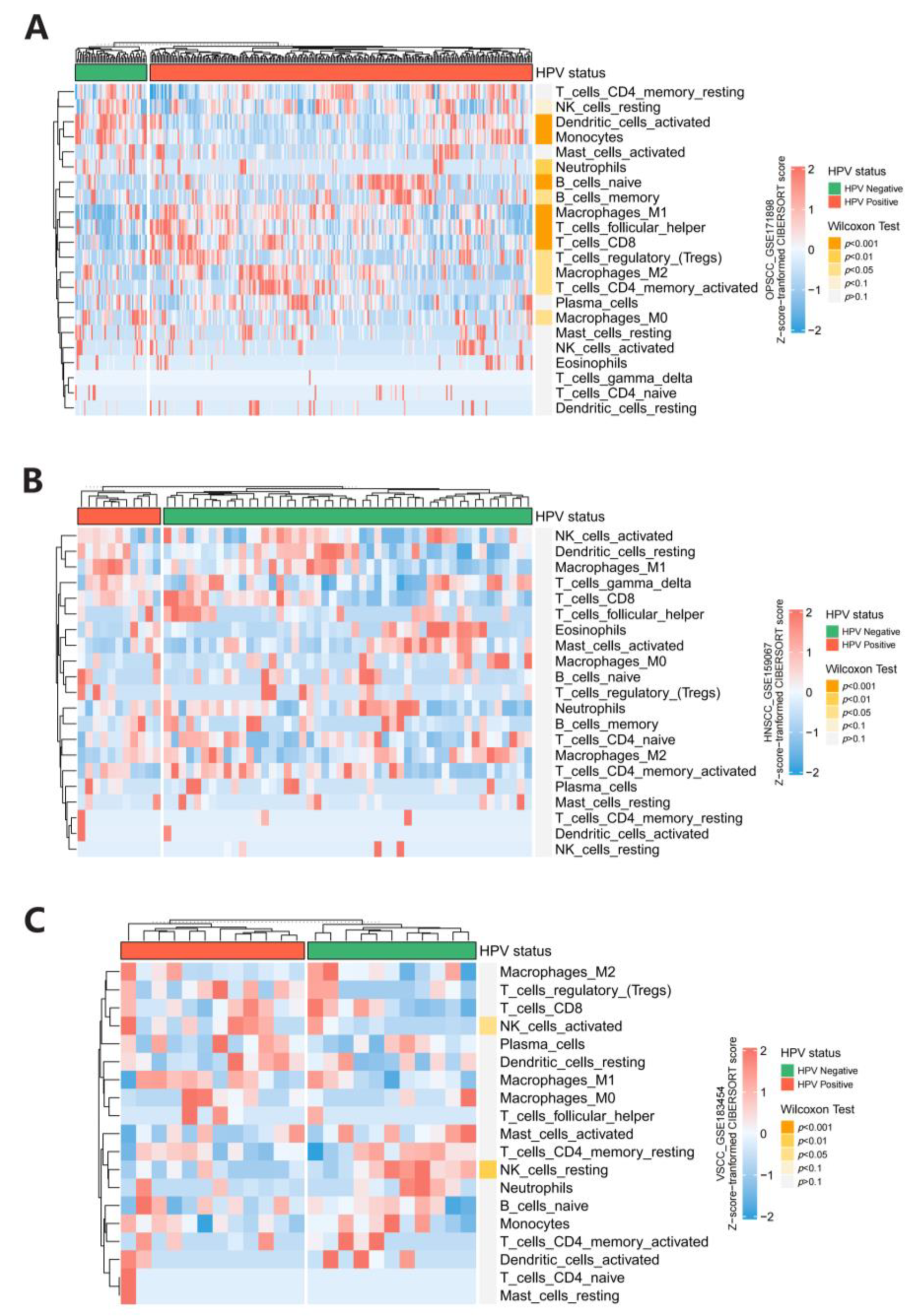
3.5. Immune Infiltration Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ardekani, A.; Sepidarkish, M.; Mollalo, A.; Afradiasbagharani, P.; Rouholamin, S.; Rezaeinejad, M.; Farid-Mojtahedi, M.; Mahjour, S.; Almukhtar, M.; Nourollahpour Shiadeh, M.; et al. Worldwide prevalence of human papillomavirus among pregnant women: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2374. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Beltran, T.H.; Song, J.W.; Klaric, J.; Choi, Y.S. Prevalence of Genital Human Papillomavirus Infection and Human Papillomavirus Vaccination Rates Among US Adult Men: National Health and Nutrition Examination Survey (NHANES) 2013–2014. JAMA Oncol. 2017, 3, 810–816. [Google Scholar] [CrossRef]
- Narisawa-Saito, M.; Kiyono, T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007, 98, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. Human papillomaviruses: Diversity, infection and host interactions. Nat. Rev. Microbiol. 2022, 20, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Sichero, L.; Pierce Campbell, C.M.; Fulp, W.; Ferreira, S.; Sobrinho, J.S.; Baggio, M.; Galan, L.; Silva, R.C.; Lazcano-Ponce, E.; Giuliano, A.R.; et al. High genital prevalence of cutaneous human papillomavirus DNA on male genital skin: The HPV Infection in Men Study. BMC Infect. Dis. 2014, 14, 677. [Google Scholar] [CrossRef] [PubMed]
- Malagutti, N.; Rotondo, J.C.; Cerritelli, L.; Melchiorri, C.; De Mattei, M.; Selvatici, R.; Oton-Gonzalez, L.; Stomeo, F.; Mazzoli, M.; Borin, M.; et al. High Human Papillomavirus DNA loads in Inflammatory Middle Ear Diseases. Pathogens 2020, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Harden, M.E.; Munger, K. Human papillomavirus molecular biology. Mutat. Res. Rev. Mutat. Res. 2017, 772, 3–12. [Google Scholar] [CrossRef]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Castellsagué, X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol. Oncol. 2008, 110 (Suppl. 2), S4–S7. [Google Scholar] [CrossRef]
- Gravitt, P.E. The known unknowns of HPV natural history. J. Clin. Investig. 2011, 121, 4593–4599. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; De Santi, G.; Rampulla, V.; Ghidini, A.; Mercurio, P.; Mariani, M.; Manara, M.; Rausa, E.; Lonati, V.; Viti, M.; et al. Human papillomavirus (HPV) types 16 and 18 infection and esophageal squamous cell carcinoma: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, K.A.; Chen, J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef]
- Wang, J.; Aldabagh, B.; Yu, J.; Arron, S.T. Role of human papillomavirus in cutaneous squamous cell carcinoma: A meta-analysis. J. Am. Acad. Dermatol. 2014, 70, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.; Carrillo-Beltrán, D.; Muñoz, J.P.; Corvalán, A.H.; Calaf, G.M.; Aguayo, F. Human Papillomavirus in Breast Carcinogenesis: A Passenger, a Cofactor, or a Causal Agent? Biology 2021, 10, 804. [Google Scholar] [CrossRef]
- Gong, X.; Chi, H.; Xia, Z.; Yang, G.; Tian, G. Advances in HPV-associated tumor management: Therapeutic strategies and emerging insights. J. Med. Virol. 2023, 95, e28950. [Google Scholar] [CrossRef] [PubMed]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Lawlor, D.A. Commentary: Two-sample Mendelian randomization: Opportunities and challenges. Int. J. Epidemiol. 2016, 45, 908–915. [Google Scholar] [CrossRef]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017, 8, 14357. [Google Scholar] [CrossRef]
- Wichmann, H.E.; Gieger, C.; Illig, T. KORA-gen--resource for population genetics, controls and a broad spectrum of disease phenotypes. Gesundheitswesen 2005, 67 (Suppl. 1), S26–S30. [Google Scholar] [CrossRef]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef] [PubMed]
- Sollis, E.; Mosaku, A.; Abid, A.; Buniello, A.; Cerezo, M.; Gil, L.; Groza, T.; Güneş, O.; Hall, P.; Hayhurst, J.; et al. The NHGRI-EBI GWAS Catalog: Knowledgebase and deposition resource. Nucleic Acids Res. 2023, 51, D977–D985. [Google Scholar] [CrossRef] [PubMed]
- Mainali, B.; Schabath, M.B.; Sudenga, S.L.; Ye, Y.; Wiener, H.W.; Villa, L.L.; Giuliano, A.R.; Shrestha, S. Variants in immune-related genes and genital HPV 16 persistence in men. Papillomavirus Res. 2019, 7, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef]
- Gill, D.; Efstathiadou, A.; Cawood, K.; Tzoulaki, I.; Dehghan, A. Education protects against coronary heart disease and stroke independently of cognitive function: Evidence from Mendelian randomization. Int. J. Epidemiol. 2019, 48, 1468–1477. [Google Scholar] [CrossRef]
- Levin, M.G.; Judy, R.; Gill, D.; Vujkovic, M.; Verma, S.S.; Bradford, Y.; Ritchie, M.D.; Hyman, M.C.; Nazarian, S.; Rader, D.J.; et al. Genetics of height and risk of atrial fibrillation: A Mendelian randomization study. PLoS Med. 2020, 17, e1003288. [Google Scholar] [CrossRef]
- Palmer, T.M.; Lawlor, D.A.; Harbord, R.M.; Sheehan, N.A.; Tobias, J.H.; Timpson, N.J.; Davey Smith, G.; Sterne, J.A.C. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 2012, 21, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, Y.; Yang, H.; Lin, A.; Dong, M.; Wang, H.; Zhang, C.; Liu, Z.; Cheng, Q.; Zhang, J.; et al. HPVTIMER: A shiny web application for tumor immune estimation in human papillomavirus-associated cancers. iMeta 2023, 2, e130. [Google Scholar] [CrossRef]
- zur Hausen, H. Condylomata acuminata and human genital cancer. Cancer Res. 1976, 36 Pt 2, 794. [Google Scholar]
- Roman, A.; Munger, K. The papillomavirus E7 proteins. Virology 2013, 445, 138–168. [Google Scholar] [CrossRef]
- Brimer, N.; Drews, C.M.; Vande Pol, S.B. Association of papillomavirus E6 proteins with either MAML1 or E6AP clusters E6 proteins by structure, function, and evolutionary relatedness. PLoS Pathog. 2017, 13, e1006781. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Han, F.; Shi, C.; Huang, Y.; Liu, Y.; Chang, X. Immunohistochemical detection of E7 human papillomavirus protein in pre-malignant and malignant lesions of the uterine cervix. Mol. Med. Rep. 2018, 18, 3949–3956. [Google Scholar] [CrossRef]
- Hammer, A.; de Koning, M.N.; Blaakaer, J.; Steiniche, T.; Doorbar, J.; Griffin, H.; Mejlgaard, E.; Svanholm, H.; Quint, W.G.; Gravitt, P.E. Whole tissue cervical mapping of HPV infection: Molecular evidence for focal latent HPV infection in humans. Papillomavirus Res. 2019, 7, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Oyouni, A.A.A. Human papillomavirus in cancer: Infection, disease transmission, and progress in vaccines. J. Infect. Public Health 2023, 16, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Chiou, H.L.; Sheu, G.T.; Hsieh, L.L.; Chen, J.T.; Chen, C.Y.; Su, J.M.; Lee, H. The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res. 2001, 61, 2799–2803. [Google Scholar]
- Khatami, A.; Salavatiha, Z.; Razizadeh, M.H. Bladder cancer and human papillomavirus association: A systematic review and meta-analysis. Infect. Agent Cancer 2022, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Muresu, N.; Di Lorenzo, B.; Saderi, L.; Sechi, I.; Del Rio, A.; Piana, A.; Sotgiu, G. Prevalence of Human Papilloma Virus Infection in Bladder Cancer: A Systematic Review. Diagnostics 2022, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Effert, P.J.; Frye, R.A.; Neubauer, A.; Liu, E.T.; Walther, P.J. Human papillomavirus types 16 and 18 are not involved in human prostate carcinogenesis: Analysis of archival human prostate cancer specimens by differential polymerase chain reaction. J. Urol. 1992, 147, 192–196. [Google Scholar] [CrossRef]
- Tachezy, R.; Hrbacek, J.; Heracek, J.; Salakova, M.; Smahelova, J.; Ludvikova, V.; Svec, A.; Urban, M.; Hamsikova, E. HPV persistence and its oncogenic role in prostate tumors. J. Med. Virol. 2012, 84, 1636–1645. [Google Scholar] [CrossRef]
- Guerendiain, D.; Mühr, L.S.A.; Grigorescu, R.; Holden, M.T.G.; Cuschieri, K. Mapping HPV 16 Sub-Lineages in Anal Cancer and Implications for Disease Outcomes. Diagnostics 2022, 12, 3222. [Google Scholar] [CrossRef]
- Guerendiain, D.; Grigorescu, R.; Kirk, A.; Stevenson, A.; Holden, M.T.G.; Pan, J.; Kavanagh, K.; Graham, S.V.; Cuschieri, K. HPV status and HPV16 viral load in anal cancer and its association with clinical outcome. Cancer Med. 2022, 11, 4193–4203. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.-Z.; Waterboer, T.; Castro, F.A.; Brenner, H. Viral infections and colorectal cancer: A systematic review of epidemiological studies. Int. J. Cancer 2015, 137, 12–24. [Google Scholar] [CrossRef]
- Kudela, E.; Kudelova, E.; Kozubík, E.; Rokos, T.; Pribulova, T.; Holubekova, V.; Biringer, K. HPV-Associated Breast Cancer: Myth or Fact? Pathogens 2022, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, P.; Luo, G.; Liu, D.; Zou, H. Cancer attributable to human papillomavirus infection in China: Burden and trends. Cancer 2020, 126, 3719–3732. [Google Scholar] [CrossRef]
- Rasmussen, C.L.; Bertoli, H.K.; Sand, F.L.; Kjaer, A.K.; Thomsen, L.T.; Kjaer, S.K. The prognostic significance of HPV, p16, and p53 protein expression in vaginal cancer: A systematic review. Acta Obstet. Gynecol. Scand. 2021, 100, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Whitmarsh, A.; Pring, M.; Thomas, S.J.; Waylen, A.; Ness, A.R.; Dudding, T.; Pawlita, M.; Brenner, N.; Waterboer, T.; Schroeder, L. Survival advantage in patients with human papillomavirus-driven oropharyngeal cancer and variation by demographic characteristics and serologic response: Findings from Head and Neck 5000. Cancer 2021, 127, 2442–2452. [Google Scholar] [CrossRef]
- Kimple, R.J.; Harari, P.M. The prognostic value of HPV in head and neck cancer patients undergoing postoperative chemoradiotherapy. Ann. Transl. Med. 2015, 3 (Suppl. 1), S14. [Google Scholar] [CrossRef]
- Ji, M.; Lin, L.; Huang, Q.; Hu, C.; Zhang, M. HPV16 status might correlate to increasing tumor-infiltrating lymphocytes in hypopharyngeal cancer. Acta Otolaryngol. 2023, 143, 543–550. [Google Scholar] [CrossRef]

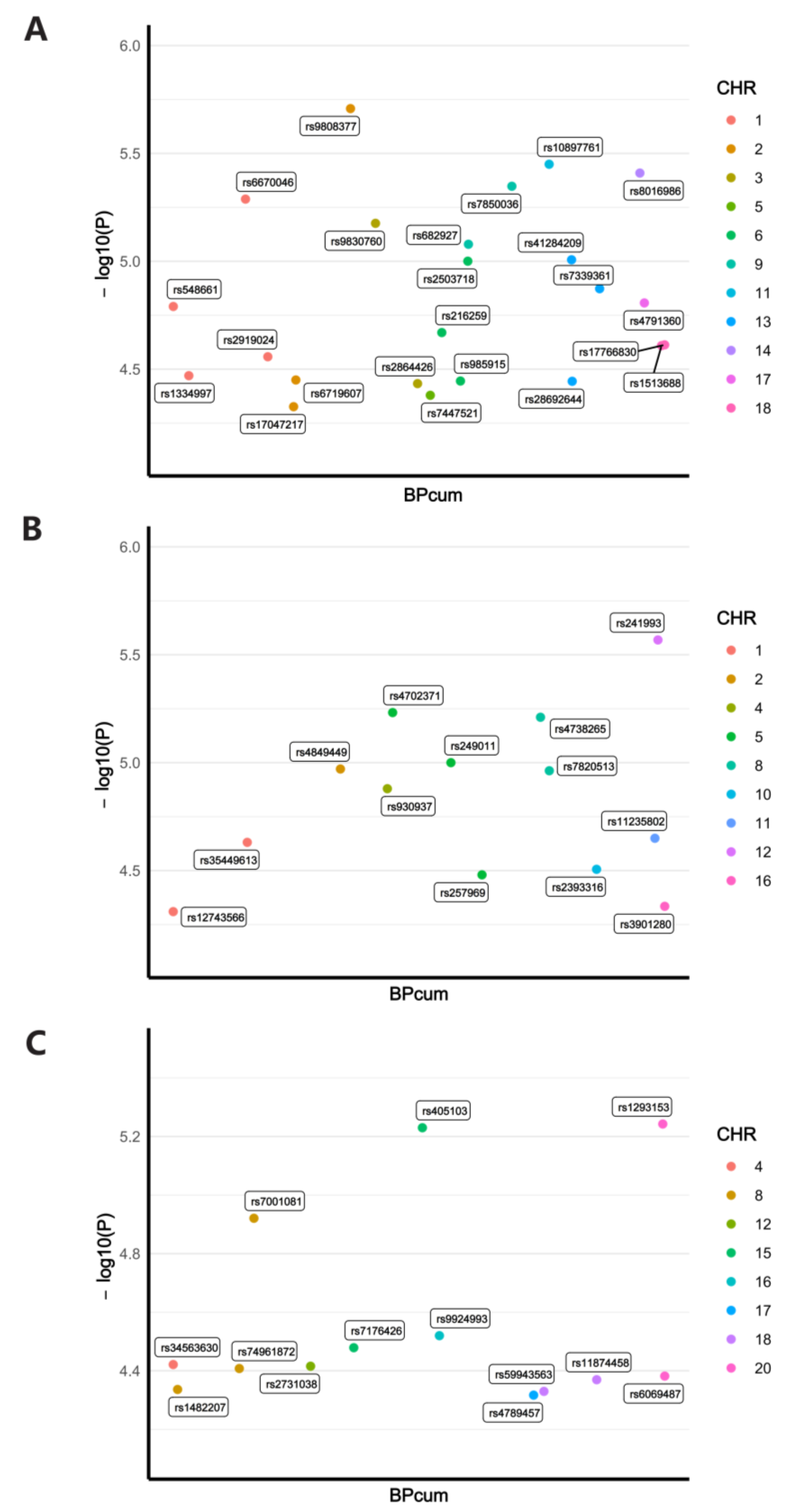
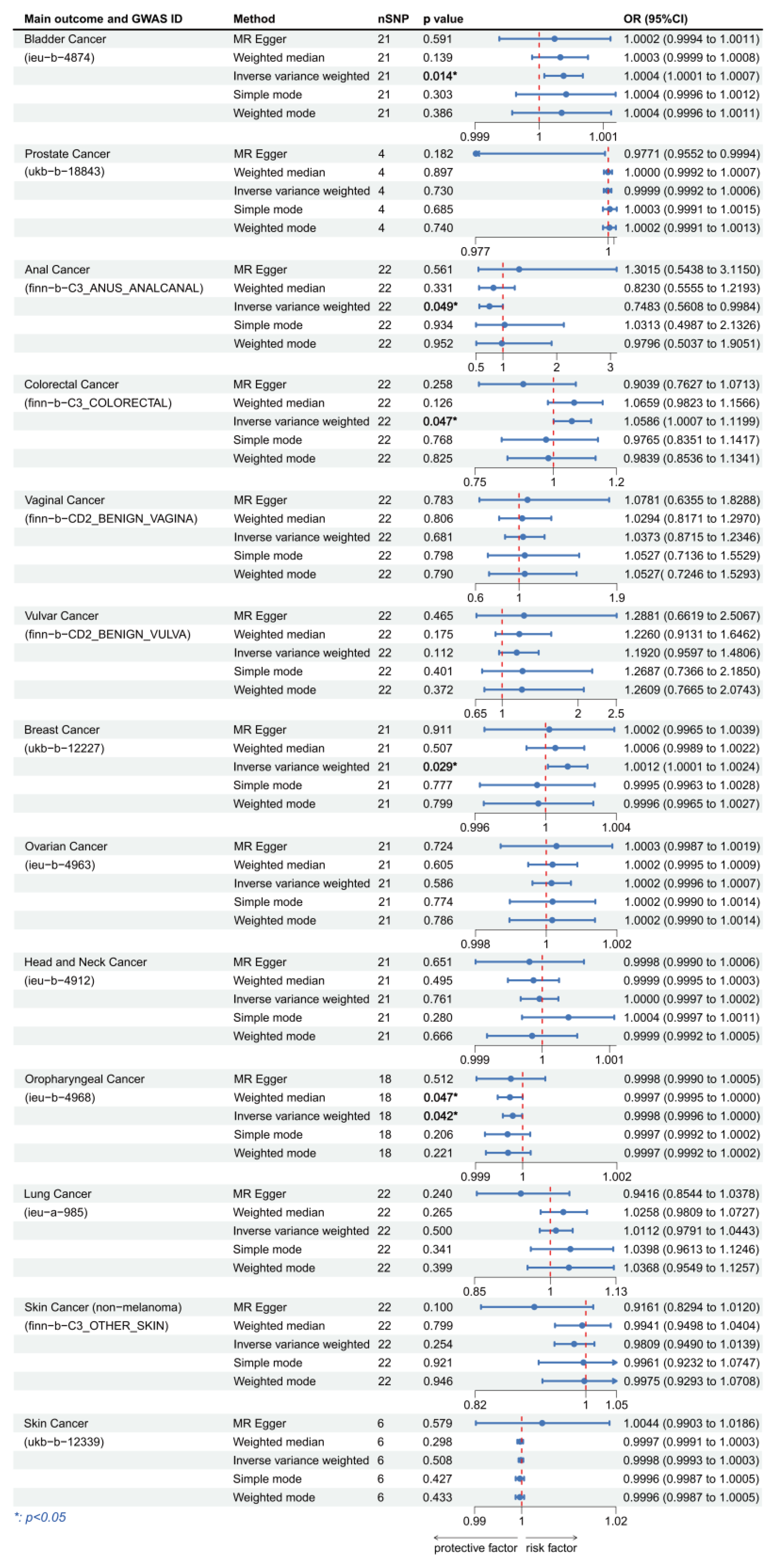

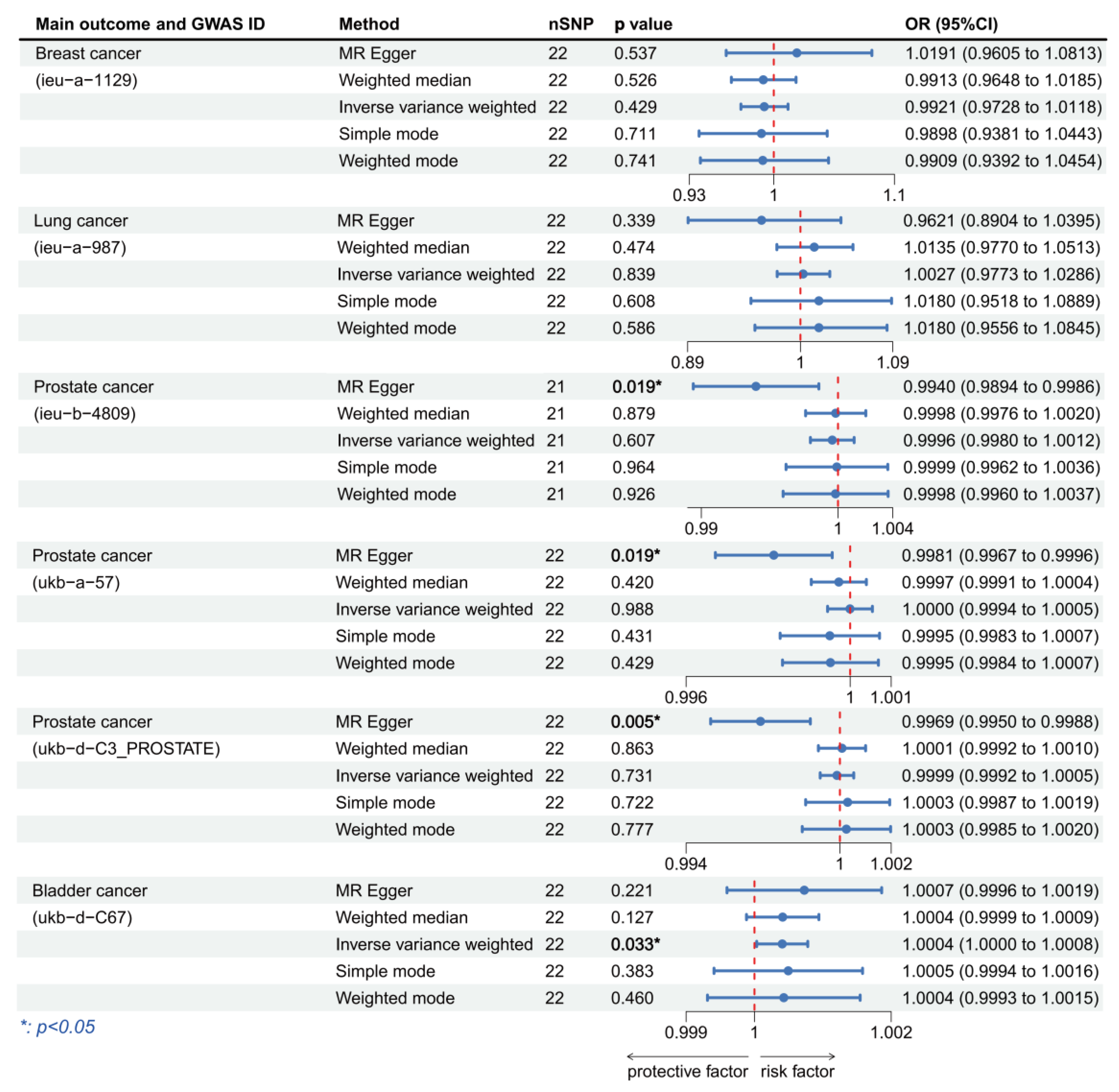
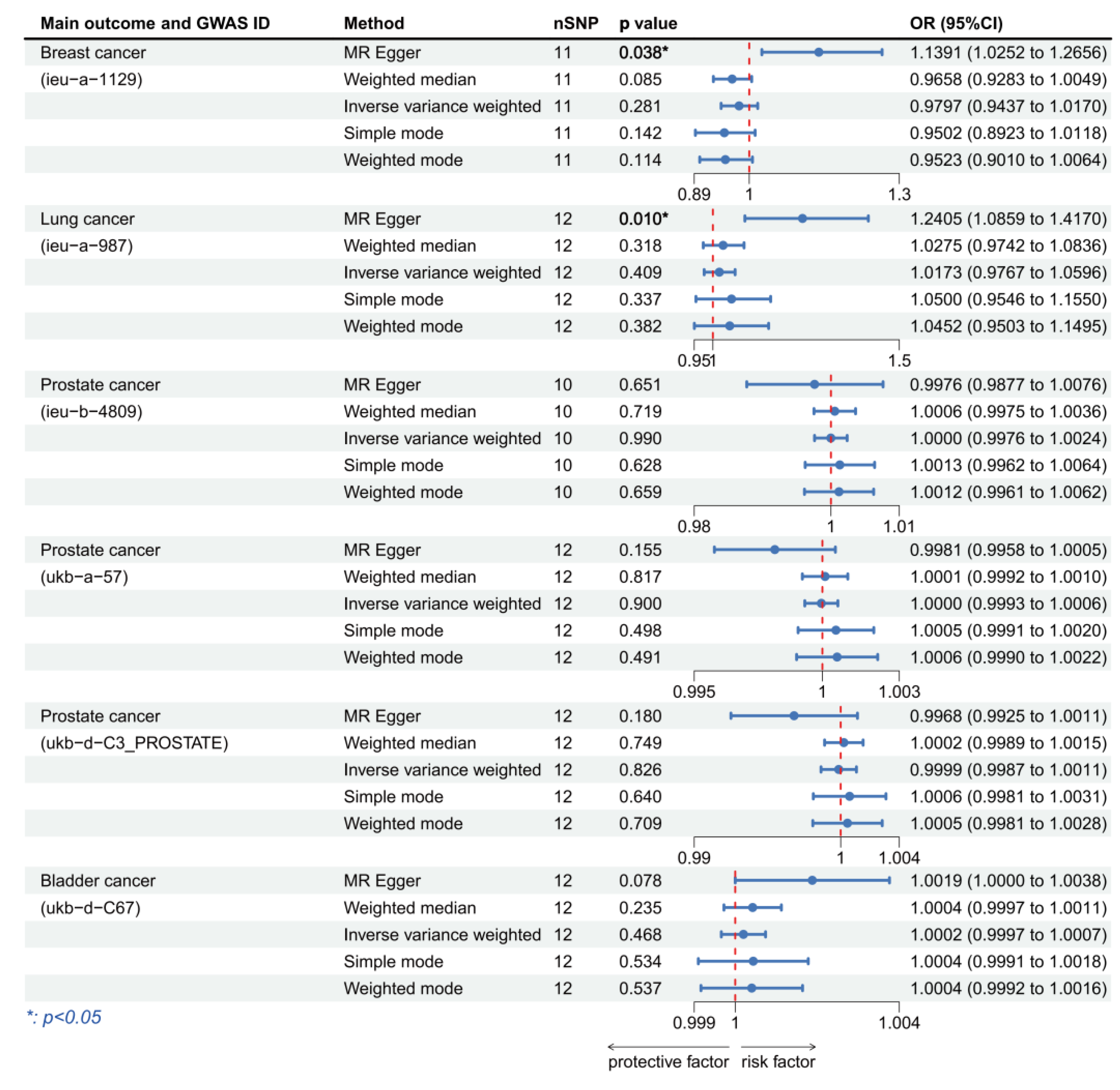

| Main Outcome | Method | Cochran’s Q-Test.p | MR.Egger.Intercept.p | MR.PRESSO.Global.Test.p |
|---|---|---|---|---|
| Bladder Cancer | MR-Egger | 0.55 | 0.747 | |
| Inverse variance weighted | 0.61 | 0.647 (raw, 0 outliers) | ||
| Prostate Cancer | MR-Egger | 0.97 | 0.184 | |
| Inverse variance weighted | 0.25 | 0.352 (raw, 0 outliers) | ||
| Anal Cancer | MR-Egger | 0.50 | 0.203 | |
| Inverse variance weighted | 0.46 | 0.504 (raw, 0 outliers) | ||
| Colorectal Cancer | MR-Egger | 0.74 | 0.068 | |
| Inverse variance weighted | 0.57 | 0.594 (raw, 0 outliers) | ||
| Vaginal Cancer | MR-Egger | 0.75 | 0.881 | |
| Inverse variance weighted | 0.80 | 0.820 (raw, 0 outliers) | ||
| Vulvar Cancer | MR-Egger | 0.43 | 0.811 | |
| Inverse variance weighted | 0.48 | 0.529 (raw, 0 outliers) | ||
| Breast Cancer | MR-Egger | 0.35 | 0.567 | |
| Inverse variance weighted | 0.38 | 0.421 (raw, 0 outliers) | ||
| Ovarian Cancer | MR-Egger | 1.00 | 0.858 | |
| Inverse variance weighted | 1.00 | 0.998 (raw, 0 outliers) | ||
| Head and Neck Cancer | MR-Egger | 0.93 | 0.710 | |
| Inverse variance weighted | 0.95 | 0.955 (raw, 0 outliers) | ||
| Oropharyngeal Cancer | MR-Egger | 0.97 | 0.922 | |
| Inverse variance weighted | 0.98 | 0.978 (raw, 0 outliers) | ||
| Lung Cancer | MR-Egger | 0.86 | 0.144 | |
| Inverse variance weighted | 0.78 | 0.771 (raw, 0 outliers) | ||
| Skin Cancer (non-melanoma) | MR-Egger | 0.50 | 0.169 | |
| Inverse variance weighted | 0.44 | 0.481 (raw, 0 outliers) | ||
| Skin Cancer | MR-Egger | 0.85 | 0.565 | |
| Inverse variance weighted | 0.88 | 0.877 (raw, 0 outliers) |
| Main Outcome | Method | Cochran’s Q-Test.p | MR.Egger.Intercept.p | MR.PRESSO.Global.Test.p |
|---|---|---|---|---|
| Bladder Cancer | MR-Egger | 0.66 | 0.775 | |
| Inverse variance weighted | 0.74 | 0.748 (raw, 0 outliers) | ||
| Prostate Cancer | MR-Egger | 0.39 | 0.548 | |
| Inverse variance weighted | 0.48 | 0.53 (raw, 0 outliers) | ||
| Anal Cancer | MR-Egger | 0.01 * | 0.430 | |
| Inverse variance weighted | 0.01 * | 0.011 (outlier corrected, 1 outlier) * | ||
| Colorectal Cancer | MR-Egger | 0.22 | 0.723 | |
| Inverse variance weighted | 0.28 | 0.289 (raw, 0 outliers) | ||
| Vaginal Cancer | MR-Egger | 0.97 | 0.394 | |
| Inverse variance weighted | 0.97 | 0.82 (raw, 0 outliers) | ||
| Vulvar Cancer | MR-Egger | 0.37 | 0.506 | |
| Inverse variance weighted | 0.41 | 0.424 (raw, 0 outliers) | ||
| Breast Cancer | MR-Egger | 0.37 | 0.963 | |
| Inverse variance weighted | 0.46 | 0.502 (raw, 0 outliers) | ||
| Ovarian Cancer | MR-Egger | 0.23 | 0.202 | |
| Inverse variance weighted | 0.15 | 0.175 (raw, 0 outliers) | ||
| Head and Neck Cancer | MR-Egger | 0.13 | 0.194 | |
| Inverse variance weighted | 0.08 | 0.082 (raw, 0 outliers) | ||
| Oropharyngeal Cancer | MR-Egger | 0.47 | 0.247 | |
| Inverse variance weighted | 0.42 | 0.439 (raw, 0 outliers) | ||
| Lung Cancer | MR-Egger | 0.79 | 0.019* | |
| Inverse variance weighted | 0.23 | 0.233 (raw, 0 outliers) | ||
| Skin Cancer (non-melanoma) | MR-Egger | 0.90 | 0.476 | |
| Inverse variance weighted | 0.91 | 0.909 (raw, 0 outliers) | ||
| Skin Cancer | MR-Egger | 0.48 | 0.407 | |
| Inverse variance weighted | 0.50 | 0.532 (raw, 0 outliers) |
| Main Outcome | Method | Cochran’s Q-Test.p | MR.Egger.Intercept.p | MR.PRESSO.Global.Test.p |
|---|---|---|---|---|
| Breast cancer | MR-Egger | 0.53 | 0.357 | 0.541 (raw, 0 outliers) |
| (ieu-a-1129) | Inverse variance weighted | 0.54 | ||
| Lung cancer | MR-Egger | 0.91 | 0.122 | 0.860 (raw, 0 outliers) |
| (ieu-a-987) | Inverse variance weighted | 0.86 | ||
| Prostate cancer | MR-Egger | 0.89 | 0.020 * | 0.543 (raw, 0 outliers) |
| (ieu-b-4809) | Inverse variance weighted | 0.57 | ||
| Prostate cancer | MR-Egger | 0.31 | 0.013 * | 0.076 (raw, 0 outliers) |
| (ukb-a-57) | Inverse variance weighted | 0.08 | ||
| Prostate cancer | MR-Egger | 0.97 | 0.005 * | 0.508 (raw, 0 outliers) |
| (ukb-d-C3_PROSTATE) | Inverse variance weighted | 0.54 | ||
| Bladder cancer | MR-Egger | 0.32 | 0.560 | 0.370 (raw, 0 outliers) |
| (ukb-d-C67) | Inverse variance weighted | 0.35 |
| Main Outcome | Method | Cochran’s Q-Test.p | MR.Egger.Intercept.p | MR.PRESSO.Global.Test.p |
|---|---|---|---|---|
| Breast cancer | MR-Egger | 0.53 | 0.017 * | 0.541 (raw, 0 outliers) |
| (ieu-a-1129) | Inverse variance weighted | 0.09 | ||
| Lung cancer | MR-Egger | 0.96 | 0.001 * | 0.134 (raw, 0 outliers) |
| (ieu-a-987) | Inverse variance weighted | 0.14 | ||
| Prostate cancer | MR-Egger | 0.71 | 0.643 | 0.790 (raw, 0 outliers) |
| (ieu-b-4809) | Inverse variance weighted | 0.77 | ||
| Prostate cancer | MR-Egger | 0.52 | 0.149 | 0.400 (raw, 0 outliers) |
| (ukb-a-57) | Inverse variance weighted | 0.40 | ||
| Prostate cancer | MR-Egger | 0.10 | 0.182 | 0.072 (raw, 0 outliers) |
| (ukb-d-C3_PROSTATE) | Inverse variance weighted | 0.06 | ||
| Bladder cancer | MR-Egger | 0.49 | 0.099 | 0.314 (raw, 0 outliers) |
| (ukb-d-C67) | Inverse variance weighted | 0.31 |
| Main Outcome | Method | Cochran’s Q-Test.p | MR.Egger.Intercept.p | MR.PRESSO.Global.Test.p |
|---|---|---|---|---|
| Breast cancer | MR-Egger | <0.01 * | 0.208 | <0.001 (outlier corrected, 1 outlier) * |
| (ebi-a-GCST007236) | Inverse variance weighted | <0.01 * | ||
| Lung cancer | MR-Egger | 0.52 | 0.196 | 0.460 (raw, 0 outliers) |
| (ukb-b-15826) | Inverse variance weighted | 0.44 | ||
| Prostate cancer | MR-Egger | 0.81 | 0.022* | 0.312 (raw, 0 outliers) |
| (ukb-d-C3_PROSTATE) | Inverse variance weighted | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Xiang, J.; An, Y.; Xu, J.; Xiong, Y.; Wang, S.; Xia, Q. Unveiling the Association between HPV and Pan-Cancers: A Bidirectional Two-Sample Mendelian Randomization Study. Cancers 2023, 15, 5147. https://doi.org/10.3390/cancers15215147
Sun J, Xiang J, An Y, Xu J, Xiong Y, Wang S, Xia Q. Unveiling the Association between HPV and Pan-Cancers: A Bidirectional Two-Sample Mendelian Randomization Study. Cancers. 2023; 15(21):5147. https://doi.org/10.3390/cancers15215147
Chicago/Turabian StyleSun, Jianxuan, Jiacheng Xiang, Ye An, Jinzhou Xu, Yifan Xiong, Shaogang Wang, and Qidong Xia. 2023. "Unveiling the Association between HPV and Pan-Cancers: A Bidirectional Two-Sample Mendelian Randomization Study" Cancers 15, no. 21: 5147. https://doi.org/10.3390/cancers15215147
APA StyleSun, J., Xiang, J., An, Y., Xu, J., Xiong, Y., Wang, S., & Xia, Q. (2023). Unveiling the Association between HPV and Pan-Cancers: A Bidirectional Two-Sample Mendelian Randomization Study. Cancers, 15(21), 5147. https://doi.org/10.3390/cancers15215147






