Simple Summary
Immunotherapy, particularly programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors, has been responsible for changing the natural history of advanced or metastatic non-small cell lung cancer. However, its use in the resectable stage is not yet fully elucidated. Therefore, we aimed to evaluate the efficacy and safety of neoadjuvant and adjuvant use of PD-1/PD-L1 inhibitors plus chemotherapy versus chemotherapy alone in resectable stage (I-III) non-small cell lung cancer. Our findings suggest that the incorporation of PD-1/PD-L1 inhibitors alongside chemotherapy offers a promising prospect for reshaping the established treatment paradigms for patients diagnosed with resectable stages of non-small cell lung cancer.
Abstract
Background: The benefit of adding programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors to the treatment of early-stage non-small cell lung cancer (NSCLC), both neoadjuvant therapy (NAT) and adjuvant therapy (AT), is not yet fully elucidated. Methods: We searched PubMed, Embase, and Cochrane databases for randomized controlled trials (RCT) that investigated PD-1/PD-L1 inhibitors plus chemotherapy for resectable stage NSCLC. We computed hazard ratios (HRs) or odds ratios (ORs) for binary endpoints, with 95% confidence intervals (CIs). Results: A total of seven RCTs comprising 3915 patients with resectable stage NSCLC were randomized to chemotherapy with or without PD-1/PD-L1 inhibitors as NAT or AT. As NAT, the PD-1/PD-L1 inhibitors plus chemotherapy group demonstrated significantly improved overall survival (HR 0.66; 95% CI 0.51–0.86) and event-free survival (HR 0.53; 95% CI 0.43–0.67) compared with the chemotherapy alone group. There was a significant increase in favor of the PD-1/PD-L1 inhibitors plus chemotherapy group for major pathological response (OR 6.40; 95% CI 3.86–10.61) and pathological complete response (OR 8.82; 95% CI 4.51–17.26). Meanwhile, as AT, disease-free survival was significant in favor of the PD-1/PD-L1 inhibitors plus chemotherapy group (HR 0.78; 95% CI 0.69–0.90). Conclusions: In this comprehensive systematic review and meta-analysis of RCTs, the incorporation of PD-1/PD-L1 inhibitors alongside chemotherapy offers a promising prospect for reshaping the established treatment paradigms for patients diagnosed with resectable stages of NSCLC. Moreover, our analyses support that neoadjuvant administration with these agents should be encouraged, in light of the fact that it was associated with an increased survival and pathological response, at the expense of a manageable safety profile.
1. Introduction
Non-small cell lung cancer (NSCLC) is the most common lung cancer, accounting for approximately 80–85% of all cases [1,2]. In about 50% of cases, the disease is either localized (stages I and II) or locally advanced (stage III) [3,4]. The standard treatment for stage I and II NSCLC, as well as specific IIIA cases, involves surgical resection. In this scenario, the 5-year survival rate for patients with stage I-II NSCLC remains at 92%; however, it drops to 36% for patients with stage IIIA [5,6,7].
Immune checkpoint inhibitors (ICIs), particularly antibodies to programmed cell death protein 1 (anti-PD1) and programmed death ligand 1 (anti-PD-L1), are used in NSCLC with the rationale that blocking programmed cell death protein 1 (PD-1) on activated T cells and programmed death-ligand 1 (PD-L1) on tumor cells could reinvigorate cytotoxic TCD8+ cells by activating host adaptive immunity [8,9]. In NSCLC, the use of anti-PD-1/PD-L1 agents has demonstrated improved overall survival (OS) and progression-free survival (PFS) following chemoradiotherapy in unresectable stage III disease. As a result, these agents have been approved for the treatment of advanced or metastatic NSCLC in cases without molecular alterations [10,11,12,13,14,15,16,17,18].
In the phase III clinical trial CheckMate 816, administration of Nivolumab together with chemotherapy as neoadjuvant therapy (NAT) improved event-free survival (EFS) compared with chemotherapy alone [19]. Additionally, in IMpower010 trial, adjuvant Atezolizumab (PD-L1 inhibitor) plus chemotherapy demonstrated a benefit for the risk of recurrence or death compared to the best supportive care for NSCLC II-IIIA, in patients with at least 1% PD-L1 expression [20].
Therefore, in this systematic review and meta-analysis of randomized controlled trials (RCTs), we aim to clarify the efficacy and safety of using neoadjuvant or adjuvant PD-1/PD-L1 inhibitors plus chemotherapy versus chemotherapy alone in resectable stage (I-III) NSCLC.
2. Methods
2.1. Protocol and Registration
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [21]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42023447777.
2.2. Eligibility Criteria
Studies that met the following eligibility criteria were included: (1) RCT; (2) comparison of neoadjuvant or adjuvant PD-1/PD-L1 inhibitors plus chemotherapy versus chemotherapy; (3) adult patients with early stage I-III NSCLC (according American Joint Committee on Cancer, 7th edition); (4) complete surgical resection including negative margins in studies with adjuvant therapy (AT); (5) no previous anti-cancer therapy in studies with NAT; and (6) Eastern Cooperative Oncology Group (ECOG) performance status score of 0, 1, or 2 (on a 5-point scale in which higher scores reflect greater disability). We excluded studies (1) with overlapping populations; (2) without outcomes of interest; and (3) with unpublished complete results. Inclusion and exclusion criteria for the RCTs included in this systematic review and meta-analysis are detailed in Supplementary Table S1.
2.3. Search Strategy and Data Extraction
PubMed, Cochrane Library, and Embase were systematically searched on 4 August 2023. The search strategy is detailed in Supplementary Table S2. In addition, backwards snowballing was performed, aimed at the inclusion of additional studies. Those found in the databases and in the references of the articles were incorporated into the reference management software (EndNote®, version X7, Thomson Reuters, Philadelphia, PA, USA). Duplicate articles were removed, using both automated and manual methods. Subsequently, two reviewers (E.P. and L.M.L.) independently analyzed the titles and abstracts of the identified articles. Disagreements were resolved by consensus between the two authors and senior author (E.P., L.M.L., and N.P.C.d.S.)
The following baseline characteristics were extracted: (1) ClinicalTrials.gov, accessed on 28 August 2023, Identifier and study design; (2) number of patients allocated for each arm; (3) regimen details in experimental and control arm; and (4) main patient’s characteristics. Two authors (A.L.S.O.R and M.E.C.S) collected pre-specified baseline characteristics and outcome data.
2.4. Endpoints
Outcomes of interest were (1) OS; (2) disease-free survival (DFS); (3) EFS; (4) major pathological response (MPR); (5) pathological complete response (pCR); patients with any grade of (6) fatigue; (7) pruritus; (8) arthralgia; (9) diarrhea; (10) increased alanine aminotransferase; (11) hypothyroidism; (12) nausea; (13) rash; (14) decreased appetite; (15) anemia; (16) constipation; (17) decreased neutrophil count; patients with grade ≥ 3 of (18) fatigue; (19) diarrhea; (20) increased alanine aminotransferase; (21) decreased neutrophil count; (22) rash; and (23) decreased appetite.
We defined (1) OS, as the period from randomization to all-cause mortality; (2) DFS, as the time from randomization to the occurrence of loco-regional or metastatic recurrence, appearance of a second primary of NSCLC or other malignancy, or death from any cause, whichever occurred first; (3) EFS, as the interval between randomization and any disease progression that would render the patient ineligible for surgery, disease progression or recurrence after surgery, disease progression in the absence of surgery, presence of unresectable tumor, or mortality from any cause. Regarding response, we defined (1) MPR as the presence of ≤10% residual viable tumor cells in the primary tumor and in the sampled lymph nodes; (2) pCR was determined by the complete absence of viable tumor cells at the primary tumor site and in the surgically removed lymph nodes after NAT.
2.5. Risk of Bias Assessment
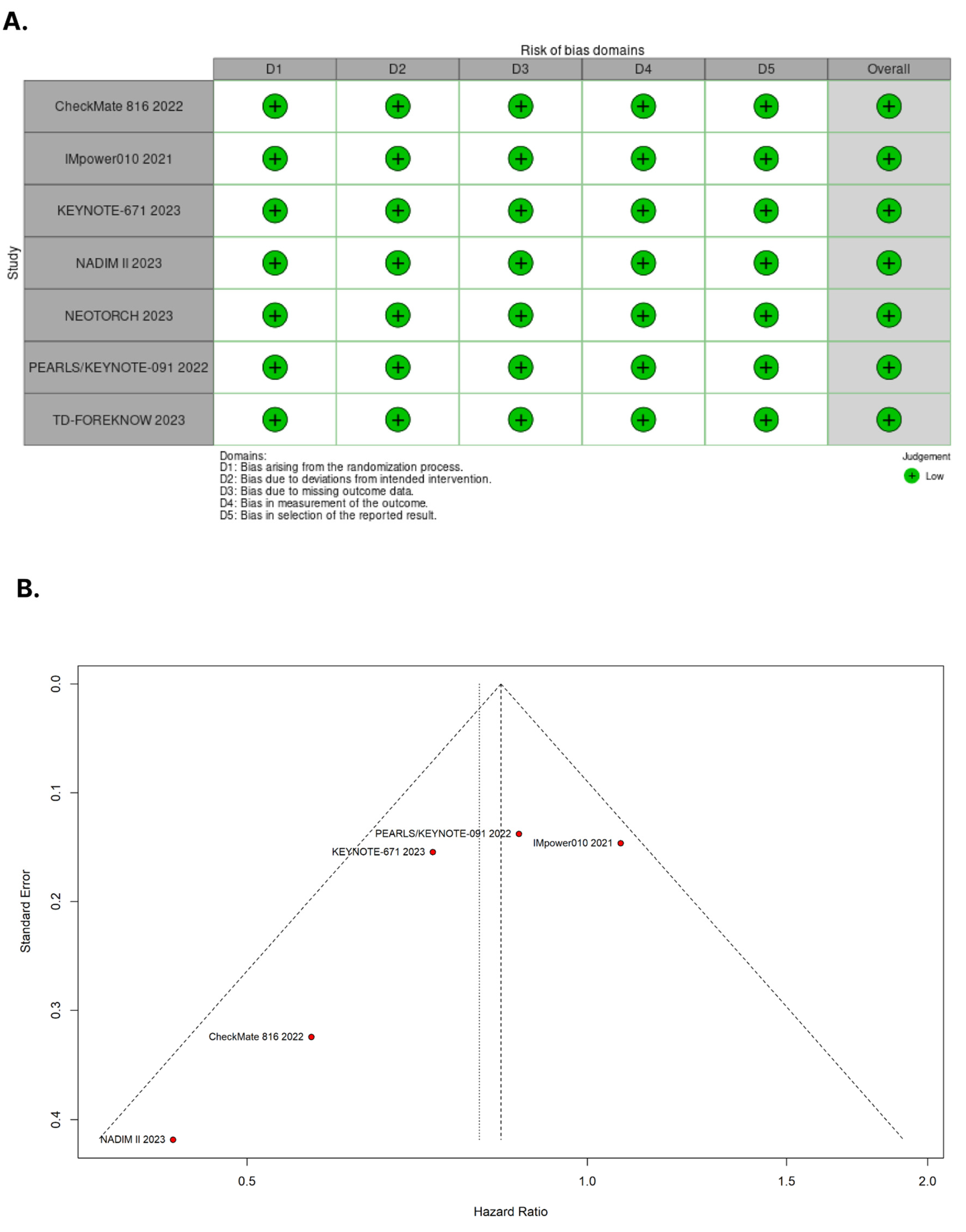
The quality assessment of individual RCTs was carried out using the Cochrane Collaboration tool for assessing risk of bias in randomized trials (RoB 2) [22]. Two authors (E.P. and R.M.O.F.) independently conducted the assessment, and disagreements were resolved by consensus. For each trial, a risk of bias score was assigned, indicating whether it was at a high, low, or unclear risk of bias across five domains: randomization process, deviations from intended interventions, missing outcomes, measurement of outcomes, and selection of reported results. To examine publication bias, contour-enhanced funnel plots [23] were visually inspected and assessed by Egger’s regression asymmetry [24] and Begg’s rank correlation test [25].
2.6. Sensitivity Analyses
2.6.1. Subgroup Analyses
Subgroup analyses included data restricted to (1) NAT and (2) AT.
2.6.2. Dominant Studies
Leave-one-out procedures were used to identify influential studies and their effect on the pooled estimates, evaluating the heterogeneity. This procedure was carried out removing data from one study and reanalyzing the remaining data, confirming that the pooled effect sizes did not result from single-study dominance.
2.7. Statistical Analysis
Binary endpoints were evaluated with hazard ratios (HRs) or odds ratios (ORs), with 95% confidence intervals (CIs). The Cochrane Q-test and I2 statistics were used to assess heterogeneity; p values > 0.10 and I2 values > 25% were considered to indicate significance for heterogeneity [26]. We used DerSimonian and Laird random-effect models for all endpoints [27]. Statistical analyses were performed using R statistical software, version 4.2.3 (R Foundation for Statistical Computing).
3. Results
3.1. Study Selection and Characteristics
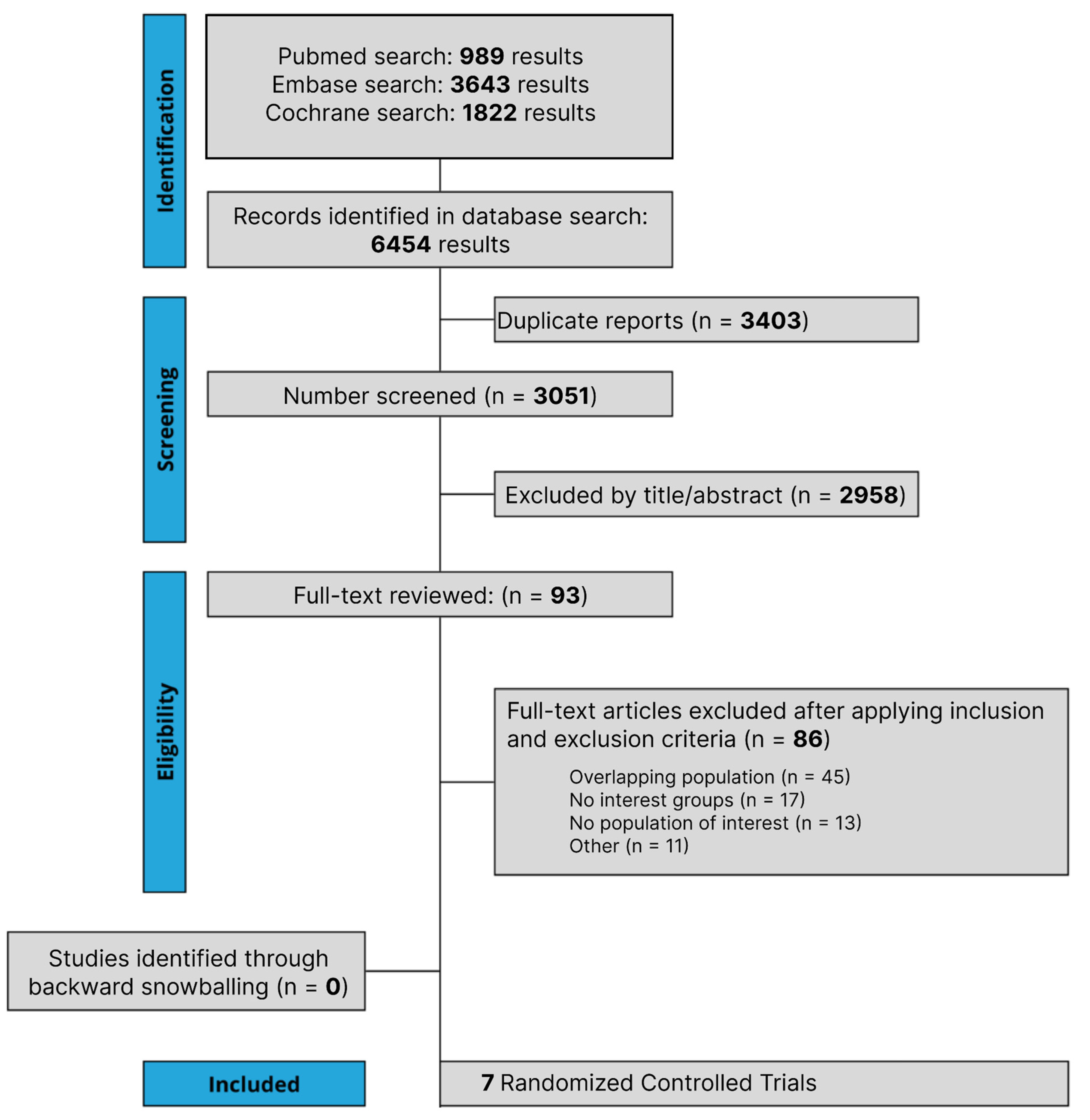
The initial search yielded 6454 results, as detailed in Figure 1. After the removal of duplicate records, and the assessment of the studies based on title and abstract, 93 full-text studies remained for full review according to prespecified criteria. Of these, seven RCTs were included comprising 3915 patients [19,20,28,29,30,31,32]. A total of 1975 patients with NSCLC were randomized to PD-1/PD-L1 inhibitors plus chemotherapy, while 1940 received chemotherapy alone. A total of 1733 patients received NAT and 2182 patients received AT. The follow-up period ranged from 14.1 to 35.5 months. The median age ranged from 61.0 to 65.0 years. A total of 223 patients had epidermal growth factor receptor (EGFR) mutation, while 68 had anaplastic lymphoma kinase (ALK) mutation. Study and participant characteristics are detailed in Table 1 and Supplementary Table S3. Different treatment regimes were carried out in the included RCT involving chemotherapy and PD-1/PD-L1 inhibitors; more details are presented in Supplementary Table S4.
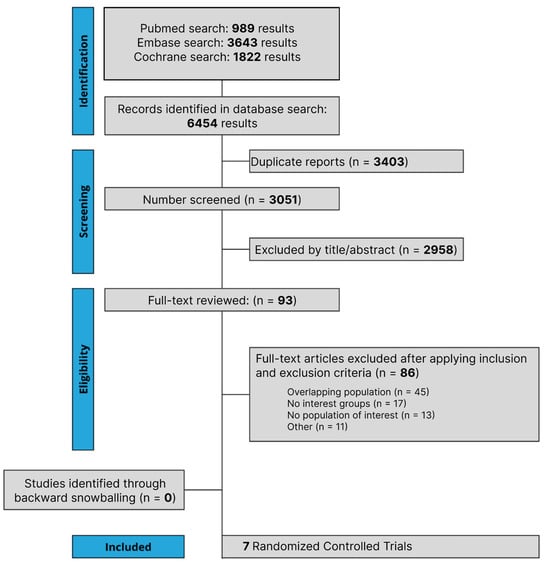
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flow diagram of study screening and selection. The search strategy in PubMed, Cochrane Library, and Embase yielded 6454 studies, of which 93 were fully reviewed for inclusion and exclusion criteria. Seven studies were included in the meta-analysis.

Table 1.
Design and characteristics of studies included in the meta-analysis.
3.2. Pooled Analysis of All Studies
3.2.1. Overall survival
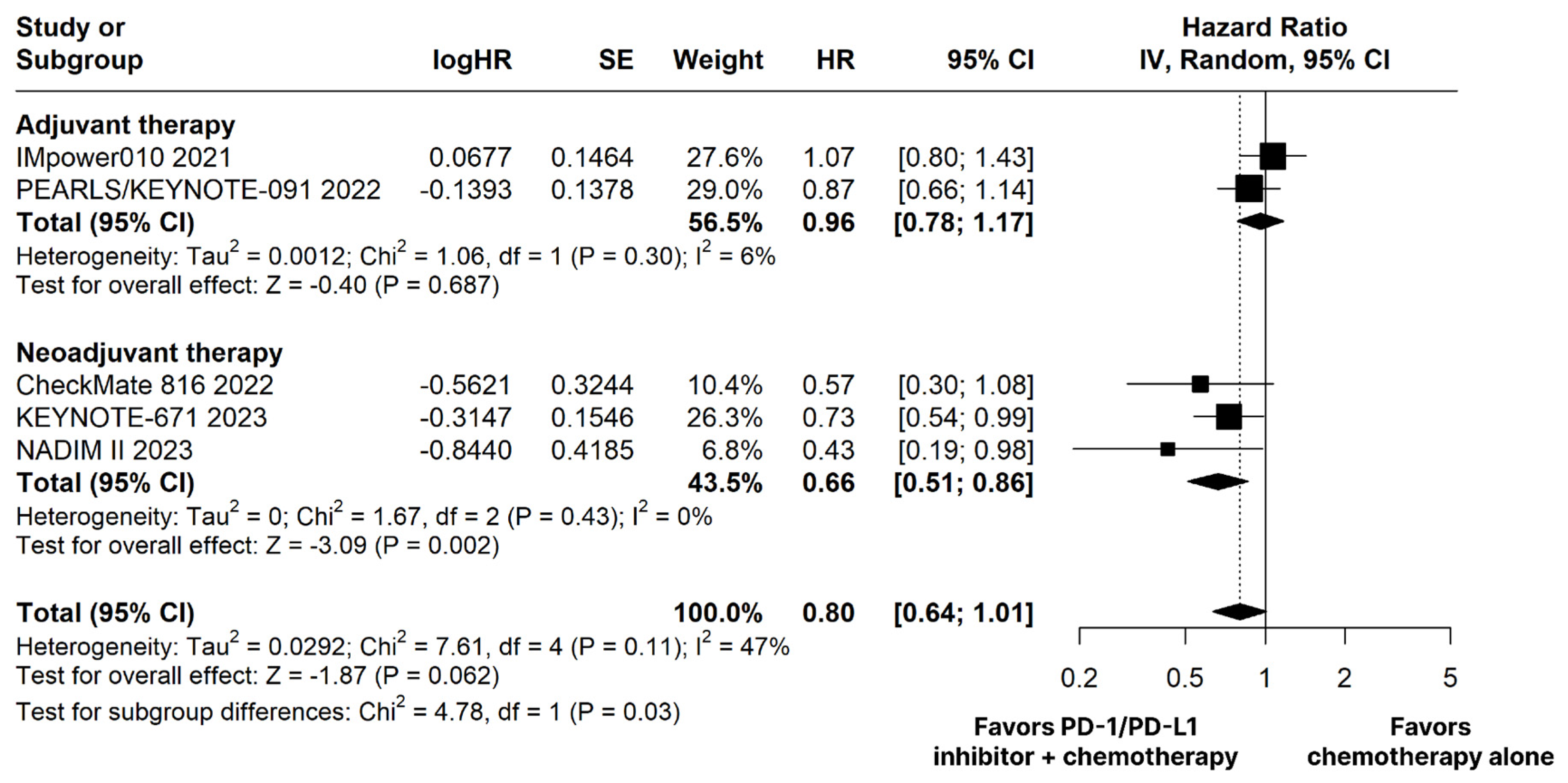
The PD-1/PD-L1 inhibitors plus chemotherapy group showed no significant difference compared to the chemotherapy alone group for OS (HR 0.80; 95% CI 0.64–1.01; p = 0.062; I2 = 47%; Figure 2).
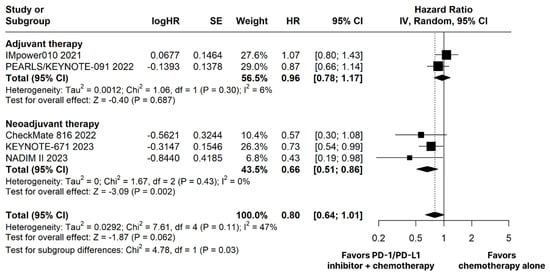
Figure 2.
Overall survival (OS) of patients with resectable stage non-small cell lung cancer treated with programmed cell death protein 1 (PD−1)/programmed death-ligand 1 (PD−L1) inhibitors plus chemotherapy versus chemotherapy alone. CI, confidence interval; HR, hazard ratio; IV, inverse variance; SE, standard error.
3.2.2. Neoadjuvant Therapy
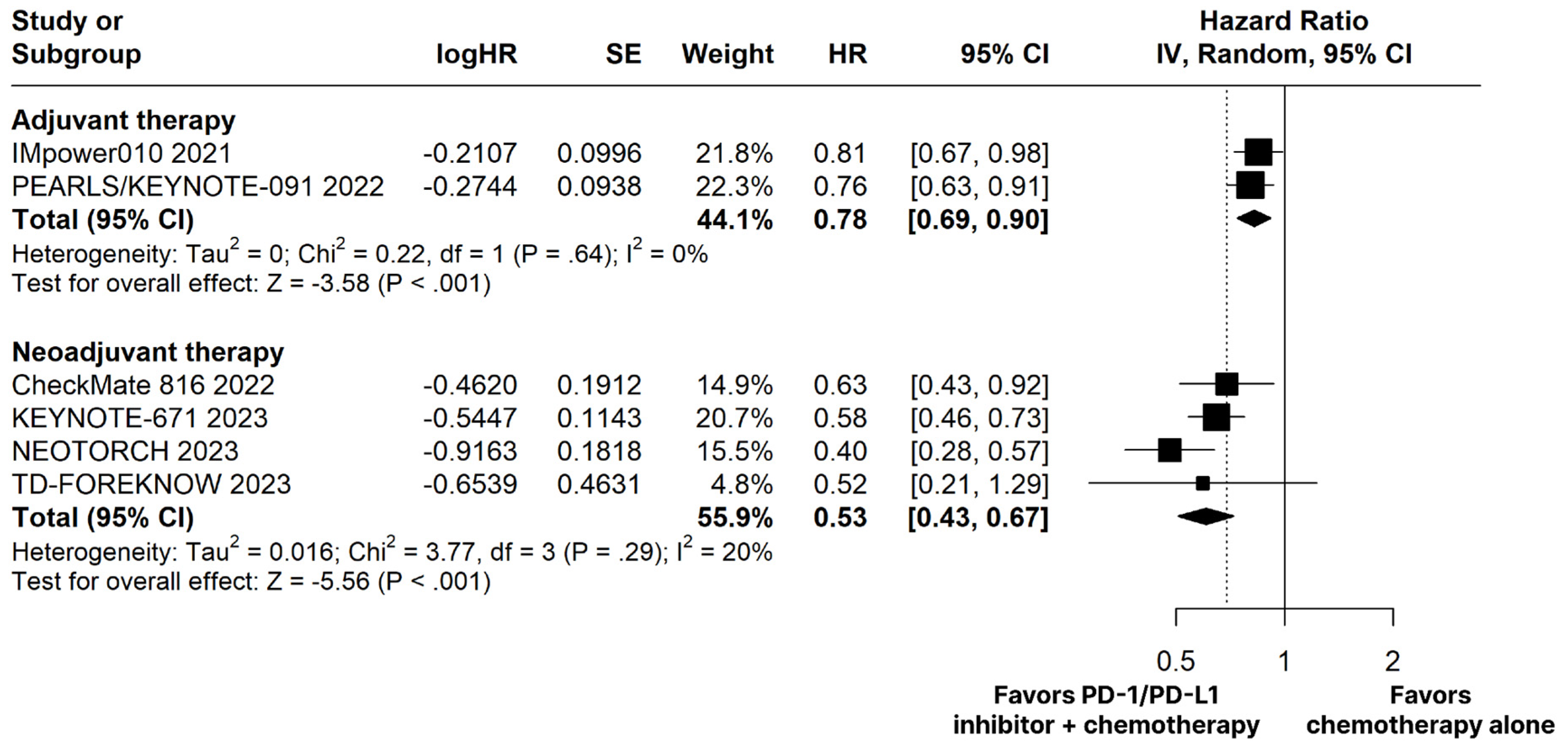
The PD-1/PD-L1 inhibitors plus chemotherapy group demonstrated significantly improved OS (HR 0.66; 95% CI 0.51–0.86; p < 0.01; I2 = 0%; Figure 2) and EFS (HR 0.53; 95% CI 0.43–0.67; p < 0.01; I2 = 20%; Figure 3) compared with the chemotherapy alone group.
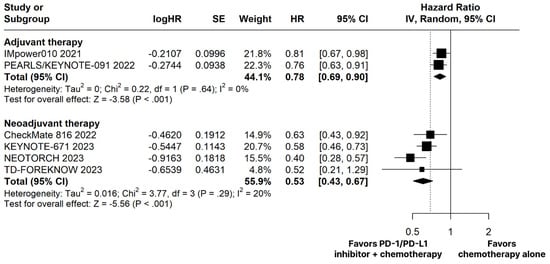
Figure 3.
Disease-free survival (DFS) in adjuvant therapy and event-free survival (EFS) in neoadjuvant therapy of patients with resectable stage non-small cell lung cancer treated with programmed cell death protein 1 (PD−1)/programmed death-ligand 1 (PD−L1) inhibitors plus chemotherapy versus chemotherapy alone. CI, confidence interval; HR, hazard ratio; IV, inverse variance; SE, standard error.
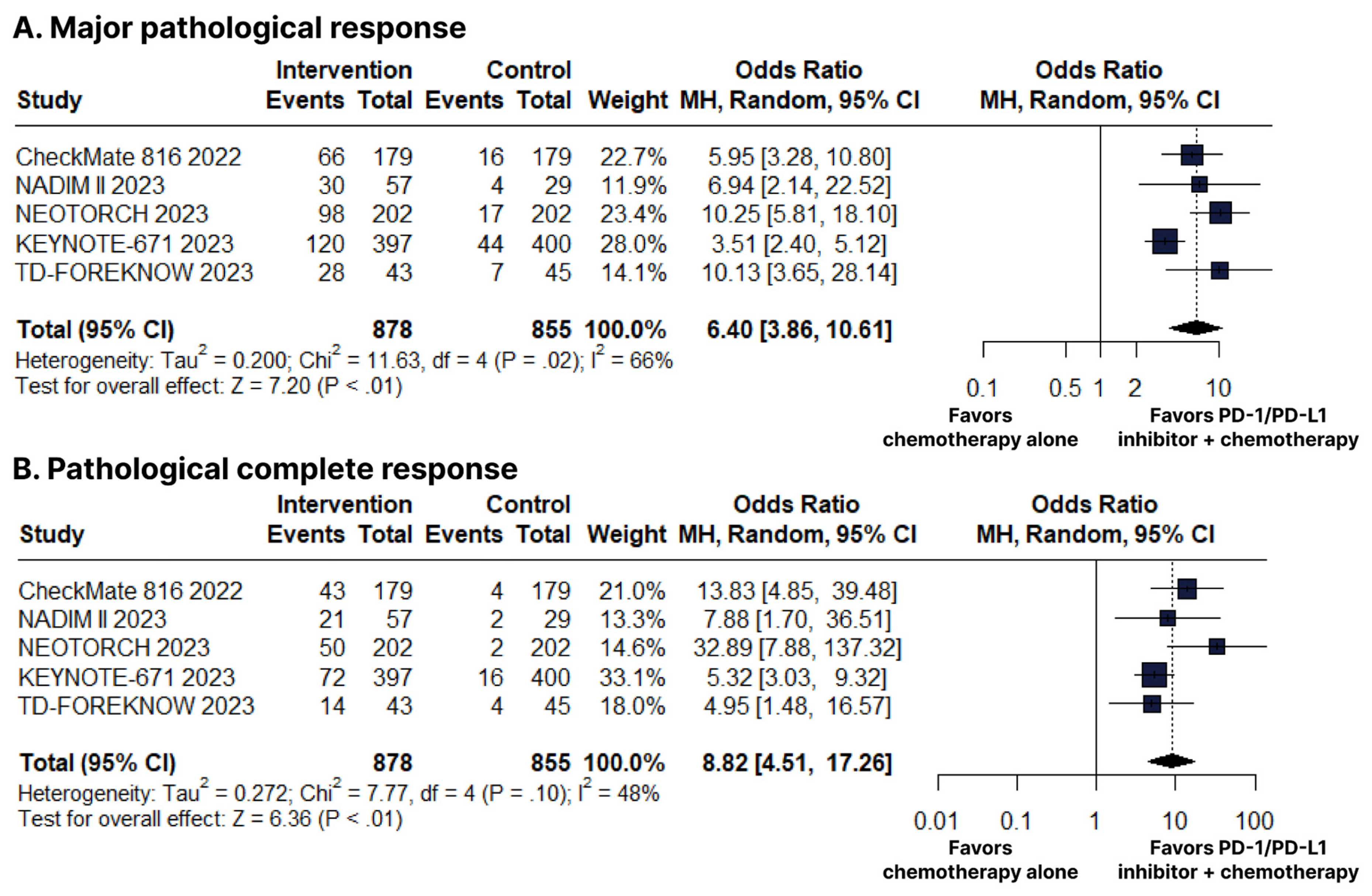
There was an increase in favor of the PD-1/PD-L1 inhibitors plus chemotherapy group for MPR (OR 6.40; 95% CI 3.86–10.61; p < 0.01; I2 = 66%; Figure 4B) and pCR (OR 8.82; 95% CI 4.51–17.26; p < 0.01; I2 = 48%; Figure 4C).
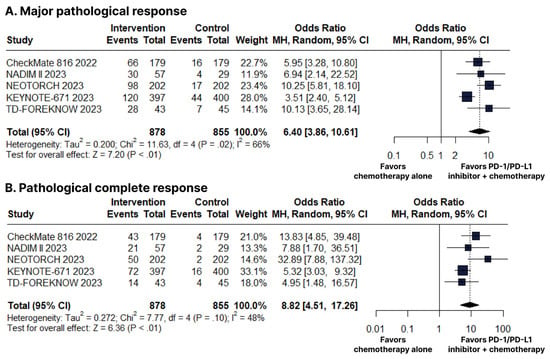
Figure 4.
(A) Major pathological response (MPR). (B) Pathological complete response (pCR). Comparison between programmed cell death protein 1 (PD−1)/programmed death-ligand 1 (PD−L1) inhibitors plus chemotherapy versus chemotherapy alone in patients with resectable stage non-small cell lung cancer. CI, confidence interval; MH, Mantel–Haenszel.
3.2.3. Adjuvant Therapy
3.2.4. Adverse Events
There was a significant increase in the PD-1/PD-L1 inhibitors plus chemotherapy group for any grade of arthralgia (OR 1.65; 95% CI 1.27–2.14; p < 0.01; I2 = 0%; Supplementary Figure S1A), increased alanine aminotransferase (OR 2.01; 95% CI 1.19–3.40; p < 0.01; I2 = 70%; Supplementary Figure S1B), hypothyroidism (OR 6.77; 95% CI 4.10–11.21; p < 0.01; I2 = 21%; Supplementary Figure S1C), and rash (OR 2.26; 95% CI 1.34–3.80; p < 0.01; I2 = 37%; Supplementary Figure S2A).
There was no significant difference between groups for any grade of fatigue (OR 1.19; 95% CI 0.96–1.48; p = 0.11; I2 = 0%; Supplementary Figure S2B), pruritus (OR 4.01; 95% CI 0.80–20.00; p = 0.09; I2 = 87%; Supplementary Figure S2C), diarrhea (OR 1.16; 95% CI 0.80–1.67; p = 0.44; I2 = 31%; Supplementary Figure S3A), nausea (OR 1.20; 95% CI 0.51–2.81; p = 0.68; I2 = 85%; Supplementary Figure S3B), decreased appetite (OR 1.07; 95% CI 0.72–1.57; p = 0.74; I2 = 55%; Supplementary Figure S3C), anemia (OR 0.94; 95% CI 0.71–1.26; p = 0.68; I2 = 14%; Supplementary Figure S4A), constipation (OR 1.01; 95% CI 0.80–1.28; p = 0.91; I2 = 0%; Supplementary Figure S4B), and decreased neutrophil count (OR 0.76; 95% CI 0.48–1.19; p = 0.23; I2 = 55%; Supplementary Figure S4C).
In addition, there was no significant difference between groups for grade ≥ 3 of fatigue (OR 1.19; 95% CI 0.41–3.46; p = 0.75; I2 = 0%; Supplementary Figure S5A), diarrhea (OR 2.60; 95% CI 0.97–6.98; p = 0.06; I2 = 0%; Supplementary Figure S5B), increased alanine aminotransferase (OR 2.12; 95% CI 0.86–5.23; p = 0.10; I2 = 6%; Supplementary Figure S5C), decreased neutrophil count (OR 0.96; 95% CI 0.71–1.31; p = 0.81; I2 = 0%; Supplementary Figure S6A), rash (OR 4.80; 95% CI 0.85–27.00; p = 0.08; I2 = 0%; Supplementary Figure S6B), decreased appetite (OR 1.50; 95% CI 0.20–11.15; p = 0.69; I2 = 52%; Supplementary Figure S6C).
3.3. Sensitivity Analyses
Subgroup analyses revealed significant differences in effect sizes attributable to AT or NAT on OS (chi2 = 4.78; df = 1; p = 0.03) and nausea (chi2 = 11.15; df = 1; p < 0.01).
We performed a leave-one-out sensitivity analysis for all outcomes. The following changes in results were found. There was a significant difference in favor of the PD-1/PD-L1 inhibitors plus chemotherapy group for OS omitting IMpower010 trial [20]. Adverse effects showed stability in the sensitivity analysis, with minimal changes. The leave-one-out sensitivity analysis of the main outcomes is detailed in Supplementary Figure S7.
3.4. Assessment of Risk of Bias
Figure 5A presents the detailed evaluation of each RCT included in the meta-analysis. Overall, all RCTs were found to have a low risk of bias [19,20,28,29,30,31,32]. In Figure 5B, the symmetrical distribution of comparable studies in the funnel plot indicates that there is no evidence of publication bias. No significant publication bias was detected by the Egger’s (p = 0.1471) and Beggs’s tests (p = 0.3272) for the OS outcome.
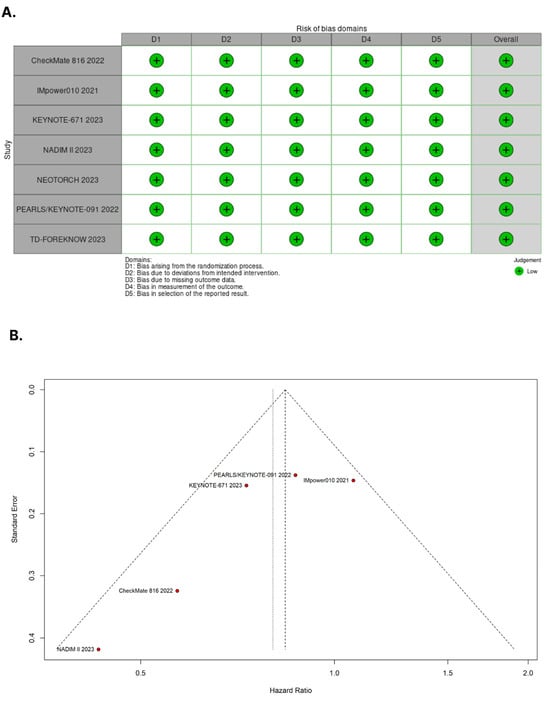
Figure 5.
(A) Critical appraisal of randomized controlled trials according to the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. (B) Funnel plot analysis of the overall survival of patients with non-small cell lung cancer shows no evidence of publication bias.
4. Discussion
In this systematic review and meta-analysis of seven RCTs including 3915 patients, we compared PD-1/PD-L1 inhibitors plus chemotherapy as NAT or AT to chemotherapy alone in patients with resectable stage NSCLC. The main findings indicate that PD-1/PD-L1 inhibitors plus chemotherapy as NAT were associated with (I) a significant improvement in OS; (II) a significant improvement in EFS; and (III) a significant increase in MPR and pCR. Furthermore, in AT, PD-1/PD-L1 inhibitors plus chemotherapy were associated with (I) a significant improvement in DFS, (II) with a manageable safety profile in both therapies.
Lung cancer is the primary cause of cancer-related death on a global scale, wherein the majority are attributed to NSCLC [1,33,34]. In this scenario, it is estimated that around 20–30% of NSCLC patients with stage I, 50% with stage II, and 60% with stage III-A die within five years [7,35,36]. The therapeutic approach with curative potential for these cases is surgical resection, providing significant benefits to patients in stages I and II of NSCLC. Additionally, a substantial improvement is observed in stage II when adjuvant chemotherapy is administered [37]. Meanwhile, in patients with locally advanced stage NSCLC (III-A), neoadjuvant chemotherapy followed by surgical resection has been the standard treatment, which may be complemented by adjuvant chemotherapy and thoracic radiotherapy in selected cases [38,39]. However, despite acting on systemic micrometastatic disease, the effect of adjuvant chemotherapy on OS remains modest, with a benefit of only 5.4% over five years [40].
Preoperative strategies involving NAT have been extensively investigated with the primary objectives of downstaging the tumor prior to surgery. This approach aims to facilitate the implementation of minimally invasive surgical procedures, suppress the early development of micrometastases, reduce the likelihood of systemic relapse, and ultimately enhance overall patient survival [35,41,42]. In this context, immunotherapy stands as a remarkable therapeutic advancement that has significantly impacted patient survival in lung cancer, especially in the context of NSCLC [41]. The elucidation of knowledge about immune mechanisms and oncogenic pathways involved in NSCLC allowed the development of new immunotherapeutic modalities [41,43,44]. Currently, PD-1 and PD-L1 inhibitors have gained approval as the first-line therapy for metastatic NSCLC patients, consistently demonstrating better results for OS and PFS [5,39]. As a result, there is a growing focus on exploring the potential of immunotherapy as a curative treatment for early-stage NSCLC [39].
NAT serves an alternative approach for managing patients with operable NSCLC and is worth considering for those with borderline resectable NSCLC [36]. In the EMERGING-CTONG 1103 trial, a NAT strategy with tyrosine kinase inhibitor was employed in patients harboring EGFR sensitivity mutations and R0-resected stage IIIA-N2 disease [45]. The group treated with Erlotinib demonstrated improved PFS compared to the group treated with gemcitabine plus cisplatin [45]. This neoadjuvant approach with anti-PD-1/PD-L1 plus antibody to cytotoxic T-lymphocyte antigen-4 (anti-CTLA-4) in the NEOSTAR trial demonstrated an improvement in pCR [46]. Hence, when specifically examining immunotherapy in the NAT setting, initial findings indicate the possibility of a prospective paradigm shift in therapy [36]. Corroborating this projection, the positive pCR rate presented in the NADIM II trial for the NAT subgroup with chemotherapy and nivolumab was accompanied by an improved OS [30]. Furthermore, the upgrade in OS was also perceived in the CheckMate 816 and KEYNOTE-671 trials [19,28]. These analyses, also applied to the EFS outcome, were confirmed in our meta-analysis, with significant results in favor of the NAT with anti PD-1/PD-L1 agents [19,28,31].
In this context, although immunotherapy for NSCLC resectable cases has been approved by the Food and Drug Administration (FDA) for both AT and NAT [47,48], AT has not always been linked to significant outcomes [20,28]. This can be seen in IMpower010 and KEYNOTE-671 trials, which presented significant improvements in DFS, but non-significant outcomes concerning the OS [20,28]. Additionally, the overall response rate in the IMpower010 was not statistically significant [20]. In our meta-analysis, similar results were encountered, with a significant improvement in the DFS and no significance in the OS outcome, when evaluating the addition of PD-1/PD-L1 inhibitors to chemotherapy in AT.
Furthermore, when analyzing subgroup treatments, the KEYNOTE-671 trial, which performed a neoadjuvant immunotherapy treatment, described a beneficial association between Pembrolizumab efficacy and the PD-L1 tumor expression. [28]. This result was remarkably confirmed in our meta-analysis, indicating a significant reduction in disease progression, disease recurrence, or death in individuals with PD-L1 tumor proportion score of ≥50% [28]. In addition, the ADAURA trial evaluated osirmetinib action on an adjuvant setting for EGFR-mutated NSCLC [49]. Its final analysis reported a significant five-year OS of 88% in the Osimertinib group versus 78% in the placebo group [49]. Moreover, in a recent trial, the adjuvant Osimertinib efficacy has been evaluated for EGFR mutations in NSCLC in association with cisplatin plus vinorelbine doublet-chemotherapy, providing valuable insights into the treatment landscape for NSCLC [50].
Regarding ALK mutations in NSCLC cases, the effectiveness of neoadjuvant crizotinib has been documented in 11 pathologically confirmed N2 ALK+ patients, of whom 91% underwent R0 resections and two presented pathologic complete responses [51]. Furthermore, a previous study reported the achievement of a significant pathological response with neoadjuvant alectinib in a patient diagnosed with stage IIIA ALK+ NSCLC [52]. This remarkable finding acted as a catalyst for the development of the current ALENO trial, which aims to investigate the activity and efficacy of alectinib as NAT in surgically resectable stage III ALK+ NSCLC [52]. In this context, there has been significant progress in the development of immunotherapies for different types of malignancies, considering the varied responses and the presence of mutations [44,53,54].
This meta-analysis showed no association for the addition of anti-PD-1/PD-L1 therapy to chemotherapy with severe toxicities (grade ≥ 3), compared to the chemotherapy alone group. Our results support that the addition of immunotherapy is associated with increased mild adverse events. Immune-mediated events, correlated to the anti-PD1/PD-L1 group, such as hypothyroidism and rash, were also present. These immune-related adverse events have already been described in the literature and are a consequence of the storm of inflammatory cytokines triggered by ICI when they activate the immune system [55,56,57,58]. This immune activation can lead to an attack on normal organs, resulting in a variety of toxic side effects [55,56,57,58]. We emphasize that all these events were manageable, thus showing the safety of the therapy.
5. Limitations
This study has limitations. First, there was moderate-to-high heterogeneity in some of the outcomes analyzed. Second, the RCTs included in this analysis involve various PD-1/PD-L1 inhibitors and chemotherapy regimens. To address this heterogeneity and assess the stability of our results, we conducted a sensitivity analysis, taking into account the different treatment regimens and potential effects of the individual studies. Third, it was not possible to perform subgroup analysis for each stage of NSCLC or types of mutations, due to studies reporting non-meaningful outcomes for each subgroup of patients. Fourth, we were unable to conduct detailed analyses on AT regimens, mostly due to only two RCTs being conducted with AT, although there is a satisfactory population.
6. Conclusions
In this comprehensive systematic review and meta-analysis of RCTs, the incorporation of PD-1/PD-L1 inhibitors alongside chemotherapy offers a promising prospect for reshaping the established treatment paradigms for patients diagnosed with resectable stages of NSCLC. Moreover, our analyses support the idea that neoadjuvant administration with these agents should be encouraged, in light of the fact that it was associated with an increased survival and pathological response, at the expense of a manageable safety profile.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15215143/s1, The online version contains Supplementary Material. Supplementary Table S1: Inclusion and exclusion criteria of included studies. Supplementary Table S2: Search strategies. Supplementary Table S3: Additional baseline characteristics of included studies. Supplementary Table S4: Treatment regimens from the randomized controlled trials included in this systematic review and meta-analysis. Supplementary Figure S1: Any grade of adverse events. A Arthralgia. B Increased alanine aminotransferase. C Hypothyroidism. Comparison between programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors plus chemotherapy versus chemotherapy alone in patients with resectable stage non-small cell lung cancer. CI, confidence interval; MH, Mantel–Haenszel. Supplementary Figure S2: Any grade of adverse events. A Rash. B Fatigue. C Pruritus. Comparison between programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors plus chemotherapy versus chemotherapy alone in patients with resectable stage non-small cell lung cancer. CI, confidence interval; MH, Mantel–Haenszel. Supplementary Figure S3: Any grade of adverse events. A Diarrhea. B Nausea. C Decreased appetite. Comparison between programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors plus chemotherapy versus chemotherapy alone in patients with resectable stage non-small cell lung cancer. CI, confidence interval; MH, Mantel–Haenszel. Supplementary Figure S4: Any grade of adverse events. A Anemia. B Constipation. C Neutrophil count decreased. Comparison between programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors plus chemotherapy versus chemotherapy alone in patients with resectable stage non-small cell lung cancer. CI, confidence interval; MH, Mantel–Haenszel. Supplementary Figure S5: Grade ≥ 3 adverse events. A Fatigue. B Diarrhea. C. Increased alanine aminotransferase. Comparison between programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors plus chemotherapy versus chemotherapy alone in patients with resectable stage non-small cell lung cancer. CI, confidence interval; MH, Mantel–Haenszel. Supplementary Figure S6: Grade ≥ 3 adverse events. A Neutrophil count decreased. B Rash. C Decreased appetite. Comparison between programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors plus chemotherapy versus chemotherapy alone in patients with resectable stage non-small cell lung cancer. CI, confidence interval; MH, Mantel–Haenszel. Supplementary Figure S7: Leave-one-out sensitivity analyses. A Overall survival. B Event-free survival and disease-free survival. CI, confidence interval; HR, Hazard-ratio; IV, inverse variance; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
Author Contributions
Conceptualization, E.P. and F.C.A.d.M.; Methodology, E.P., F.C.A.d.M. and M.P.C.; Software, E.P., M.P.C. and R.O.M.F.; Validation, E.P., L.M.L., M.E.C.S. and A.L.S.d.O.R.; Formal Analysis, E.P., L.M.L. and R.O.M.F.; Investigation, E.P., F.C.A.d.M. and M.P.C.; Resources, E.P. and L.M.L.; Data Curation, E.P., M.E.C.S. and A.L.S.d.O.R.; Writing—Original Draft Preparation, E.P., F.C.A.d.M., M.P.C., R.O.M.F., L.M.L., M.E.C.S., A.L.S.d.O.R., A.M.d.A., M.R.F. and N.P.C.d.S.; Writing—Review & Editing, E.P., F.C.A.d.M., M.P.C., R.O.M.F., L.M.L., M.E.C.S., A.L.S.d.O.R., A.M.d.A., M.R.F. and N.P.C.d.S.; Visualization, E.P.; Supervision, E.P., M.R.F. and N.P.C.d.S.; Project Administration, E.P. and N.P.C.d.S.; Funding Acquisition, N.P.C.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Pró-Reitoria de Pesquisa e Pós-Graduação da UFPA (PROPESP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for this study were systematically collected and organized into a comprehensive database. Access to the data can be granted upon request from the corresponding author.
Acknowledgments
We thank the Federal University of Pará (UFPA) and the Center for Research in Oncology (NPO/UFPA). The design of the study, sample collection, data analysis, interpretation, and manuscript writing were conducted independently of any influence or involvement from the funding agencies.
Conflicts of Interest
The authors declare no competing interests.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Liang, Y.; Wakelee, H.A. Adjuvant Chemotherapy of Completely Resected Early Stage Non-Small Cell Lung Cancer (NSCLC). Transl. Lung Cancer Res. 2013, 2, 403–410. [Google Scholar]
- Uramoto, H.; Tanaka, F. Recurrence after Surgery in Patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar]
- Taylor, M.D.; Nagji, A.S.; Bhamidipati, C.M.; Theodosakis, N.; Kozower, B.D.; Lau, C.L.; Jones, D.R. Tumor Recurrence after Complete Resection for Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2012, 93, 1813–1821. [Google Scholar] [CrossRef]
- Singh, N.; Daly, M.E.; Ismaila, N.; For the Management of Stage III NSCLC Guideline Expert Panel; Daly, M.E.; Singh, N.; Antonoff, M.B.; Arenberg, D.A.; Bradley, J.; David, E.; et al. Management of Stage III Non–Small-Cell Lung Cancer: ASCO Guideline Rapid Recommendation Update. JCO 2023, 41, 4430–4432. [Google Scholar] [CrossRef]
- Ramnath, N.; Dilling, T.J.; Harris, L.J.; Kim, A.W.; Michaud, G.C.; Balekian, A.A.; Diekemper, R.; Detterbeck, F.C.; Arenberg, D.A. Treatment of Stage III Non-Small Cell Lung Cancer. Chest 2013, 143, e314S–e340S. [Google Scholar] [CrossRef]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for Locally Advanced and Metastatic Non–Small Cell Lung Cancer: A Review. JAMA 2019, 322, 764. [Google Scholar] [CrossRef]
- Brito, A.B.C.; Camandaroba, M.P.G.; de Lima, V.C.C. Anti-PD1 versus Anti-PD-L1 Immunotherapy in First-Line Therapy for Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Thorac. Cancer 2021, 12, 1058–1066. [Google Scholar] [CrossRef]
- Langer, C.; Gadgeel, S.; Borghaei, H.; Papadimitrakopoulou, V.; Patnaik, A.; Powell, S.; Gentzler, R.; Martins, R.; Stevenson, J.; Jalal, S.; et al. Carboplatin and Pemetrexed with or without Pembrolizumab for Advanced, Non-Squamous Non-Small-Cell Lung Cancer: A Randomised, Phase 2 Cohort of the Open-Label KEYNOTE-021 Study. Lancet. Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.; Ciuleanu, T.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Nishio, M.; Cobo, M.; Steele, N.; Paramonov, V.; Parente, B.; Dear, R.; Berard, H.; Peled, N.; Seneviratne, L.C.; et al. IMpower132: Efficacy of Atezolizumab (Atezo) + Carboplatin (Carbo)/Cisplatin (Cis) + Pemetrexed (Pem) as 1L Treatment in Key Subgroups with Stage IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC). Ann. Oncol. 2018, 29, viii743–viii744. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.; Powell, S.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Socinski, M.; Jotte, R.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Hellmann, M.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Rizvi, N.; Cho, B.; Reinmuth, N.; Lee, K.; Luft, A.; Ahn, M.; van den Heuvel, M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab with or without Tremelimumab vs Standard Chemotherapy in First-Line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef]
- Herbst, R.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Forde, P.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.; Felip, E.; Broderick, S.; Brahmer, J.; Swanson, S.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csoszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant Atezolizumab after Adjuvant Chemotherapy in Resected Stage IB? IIIA Non-Small-Cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing Risk of Bias Due to Missing Results in a Synthesis. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022; Available online: https://www.training.cochrane.org/handbook (accessed on 3 August 2023).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Gjerdevik, M.; Heuch, I. Improving the Error Rates of the Begg and Mazumdar Test for Publication Bias in Fixed Effects Meta-Analysis. BMC Med. Res. Methodol. 2014, 14, 109. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus Placebo as Adjuvant Therapy for Completely Resected Stage IB-IIIA Non-Small-Cell Lung Cancer (PEARLS/KEYNOTE-091): An Interim Analysis of a Randomised, Triple-Blind, Phase 3 Trial. Lancet. Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; González-Larriba, J.L.; Martínez-Martí, A.; Bernabé, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Perioperative Nivolumab and Chemotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef]
- Lu, S.; Wu, L.; Zhang, W.; Zhang, P.; Wang, W.; Fang, W.; Xing, W.; Chen, Q.; Mei, J.; Yang, L.; et al. Perioperative Toripalimab + Platinum-Doublet Chemotherapy vs Chemotherapy in Resectable Stage II/III Non-Small Cell Lung Cancer (NSCLC): Interim Event-Free Survival (EFS) Analysis of the Phase III NEOTORCH Study. JCO 2023, 41, 8501. [Google Scholar] [CrossRef]
- Lei, J.; Zhao, J.; Gong, L.; Ni, Y.; Zhou, Y.; Tian, F.; Liu, H.; Gu, Z.; Huang, L.; Lu, Q.; et al. Neoadjuvant Camrelizumab Plus Platinum-Based Chemotherapy vs Chemotherapy Alone for Chinese Patients with Resectable Stage IIIA or IIIB (T3N2) Non–Small Cell Lung Cancer: The TD-FOREKNOW Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1348–1355. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ganti, A.K.; Klein, A.B.; Cotarla, I.; Seal, B.; Chou, E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients with Non–Small Cell Lung Cancer in the US. JAMA Oncol. 2021, 7, 1824. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, C.; Spagnolo, C.C.; Ciappina, G.; Di Pietro, M.; Squeri, A.; Passalacqua, M.I.; Marchesi, S.; Gregorc, V.; Santarpia, M. Immunotherapy in Early-Stage Non-Small Cell Lung Cancer (NSCLC): Current Evidence and Perspectives. Curr. Oncol. 2023, 30, 3684–3696. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, A.; Naidoo, J.; Banna, G.L.; Metro, G.; Forde, P.; Addeo, A. Role and Impact of Immune Checkpoint Inhibitors in Neoadjuvant Treatment for NSCLC. Cancer Treat. Rev. 2022, 104, 102350. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. JCO 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.-L.; Zielinski, M.; Ball, D.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, H.; Xu, F.; Lu, C.; Zhu, Q.; Grossi, F.; Divisi, D.; Ma, T.; Gu, J.; Ge, D. Neoadjuvant Pembrolizumab and Chemotherapy in Resectable Clinical Stage III Non-Small-Cell Lung Cancer: A Retrospective Cohort Study. Transl. Lung Cancer Res. 2023, 12, 141–149. [Google Scholar] [CrossRef]
- NSCLC Meta-analysis Collaborative Group. Preoperative Chemotherapy for Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Individual Participant Data. Lancet 2014, 383, 1561–1571. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung Cancer Immunotherapy: Progress, Pitfalls, and Promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- Genova, C.; Dellepiane, C.; Carrega, P.; Sommariva, S.; Ferlazzo, G.; Pronzato, P.; Gangemi, R.; Filaci, G.; Coco, S.; Croce, M. Therapeutic Implications of Tumor Microenvironment in Lung Cancer: Focus on Immune Checkpoint Blockade. Front. Immunol. 2022, 12, 799455. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Rad, R.; Li, R.; Paul, M.K.; Dubinett, S.M.; Liu, B. The Biology of Lung Cancer. Clin. Chest Med. 2020, 41, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Caglevic, C.; Santarpia, M.; Araujo, A.; Giovannetti, E.; Gallardo, C.D.; Pauwels, P.; Mahave, M. Immunotherapy in NSCLC: A Promising and Revolutionary Weapon. Adv. Exp. Med. Biol. 2017, 995, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.-Z.; Yan, H.-H.; Chen, K.-N.; Chen, C.; Gu, C.-D.; Wang, J.; Yang, X.-N.; Mao, W.-M.; Wang, Q.; Qiao, G.-B.; et al. Erlotinib versus Gemcitabine plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer: Final Overall Survival Analysis of the EMERGING-CTONG 1103 Randomised Phase II Trial. Sig Transduct. Target Ther. 2023, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; William, W.; Weissferdt, A.; Leung, C.; Lin, H.; Pataer, A.; Godoy, M.; Carter, B.; Federico, L.; Reuben, A.; et al. Neoadjuvant Nivolumab or Nivolumab plus Ipilimumab in Operable Non-Small Cell Lung Cancer: The Phase 2 Randomized NEOSTAR Trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef]
- De Scordilli, M.; Michelotti, A.; Bertoli, E.; De Carlo, E.; Del Conte, A.; Bearz, A. Targeted Therapy and Immunotherapy in Early-Stage Non-Small Cell Lung Cancer: Current Evidence and Ongoing Trials. IJMS 2022, 23, 7222. [Google Scholar] [CrossRef] [PubMed]
- Leal, T.; Ramalingam, S. Neoadjuvant Therapy Gains FDA Approval in Non-Small Cell Lung Cancer. Cell Rep. Med. 2022, 3, 100691. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall Survival with Osimertinib in Resected EGFR -Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Shibaki, R.; Akamatsu, H.; Kato, T.; Nishino, K.; Okada, M.; Mitsudomi, T.; Wakuda, K.; Yoshimura, K.; Yamamoto, N.; Nakagawa, K. A Phase II Study of Cisplatin plus Vinorelbine Combined with Atezolizumab as Adjuvant Therapy for Completely Resected Non-Small-Cell Lung Cancer with EGFR Mutation (West Japan Oncology Group 11719L/ADJUST Study). Ther. Adv. Med. Oncol. 2021, 13, 175883592098764. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Nie, Q.; Dong, S.; Shao, Y.; Yang, X.; Wu, Y.; Yang, Y.; Zhong, W. Neoadjuvant Crizotinib in Resectable Locally Advanced Non–Small Cell Lung Cancer with ALK Rearrangement. J. Thorac. Oncol. 2019, 14, 726–731. [Google Scholar] [CrossRef]
- Leonetti, A.; Minari, R.; Boni, L.; Gnetti, L.; Verzè, M.; Ventura, L.; Musini, L.; Tognetto, M.; Tiseo, M. Phase II, Open-Label, Single-Arm, Multicenter Study to Assess the Activity and Safety of Alectinib as Neoadjuvant Treatment in Surgically Resectable Stage III ALK-Positive NSCLC: ALNEO Trial. Clin. Lung Cancer 2021, 22, 473–477. [Google Scholar] [CrossRef]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, D.; Traversa, D.; Venuto, S.; Ebbesen, K.K.; Rodríguez, J.L.G.; Tamma, G.; Ranieri, M.; Simonetti, G.; Ghetti, M.; Paganelli, M.; et al. circPVT1 and PVT1/AKT3 show a role in cell proliferation, apoptosis, and tumor subtype-definition in small cell lung cancer. Genes Chromosomes Cancer 2023, 62, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Teulings, H.-E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-Like Depigmentation in Patients with Stage III-IV Melanoma Receiving Immunotherapy and Its Association with Survival: A Systematic Review and Meta-Analysis. JCO 2015, 33, 773–781. [Google Scholar] [CrossRef]
- Chen, C.; Wu, B.; Zhang, C.; Xu, T. Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors: An Updated Comprehensive Disproportionality Analysis of the FDA Adverse Event Reporting System. Int. Immunopharmacol. 2021, 95, 107498. [Google Scholar] [CrossRef]
- Zhong, L.; Wu, Q.; Chen, F.; Liu, J.; Xie, X. Immune-Related Adverse Events: Promising Predictors for Efficacy of Immune Checkpoint Inhibitors. Cancer Immunol. Immunother. 2021, 70, 2559–2576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).