CD47: The Next Frontier in Immune Checkpoint Blockade for Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Lung Cancer and the Evolving Treatment Landscape
2. Immunotherapy in Non-Small-Cell Lung Cancer
2.1. Disruption of the Cancer Immunity Cycle Enables Tumor Immune Evasion
2.2. PD-1/PD-L1 and CTLA-4 Immune Checkpoint Inhibitors (ICIs) in NSCLC
2.3. Limitations of Current Immune Checkpoint Inhibitors
3. Innate Immune Checkpoints as Targets for Cancer Immunotherapy
4. Cellular Functions of CD47 and Implications in Tumor Biology
4.1. CD47 Is a Ubiquitously Expressed Transmembrane Protein Upregulated in Cancer
4.2. Regulation of CD47 Expression in Cancer
4.3. CD47: Molecular Interactions, Signaling Pathways, and Malignant Phenotypes
4.3.1. Thrombospondin-1 (TSP-1)—Proliferation, Migration, Cell Death, and Angiogenesis
4.3.2. Integrins—Migration, Invasion, and Inflammation
4.3.3. SIRPα/γ—Phagocytosis and Tumor Immune Evasion
4.3.4. Intracellular Interactions and Signaling
5. CD47 Is a Clinically Relevant Therapeutic Vulnerability in Non-Small Cell Lung Cancer
5.1. CD47 Expression and Clinical Significance in NSCLC
5.2. Pathways to CD47 Upregulation in NSCLC
5.3. CD47 Promotes NSCLC Growth and Progression
5.3.1. Tumor Growth
5.3.2. Migration and Metastasis
5.3.3. Cancer Stem Cells
5.3.4. Therapy Resistance
6. CD47-Targeted Immunotherapies for Cancer
6.1. Assessment of CD47 Blockade in Preclinical Models
6.2. Clinical Strategies for Augmenting Tumor Immunity with CD47 Blockade
7. Challenges to Overcome for the Success of CD47 Blockade in Cancer
7.1. Mechanisms Contributing to the Anti-Tumor Effects of CD47 Blockade: How DOES It Work?
7.2. Response-Predictive Biomarkers: How to Select Patients for CD47-Targeted Therapy?
7.3. Mitigating Toxicity: How to Circumvent Challenges Associated with the CD47 Antigen Sink?
7.4. Therapeutic Combinations: What Standard of Care Therapies and Newly Discovered Targets Could Be Integrated with CD47 Blockade to Maximize Efficacy?
8. Perspective and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The Global Burden of Lung Cancer: Current Status and Future Trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Politi, K.; Herbst, R.S. Lung Cancer in the Era of Precision Medicine. Clin. Cancer Res. 2015, 21, 2213–2220. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive Molecular Profiling of Lung Adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive Genomic Characterization of Squamous Cell Lung Cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Otano, I.; Ucero, A.C.; Zugazagoitia, J.; Paz-Ares, L. At the Crossroads of Immunotherapy for Oncogene-Addicted Subsets of NSCLC. Nat. Rev. Clin. Oncol. 2023, 20, 143–159. [Google Scholar] [CrossRef]
- Stewart, E.L.; Tan, S.Z.; Liu, G.; Tsao, M.-S. Known and Putative Mechanisms of Resistance to EGFR Targeted Therapies in NSCLC Patients with EGFR Mutations-a Review. Transl. Lung Cancer Res. 2015, 4, 67–81. [Google Scholar]
- Maynard, A.; McCoach, C.E.; Rotow, J.K.; Harris, L.; Haderk, F.; Kerr, D.L.; Yu, E.A.; Schenk, E.L.; Tan, W.; Zee, A.; et al. Therapy-Induced Evolution of Human Lung Cancer Revealed by Single-Cell RNA Sequencing. Cell 2020, 182, 1232–1251.e22. [Google Scholar] [CrossRef]
- Lim, Z.-F.; Ma, P.C. Emerging Insights of Tumor Heterogeneity and Drug Resistance Mechanisms in Lung Cancer Targeted Therapy. J. Hematol. Oncol. 2019, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S. Chemotherapy Resistance in Lung Cancer. In Lung Cancer and Personalized Medicine: Current Knowledge and Therapies; Ahmad, A., Gadgeel, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–209. ISBN 9783319242231. [Google Scholar]
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.-L.; Paz-Ares, L. Lung Cancer: Current Therapies and New Targeted Treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant Atezolizumab after Adjuvant Chemotherapy in Resected Stage IB-IIIA Non-Small-Cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Heymach, J.V.; Mitsudomi, T.; Harpole, D.; Aperghis, M.; Jones, S.; Mann, H.; Fouad, T.M.; Reck, M. Design and Rationale for a Phase III, Double-Blind, Placebo-Controlled Study of Neoadjuvant Durvalumab + Chemotherapy Followed by Adjuvant Durvalumab for the Treatment of Patients with Resectable Stages II and III Non-Small-Cell Lung Cancer: The AEGEAN Trial. Clin. Lung Cancer 2022, 23, e247–e251. [Google Scholar] [PubMed]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus Placebo as Adjuvant Therapy for Completely Resected Stage IB-IIIA Non-Small-Cell Lung Cancer (PEARLS/KEYNOTE-091): An Interim Analysis of a Randomised, Triple-Blind, Phase 3 Trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Cascone, T.; Leung, C.H.; Weissferdt, A.; Pataer, A.; Carter, B.W.; Godoy, M.C.B.; Feldman, H.; William, W.N., Jr.; Xi, Y.; Basu, S.; et al. Neoadjuvant Chemotherapy plus Nivolumab with or without Ipilimumab in Operable Non-Small Cell Lung Cancer: The Phase 2 Platform NEOSTAR Trial. Nat. Med. 2023, 29, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, W.; Zhou, B.; Wang, S.; Li, N.; Qiu, B.; Lv, F.; Zhao, L.; Li, J.; Shao, K.; et al. Three-Year Follow-Up of Neoadjuvant Programmed Cell Death Protein-1 Inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. 2022, 17, 909–920. [Google Scholar] [CrossRef]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant Atezolizumab and Chemotherapy in Patients with Resectable Non-Small-Cell Lung Cancer: An Open-Label, Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Deb, D.; Moore, A.C.; Roy, U.B. The 2021 Global Lung Cancer Therapy Landscape. J. Thorac. Oncol. 2022, 17, 931–936. [Google Scholar] [CrossRef]
- Li, Z.; Feiyue, Z.; Gaofeng, L.; Haifeng, L. Lung Cancer and Oncolytic Virotherapy—Enemy’s Enemy. Transl. Oncol. 2023, 27, 101563. [Google Scholar] [CrossRef]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer Vaccines: The next Immunotherapy Frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, F.; Li, J.; Pu, Y.; Yang, C.; Wang, Y.; Lei, Y.; Huang, Y. CAR-T Cell Therapy for Lung Cancer: Potential and Perspective. Thorac. Cancer 2022, 13, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Jiang, W.; Kim, B.Y.S.; Zhang, C.C.; Fu, Y.-X.; Weissman, I.L. Phagocytosis Checkpoints as New Targets for Cancer Immunotherapy. Nat. Rev. Cancer 2019, 19, 568–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune Evasion in Cancer: Mechanistic Basis and Therapeutic Strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Beatty, G.L.; Gladney, W.L. Immune Escape Mechanisms as a Guide for Cancer Immunotherapy. Clin. Cancer Res. 2015, 21, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Anichini, A.; Perotti, V.E.; Sgambelluri, F.; Mortarini, R. Immune Escape Mechanisms in Non Small Cell Lung Cancer. Cancers 2020, 12, 3605. [Google Scholar] [CrossRef]
- McGranahan, N.; Rosenthal, R.; Hiley, C.T.; Rowan, A.J.; Watkins, T.B.K.; Wilson, G.A.; Birkbak, N.J.; Veeriah, S.; Van Loo, P.; Herrero, J.; et al. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell 2017, 171, 1259–1271.e11. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, V.; Smith, K.N.; Forde, P.M.; Niknafs, N.; Bhattacharya, R.; White, J.; Zhang, T.; Adleff, V.; Phallen, J.; Wali, N.; et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov. 2017, 7, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Cadieux, E.L.; Salgado, R.; Bakir, M.A.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanić, M.; Reading, J.L.; Joshi, K.; et al. Neoantigen-Directed Immune Escape in Lung Cancer Evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Beane, J.E.; Mazzilli, S.A.; Campbell, J.D.; Duclos, G.; Krysan, K.; Moy, C.; Perdomo, C.; Schaffer, M.; Liu, G.; Zhang, S.; et al. Molecular Subtyping Reveals Immune Alterations Associated with Progression of Bronchial Premalignant Lesions. Nat. Commun. 2019, 10, 1856. [Google Scholar] [CrossRef] [PubMed]
- Mascaux, C.; Angelova, M.; Vasaturo, A.; Beane, J.; Hijazi, K.; Anthoine, G.; Buttard, B.; Rothe, F.; Willard-Gallo, K.; Haller, A.; et al. Immune Evasion before Tumour Invasion in Early Lung Squamous Carcinogenesis. Nature 2019, 571, 570–575. [Google Scholar] [CrossRef]
- Sorin, M.; Rezanejad, M.; Karimi, E.; Fiset, B.; Desharnais, L.; Perus, L.J.M.; Milette, S.; Yu, M.W.; Maritan, S.M.; Doré, S.; et al. Single-Cell Spatial Landscapes of the Lung Tumour Immune Microenvironment. Nature 2023, 614, 548–554. [Google Scholar] [CrossRef]
- Lehtiö, J.; Arslan, T.; Siavelis, I.; Pan, Y.; Socciarelli, F.; Berkovska, O.; Umer, H.M.; Mermelekas, G.; Pirmoradian, M.; Jönsson, M.; et al. Proteogenomics of Non-Small Cell Lung Cancer Reveals Molecular Subtypes Associated with Specific Therapeutic Targets and Immune Evasion Mechanisms. Nat. Cancer 2021, 2, 1224–1242. [Google Scholar] [CrossRef]
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.-A.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17. [Google Scholar] [CrossRef]
- Arrieta, O.; Aviles-Salas, A.; Orozco-Morales, M.; Hernández-Pedro, N.; Cardona, A.F.; Cabrera-Miranda, L.; Barrios-Bernal, P.; Soca-Chafre, G.; Cruz-Rico, G.; Peña-Torres, M.d.L.; et al. Association between CD47 Expression, Clinical Characteristics and Prognosis in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Med. 2020, 9, 2390–2402. [Google Scholar] [CrossRef]
- Donini, C.; D’Ambrosio, L.; Grignani, G.; Aglietta, M.; Sangiolo, D. Next Generation Immune-Checkpoints for Cancer Therapy. J. Thorac. Dis. 2018, 10, S1581–S1601. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next Generation of Immune Checkpoint Therapy in Cancer: New Developments and Challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Lentz, R.W.; Colton, M.D.; Mitra, S.S.; Messersmith, W.A. Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol. Cancer Ther. 2021, 20, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The Future of Immune Checkpoint Therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced Expression of PD-1, a Novel Member of the Immunoglobulin Gene Superfamily, upon Programmed Cell Death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on Tumor Cells in the Escape from Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 Have Opposing Effects on the Response of T Cells to Stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Two Win Nobel for Immune Regulation Discoveries. Cancer Discov. 2018, 8, 1338–1339. [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Fumet, J.-D.; Richard, C.; Ledys, F.; Klopfenstein, Q.; Joubert, P.; Routy, B.; Truntzer, C.; Gagné, A.; Hamel, M.-A.; Guimaraes, C.F.; et al. Prognostic and Predictive Role of CD8 and PD-L1 Determination in Lung Tumor Tissue of Patients under Anti-PD-1 Therapy. Br. J. Cancer 2018, 119, 950–960. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Choi, J.; Mani, N.; Sanmamed, M.F.; Datar, I.; Sowell, R.; Du, V.Y.; Kaftan, E.; Goldberg, S.; Dong, W.; et al. A Dormant TIL Phenotype Defines Non-Small Cell Lung Carcinomas Sensitive to Immune Checkpoint Blockers. Nat. Commun. 2018, 9, 3196. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive Correlates of Response to the Anti-PD-L1 Antibody MPDL3280A in Cancer Patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab plus Ipilimumab Combined with Two Cycles of Chemotherapy in Patients with Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients with Advanced Non–Small-Cell Lung Cancer Treated with Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The Role of PD-L1 Expression as a Predictive Biomarker: An Analysis of All US Food and Drug Administration (FDA) Approvals of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High Tumor Mutation Burden Fails to Predict Immune Checkpoint Blockade Response across All Cancer Types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Haslam, A.; Gill, J.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw. Open 2020, 3, e200423. [Google Scholar] [CrossRef]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [PubMed]
- Addeo, A.; Passaro, A.; Malapelle, U.; Banna, G.L.; Subbiah, V.; Friedlaender, A. Immunotherapy in Non-Small Cell Lung Cancer Harbouring Driver Mutations. Cancer Treat. Rev. 2021, 96, 102179. [Google Scholar] [CrossRef] [PubMed]
- Hastings, K.; Yu, H.A.; Wei, W.; Sanchez-Vega, F.; DeVeaux, M.; Choi, J.; Rizvi, H.; Lisberg, A.; Truini, A.; Lydon, C.A.; et al. EGFR Mutation Subtypes and Response to Immune Checkpoint Blockade Treatment in Non-Small-Cell Lung Cancer. Ann. Oncol. 2019, 30, 1311–1320. [Google Scholar] [CrossRef]
- Lee, C.K.; Man, J.; Lord, S.; Links, M.; Gebski, V.; Mok, T.; Yang, J.C.-H. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non–Small Cell Lung Cancer—A Meta-Analysis. J. Thorac. Oncol. 2017, 12, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive Resistance to Therapeutic PD-1 Blockade Is Associated with Upregulation of Alternative Immune Checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nixon, M.J.; Wang, Y.; Wang, D.Y.; Castellanos, E.; Estrada, M.V.; Ericsson-Gonzalez, P.I.; Cote, C.H.; Salgado, R.; Sanchez, V.; et al. Tumor-Specific MHC-II Expression Drives a Unique Pattern of Resistance to Immunotherapy via LAG-3/FCRL6 Engagement. JCI Insight 2018, 3, e120360. [Google Scholar] [CrossRef]
- Barkal, A.A.; Weiskopf, K.; Kao, K.S.; Gordon, S.R.; Rosental, B.; Yiu, Y.Y.; George, B.M.; Markovic, M.; Ring, N.G.; Tsai, J.M.; et al. Engagement of MHC Class I by the Inhibitory Receptor LILRB1 Suppresses Macrophages and Is a Target of Cancer Immunotherapy. Nat. Immunol. 2018, 19, 76–84. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Haberberger, J.; Severson, E.; Duncan, D.L.; Hemmerich, A.; Edgerly, C.; Ferguson, N.L.; Williams, E.; Elvin, J.; Vergilio, J.-A.; et al. A Pan-Cancer Analysis of PD-L1 Immunohistochemistry and Gene Amplification, Tumor Mutation Burden and Microsatellite Instability in 48,782 Cases. Mod. Pathol. 2021, 34, 252–263. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jamieson, C.H.M.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef]
- Chu, J.; Gao, F.; Yan, M.; Zhao, S.; Yan, Z.; Shi, B.; Liu, Y. Natural Killer Cells: A Promising Immunotherapy for Cancer. J. Transl. Med. 2022, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, C. The Rise of NK Cell Checkpoints as Promising Therapeutic Targets in Cancer Immunotherapy. Front. Immunol. 2019, 10, 2354. [Google Scholar] [CrossRef] [PubMed]
- De Louche, C.D.; Roghanian, A. Human Inhibitory Leukocyte Ig-like Receptors: From Immunotolerance to Immunotherapy. JCI Insight 2022, 7, e151553. [Google Scholar] [CrossRef] [PubMed]
- Carosella, E.D.; Gregori, S.; Tronik-Le Roux, D. HLA-G/LILRBs: A Cancer Immunotherapy Challenge. Trends Cancer Res. 2021, 7, 389–392. [Google Scholar] [CrossRef]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor That Promotes Anti-Tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef]
- Romagné, F.; André, P.; Spee, P.; Zahn, S.; Anfossi, N.; Gauthier, L.; Capanni, M.; Ruggeri, L.; Benson, D.M., Jr.; Blaser, B.W.; et al. Preclinical Characterization of 1-7F9, a Novel Human Anti-KIR Receptor Therapeutic Antibody That Augments Natural Killer-Mediated Killing of Tumor Cells. Blood 2009, 114, 2667–2677. [Google Scholar] [CrossRef]
- Marie-Cardine, A.; Viaud, N.; Thonnart, N.; Joly, R.; Chanteux, S.; Gauthier, L.; Bonnafous, C.; Rossi, B.; Bléry, M.; Paturel, C.; et al. IPH4102, a Humanized KIR3DL2 Antibody with Potent Activity against Cutaneous T-Cell Lymphoma. Cancer Res. 2014, 74, 6060–6070. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the Checkpoint Receptor TIGIT Prevents NK Cell Exhaustion and Elicits Potent Anti-Tumor Immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Chen, H.-M.; van der Touw, W.; Wang, Y.S.; Kang, K.; Mai, S.; Zhang, J.; Alsina-Beauchamp, D.; Duty, J.A.; Mungamuri, S.K.; Zhang, B.; et al. Blocking Immunoinhibitory Receptor LILRB2 Reprograms Tumor-Associated Myeloid Cells and Promotes Antitumor Immunity. J. Clin. Investig. 2018, 128, 5647–5662. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Deng, M.; John, S.; Gui, X.; Kansagra, A.; Chen, W.; Kim, J.; Lewis, C.; Wu, G.; et al. Antagonistic Anti-LILRB1 Monoclonal Antibody Regulates Antitumor Functions of Natural Killer Cells. J. Immunother. Cancer 2020, 8, e000515. [Google Scholar] [CrossRef]
- Siu, L.L.; Wang, D.; Hilton, J.; Geva, R.; Rasco, D.; Perets, R.; Abraham, A.K.; Wilson, D.C.; Markensohn, J.F.; Lunceford, J.; et al. First-in-Class Anti-Immunoglobulin-like Transcript 4 Myeloid-Specific Antibody MK-4830 Abrogates a PD-1 Resistance Mechanism in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2022, 28, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, M.I.; Lindberg, F.P.; Plas, D.; Reynolds, S.; Peters, M.G.; Brown, E.J. In Vivo Expression of Alternatively Spliced Forms of Integrin-Associated Protein (CD47). J. Cell Sci. 1995, 108 Pt 11, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Hatherley, D.; Graham, S.C.; Turner, J.; Harlos, K.; Stuart, D.I.; Barclay, A.N. Paired Receptor Specificity Explained by Structures of Signal Regulatory Proteins Alone and Complexed with CD47. Mol. Cell 2008, 31, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Rebres, R.A.; Vaz, L.E.; Green, J.M.; Brown, E.J. Normal Ligand Binding and Signaling by CD47 (Integrin-Associated Protein) Requires a Long Range Disulfide Bond between the Extracellular and Membrane-Spanning Domains. J. Biol. Chem. 2001, 276, 34607–34616. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kuznetsova, S.A.; Pendrak, M.L.; Sipes, J.M.; Romeo, M.J.; Li, Z.; Zhang, L.; Roberts, D.D. Heparan Sulfate Modification of the Transmembrane Receptor CD47 Is Necessary for Inhibition of T Cell Receptor Signaling by Thrombospondin-1. J. Biol. Chem. 2011, 286, 14991–15002. [Google Scholar] [CrossRef]
- Kim, W.; Bennett, E.J.; Huttlin, E.L.; Guo, A.; Li, J.; Possemato, A.; Sowa, M.E.; Rad, R.; Rush, J.; Comb, M.J.; et al. Systematic and Quantitative Assessment of the Ubiquitin-Modified Proteome. Mol. Cell 2011, 44, 325–340. [Google Scholar] [CrossRef]
- Logtenberg, M.E.W.; Jansen, J.H.M.; Raaben, M.; Toebes, M.; Franke, K.; Brandsma, A.M.; Matlung, H.L.; Fauster, A.; Gomez-Eerland, R.; Bakker, N.A.M.; et al. Glutaminyl Cyclase Is an Enzymatic Modifier of the CD47- SIRPα Axis and a Target for Cancer Immunotherapy. Nat. Med. 2019, 25, 612–619. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Subramanian, S.; Boder, E.T.; Discher, D.E. Post-Translational Regulation of Expression and Conformation of an Immunoglobulin Domain in Yeast Surface Display. Biotechnol. Bioeng. 2006, 93, 159–168. [Google Scholar] [CrossRef]
- Du, L.; Su, Z.; Wang, S.; Meng, Y.; Xiao, F.; Xu, D.; Li, X.; Qian, X.; Lee, S.B.; Lee, J.-H.; et al. EGFR-Induced and c-Src-Mediated CD47 Phosphorylation Inhibits TRIM21-Dependent Polyubiquitylation and Degradation of CD47 to Promote Tumor Immune Evasion. Adv. Sci. 2023, 10, e2206380. [Google Scholar] [CrossRef]
- Murata, Y.; Kotani, T.; Ohnishi, H.; Matozaki, T. The CD47-SIRPα Signalling System: Its Physiological Roles and Therapeutic Application. J. Biochem. 2014, 155, 335–344. [Google Scholar] [CrossRef]
- Brown, E.J.; Frazier, W.A. Integrin-Associated Protein (CD47) and Its Ligands. Trends Cell Biol. 2001, 11, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.-T.; Shen, Y.-W.; Zhou, Y.-D.; Nagle, D.G.; Guan, Y.-Y.; Zhang, W.-D.; Luan, X. CD47: Beyond an Immune Checkpoint in Cancer Treatment. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188771. [Google Scholar] [CrossRef] [PubMed]
- Soto-Pantoja, D.R.; Kaur, S.; Roberts, D.D. CD47 Signaling Pathways Controlling Cellular Differentiation and Responses to Stress. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 212–230. [Google Scholar] [CrossRef]
- Khandelwal, S.; van Rooijen, N.; Saxena, R.K. Reduced Expression of CD47 during Murine Red Blood Cell (RBC) Senescence and Its Role in RBC Clearance from the Circulation. Transfusion 2007, 47, 1725–1732. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Mawby, W.J.; Holmes, C.H.; Anstee, D.J.; Spring, F.A.; Tanner, M.J. Isolation and Characterization of CD47 Glycoprotein: A Multispanning Membrane Protein Which Is the Same as Integrin-Associated Protein (IAP) and the Ovarian Tumour Marker OA3. Biochem. J. 1994, 304 Pt 2, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A Subcellular Map of the Human Proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Fossati-Jimack, L.; Azeredo da Silveira, S.; Moll, T.; Kina, T.; Kuypers, F.A.; Oldenborg, P.-A.; Reininger, L.; Izui, S. Selective Increase of Autoimmune Epitope Expression on Aged Erythrocytes in Mice: Implications in Anti-Erythrocyte Autoimmune Responses. J. Autoimmun. 2002, 18, 17–25. [Google Scholar] [CrossRef]
- Olsson, M.; Bruhns, P.; Frazier, W.A.; Ravetch, J.V.; Oldenborg, P.-A. Platelet Homeostasis Is Regulated by Platelet Expression of CD47 under Normal Conditions and in Passive Immune Thrombocytopenia. Blood 2005, 105, 3577–3582. [Google Scholar] [CrossRef]
- Brown, S.; Heinisch, I.; Ross, E.; Shaw, K.; Buckley, C.D.; Savill, J. Apoptosis Disables CD31-Mediated Cell Detachment from Phagocytes Promoting Binding and Engulfment. Nature 2002, 418, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Massuger, L.F.; Claessens, R.A.; Kenemans, P.; Verheijen, R.H.; Boerman, O.C.; Meeuwis, A.P.; Schijf, C.P.; Buijs, W.C.; Hanselaar, T.G.; Corstens, F.H. Kinetics and Biodistribution in Relation to Tumour Detection with 111In-Labelled OV-TL 3 F(ab’)2 in Patients with Ovarian Cancer. Nucl. Med. Commun. 1991, 12, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.G.; Freemont, P.S.; Foulkes, W.; Trowsdale, J. An Ovarian Tumor Marker with Homology to Vaccinia Virus Contains an IgV-like Region and Multiple Transmembrane Domains. Cancer Res. 1992, 52, 5416–5420. [Google Scholar] [PubMed]
- Xu, Y.; Li, J.; Tong, B.; Chen, M.; Liu, X.; Zhong, W.; Zhao, J.; Wang, M. Positive Tumour CD47 Expression Is an Independent Prognostic Factor for Recurrence in Resected Non-Small Cell Lung Cancer. ESMO Open 2020, 5, e000823. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, J.; Kong, X.; Li, E.; Liu, Y.; Du, X.; Kang, Z.; Tang, Y.; Kuang, Y.; Yang, Z.; et al. CD47 Promotes Tumor Invasion and Metastasis in Non-Small Cell Lung Cancer. Sci. Rep. 2016, 6, 29719. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Zhang, Y.; Gao, Z.; Zhao, Y.; Wen, Z.; Han, H.; Li, Y.; Hu, H.; Chen, H. Combination of CD47 and CD68 Expression Predicts Survival in Eastern-Asian Patients with Non-Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 739–747. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.; Zheng, M.; Zhen, H.; Xie, Q.; Zhang, C.; Zhou, Z.; Jin, G. RAGA Prevents Tumor Immune Evasion of LUAD by Promoting CD47 Lysosome Degradation. Commun. Biol. 2023, 6, 211. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Mitrakas, A.; Anestopoulos, I.; Kontosis, A.; Koukourakis, I.M.; Pappa, A.; Panayiotidis, M.I.; Koukourakis, M.I. Expression of CD47 and SIRPα Macrophage Immune-Checkpoint Pathway in Non-Small-Cell Lung Cancer. Cancers 2022, 14, 1801. [Google Scholar] [CrossRef]
- Qu, S.; Jiao, Z.; Lu, G.; Xu, J.; Yao, B.; Wang, T.; Wang, J.; Yao, Y.; Yan, X.; Wang, T.; et al. Human Lung Adenocarcinoma CD47 Is Upregulated by Interferon-γ and Promotes Tumor Metastasis. Mol. Ther. Oncolytics 2022, 25, 276–287. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, Y.; Guo, W.; Xu, J.; Li, L.; Tian, H.; Li, R.; Liu, L.; Tan, F.; Gao, S.; et al. PD-L1 and CD47 Co-Expression Predicts Survival and Enlightens Future Dual-Targeting Immunotherapy in Non-Small Cell Lung Cancer. Thorac. Cancer 2021, 12, 1743–1751. [Google Scholar] [CrossRef]
- Lang, C.; Lantos, A.; Megyesfalvi, Z.; Egger, F.; Hoda, M.A.; Mosleh, B.; Klikovits, T.; Oberndorfer, F.; Timelthaler, G.; Ferencz, B.; et al. Clinical and Prognostic Implications of CD47 and PD-L1 Expression in Surgically Resected Small-Cell Lung Cancer. ESMO Open 2022, 7, 100631. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Morales, M.; Avilés-Salas, A.; Hernández-Pedro, N.; Catalán, R.; Cruz-Rico, G.; Colín-González, A.L.; Dosal-Mancilla, E.; Barrios-Bernal, P.; Arrieta, O. Clinicopathological and Prognostic Significance of CD47 Expression in Lung Neuroendocrine Tumors. J. Immunol. Res. 2021, 2021, 6632249. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.; Zlobec, I.; Schürch, C.; Perren, A.; Ochsenbein, A.F.; Banz, Y. CD47 Protein Expression in Acute Myeloid Leukemia: A Tissue Microarray-Based Analysis. Leuk. Res. 2015, 39, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Myint, Z.W.; Chahine, Z.; Jayswal, R.; Bachert, E.; McDonald, R.J.; Strup, S.E.; James, A.C.; Hensley, P.J.; Allison, D.B. Association of CD47 Expression with Clinicopathologic Characteristics and Survival Outcomes in Muscle Invasive Bladder Cancer. J. Pers. Med. 2023, 13, 885. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, S.; Li, T.; Peng, C.; Yang, Y.; Lin, Y.; Ma, Y.; Dong, C. Prognostic Implications of Combined High Expression of CD47 and MCT1 in Breast Cancer: A Retrospective Study during a 10-Year Period. Transl. Cancer Res. 2022, 11, 29–42. [Google Scholar] [CrossRef] [PubMed]
- López-Pereira, B.; Fernández-Velasco, A.A.; Fernández-Vega, I.; Corte-Torres, D.; Quirós, C.; Villegas, J.A.; Palomo, P.; González, S.; González, A.P.; Payer, Á.; et al. Expression of CD47 Antigen in Reed-Sternberg Cells as a New Potential Biomarker for Classical Hodgkin Lymphoma. Clin. Transl. Oncol. 2020, 22, 782–785. [Google Scholar] [CrossRef]
- Oh, H.-H.; Park, Y.-L.; Park, S.-Y.; Myung, E.; Im, C.-M.; Yu, H.-J.; Han, B.; Seo, Y.-J.; Kim, K.-H.; Myung, D.-S.; et al. CD47 Mediates the Progression of Colorectal Cancer by Inducing Tumor Cell Apoptosis and Angiogenesis. Pathol. Res. Pract. 2022, 240, 154220. [Google Scholar] [CrossRef]
- Hu, T.; Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Zhang, Y.; Song, Y.; Hu, J.; He, X.; et al. Tumor-Intrinsic CD47 Signal Regulates Glycolysis and Promotes Colorectal Cancer Cell Growth and Metastasis. Theranostics 2020, 10, 4056–4072. [Google Scholar] [CrossRef]
- Ren, S.; Cai, Y.; Hu, S.; Liu, J.; Zhao, Y.; Ding, M.; Chen, X.; Zhan, L.; Zhou, X.; Wang, X. Berberine Exerts Anti-Tumor Activity in Diffuse Large B-Cell Lymphoma by Modulating c-myc/CD47 Axis. Biochem. Pharmacol. 2021, 188, 114576. [Google Scholar] [CrossRef]
- Kazama, R.; Miyoshi, H.; Takeuchi, M.; Miyawaki, K.; Nakashima, K.; Yoshida, N.; Kawamoto, K.; Yanagida, E.; Yamada, K.; Umeno, T.; et al. Combination of CD47 and Signal-Regulatory Protein-α Constituting the “Don’t Eat Me Signal” Is a Prognostic Factor in Diffuse Large B-Cell Lymphoma. Cancer Sci. 2020, 111, 2608–2619. [Google Scholar] [CrossRef]
- Cho, J.; Yoon, S.E.; Kim, S.J.; Ko, Y.H.; Kim, W.S. CD47 Overexpression Is Common in Intestinal Non-GCB Type Diffuse Large B-Cell Lymphoma and Associated with 18q21 Gain. Blood Adv. 2022, 6, 6120–6130. [Google Scholar] [CrossRef] [PubMed]
- Sercan, Ç.; Haberal Reyhan, A.N.; Özen, Ö.; Ayhan, A. Clinicopathologic and Prognostic Significance of CD47 Expression and Tumor-Associated Macrophages in Endometrial Carcinoma. Int. J. Gynecol. Pathol. 2022, 41, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Yokobori, T.; Tanaka, N.; Sakai, M.; Sano, A.; Inose, T.; Sohda, M.; Nakajima, M.; Miyazaki, T.; Kato, H.; et al. CD47 Expression Regulated by the miR-133a Tumor Suppressor Is a Novel Prognostic Marker in Esophageal Squamous Cell Carcinoma. Oncol. Rep. 2012, 28, 465–472. [Google Scholar] [CrossRef]
- Jiang, N.; Xie, B.; Xiao, W.; Fan, M.; Xu, S.; Duan, Y.; Hamsafar, Y.; Evans, A.C.; Huang, J.; Zhou, W.; et al. Fatty Acid Oxidation Fuels Glioblastoma Radioresistance with CD47-Mediated Immune Evasion. Nat. Commun. 2022, 13, 1511. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bang, S.; Jee, S.; Paik, S.S.; Jang, K. Clinicopathological Significance of CD47 Expression in Hepatocellular Carcinoma. J. Clin. Pathol. 2021, 74, 111–115. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, D.-X.; Yu, X.-J.; Sun, H.-W.; Xu, Y.-T.; Zhang, Y.-J.; Xu, J. Macrophages Induce CD47 Upregulation via IL-6 and Correlate with Poor Survival in Hepatocellular Carcinoma Patients. Oncoimmunology 2019, 8, e1652540. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Pei, X.-F.; Zhu, Z.-Q.; Lin, Z.; Mao, Y.-Y.; Xu, X.-L.; Luo, Y.-L.; Zhang, L.; Peng, P.-J. CD47 Overexpression Is Associated with Epstein-Barr Virus Infection and Poor Prognosis in Patients with Nasopharyngeal Carcinoma. OncoTargets Ther. 2020, 13, 3325–3334. [Google Scholar] [CrossRef]
- Xu, J.-F.; Pan, X.-H.; Zhang, S.-J.; Zhao, C.; Qiu, B.-S.; Gu, H.-F.; Hong, J.-F.; Cao, L.; Chen, Y.; Xia, B.; et al. CD47 Blockade Inhibits Tumor Progression Human Osteosarcoma in Xenograft Models. Oncotarget 2015, 6, 23662–23670. [Google Scholar] [CrossRef]
- Shimizu, A.; Sawada, K.; Kobayashi, M.; Yamamoto, M.; Yagi, T.; Kinose, Y.; Kodama, M.; Hashimoto, K.; Kimura, T. Exosomal CD47 Plays an Essential Role in Immune Evasion in Ovarian Cancer. Mol. Cancer Res. 2021, 19, 1583–1595. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, L.; Zhuang, H.; Hao, Y.; Gao, S.; Liu, S.; Liu, Q.; Liu, D.; Liu, J.; Lin, B. Lewis Y Antigen Modified CD47 Is an Independent Risk Factor for Poor Prognosis and Promotes Early Ovarian Cancer Metastasis. Am. J. Cancer Res. 2015, 5, 2777–2787. [Google Scholar]
- Wang, H.; Tan, M.; Zhang, S.; Li, X.; Gao, J.; Zhang, D.; Hao, Y.; Gao, S.; Liu, J.; Lin, B. Expression and Significance of CD44, CD47 and c-Met in Ovarian Clear Cell Carcinoma. Int. J. Mol. Sci. 2015, 16, 3391–3404. [Google Scholar] [CrossRef]
- Imam, R.; Chang, Q.; Black, M.; Yu, C.; Cao, W. CD47 Expression and CD163+ Macrophages Correlated with Prognosis of Pancreatic Neuroendocrine Tumor. BMC Cancer 2021, 21, 320. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Shi, X.; Chen, C.; He, H.; Liu, L.; Wu, J.; Yan, H. High Expression of CD47 in Triple Negative Breast Cancer Is Associated with Epithelial-Mesenchymal Transition and Poor Prognosis. Oncol. Lett. 2019, 18, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Ye, Z.-H.; Huang, M.-Y.; Lu, J.-J. Regulation of CD47 Expression in Cancer Cells. Transl. Oncol. 2020, 13, 100862. [Google Scholar] [CrossRef] [PubMed]
- Betancur, P.A.; Abraham, B.J.; Yiu, Y.Y.; Willingham, S.B.; Khameneh, F.; Zarnegar, M.; Kuo, A.H.; McKenna, K.; Kojima, Y.; Leeper, N.J.; et al. A CD47-Associated Super-Enhancer Links pro-Inflammatory Signalling to CD47 Upregulation in Breast Cancer. Nat. Commun. 2017, 8, 14802. [Google Scholar] [CrossRef]
- Lo, J.; Lau, E.Y.T.; Ching, R.H.H.; Cheng, B.Y.L.; Ma, M.K.F.; Ng, I.O.L.; Lee, T.K.W. Nuclear Factor Kappa B-Mediated CD47 up-Regulation Promotes Sorafenib Resistance and Its Blockade Synergizes the Effect of Sorafenib in Hepatocellular Carcinoma in Mice. Hepatology 2015, 62, 534–545. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Dougan, M.; Ingram, J.R.; Ho, C.C.M.; Kauke, M.J.; Almo, S.C.; Ploegh, H.L.; Garcia, K.C. Durable Antitumor Responses to CD47 Blockade Require Adaptive Immune Stimulation. Proc. Natl. Acad. Sci. USA 2016, 113, E2646–E2654. [Google Scholar] [CrossRef]
- Basile, M.S.; Mazzon, E.; Russo, A.; Mammana, S.; Longo, A.; Bonfiglio, V.; Fallico, M.; Caltabiano, R.; Fagone, P.; Nicoletti, F.; et al. Differential Modulation and Prognostic Values of Immune-Escape Genes in Uveal Melanoma. PLoS ONE 2019, 14, e0210276. [Google Scholar] [CrossRef]
- Liu, F.; Dai, M.; Xu, Q.; Zhu, X.; Zhou, Y.; Jiang, S.; Wang, Y.; Ai, Z.; Ma, L.; Zhang, Y.; et al. SRSF10-Mediated IL1RAP Alternative Splicing Regulates Cervical Cancer Oncogenesis via mIL1RAP-NF-κB-CD47 Axis. Oncogene 2018, 37, 2394–2409. [Google Scholar] [CrossRef]
- Johnson, L.D.S.; Banerjee, S.; Kruglov, O.; Viller, N.N.; Horwitz, S.M.; Lesokhin, A.; Zain, J.; Querfeld, C.; Chen, R.; Okada, C.; et al. Targeting CD47 in Sézary Syndrome with SIRPαFc. Blood Adv. 2019, 3, 1145–1153. [Google Scholar] [CrossRef]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC Regulates the Antitumor Immune Response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Xiang, L.; Bullen, J.W.; Zhang, C.; Samanta, D.; Gilkes, D.M.; He, J.; Semenza, G.L. HIF-1 Regulates CD47 Expression in Breast Cancer Cells to Promote Evasion of Phagocytosis and Maintenance of Cancer Stem Cells. Proc. Natl. Acad. Sci. USA 2015, 112, E6215–E6223. [Google Scholar] [CrossRef]
- Hu, H.; Cheng, R.; Wang, Y.; Wang, X.; Wu, J.; Kong, Y.; Zhan, S.; Zhou, Z.; Zhu, H.; Yu, R.; et al. Oncogenic KRAS Signaling Drives Evasion of Innate Immune Surveillance in Lung Adenocarcinoma by Activating CD47. J. Clin. Investig. 2022, 133, e153470. [Google Scholar] [CrossRef]
- Kale, A.; Rogers, N.M.; Ghimire, K. Thrombospondin-1 CD47 Signalling: From Mechanisms to Medicine. Int. J. Mol. Sci. 2021, 22, 4062. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bronson, S.M.; Pal-Nath, D.; Miller, T.W.; Soto-Pantoja, D.R.; Roberts, D.D. Functions of Thrombospondin-1 in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 4570. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, H.; Miyagaki, T.; Takahashi-Shishido, N.; Nakajima, R.; Oka, T.; Suga, H.; Sugaya, M.; Sato, S. Thrombospondin-1 Promotes Tumor Progression in Cutaneous T-Cell Lymphoma via CD47. Leukemia 2020, 34, 845–856. [Google Scholar] [CrossRef]
- Ticchioni, M.; Deckert, M.; Mary, F.; Bernard, G.; Brown, E.J.; Bernard, A. Integrin-Associated Protein (CD47) Is a Comitogenic Molecule on CD3-Activated Human T Cells. J. Immunol. 1997, 158, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Waclavicek, M.; Majdic, O.; Stulnig, T.; Berger, M.; Baumruker, T.; Knapp, W.; Pickl, W.F. T Cell Stimulation via CD47: Agonistic and Antagonistic Effects of CD47 Monoclonal Antibody 1/1A4. J. Immunol. 1997, 159, 5345–5354. [Google Scholar] [CrossRef] [PubMed]
- Saumet, A.; Slimane, M.B.; Lanotte, M.; Lawler, J.; Dubernard, V. Type 3 repeat/C-Terminal Domain of Thrombospondin-1 Triggers Caspase-Independent Cell Death through CD47/alphavbeta3 in Promyelocytic Leukemia NB4 Cells. Blood 2005, 106, 658–667. [Google Scholar] [CrossRef]
- Denèfle, T.; Boullet, H.; Herbi, L.; Newton, C.; Martinez-Torres, A.-C.; Guez, A.; Pramil, E.; Quiney, C.; Pourcelot, M.; Levasseur, M.D.; et al. Thrombospondin-1 Mimetic Agonist Peptides Induce Selective Death in Tumor Cells: Design, Synthesis, and Structure-Activity Relationship Studies. J. Med. Chem. 2016, 59, 8412–8421. [Google Scholar] [CrossRef]
- Mateo, V.; Lagneaux, L.; Bron, D.; Biron, G.; Armant, M.; Delespesse, G.; Sarfati, M. CD47 Ligation Induces Caspase-Independent Cell Death in Chronic Lymphocytic Leukemia. Nat. Med. 1999, 5, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Kaur, S.; Ivins-O’Keefe, K.; Roberts, D.D. Thrombospondin-1 Is a CD47-Dependent Endogenous Inhibitor of Hydrogen Sulfide Signaling in T Cell Activation. Matrix Biol. 2013, 32, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, L.; Wilson, K.; Roberts, D. Thrombospondin-1 Inhibits TCR-Mediated T Lymphocyte Early Activation. J. Immunol. 2001, 166, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Lamy, L.; Ticchioni, M.; Rouquette-Jazdanian, A.K.; Samson, M.; Deckert, M.; Greenberg, A.H.; Bernard, A. CD47 and the 19 kDa Interacting Protein-3 (BNIP3) in T Cell Apoptosis. J. Biol. Chem. 2003, 278, 23915–23921. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Forslöw, A.; Sundqvist, K.-G. Autocrine Regulation of T Cell Motility by Calreticulin-Thrombospondin-1 Interaction. J. Immunol. 2005, 174, 654–661. [Google Scholar] [CrossRef]
- Guillon, J.; Petit, C.; Moreau, M.; Toutain, B.; Henry, C.; Roché, H.; Bonichon-Lamichhane, N.; Salmon, J.P.; Lemonnier, J.; Campone, M.; et al. Regulation of Senescence Escape by TSP1 and CD47 Following Chemotherapy Treatment. Cell Death Dis. 2019, 10, 199. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Maxhimer, J.B.; Hyodo, F.; Pendrak, M.L.; Ridnour, L.A.; DeGraff, W.G.; Tsokos, M.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 and CD47 Limit Cell and Tissue Survival of Radiation Injury. Am. J. Pathol. 2008, 173, 1100–1112. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Ridnour, L.A.; Wink, D.A.; Roberts, D.D. Blockade of CD47 Increases Survival of Mice Exposed to Lethal Total Body Irradiation. Sci. Rep. 2013, 3, 1038. [Google Scholar] [CrossRef]
- Sick, E.; Jeanne, A.; Schneider, C.; Dedieu, S.; Takeda, K.; Martiny, L. CD47 Update: A Multifaceted Actor in the Tumour Microenvironment of Potential Therapeutic Interest. Br. J. Pharmacol. 2012, 167, 1415–1430. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Ridnour, L.A.; Dimitry, J.; Frazier, W.A.; Wink, D.A.; Roberts, D.D. CD47 Is Necessary for Inhibition of Nitric Oxide-Stimulated Vascular Cell Responses by Thrombospondin-1. J. Biol. Chem. 2006, 281, 26069–26080. [Google Scholar] [CrossRef]
- Kaur, S.; Martin-Manso, G.; Pendrak, M.L.; Garfield, S.H.; Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 Inhibits VEGF Receptor-2 Signaling by Disrupting Its Association with CD47. J. Biol. Chem. 2010, 285, 38923–38932. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Chang, T.; Singh, S.P.; Lim, L.; Mannan, P.; Garfield, S.H.; Pendrak, M.L.; Soto-Pantoja, D.R.; Rosenberg, A.Z.; Jin, S.; et al. CD47 Signaling Regulates the Immunosuppressive Activity of VEGF in T Cells. J. Immunol. 2014, 193, 3914–3924. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Martin-Manso, G.; Maxhimer, J.B.; Roberts, D.D. Regulation of Nitric Oxide Signalling by Thrombospondin 1: Implications for Anti-Angiogenic Therapies. Nat. Rev. Cancer 2009, 9, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Hyodo, F.; Ridnour, L.A.; Shannon, C.S.; Wink, D.A.; Krishna, M.C.; Roberts, D.D. Thrombospondin 1 and Vasoactive Agents Indirectly Alter Tumor Blood Flow. Neoplasia 2008, 10, 886–896. [Google Scholar] [CrossRef]
- Gao, L.; Chen, K.; Gao, Q.; Wang, X.; Sun, J.; Yang, Y.-G. CD47 Deficiency in Tumor Stroma Promotes Tumor Progression by Enhancing Angiogenesis. Oncotarget 2017, 8, 22406–22413. [Google Scholar] [CrossRef] [PubMed]
- Jeanne, A.; Martiny, L.; Dedieu, S. Thrombospondin-Targeting TAX2 Peptide Impairs Tumor Growth in Preclinical Mouse Models of Childhood Neuroblastoma. Pediatr. Res. 2017, 81, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Cao, Y.; Wang, F.; Zhang, N.; Qi, Z.; Mao, X.; Guo, S.; Li, F.; Guo, Y.; Lin, Y.; et al. Bortezomib-Resistant Multiple Myeloma Patient-Derived Xenograft Is Sensitive to Anti-CD47 Therapy. Leuk. Res. 2022, 122, 106949. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Lindberg, F.P.; Gresham, H.D.; Reinhold, M.I.; Brown, E.J. Integrin-Associated Protein Immunoglobulin Domain Is Necessary for Efficient Vitronectin Bead Binding. J. Cell Biol. 1996, 134, 1313–1322. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Guo, N.H.; Rodrigues, R.G.; Kaiser, J.; Roberts, D.D. Pro-Adhesive and Chemotactic Activities of Thrombospondin-1 for Breast Carcinoma Cells Are Mediated by alpha3beta1 Integrin and Regulated by Insulin-like Growth Factor-1 and CD98. J. Biol. Chem. 1999, 274, 11408–11416. [Google Scholar] [CrossRef]
- Gao, A.G.; Lindberg, F.P.; Dimitry, J.M.; Brown, E.J.; Frazier, W.A. Thrombospondin Modulates Alpha v Beta 3 Function through Integrin-Associated Protein. J. Cell Biol. 1996, 135, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Shahan, T.A.; Fawzi, A.; Bellon, G.; Monboisse, J.C.; Kefalides, N.A. Regulation of Tumor Cell Chemotaxis by Type IV Collagen Is Mediated by a Ca(2+)-Dependent Mechanism Requiring CD47 and the Integrin alpha(V)beta(3). J. Biol. Chem. 2000, 275, 4796–4802. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Tomiyama, Y.; Ishikawa, J.; Oritani, K.; Matsumura, I.; Shiraga, M.; Yokota, T.; Okajima, Y.; Ogawa, M.; Miyagawa, J.I.; et al. Integrin-Associated protein/CD47 Regulates Motile Activity in Human B-Cell Lines through CDC42. Blood 2000, 96, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.E.; Li, Z.; Kara, M.; Gardner, K.L.; Roberts, D.D. Beta 1 Integrin- and Proteoglycan-Mediated Stimulation of T Lymphoma Cell Adhesion and Mitogen-Activated Protein Kinase Signaling by Thrombospondin-1 and Thrombospondin-1 Peptides. J. Immunol. 1999, 163, 3621–3628. [Google Scholar] [CrossRef]
- Barazi, H.O.; Li, Z.; Cashel, J.A.; Krutzsch, H.C.; Annis, D.S.; Mosher, D.F.; Roberts, D.D. Regulation of Integrin Function by CD47 Ligands. Differential Effects on Alpha Vbeta 3 and Alpha 4beta1 Integrin-Mediated Adhesion. J. Biol. Chem. 2002, 277, 42859–42866. [Google Scholar] [CrossRef]
- Hermann, P.; Armant, M.; Brown, E.; Rubio, M.; Ishihara, H.; Ulrich, D.; Caspary, R.G.; Lindberg, F.P.; Armitage, R.; Maliszewski, C.; et al. The Vitronectin Receptor and Its Associated CD47 Molecule Mediates Proinflammatory Cytokine Synthesis in Human Monocytes by Interaction with Soluble CD23. J. Cell Biol. 1999, 144, 767–775. [Google Scholar] [CrossRef]
- Armant, M.; Avice, M.N.; Hermann, P.; Rubio, M.; Kiniwa, M.; Delespesse, G.; Sarfati, M. CD47 Ligation Selectively Downregulates Human Interleukin 12 Production. J. Exp. Med. 1999, 190, 1175–1182. [Google Scholar] [CrossRef]
- Avice, M.N.; Rubio, M.; Sergerie, M.; Delespesse, G.; Sarfati, M. CD47 Ligation Selectively Inhibits the Development of Human Naive T Cells into Th1 Effectors. J. Immunol. 2000, 165, 4624–4631. [Google Scholar] [CrossRef]
- Barclay, A.N.; Brown, M.H. The SIRP Family of Receptors and Immune Regulation. Nat. Rev. Immunol. 2006, 6, 457–464. [Google Scholar] [CrossRef]
- Catalán, R.; Orozco-Morales, M.; Hernández-Pedro, N.Y.; Guijosa, A.; Colín-González, A.L.; Ávila-Moreno, F.; Arrieta, O. CD47-SIRPα Axis as a Biomarker and Therapeutic Target in Cancer: Current Perspectives and Future Challenges in Nonsmall Cell Lung Cancer. J. Immunol. Res. 2020, 2020, 9435030. [Google Scholar] [CrossRef]
- Hatherley, D.; Harlos, K.; Dunlop, D.C.; Stuart, D.I.; Barclay, A.N. The Structure of the Macrophage Signal Regulatory Protein α (SIRPα) Inhibitory Receptor Reveals a Binding Face Reminiscent of That Used by T Cell Receptors. J. Biol. Chem. 2007, 282, 14567–14575. [Google Scholar] [CrossRef]
- Brooke, G.; Holbrook, J.D.; Brown, M.H.; Barclay, A.N. Human Lymphocytes Interact Directly with CD47 through a Novel Member of the Signal Regulatory Protein (SIRP) Family. J. Immunol. 2004, 173, 2562–2570. [Google Scholar] [CrossRef]
- Stefanidakis, M.; Newton, G.; Lee, W.Y.; Parkos, C.A.; Luscinskas, F.W. Endothelial CD47 Interaction with SIRPgamma Is Required for Human T-Cell Transendothelial Migration under Shear Flow Conditions in Vitro. Blood 2008, 112, 1280–1289. [Google Scholar] [CrossRef]
- Dehmani, S.; Nerrière-Daguin, V.; Néel, M.; Elain-Duret, N.; Heslan, J.-M.; Belarif, L.; Mary, C.; Thepenier, V.; Biteau, K.; Poirier, N.; et al. SIRPγ-CD47 Interaction Positively Regulates the Activation of Human T Cells in Situation of Chronic Stimulation. Front. Immunol. 2021, 12, 732530. [Google Scholar] [CrossRef]
- Gauttier, V.; Pengam, S.; Durand, J.; Biteau, K.; Mary, C.; Morello, A.; Néel, M.; Porto, G.; Teppaz, G.; Thepenier, V.; et al. Selective SIRPα Blockade Reverses Tumor T Cell Exclusion and Overcomes Cancer Immunotherapy Resistance. J. Clin. Investig. 2020, 130, 6109–6123. [Google Scholar] [CrossRef]
- Frazier, W.A.; Gao, A.G.; Dimitry, J.; Chung, J.; Brown, E.J.; Lindberg, F.P.; Linder, M.E. The Thrombospondin Receptor Integrin-Associated Protein (CD47) Functionally Couples to Heterotrimeric Gi. J. Biol. Chem. 1999, 274, 8554–8560. [Google Scholar] [CrossRef]
- Zhang, C.; Saunders, A.J. An Emerging Role for Ubiquilin 1 in Regulating Protein Quality Control System and in Disease Pathogenesis. Discov. Med. 2009, 8, 18–22. [Google Scholar]
- Wu, A.L.; Wang, J.; Zheleznyak, A.; Brown, E.J. Ubiquitin-Related Proteins Regulate Interaction of Vimentin Intermediate Filaments with the Plasma Membrane. Mol. Cell 1999, 4, 619–625. [Google Scholar] [CrossRef]
- N’Diaye, E.-N.; Brown, E.J. The Ubiquitin-Related Protein PLIC-1 Regulates Heterotrimeric G Protein Function through Association with Gbetagamma. J. Cell Biol. 2003, 163, 1157–1165. [Google Scholar] [CrossRef]
- Sick, E.; Boukhari, A.; Deramaudt, T.; Rondé, P.; Bucher, B.; André, P.; Gies, J.-P.; Takeda, K. Activation of CD47 Receptors Causes Proliferation of Human Astrocytoma but Not Normal Astrocytes via an Akt-Dependent Pathway. Glia 2011, 59, 308–319. [Google Scholar] [CrossRef]
- Chung, J.; Gao, A.G.; Frazier, W.A. Thrombspondin Acts via Integrin-Associated Protein to Activate the Platelet Integrin alphaIIbbeta3. J. Biol. Chem. 1997, 272, 14740–14746. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lindberg, F.P.; Frazier, W.A. Integrin-Associated Protein Stimulates alpha2beta1-Dependent Chemotaxis via Gi-Mediated Inhibition of Adenylate Cyclase and Extracellular-Regulated Kinases. J. Cell Biol. 1999, 147, 389–400. [Google Scholar] [CrossRef]
- Manna, P.P.; Frazier, W.A. The Mechanism of CD47-Dependent Killing of T Cells: Heterotrimeric Gi-Dependent Inhibition of Protein Kinase A. J. Immunol. 2003, 170, 3544–3553. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Miller, T.W.; Pendrak, M.L.; DeGraff, W.G.; Sullivan, C.; Ridnour, L.A.; Abu-Asab, M.; Wink, D.A.; Tsokos, M.; Roberts, D.D. CD47 Deficiency Confers Cell and Tissue Radioprotection by Activation of Autophagy. Autophagy 2012, 8, 1628–1642. [Google Scholar] [CrossRef]
- Kalas, W.; Swiderek, E.; Switalska, M.; Wietrzyk, J.; Rak, J.; Strzadala, L. Thrombospondin-1 Receptor Mediates Autophagy of RAS-Expressing Cancer Cells and Triggers Tumour Growth Inhibition. Anticancer. Res. 2013, 33, 1429–1438. [Google Scholar] [PubMed]
- Pedersen, N.; Mortensen, S.; Sørensen, S.B.; Pedersen, M.W.; Rieneck, K.; Bovin, L.F.; Poulsen, H.S. Transcriptional Gene Expression Profiling of Small Cell Lung Cancer Cells. Cancer Res. 2003, 63, 1943–1953. [Google Scholar]
- Weiskopf, K.; Jahchan, N.S.; Schnorr, P.J.; Cristea, S.; Ring, A.M.; Maute, R.L.; Volkmer, A.K.; Volkmer, J.-P.; Liu, J.; Lim, J.S.; et al. CD47-Blocking Immunotherapies Stimulate Macrophage-Mediated Destruction of Small-Cell Lung Cancer. J. Clin. Investig. 2016, 126, 2610–2620. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Yang, L.; Li, H.; Li, R.; Yu, J.; Yang, L.; Wei, F.; Yan, C.; Sun, Q.; et al. Anti-CD47 Antibody As a Targeted Therapeutic Agent for Human Lung Cancer and Cancer Stem Cells. Front. Immunol. 2017, 8, 404. [Google Scholar] [CrossRef]
- Peng, Y.; Qiu, B.; Tan, F.; Xu, J.; Bie, F.; He, H.; Liu, L.; Tian, H.; Bai, G.; Zhou, B.; et al. TIGIT/CD47 Dual High Expression Predicts Prognosis and Is Associated with Immunotherapy Response in Lung Squamous Cell Carcinoma. Thorac. Cancer 2022, 13, 2014–2023. [Google Scholar] [CrossRef]
- Luo, W.; Zeng, Z.; Jin, Y.; Yang, L.; Fan, T.; Wang, Z.; Pan, Y.; Yang, Y.; Yao, M.; Li, Y.; et al. Distinct Immune Microenvironment of Lung Adenocarcinoma in Never-Smokers from Smokers. Cell Rep. Med. 2023, 4, 101078. [Google Scholar] [CrossRef]
- Nigro, A.; Ricciardi, L.; Salvato, I.; Sabbatino, F.; Vitale, M.; Crescenzi, M.A.; Montico, B.; Triggiani, M.; Pepe, S.; Stellato, C.; et al. Enhanced Expression of CD47 Is Associated with Off-Target Resistance to Tyrosine Kinase Inhibitor Gefitinib in NSCLC. Front. Immunol. 2019, 10, 3135. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Heymach, J.V. Co-Occurring Genomic Alterations in Non-Small-Cell Lung Cancer Biology and Therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari Kenzerki, M.; Ahmadi, M.; Mousavi, P.; Ghafouri-Fard, S. MYC and Non-Small Cell Lung Cancer: A Comprehensive Review. Hum. Genet. 2023, 37, 201185. [Google Scholar] [CrossRef]
- Wang, L.-L.; Wan, X.-Y.; Wang, T.-L.; Liu, C.-Q.; Zheng, F.-M. NDR1 Activates CD47 Transcription by Increasing Protein Stability and Nuclear Location of ASCL1 to Enhance Cancer Stem Cell Properties and Evasion of Phagocytosis in Small Cell Lung Cancer. Med. Oncol. 2022, 39, 254. [Google Scholar] [CrossRef]
- Ye, Z.-H.; Jiang, X.-M.; Huang, M.-Y.; Xu, Y.-L.; Chen, Y.-C.; Yuan, L.-W.; Huang, C.-Y.; Yu, W.-B.; Chen, X.; Lu, J.-J. Regulation of CD47 Expression by Interferon-Gamma in Cancer Cells. Transl. Oncol. 2021, 14, 101162. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Cui, Z.; Xu, D.; Zhang, F.; Sun, J.; Song, L.; Ye, W.; Zeng, J.; Zhou, M.; Ruan, Z.; Zhang, L.; et al. CD47 Blockade Enhances Therapeutic Efficacy of Cisplatin against Lung Carcinoma in a Murine Model. Exp. Cell Res. 2021, 405, 112677. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Fang, M.; Chen, T.; Wang, H.; Zhou, Q.; Wei, Y.; Zheng, L.; Zhou, Y.; Chen, K. The Mechanism of Low-Dose Radiation-Induced Upregulation of Immune Checkpoint Molecule Expression in Lung Cancer Cells. Biochem. Biophys. Res. Commun. 2022, 608, 102–107. [Google Scholar] [CrossRef]
- Lugade, A.A.; Sorensen, E.W.; Gerber, S.A.; Moran, J.P.; Frelinger, J.G.; Lord, E.M. Radiation-Induced IFN-Gamma Production within the Tumor Microenvironment Influences Antitumor Immunity. J. Immunol. 2008, 180, 3132–3139. [Google Scholar] [CrossRef]
- Gerber, S.A.; Sedlacek, A.L.; Cron, K.R.; Murphy, S.P.; Frelinger, J.G.; Lord, E.M. IFN-γ Mediates the Antitumor Effects of Radiation Therapy in a Murine Colon Tumor. Am. J. Pathol. 2013, 182, 2345–2354. [Google Scholar] [CrossRef]
- Lim, J.Y.H.; Gerber, S.A.; Murphy, S.P.; Lord, E.M. Type I Interferons Induced by Radiation Therapy Mediate Recruitment and Effector Function of CD8(+) T Cells. Cancer Immunol. Immunother. 2014, 63, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, T.; Yang, H.; Chen, J.; Wen, X.; Jiang, Z.; Yi, H.; Luo, Y. Interferon Regulatory Factor-1 Regulates Cisplatin-Induced Apoptosis and Autophagy in A549 Lung Cancer Cells. Med. Oncol. 2022, 39, 38. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Olivero, M.; Corà, D.; Di Renzo, M.F. IRF-1 Expression Is Induced by Cisplatin in Ovarian Cancer Cells and Limits Drug Effectiveness. Eur. J. Cancer 2013, 49, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Storozynsky, Q.; Hitt, M.M. The Impact of Radiation-Induced DNA Damage on cGAS-STING-Mediated Immune Responses to Cancer. Int. J. Mol. Sci. 2020, 21, 8877. [Google Scholar] [CrossRef]
- Liu, X.; Pu, Y.; Cron, K.; Deng, L.; Kline, J.; Frazier, W.A.; Xu, H.; Peng, H.; Fu, Y.-X.; Xu, M.M. CD47 Blockade Triggers T Cell-Mediated Destruction of Immunogenic Tumors. Nat. Med. 2015, 21, 1209–1215. [Google Scholar] [CrossRef]
- Hassan, E.M.; McWhirter, S.; Walker, G.C.; Martinez-Rubi, Y.; Zou, S. Elimination of Cancer Cells in Co-Culture: Role of Different Nanocarriers in Regulation of CD47 and Calreticulin-Induced Phagocytosis. ACS Appl. Mater. Interfaces 2023, 15, 3791–3803. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Fan, J.; Chen, W.; Luan, J.; Mei, X.; Wang, S.; Li, Y.; Ye, L.; Li, S.; et al. Blocking CD47 Efficiently Potentiated Therapeutic Effects of Anti-Angiogenic Therapy in Non-Small Cell Lung Cancer. J. Immunother. Cancer 2019, 7, 346. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Wang, S.; Li, Y.; Wang, Y.; Li, S.; Luan, J.; Wang, Z.; Song, P.; Chen, Q.; et al. Targeting CD47 and Autophagy Elicited Enhanced Antitumor Effects in Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2017, 5, 363–375. [Google Scholar] [CrossRef]
- Raniszewska, A.; Kwiecień, I.; Sokołowski, R.; Rutkowska, E.; Domagała-Kulawik, J. Immunomodulatory Molecules On Lung Cancer Stem Cells From Lymph Nodes Aspirates. Cancers 2020, 12, 838. [Google Scholar] [CrossRef]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef]
- Blaquier, J.B.; Cardona, A.F.; Recondo, G. Resistance to KRASG12C Inhibitors in Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 787585. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Volkmer, J.-P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-Signal Regulatory Protein Alpha (SIRPa) Interaction Is a Therapeutic Target for Human Solid Tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Jan, M.; Weissman-Tsukamoto, R.; Zhao, F.; Park, C.Y.; Weissman, I.L.; Majeti, R. Therapeutic Antibody Targeting of CD47 Eliminates Human Acute Lymphoblastic Leukemia. Cancer Res. 2011, 71, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.; Volkmer, J.-P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 Antibody-Mediated Phagocytosis of Cancer by Macrophages Primes an Effective Antitumor T-Cell Response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kwon, H.; Li, Z.; Fu, Y.-X. Is CD47 an Innate Immune Checkpoint for Tumor Evasion? J. Hematol. Oncol. 2017, 10, 12. [Google Scholar] [CrossRef]
- Kwong, L.S.; Brown, M.H.; Barclay, A.N.; Hatherley, D. Signal-Regulatory Protein α from the NOD Mouse Binds Human CD47 with an Exceptionally High Affinity—Implications for Engraftment of Human Cells. Immunology 2014, 143, 61–67. [Google Scholar] [CrossRef]
- Veillette, A.; Chen, J. SIRPα-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Xiao, W.; Zhao, Y.; Pi, J.; Xu, H.; Zhao, H.; Xu, J.; Evans, C.E.; Jin, H. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis. Front. Immunol. 2020, 11, 18. [Google Scholar] [CrossRef]
- Yang, H.; Xun, Y.; You, H. The Landscape Overview of CD47-Based Immunotherapy for Hematological Malignancies. Biomark. Res. 2023, 11, 15. [Google Scholar] [CrossRef]
- Eladl, E.; Tremblay-LeMay, R.; Rastgoo, N.; Musani, R.; Chen, W.; Liu, A.; Chang, H. Role of CD47 in Hematological Malignancies. J. Hematol. Oncol. 2020, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’Rourke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients with Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, N.J.; Chow, L.Q.M.; Gainor, J.F.; LoRusso, P.; Lee, K.-W.; Chung, H.C.; Lee, J.; Bang, Y.-J.; Hodi, F.S.; Kim, W.S.; et al. Evorpacept Alone and in Combination with Pembrolizumab or Trastuzumab in Patients with Advanced Solid Tumours (ASPEN-01): A First-in-Human, Open-Label, Multicentre, Phase 1 Dose-Escalation and Dose-Expansion Study. Lancet Oncol. 2021, 22, 1740–1751. [Google Scholar] [CrossRef] [PubMed]
- Andrejeva, G.; Capoccia, B.J.; Hiebsch, R.R.; Donio, M.J.; Darwech, I.M.; Puro, R.J.; Pereira, D.S. Novel SIRPα Antibodies That Induce Single-Agent Phagocytosis of Tumor Cells While Preserving T Cells. J. Immunol. 2021, 206, 712–721. [Google Scholar] [CrossRef]
- Ansell, S.M.; Maris, M.B.; Lesokhin, A.M.; Chen, R.W.; Flinn, I.W.; Sawas, A.; Minden, M.D.; Villa, D.; Percival, M.-E.M.; Advani, A.S.; et al. Phase I Study of the CD47 Blocker TTI-621 in Patients with Relapsed or Refractory Hematologic Malignancies. Clin. Cancer Res. 2021, 27, 2190–2199. [Google Scholar] [CrossRef]
- Yu, J.; Song, Y.; Tian, W. How to Select IgG Subclasses in Developing Anti-Tumor Therapeutic Antibodies. J. Hematol. Oncol. 2020, 13, 45. [Google Scholar] [CrossRef]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef]
- Weiskopf, K.; Ring, A.M.; Ho, C.C.M.; Volkmer, J.-P.; Levin, A.M.; Volkmer, A.K.; Ozkan, E.; Fernhoff, N.B.; van de Rijn, M.; Weissman, I.L.; et al. Engineered SIRPα Variants as Immunotherapeutic Adjuvants to Anticancer Antibodies. Science 2013, 341, 88–91. [Google Scholar] [CrossRef]
- Petrova, P.S.; Viller, N.N.; Wong, M.; Pang, X.; Lin, G.H.Y.; Dodge, K.; Chai, V.; Chen, H.; Lee, V.; House, V.; et al. TTI-621 (SIRPαFc): A CD47-Blocking Innate Immune Checkpoint Inhibitor with Broad Antitumor Activity and Minimal Erythrocyte Binding. Clin. Cancer Res. 2017, 23, 1068–1079. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Guo, S.; Zhang, L.; Sharma, A.; Robertson, G.P.; Huang, L. Intravenous Delivery of siRNA Targeting CD47 Effectively Inhibits Melanoma Tumor Growth and Lung Metastasis. Mol. Ther. 2013, 21, 1919–1929. [Google Scholar] [CrossRef]
- Rastgoo, N.; Wu, J.; Liu, A.; Pourabdollah, M.; Atenafu, E.G.; Reece, D.; Chen, W.; Chang, H. Targeting CD47/TNFAIP8 by miR-155 Overcomes Drug Resistance and Inhibits Tumor Growth through Induction of Phagocytosis and Apoptosis in Multiple Myeloma. Haematologica 2020, 105, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Uscanga-Palomeque, A.C.; Calvillo-Rodríguez, K.M.; Gómez-Morales, L.; Lardé, E.; Denèfle, T.; Caballero-Hernández, D.; Merle-Béral, H.; Susin, S.A.; Karoyan, P.; Martínez-Torres, A.C.; et al. CD47 Agonist Peptide PKHB1 Induces Immunogenic Cell Death in T-Cell Acute Lymphoblastic Leukemia Cells. Cancer Sci. 2019, 110, 256–268. [Google Scholar] [CrossRef]
- Daginakatte, G.C.; Pottayil, S.; Naremaddepalli, S.; Chennakrishnareddy, G.; Bilugudi, P.; Tangella, S.K.; Patil, S.; Gowda, N.; Aithal, K.; Balasubramanian, W.R.; et al. Abstract 3500: AUR103 an Oral Small Molecule CD47 Antagonist in Combination with Azacytidine and Bortezomib Exhibits Potent Anti-Tumor Activity in Myeloma and Leukemia Models in Vitro and in Vivo. Cancer Res. 2022, 82, 3500. [Google Scholar] [CrossRef]
- Chiang, Z.-C.; Fang, S.; Shen, Y.-K.; Cui, D.; Weng, H.; Wang, D.; Zhao, Y.; Lin, J.; Chen, Q. Development of Novel CD47-Specific ADCs Possessing High Potency Against Non-Small Cell Lung Cancer in Vitro and in Vivo. Front. Oncol. 2022, 12, 857927. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Zhang, Y.; Guan, J.-S.; Ngo, H.G.; Totoro, A.; Singh, A.P.; Chen, K.; Xu, Y.; Yang, E.S.; Zhou, L.; et al. Anti-CD47 Monoclonal Antibody-Drug Conjugate: A Targeted Therapy to Treat Triple-Negative Breast Cancers. Vaccines 2021, 9, 882. [Google Scholar] [CrossRef]
- Son, J.; Hsieh, R.C.-E.; Lin, H.Y.; Krause, K.J.; Yuan, Y.; Biter, A.B.; Welsh, J.; Curran, M.A.; Hong, D.S. Inhibition of the CD47-SIRPα Axis for Cancer Therapy: A Systematic Review and Meta-Analysis of Emerging Clinical Data. Front. Immunol. 2022, 13, 1027235. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Agbor-Enoh, S.; Jang, M.K.; Alawi, M.; Saygi, C.; Gravina, A.; Tediashvili, G.; Nguyen, V.Q.; Liu, Y.; et al. The SIRPα-CD47 Immune Checkpoint in NK Cells. J. Exp. Med. 2021, 218, e20200839. [Google Scholar] [CrossRef]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep. 2018, 23, 3946–3959.e6. [Google Scholar] [CrossRef]
- Li, H.; van der Merwe, P.A.; Sivakumar, S. Biomarkers of Response to PD-1 Pathway Blockade. Br. J. Cancer 2022, 126, 1663–1675. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Nagy, J.A.; Dvorak, A.M. Structure of Solid Tumors and Their Vasculature: Implications for Therapy with Monoclonal Antibodies. Cancer Cells 1991, 3, 77–85. [Google Scholar]
- Abrisqueta, P.; Sancho, J.-M.; Cordoba, R.; Persky, D.O.; Andreadis, C.; Huntington, S.F.; Carpio, C.; Morillo Giles, D.; Wei, X.; Li, Y.F.; et al. Anti-CD47 Antibody, CC-90002, in Combination with Rituximab in Subjects with Relapsed And/or Refractory Non-Hodgkin Lymphoma (R/R NHL). Blood 2019, 134, 4089. [Google Scholar] [CrossRef]
- Berlin, J.; Harb, W.; Adjei, A.; Xing, Y.; Swiecicki, P.; Seetharam, M.; Nandagopal, L.; Gopal, A.; Xu, C.; Meng, Y.; et al. 385 A First-in-Human Study of Lemzoparlimab, a Differentiated Anti-CD47 Antibody, in Subjects with Relapsed/refractory Malignancy: Initial Monotherapy Results. J. Immunother. Cancer 2020, 8, A233–A234. [Google Scholar] [CrossRef]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.Y.X.; Ho, J.; Demaria, S.; Ferrari, M.; Grattoni, A. Emerging Technologies for Local Cancer Treatment. Adv. Ther. 2020, 3, 2000027. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, C.; Thompson, J.A.; Taylor, M.H.; DeSimone, J.A.; Zain, J.M.; Shustov, A.R.; Johns, C.; McCann, S.; Lin, G.H.Y.; Petrova, P.S.; et al. Intralesional TTI-621, a Novel Biologic Targeting the Innate Immune Checkpoint CD47, in Patients with Relapsed or Refractory Mycosis Fungoides or Sézary Syndrome: A Multicentre, Phase 1 Study. Lancet Haematol. 2021, 8, e808–e817. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Li, S.; Zhao, L.; Li, D.; Cao, Z.; Xu, X.; Yang, X. A siRNA-Assisted Assembly Strategy to Simultaneously Suppress “Self” and Upregulate “Eat-Me” Signals for Nanoenabled Chemo-Immunotherapy. ACS Nano 2021, 15, 16030–16042. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Ma, R.; Du, Y.; Han, B. Cationic Lipid-Assisted Nanoparticles for Simultaneous Delivery of CD47 siRNA and R848 to Promote Antitumor Immune Responses. Front. Pharmacol. 2023, 14, 1142374. [Google Scholar] [CrossRef]
- Li, Y.; Meng, X.; Chen, G.; Hou, Y.; Wu, X.; Wang, J.; Cong, X.; Mao, K.; Wu, C.; Chen, H.; et al. Lipid-Mediated Delivery of CD47 siRNA Aids JQ1 in Ensuring Simultaneous Downregulation of PD-L1 and CD47 and Improves Antitumor Immunotherapy Efficacy. Biomater. Sci. 2022, 10, 6755–6767. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Li, H.; Mao, K.; Wang, H.; Meng, X.; Wang, J.; Wu, C.; Chen, H.; Wang, X.; et al. An Optimized Ionizable Cationic Lipid for Brain Tumor-Targeted siRNA Delivery and Glioblastoma Immunotherapy. Biomaterials 2022, 287, 121645. [Google Scholar] [CrossRef]
- Lian, S.; Xie, R.; Ye, Y.; Xie, X.; Li, S.; Lu, Y.; Li, B.; Cheng, Y.; Katanaev, V.L.; Jia, L. Simultaneous Blocking of CD47 and PD-L1 Increases Innate and Adaptive Cancer Immune Responses and Cytokine Release. EBioMedicine 2019, 42, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-Q.; Liu, R.; Chen, F.-M.; Zhang, J.-Y.; Zheng, S.-J.; Shao, D.; Du, J.-Z. Nanoparticle-Mediated CD47-SIRPα Blockade and Calreticulin Exposure for Improved Cancer Chemo-Immunotherapy. ACS Nano 2023, 17, 8966–8979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, Y.; Chen, L.; Zhang, X.; Wu, W.; Yang, Z.; Li, X.; Wang, Y.; Hu, Z.; Wang, Z. Co-Delivery of Phagocytosis Checkpoint and STING Agonist by a Trojan Horse Nanocapsule for Orthotopic Glioma Immunotherapy. Theranostics 2022, 12, 5488–5503. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Wu, L.; Liu, Z.; Tian, R.; Yu, G.; Zhou, Z.; Yang, K.; Xiong, H.-G.; Zhang, A.; Yu, G.-T.; et al. Hybrid Cellular Membrane Nanovesicles Amplify Macrophage Immune Responses against Cancer Recurrence and Metastasis. Nat. Commun. 2020, 11, 4909. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, R.; van Meerten, T.; Bremer, E. CD47-SIRPα Blocking-Based Immunotherapy: Current and Prospective Therapeutic Strategies. Clin. Transl. Med. 2022, 12, e943. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic Cell Death in Cancer and Infectious Disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Yu, W.-B.; Chen, Y.-C.; Huang, C.-Y.; Ye, Z.-H.; Shi, W.; Zhu, H.; Shi, J.-J.; Chen, J.; Lu, J.-J. CD47 Blockade Improves the Therapeutic Effect of Osimertinib in Non-Small Cell Lung Cancer. Front. Med. 2023, 17, 105–118. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Ren, Z.; Yang, K.; Xu, H.; Luan, Y.; Fu, K.; Guo, J.; Peng, H.; Zhu, M.; et al. Dual Targeting of Innate and Adaptive Checkpoints on Tumor Cells Limits Immune Evasion. Cell Rep. 2018, 24, 2101–2111. [Google Scholar] [CrossRef]
- Feliz-Mosquea, Y.R.; Christensen, A.A.; Wilson, A.S.; Westwood, B.; Varagic, J.; Meléndez, G.C.; Schwartz, A.L.; Chen, Q.-R.; Mathews Griner, L.; Guha, R.; et al. Combination of Anthracyclines and Anti-CD47 Therapy Inhibit Invasive Breast Cancer Growth While Preventing Cardiac Toxicity by Regulation of Autophagy. Breast Cancer Res. Treat. 2018, 172, 69–82. [Google Scholar] [CrossRef]
- Lu, J.; Li, J.; Lin, Z.; Li, H.; Lou, L.; Ding, W.; Ouyang, S.; Wu, Y.; Wen, Y.; Chen, X.; et al. Reprogramming of TAMs via the STAT3/CD47-SIRPα Axis Promotes Acquired Resistance to EGFR-TKIs in Lung Cancer. Cancer Lett. 2023, 564, 216205. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Chung, H.; Kim, T.M.; Lakhani, N.; Messersmith, W.; Santana-Davila, R.; Kim, W.S.; LoRusso, P.; Bang, Y.-J.; Chow, L.; et al. 498 Evorpacept (ALX148), a CD47 Myeloid Checkpoint Inhibitor, in Patients with Head and Neck Squamous Cell Carcinoma (HNSCC) and with Gastric/gastroesophageal Cancer (GC); ASPEN-01. J. Immunother. Cancer 2021, 9, A530. [Google Scholar] [CrossRef]
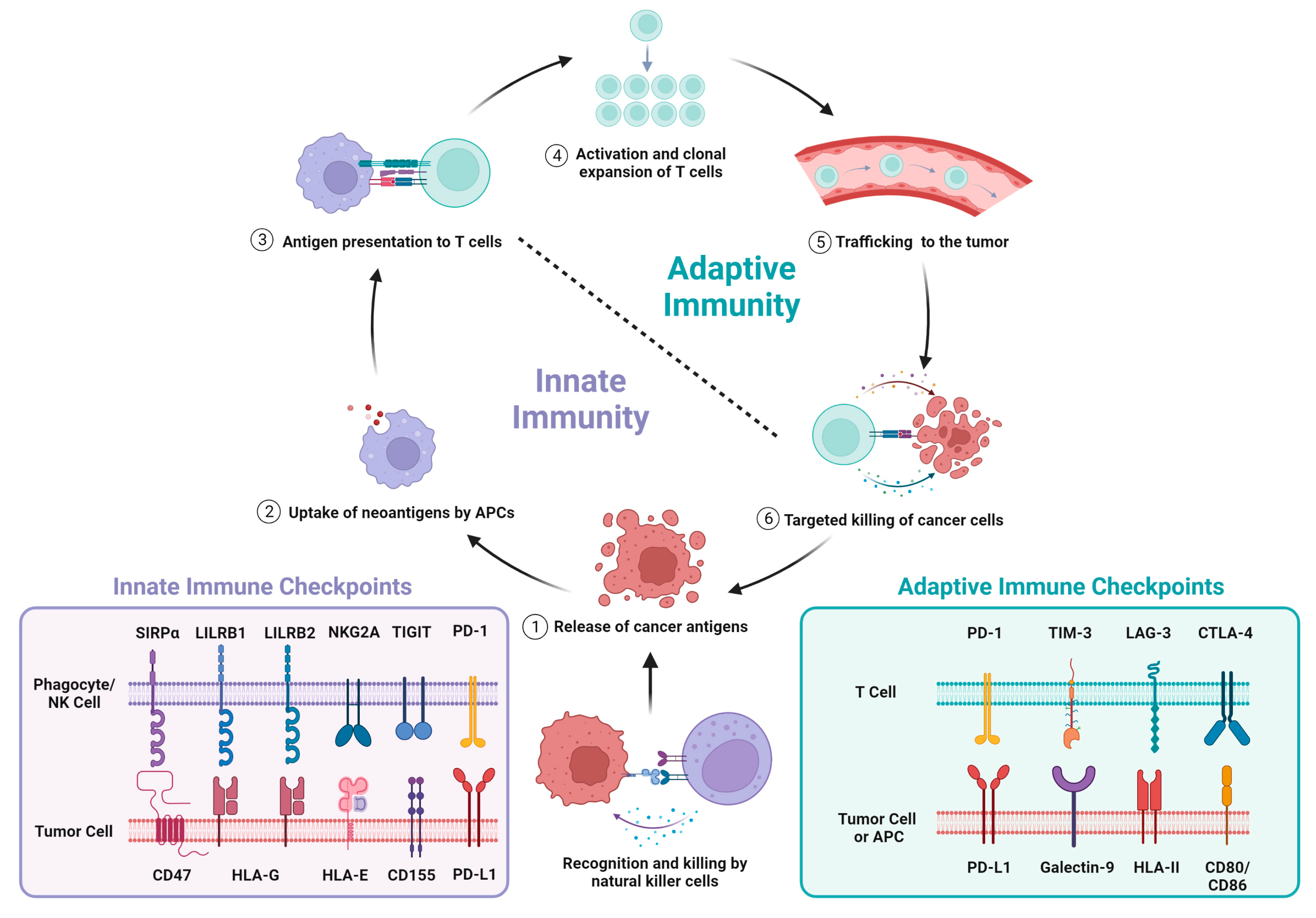

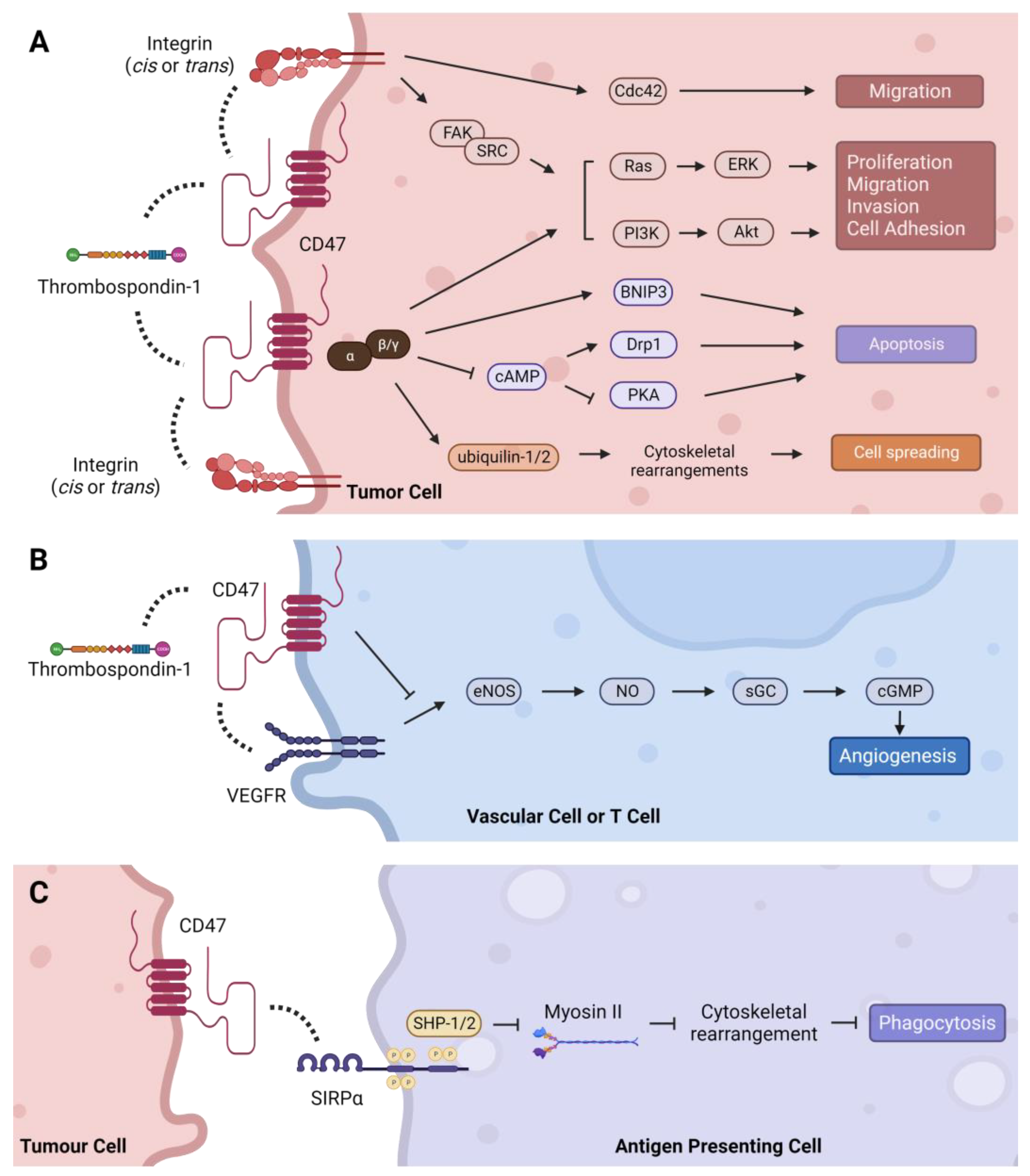
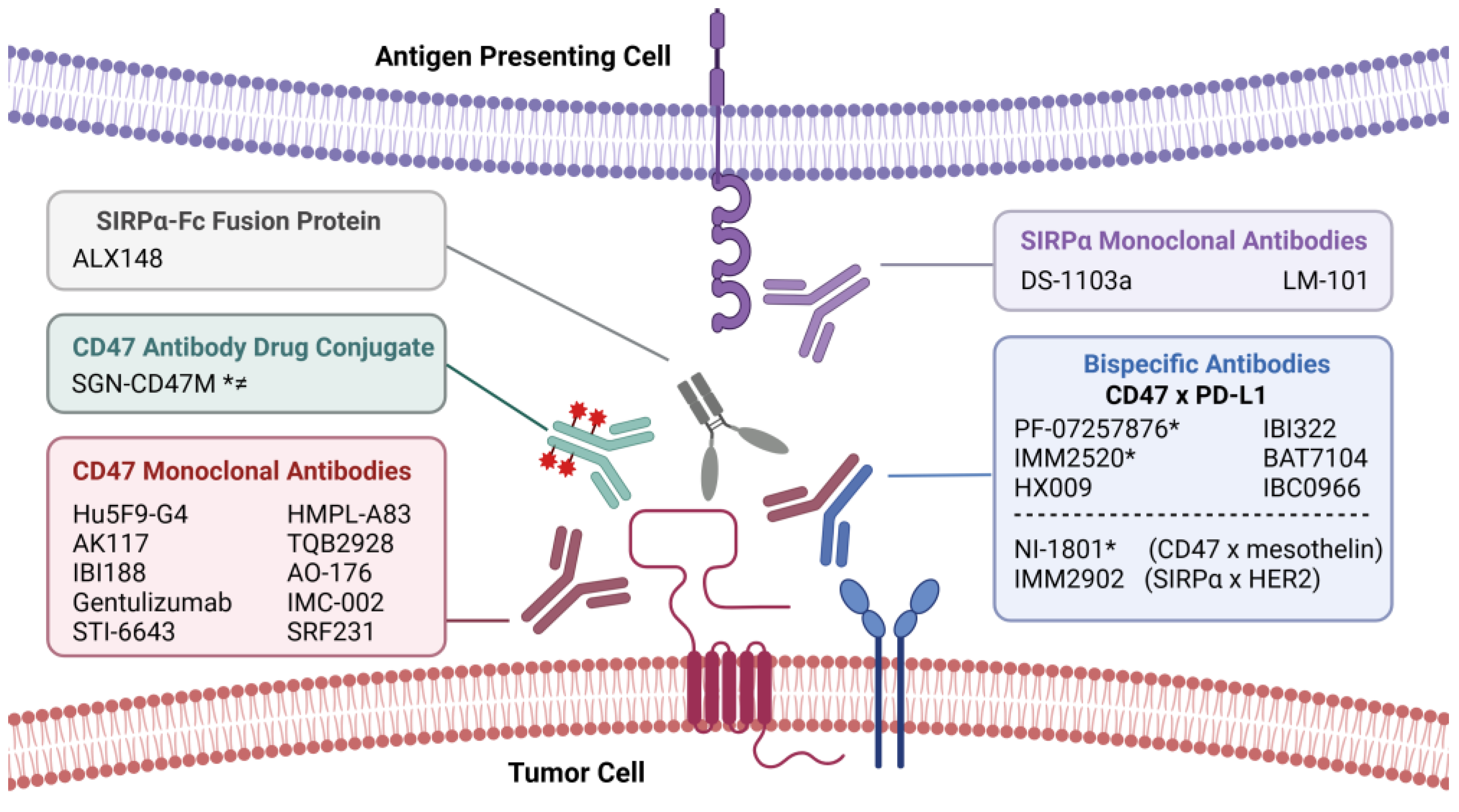
| Lung Cancer | ||||
|---|---|---|---|---|
| Cancer Type | Sample Size | % CD47 Expression | Prognostic Association or Clinical Significance | References |
| Non-Small Cell Lung Cancer | 169 | 84 (>1% cells) 65.7 (>50% cells) | Worse PFS and OS | [50] |
| 191 | 53.4 (>10% cells) 33 (>10% strong staining) | Worse OS and DFS | [125] | |
| 80 | 57.5 (IRS ≥ 6) | Advanced TNM stage, metastasis, ≠ | [126] | |
| 384 | 49.7 (IRS ≥ 3) | Advanced TNM stage and histological subtype, tumor size; worse RFS | [127] | |
| 84 | 50 (>median staining) | Worse OS | [128] | |
| 98 | 29.6 (>50% cells) | Advanced tumor stage, € | [129] | |
| 120 | 75 (IRS > 1) | LN metastasis, ≠ | [130] | |
| 430 | 68.8 (TPS > 5%) | Worse OS, TNM stage | [131] | |
| Small-Cell Lung Cancer | 104 | 84.6 (≥10% cells) | Worse OS and DFS | [132] |
| 29 | 27.5 (>1% cells) | None reported, € | [133] | |
| Other Cancers | ||||
| Cancer Type | Sample Size | % CD47 Expression | Prognostic Association or Clinical Significance | References |
| Acute Myeloid Leukemia | 171 | 94.7 (detectable staining) 18.7 (≥3) | Higher tumor load, € | [134] |
| Bladder Cancer | 87 | 43.7 (IRS ≥ 1) 9.2 (IRS ≥ 8) | None reported, € | [135] |
| Breast Invasive Ductal Carcinoma | 137 | 61.3 (≥0.8) ↟ | Advanced TNM stage and histological grade; worse OS and DFS | [136] |
| Classical Hodgkin Lymphoma | 16 | 100 (≥1+) 50 (≥2+) | None reported, ≠ | [137] |
| Colorectal Cancer | 468 | 53.4 (IRS >5) | Distant metastasis; advanced TNM stage; worse OS | [138] |
| 293 | 53 (IRS ≥4) | Advanced TNM stage; worse OS and DFS | [139] | |
| Diffuse Large B-cell Lymphoma | 55 | 54.5 (cutoff not specified) | Worse OS | [140] |
| 120 | 59 (>median staining) | MYC+, € | [141] | |
| 238 | 63.1 (≥1) ↡ 16.4 (≥3) ↡ | None reported, € | [142] | |
| Endometrial Carcinoma | 165 | 94.6 (>1% cells) 21.2 (>50% cells) | Advanced histological grade, € | [143] |
| Esophageal Squamous Cell Cancer | 102 | 54.9 (>median cells) | LN metastasis; worse OS and disease-specific survival | [144] |
| Glioblastoma | 46 | 82.6 (cutoff not specified) | Disease recurrence; worse OS | [145] |
| Hepatocellular Carcinoma | 166 | 21.7 (>10% cells) | Advanced stage; frequent vessel invasion, € | [146] |
| 390 | 49.5 (>median cells) | Advanced TNM stage; increased vascular invasion; worse OS and RFS | [147] | |
| Nasopharyngeal Carcinoma | 66 | 56.1 (≥10% cells) | Worse OS; increased disease recurrence | [148] |
| Osteosarcoma | 20 | 85 (cutoff not specified) | Metastasis; worse OS and PFS | [149] |
| Ovarian cancer | 26 | 46.2 (≥2+) | Worse PFS | [150] |
| 116 | 90.5 (IRS ≥ 1) 60.3 (IRS ≥ 5) | Advanced stage; LN metastasis; worse OS | [151] | |
| 86 | 91.9 (IRS ≥ 1) 60.5 (IRS ≥ 5) | Tumor stage; chemo-resistance; worse OS | [152] | |
| Pancreatic Neuroendocrine Tumor | 47 | 100 (H-score ≥ 1) | Disease recurrence/death; lymph node metastasis; lymph node and perineural invasion, * | [153] |
| Triple Negative Breast Cancer | 57 | 77.2 (IRS ≥ 7) | Advanced TNM stage; LN invasion; increased recurrence; worse DFS | [154] |
| Trial | Treatment(s) | Indication | Phase | Status |
|---|---|---|---|---|
| CD47 Monoclonal Antibody | ||||
| NCT04349969 | AK117 | Malignant Neoplasms | I | Completed |
| NCT05235542 | AK117 ± AK104, Oxaliplatin, Cisplatin, Paclitaxel, Irinotecan, Docetaxel, 5-FU | Advanced Malignant Tumors | I, II | Ongoing |
| NCT05214482 | AK117 + AK112 ± Chemotherapy | Advanced Malignant Tumors | I, II | Ongoing |
| NCT05229497 | AK117 + AK112 ± Carboplatin, Cisplatin, 5-FU | Advanced Malignant Tumors | I, II | Ongoing |
| NCT03763149 | IBI188 | Advanced Malignancies | I | Ongoing |
| NCT05221385 | Gentulizumab | Solid Tumors, Non-Hodgkin Lymphoma | I | Ongoing |
| NCT04900519 | STI-6643 | Solid Tumors, Relapsed Solid Neoplasms, Refractory Tumors | I | Ongoing |
| NCT05429008 | HMPL-A83 | Advanced Tumors | I | Ongoing |
| NCT05192512 | TQB2928 | Advanced Cancers | I | Ongoing |
| NCT03834948 | AO-176 ± Paclitaxel, Pembrolizumab | Solid Tumors | I, II | Completed |
| NCT05276310 | IMC-002 | Advanced Cancer | I | Ongoing |
| NCT03512340 | SRF231 | Advanced Solid Cancers, Hematologic Cancers | I | Completed |
| NCT02216409 | Hu5F9-G4 (Magrolimab) | Solid Tumors | I | Completed |
| CD47–PD-L1 Bispecific Antibody | ||||
| NCT05780307 | IMM2520 | Advanced Solid Tumors, NSCLC, SCLC, Breast Cancer, SCC Head and Neck, CRC | I | Ongoing |
| NCT05200013 | BAT7104 | Advanced Solid Tumors | I | Ongoing |
| NCT04881045 | PF-07257876 | NSCLC, SSC Head and Neck, Ovarian | I | Ongoing |
| NCT04886271 | HX009 | Advanced Solid Tumors | II | Ongoing |
| NCT04097769 | HX009 | Advanced Solid Tumors | I | Completed |
| NCT04980690 | IBC0966 | Advanced Malignant Tumors | I, II | Ongoing |
| CD47–Mesothelin Bispecific Antibody | ||||
| NCT05403554 | NI-1801 | Non-Squamous NSCLC, Epithelial Ovarian, TNBC | I | Ongoing |
| SIRPα–HER2 Bispecific Antibody | ||||
| NCT05076591 | IMM2902 | Advanced Solid Tumors, Advanced Breast Cancer, Advanced Gastric Cancer | I | Ongoing |
| SIRPα–Fc Fusion Protein | ||||
| NCT03013218 | ALX148 (Evorpacept) ± Pembrolizumab, Trastuzumab, Rituximab, Ramucirumab + Paclitaxel, 5-FU + Cisplatin | Advanced Solid Tumors, Lymphoma | I | Ongoing |
| SIRPα Monoclonal Antibody | ||||
| NCT05765851 | DS-1103a + T-DXd | Advanced Solid Tumors, Breast Cancer | I | Ongoing |
| NCT05615974 | LM-101 ± Toripalimab, Rituximab, Envafolimab | Malignant Tumors | I, II | Ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, A.P.Y.; Khavkine Binstock, S.S.; Thu, K.L. CD47: The Next Frontier in Immune Checkpoint Blockade for Non-Small Cell Lung Cancer. Cancers 2023, 15, 5229. https://doi.org/10.3390/cancers15215229
Lau APY, Khavkine Binstock SS, Thu KL. CD47: The Next Frontier in Immune Checkpoint Blockade for Non-Small Cell Lung Cancer. Cancers. 2023; 15(21):5229. https://doi.org/10.3390/cancers15215229
Chicago/Turabian StyleLau, Asa P. Y., Sharon S. Khavkine Binstock, and Kelsie L. Thu. 2023. "CD47: The Next Frontier in Immune Checkpoint Blockade for Non-Small Cell Lung Cancer" Cancers 15, no. 21: 5229. https://doi.org/10.3390/cancers15215229
APA StyleLau, A. P. Y., Khavkine Binstock, S. S., & Thu, K. L. (2023). CD47: The Next Frontier in Immune Checkpoint Blockade for Non-Small Cell Lung Cancer. Cancers, 15(21), 5229. https://doi.org/10.3390/cancers15215229







