Decoding the Impact of Tumor Microenvironment in Osteosarcoma Progression and Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
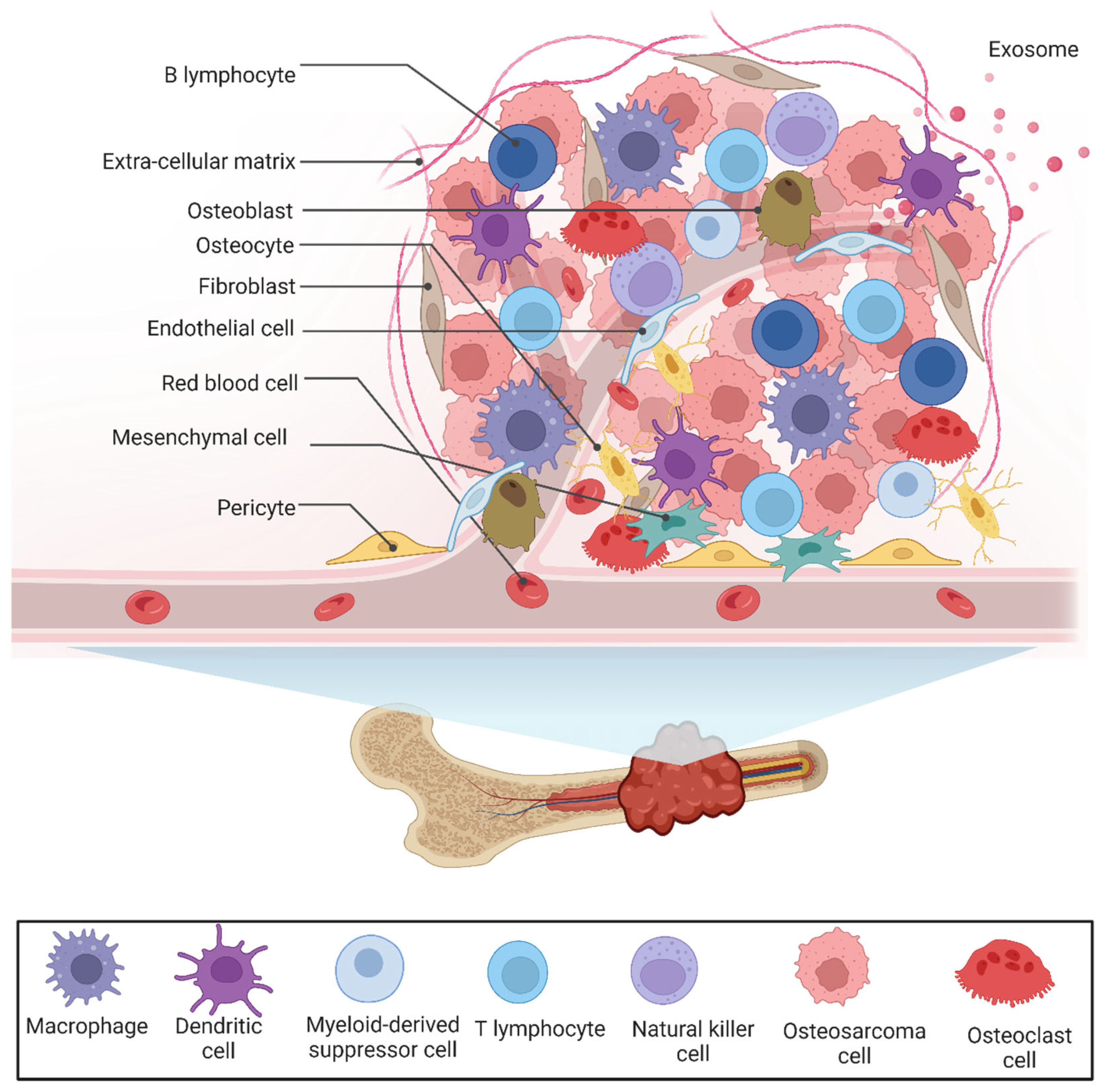
2. The Tumor Microenvironment of OS
2.1. Bone Cells
2.1.1. Osteoblast Cells
2.1.2. Osteoclast Cells
2.1.3. Osteocytes
2.2. Mesenchymal Stem Cells
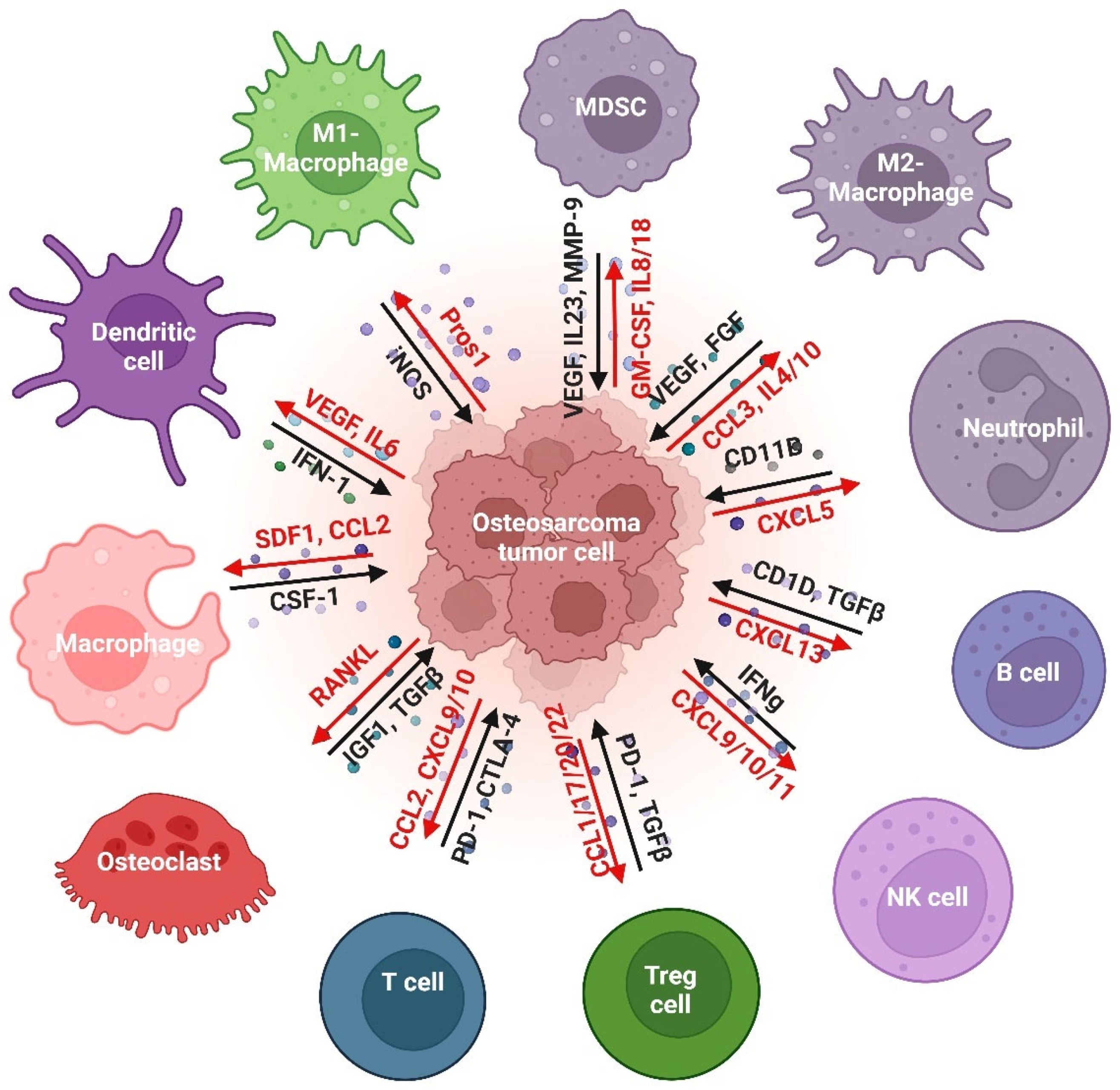
2.3. The Immune Landscape of OS
2.3.1. Lymphoid Cells
T Cells
B Cells
NK Cells
2.3.2. Myeloid Cells
Monocytes
Macrophages
- M1-Macrophages
- M2-Macrophages
Dendritic Cells
Neutrophils
MDSCs
2.4. Mast Cells
2.5. The Vasculature of the OS
2.6. The ECM of the OS Tumor
2.7. Extracellular Vesicles in OS (EVs)
2.8. Hypoxia and the Tumor Microenvironment of Osteosarcoma
2.9. Epithelial-to-Mesenchymal Transformation and the Osteosarcoma Tumor Microenvironment
2.10. Ferroptosis and the Osteosarcoma Tumor Microenvironment
3. Tumor Microenvironment Modulating and Targeting Therapies in Osteosarcoma
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma Overview. Rheumatol. Ther. 2017, 4, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Gianferante, D.M.; Zhu, B.; Mirabello, L. Osteosarcoma: A Surveillance, Epidemiology, and End Results program-based analysis from 1975 to 2017. Cancer 2022, 128, 2107–2118. [Google Scholar] [CrossRef]
- Odri, G.A.; Tchicaya-Bouanga, J.; Yoon, D.J.Y.; Modrowski, D. Metastatic Progression of Osteosarcomas: A Review of Current Knowledge of Environmental versus Oncogenic Drivers. Cancers 2022, 14, 360. [Google Scholar] [CrossRef]
- Martin, J.W.; Squire, J.A.; Zielenska, M. The genetics of osteosarcoma. Sarcoma 2012, 2012, 627254. [Google Scholar] [CrossRef] [PubMed]
- Nirala, B.K.; Yamamichi, T.; Yustein, J.T. Deciphering the Signaling Mechanisms of Osteosarcoma Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 11367. [Google Scholar] [CrossRef] [PubMed]
- Nirala, B.K.; Patel, T.D.; Kurenbekova, L.; Shuck, R.; Dasgupta, A.; Rainusso, N.; Coarfa, C.; Yustein, J.T. MYC regulates CSF1 expression via microRNA 17/20a to modulate tumor-associated macrophages in osteosarcoma. JCI Insight 2023, 8, e164947. [Google Scholar] [CrossRef]
- Bartholf DeWitt, S.; Hoskinson Plumlee, S.; Brighton, H.E.; Sivaraj, D.; Martz, E.J.; Zand, M.; Kumar, V.; Sheth, M.U.; Floyd, W.; Spruance, J.V.; et al. Loss of ATRX promotes aggressive features of osteosarcoma with increased NF-κB signaling and integrin binding. JCI Insight 2022, 7, e151583. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.W.; Wood, G.A.; Lu, J.; Tang, Q.-L.; Liu, J.; Molyneux, S.; Chen, Y.; Fang, H.; Adissu, H.; McKee, T.; et al. Cross-species genomics identifies DLG2 as a tumor suppressor in osteosarcoma. Oncogene 2019, 38, 291–298. [Google Scholar] [CrossRef]
- Tsagozis, P.; Gonzalez-Molina, J.; Georgoudaki, A.M.; Lehti, K.; Carlson, J.; Lundqvist, A.; Haglund, F.; Ehnman, M. Sarcoma Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1296, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Han, J.; Yang, L.; Cai, Z.; Sun, W.; Hua, Y.; Xu, J. Immune Microenvironment in Osteosarcoma: Components, Therapeutic Strategies and Clinical Applications. Front. Immunol. 2022, 13, 907550. [Google Scholar] [CrossRef]
- Liu, W.; Hu, H.; Shao, Z.; Lv, X.; Zhang, Z.; Deng, X.; Song, Q.; Han, Y.; Guo, T.; Xiong, L.; et al. Characterizing the tumor microenvironment at the single-cell level reveals a novel immune evasion mechanism in osteosarcoma. Bone Res. 2023, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, V.; Villamayor, M.L.; Pozas, J.; Chamorro, J.; Rosero, D.I.; San Román, M.; Guerrero, P.; Pérez de Aguado, P.; Calvo, J.C.; García de Quevedo, C.; et al. Current Landscape of Immunotherapy for Advanced Sarcoma. Cancers 2023, 15, 2287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jian, A.; Zhang, Y.; Zhang, X. A New Signature of Sarcoma Based on the Tumor Microenvironment Benefits Prognostic Prediction. Int. J. Mol. Sci. 2023, 24, 2961. [Google Scholar] [CrossRef]
- Cui, J.; Dean, D.; Hornicek, F.J.; Chen, Z.; Duan, Z. The role of extracelluar matrix in osteosarcoma progression and metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 178. [Google Scholar] [CrossRef] [PubMed]
- Cortini, M.; Macchi, F.; Reggiani, F.; Vitale, E.; Lipreri, M.V.; Perut, F.; Ciarrocchi, A.; Baldini, N.; Avnet, S. Endogenous Extracellular Matrix Regulates the Response of Osteosarcoma 3D Spheroids to Doxorubicin. Cancers 2023, 15, 1221. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Wu, B.; Wang, X.; Jiang, Y.; Zhu, D. Role of extracellular vesicles in osteosarcoma. Int. J. Med. Sci. 2022, 19, 1216–1226. [Google Scholar] [CrossRef]
- Jerez, S.; Araya, H.; Hevia, D.; Irarrázaval, C.E.; Thaler, R.; van Wijnen, A.J.; Galindo, M. Extracellular vesicles from osteosarcoma cell lines contain miRNAs associated with cell adhesion and apoptosis. Gene 2019, 710, 246–257. [Google Scholar] [CrossRef]
- Osaki, M.; Takeshita, F.; Sugimoto, Y.; Kosaka, N.; Yamamoto, Y.; Yoshioka, Y.; Kobayashi, E.; Yamada, T.; Kawai, A.; Inoue, T.; et al. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol. Ther. 2011, 19, 1123–1130. [Google Scholar] [CrossRef]
- Li, W.H.; Wu, H.J.; Li, Y.X.; Pan, H.G.; Meng, T.; Wang, X. MicroRNA-143 promotes apoptosis of osteosarcoma cells by caspase-3 activation via targeting Bcl-2. Biomed. Pharmacother. 2016, 80, 8–15. [Google Scholar] [CrossRef]
- Chen, F.; Liu, J.; Yang, T.; Sun, J.; He, X.; Fu, X.; Qiao, S.; An, J.; Yang, J. Analysis of intercellular communication in the osteosarcoma microenvironment based on single cell sequencing data. J. Bone Oncol. 2023, 41, 100493. [Google Scholar] [CrossRef]
- Zeng, J.; Peng, Y.; Wang, D.; Ayesha, K.; Chen, S. The interaction between osteosarcoma and other cells in the bone microenvironment: From mechanism to clinical applications. Front. Cell Dev. Biol. 2023, 11, 1123065. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Wang, Z.; Tao, Y.; Bai, J.; Yu, B.; Shen, J.; Sun, H.; Xiao, L.; Xu, Y.; Zhou, J.; et al. KLF2 regulates osteoblast differentiation by targeting of Runx2. Lab. Investig. 2019, 99, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, J.H.; Kim, K.; Seong, S.; Kim, N. The IRF2BP2-KLF2 axis regulates osteoclast and osteoblast differentiation. BMB Rep. 2019, 52, 469–474. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Ponzetti, M.; Ucci, A.; Puri, C.; Giacchi, L.; Flati, I.; Capece, D.; Zazzeroni, F.; Cappariello, A.; Rucci, N.; Falone, S. Effects of osteoblast-derived extracellular vesicles on aggressiveness, redox status and mitochondrial bioenergetics of MNNG/HOS osteosarcoma cells. Front. Oncol. 2022, 12, 983254. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Atkinson, E.G.; Delgado-Calle, J. The Emerging Role of Osteocytes in Cancer in Bone. JBMR Plus 2019, 3, e10186. [Google Scholar] [CrossRef]
- Behzatoglu, K. Osteoclasts in Tumor Biology: Metastasis and Epithelial-Mesenchymal-Myeloid Transition. Pathol. Oncol. Res. 2021, 27, 609472. [Google Scholar] [CrossRef]
- Lampiasi, N.; Russo, R.; Zito, F. The Alternative Faces of Macrophage Generate Osteoclasts. Biomed. Res. Int. 2016, 2016, 9089610. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Nirala, B.; Kurenbekova, L.; Patel, T.; Shuck, R.; Dasgupta, A.; Rainusso, N.; Yustein, J. Abstract 6713: Myc-regulated miR17, 20a modulate RANK expression in osteosarcoma. Cancer Res. 2023, 83, 6713. [Google Scholar] [CrossRef]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhou, X.; Jiang, E.; Wang, L.; Ji, Y.; Shang, Z. Osteosarcoma Cell-Derived Small Extracellular Vesicles Enhance Osteoclastogenesis and Bone Resorption Through Transferring MicroRNA-19a-3p. Front. Oncol. 2021, 11, 618662. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Higuchi, T.; Abe, K.; Taniguchi, Y.; Yonezawa, H.; et al. The number of osteoclasts in a biopsy specimen can predict the efficacy of neoadjuvant chemotherapy for primary osteosarcoma. Sci. Rep. 2021, 11, 1989. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Yang, Q.; Lv, X.; Huang, W.; Zhou, Z.; Wang, Y.; Zhang, Z.; Yuan, T.; Ding, X.; et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020, 11, 6322. [Google Scholar] [CrossRef]
- Li, H.; Hong, S.; Qian, J.; Zheng, Y.; Yang, J.; Yi, Q. Cross talk between the bone and immune systems: Osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells. Blood 2010, 116, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tang, H.; Luo, X.; Li, X.; Luo, K.; Liu, S.; Liang, J.; Liao, S.; Zhong, C.; Zhan, X.; et al. Interaction gene set between osteoclasts and regulatory CD4+ T cells can accurately predict the prognosis of patients with osteosarcoma. Cancer Sci. 2023, 114, 3014–3026. [Google Scholar] [CrossRef]
- Liu, L.; Geng, H.; Mei, C.; Chen, L. Zoledronic Acid Enhanced the Antitumor Effect of Cisplatin on Orthotopic Osteosarcoma by ROS-PI3K/AKT Signaling and Attenuated Osteolysis. Oxidative Med. Cell. Longev. 2021, 2021, 6661534. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-H.; Lu, E.W.-H.; Lin, C.-W.; Yang, J.-S.; Yang, S.-F. New insights into molecular and cellular mechanisms of zoledronate in human osteosarcoma. Pharmacol. Ther. 2020, 214, 107611. [Google Scholar] [CrossRef] [PubMed]
- Endo-Munoz, L.; Cumming, A.; Rickwood, D.; Wilson, D.; Cueva, C.; Ng, C.; Strutton, G.; Cassady, A.I.; Evdokiou, A.; Sommerville, S. Loss of osteoclasts contributes to development of osteosarcoma pulmonary metastases. Cancer Res. 2010, 70, 7063–7072. [Google Scholar] [CrossRef] [PubMed]
- Endo-Munoz, L.; Evdokiou, A.; Saunders, N.A. The role of osteoclasts and tumour-associated macrophages in osteosarcoma metastasis. Biochim. Biophys. Acta BBA—Rev. Cancer 2012, 1826, 434–442. [Google Scholar] [CrossRef]
- Anloague, A.; Delgado-Calle, J. Osteocytes: New Kids on the Block for Cancer in Bone Therapy. Cancers 2023, 15, 2645. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, G.; Chen, R.; Hua, Y.; Cai, Z. Mesenchymal stem cells in the osteosarcoma microenvironment: Their biological properties, influence on tumor growth, and therapeutic implications. Stem Cell Res. Ther. 2018, 9, 22. [Google Scholar] [CrossRef]
- Li, F.; Chen, X.; Shang, C.; Ying, Q.; Zhou, X.; Zhu, R.; Lu, H.; Hao, X.; Dong, Q.; Jiang, Z. Bone Marrow Mesenchymal Stem Cells-Derived Extracellular Vesicles Promote Proliferation, Invasion and Migration of Osteosarcoma Cells via the lncRNA MALAT1/miR-143/NRSN2/Wnt/β-Catenin Axis. OncoTargets Ther. 2021, 14, 737–749. [Google Scholar] [CrossRef]
- Zhu, G.; Xia, Y.; Zhao, Z.; Li, A.; Li, H.; Xiao, T. LncRNA XIST from the bone marrow mesenchymal stem cell derived exosome promotes osteosarcoma growth and metastasis through miR-655/ACLY signal. Cancer Cell Int. 2022, 22, 330. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, Q.; Zhao, Y.; Zhao, L.; Shan, B. Leptin acts on mesenchymal stem cells to promote chemoresistance in osteosarcoma cells. Aging 2020, 12, 6340–6351. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; He, B.; Wang, L.; Zhang, F.; Shu, H.; Sun, L. Exosomes derived from bone marrow mesenchymal stem cells promote osteosarcoma development by activating oncogenic autophagy. J. Bone Oncol. 2020, 21, 100280. [Google Scholar] [CrossRef]
- Chang, X.; Ma, Z.; Zhu, G.; Lu, Y.; Yang, J. New perspective into mesenchymal stem cells: Molecular mechanisms regulating osteosarcoma. J. Bone Oncol. 2021, 29, 100372. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Tang, H.; Zhang, Y.; Zhang, Z.; Huang, P.; Zhu, J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J. Cell Physiol. 2020, 235, 4734–4745. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, R.; Wang, Y. Exosomal miR-21-5p derived from bone marrow mesenchymal stem cells promote osteosarcoma cell proliferation and invasion by targeting PIK3R1. J. Cell Mol. Med. 2021, 25, 11016–11030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Ren, T.; Huang, Y.; Liang, X.; Yu, Y.; Wang, W.; Niu, J.; Guo, W. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020, 490, 54–65. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, L.; Zhang, Y.; Li, F.-F. A comprehensive analysis of immune infiltration in the tumor microenvironment of osteosarcoma. Cancer Med. 2021, 10, 5696–5711. [Google Scholar] [CrossRef] [PubMed]
- Mani, N.; Andrews, D.; Obeng, R.C. Modulation of T cell function and survival by the tumor microenvironment. Front. Cell Dev. Biol. 2023, 11, 1191774. [Google Scholar] [CrossRef]
- Wu, C.-C.; Beird, H.C.; Andrew Livingston, J.; Advani, S.; Mitra, A.; Cao, S.; Reuben, A.; Ingram, D.; Wang, W.-L.; Ju, Z.; et al. Immuno-genomic landscape of osteosarcoma. Nat. Commun. 2020, 11, 1008. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, S.; Zhang, R.; Wang, H.; Ye, Z.; Zhang, Z.; Li, K.; Zuo, G. CTGF/CCN2 promotes the proliferation of human osteosarcoma cells via cross-talking with the stromal CXCL12/CXCR4-AKT-αvβ3 signaling axis in tumor microenvironment. Genes Dis. 2023, 10, 356–358. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Heymann, M.F.; Lézot, F.; Heymann, D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell. Immunol. 2019, 343, 103711. [Google Scholar] [CrossRef]
- Kendal, J.K.; Shehata, M.S.; Lofftus, S.Y.; Crompton, J.G. Cancer-Associated B Cells in Sarcoma. Cancers 2023, 15, 622. [Google Scholar] [CrossRef]
- Zhao, G.; Liang, J.; Cao, J.; Jiang, S.; Lu, J.; Jiang, B. Abnormal Function of Circulating Follicular Helper T Cells Leads to Different Manifestations of B Cell Maturation and Differentiation in Patients with Osteosarcoma. J. Healthc. Eng. 2022, 2022, 3724033. [Google Scholar] [CrossRef]
- Yang, M.; Cao, Y.; Wang, Z.; Zhang, T.; Hua, Y.; Cai, Z. Identification of two immune subtypes in osteosarcoma based on immune gene sets. Int. Immunopharmacol. 2021, 96, 107799. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, J.; Jiang, S.; Zhao, G.; Lu, J.; Jiang, B. The Effect of miR-138 on the Function of Follicular Helper T Cells and the Differentiation of B Cells in Osteosarcoma. Comput. Math. Methods Med. 2021, 2021, 2057782. [Google Scholar] [CrossRef]
- Bod, L.; Kye, Y.-C.; Shi, J.; Torlai Triglia, E.; Schnell, A.; Fessler, J.; Ostrowski, S.M.; Von-Franque, M.Y.; Kuchroo, J.R.; Barilla, R.M.; et al. B-cell-specific checkpoint molecules that regulate anti-tumour immunity. Nature 2023, 619, 348–356. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Duan, Y.; Li, X.; Pan, J.; Wang, G.; Shen, B. Identification of B cell marker genes based on single-cell sequencing to establish a prognostic model and identify immune infiltration in osteosarcoma. Front. Immunol. 2022, 13, 1026701. [Google Scholar] [CrossRef]
- Lachota, M.; Vincenti, M.; Winiarska, M.; Boye, K.; Zagożdżon, R.; Malmberg, K.-J. Prospects for NK Cell Therapy of Sarcoma. Cancers 2020, 12, 3719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Li, B.; Wang, S.; Chen, T.; Ye, Z. Innate Immune Cells: A Potential and Promising Cell Population for Treating Osteosarcoma. Front. Immunol. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Quamine, A.E.; Olsen, M.R.; Cho, M.M.; Capitini, C.M. Approaches to Enhance Natural Killer Cell-Based Immunotherapy for Pediatric Solid Tumors. Cancers 2021, 13, 2796. [Google Scholar] [CrossRef] [PubMed]
- Marchais, A.; Marques da Costa, M.E.; Job, B.; Abbas, R.; Drubay, D.; Piperno-Neumann, S.; Fromigué, O.; Gomez-Brouchet, A.; Redini, F.; Droit, R.; et al. Immune Infiltrate and Tumor Microenvironment Transcriptional Programs Stratify Pediatric Osteosarcoma into Prognostic Groups at Diagnosis. Cancer Res. 2022, 82, 974–985. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, W.; Huang, J.; Zhang, Y.; Zhou, Y.; Zhang, J.; Dong, Y.; Yuan, T.; Yang, Q.; Ding, X.; et al. Characterization of the tumour microenvironment phenotypes in malignant tissues and pleural effusion from advanced osteoblastic osteosarcoma patients. Clin. Transl. Med. 2022, 12, e1072. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Tian, Z. NK Cell Exhaustion. Front. Immunol. 2017, 8, 760. [Google Scholar] [CrossRef]
- Rademacher, M.J.; Cruz, A.; Faber, M.; Oldham, R.A.A.; Wang, D.; Medin, J.A.; Schloemer, N.J. Sarcoma IL-12 overexpression facilitates NK cell immunomodulation. Sci. Rep. 2021, 11, 8321. [Google Scholar] [CrossRef] [PubMed]
- Omer, N.; Nicholls, W.; Ruegg, B.; Souza-Fonseca-Guimaraes, F.; Rossi, G.R. Enhancing Natural Killer Cell Targeting of Pediatric Sarcoma. Front. Immunol. 2021, 12, 791206. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Nayyar, G.; Jiang, S.; Rosenblum, J.M.; Soon-Shiong, P.; Safrit, J.T.; Lee, D.A.; Cairo, M.S. Combinatorial immunotherapy of N-803 (IL-15 superagonist) and dinutuximab with ex vivo expanded natural killer cells significantly enhances in vitro cytotoxicity against GD2+ pediatric solid tumors and in vivo survival of xenografted immunodeficient NSG mice. J. Immunother. Cancer 2021, 9, e002267. [Google Scholar] [CrossRef]
- Judge, S.J.; Darrow, M.A.; Thorpe, S.W.; Gingrich, A.A.; O’Donnell, E.F.; Bellini, A.R.; Sturgill, I.S.; Vick, L.V.; Dunai, C.; Stoffel, K.M.; et al. Analysis of tumor-infiltrating NK and T cells highlights IL-15 stimulation and TIGIT blockade as a combination immunotherapy strategy for soft tissue sarcomas. J. ImmunoTherapy Cancer 2020, 8, e001355. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, M.; Yang, J.; Que, Y.; Zhang, X. Panobinostat enhances NK cell cytotoxicity in soft tissue sarcoma. Clin. Exp. Immunol. 2022, 209, 127–139. [Google Scholar] [CrossRef]
- Kawamoto, H.; Minato, N. Myeloid cells. Int. J. Biochem. Cell Biol. 2004, 36, 1374–1379. [Google Scholar] [CrossRef]
- De Kleer, I.; Willems, F.; Lambrecht, B.; Goriely, S. Ontogeny of myeloid cells. Front. Immunol. 2014, 5, 423. [Google Scholar] [CrossRef]
- Pratt, H.G.; Justin, E.M.; Lindsey, B.A. Applying Osteosarcoma Immunology to Understand Disease Progression and Assess Immunotherapeutic Response. Adv. Exp. Med. Biol. 2020, 1258, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Chen, P.C.; Chang, T.M.; Hou, C.H. Monocyte Chemoattractant Protein-1 promotes cancer cell migration via c-Raf/MAPK/AP-1 pathway and MMP-9 production in osteosarcoma. J. Exp. Clin. Cancer Res. 2020, 39, 254. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.P.; Coy, J.W.; Chahal, K.K.; Chow, L.; Kurihara, J.N.; Guth, A.M.; Kufareva, I.; Dow, S.W. The Angiotensin Receptor Blocker Losartan Suppresses Growth of Pulmonary Metastases via AT1R-Independent Inhibition of CCR2 Signaling and Monocyte Recruitment. J. Immunol. 2019, 202, 3087–3102. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.P.; Chow, L.; Das, S.; Haines, L.; Palmer, E.; Kurihara, J.N.; Coy, J.W.; Mathias, A.; Thamm, D.H.; Gustafson, D.L.; et al. Losartan Blocks Osteosarcoma-Elicited Monocyte Recruitment, and Combined With the Kinase Inhibitor Toceranib, Exerts Significant Clinical Benefit in Canine Metastatic Osteosarcoma. Clin. Cancer Res. 2022, 28, 662–676. [Google Scholar] [CrossRef]
- Kelleher, F.C.; O’Sullivan, H. Monocytes, Macrophages, and Osteoclasts in Osteosarcoma. J. Adolesc. Young Adult Oncol. 2017, 6, 396–405. [Google Scholar] [CrossRef]
- Chazaud, B. Macrophages: Supportive cells for tissue repair and regeneration. Immunobiology 2014, 219, 172–178. [Google Scholar] [CrossRef]
- Luo, Z.-W.; Liu, P.-P.; Wang, Z.-X.; Chen, C.-Y.; Xie, H. Macrophages in Osteosarcoma Immune Microenvironment: Implications for Immunotherapy. Front. Oncol. 2020, 10, 586580. [Google Scholar] [CrossRef]
- Nirala, B.; Kurenbekova, L.; Shuck, R.; Patel, T.; Rajapakshe, K.; Yustein, J. Abstract 1668: Development and characterization of a c-Myc-driven preclinical mouse model of osteosarcoma to investigate the tumor immune microenvironment. Cancer Res. 2022, 82, 1668. [Google Scholar] [CrossRef]
- Nirala, B.; Lyazat, K.; Patel, T.; Shuck, R.; Kimal, R.; Yustein, J. Myc-driven osteosarcoma murine model for dissecting the molecular genetics and novel therapeutic strategies for OS. Cancer Res. 2020, 80, 6147. [Google Scholar] [CrossRef]
- Buddingh, E.P.; Kuijjer, M.L.; Duim, R.A.; Bürger, H.; Agelopoulos, K.; Myklebost, O.; Serra, M.; Mertens, F.; Hogendoorn, P.C.; Lankester, A.C.; et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: A rationale for treatment with macrophage activating agents. Clin. Cancer Res. 2011, 17, 2110–2119. [Google Scholar] [CrossRef]
- Duan, Z.; Luo, Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 127. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, K.; Vegna, S.; Ramirez, C.; Akkari, L. Understanding the Origin and Diversity of Macrophages to Tailor Their Targeting in Solid Cancers. Front. Immunol. 2019, 10, 2215. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Hughes, R.; Lewis, C.E. Tumor-associated macrophages: Effectors of angiogenesis and tumor progression. Biochim. Biophys. Acta 2009, 1796, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Song, F.; Jin, J.; Zou, B.; Dai, P.; Sun, M.; Xu, W.; Wang, L.; Kang, Y. Prognostic and immunological significance of an M1 macrophage-related gene signature in osteosarcoma. Front. Immunol. 2023, 14, 1202725. [Google Scholar] [CrossRef]
- Anand, N.; Peh, K.H.; Kolesar, J.M. Macrophage Repolarization as a Therapeutic Strategy for Osteosarcoma. Int. J. Mol. Sci. 2023, 24, 2858. [Google Scholar] [CrossRef]
- Su, Y.; Zhou, Y.; Sun, Y.-J.; Wang, Y.-L.; Yin, J.-Y.; Huang, Y.-J.; Zhang, J.-J.; He, A.-N.; Han, K.; Zhang, H.-Z.; et al. Macrophage-derived CCL18 promotes osteosarcoma proliferation and migration by upregulating the expression of UCA1. J. Mol. Med. 2019, 97, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xian, M.; Xiang, S.; Xiang, D.; Shao, X.; Wang, J.; Cao, J.; Yang, X.; Yang, B.; Ying, M.; et al. All-Trans Retinoic Acid Prevents Osteosarcoma Metastasis by Inhibiting M2 Polarization of Tumor-Associated Macrophages. Cancer Immunol. Res. 2017, 5, 547–559. [Google Scholar] [CrossRef]
- Han, Y.; Guo, W.; Ren, T.; Huang, Y.; Wang, S.; Liu, K.; Zheng, B.; Yang, K.; Zhang, H.; Liang, X. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019, 440–441, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, L.; Wu, C.; Huang, L.; Ruan, Y.; Xue, W. Tumor-derived Exosomes Induced M2 Macrophage Polarization and Promoted the Metastasis of Osteosarcoma Cells Through Tim-3. Arch. Med. Res. 2021, 52, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.P.; Liu, D.B.; Wu, L.Q.; Li, Q.; Wang, Z.G.; Zang, X.F. IL-1beta secreted by macrophage M2 promotes metastasis of osteosarcoma via NF-kappaB/miR-181alpha-5p/RASSF1A/Wnt pathway. Transl. Cancer Res. 2020, 9, 2721–2733. [Google Scholar] [CrossRef]
- Shao, X.J.; Xiang, S.F.; Chen, Y.Q.; Zhang, N.; Cao, J.; Zhu, H.; Yang, B.; Zhou, Q.; Ying, M.D.; He, Q.J. Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells. Acta Pharmacol. Sin. 2019, 40, 1343–1350. [Google Scholar] [CrossRef]
- Li, D.K.; Wang, G.H. Asiaticoside reverses M2 phenotype macrophage polarization-evoked osteosarcoma cell malignant behaviour by TRAF6/NF-kappaB inhibition. Pharm. Biol. 2022, 60, 1635–1645. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Anandasabapathy, N. The role of dendritic cells in cancer and anti-tumor immunity. Semin. Immunol. 2021, 52, 101481. [Google Scholar] [CrossRef]
- Soto, J.A.; Gálvez, N.M.S.; Andrade, C.A.; Pacheco, G.A.; Bohmwald, K.; Berrios, R.V.; Bueno, S.M.; Kalergis, A.M. The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses. Front. Immunol. 2020, 11, 1513. [Google Scholar] [CrossRef]
- Kawano, M.; Tanaka, K.; Itonaga, I.; Iwasaki, T.; Miyazaki, M.; Ikeda, S.; Tsumura, H. Dendritic cells combined with doxorubicin induces immunogenic cell death and exhibits antitumor effects for osteosarcoma. Oncol. Lett. 2016, 11, 2169–2175. [Google Scholar] [CrossRef]
- Kawano, M.; Itonaga, I.; Iwasaki, T.; Tsuchiya, H.; Tsumura, H. Anti-TGF-beta antibody combined with dendritic cells produce antitumor effects in osteosarcoma. Clin. Orthop. Relat. Res. 2012, 470, 2288–2294. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Y.; Fan, G.; Hu, S. Photodynamic therapy reduces the inhibitory effect of osteosarcoma cells on dendritic cells by upregulating HSP70. Oncol. Lett. 2018, 16, 5034–5040. [Google Scholar] [CrossRef]
- Pan, R.; Pan, F.; Zeng, Z.; Lei, S.; Yang, Y.; Yang, Y.; Hu, C.; Chen, H.; Tian, X. A novel immune cell signature for predicting osteosarcoma prognosis and guiding therapy. Front. Immunol. 2022, 13, 1017120. [Google Scholar] [CrossRef]
- Tan, S.; Chao, R. An Exploration of Osteosarcoma Metastasis Diagnostic Markers Based on Tumor-Associated Neutrophils. Discov. Med. 2023, 35, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, D.; Lu, J.; He, J.; Ji, S.; Liao, S.; Wei, Q.; Lu, S.; Liu, Y. Significance of the neutrophil-to-lymphocyte ratio in predicting the response to neoadjuvant chemotherapy in extremity osteosarcoma: A multicentre retrospective study. BMC Cancer 2022, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Yang, C.; Tan, J.; Dou, C.; Chen, Y. Beyond immunosuppressive effects: Dual roles of myeloid-derived suppressor cells in bone-related diseases. Cell Mol. Life Sci. 2021, 78, 7161–7183. [Google Scholar] [CrossRef]
- Joshi, S.; Sharabi, A. Targeting myeloid-derived suppressor cells to enhance natural killer cell-based immunotherapy. Pharmacol. Ther. 2022, 235, 108114. [Google Scholar] [CrossRef] [PubMed]
- Novitskiy, S.V.; Pickup, M.W.; Gorska, A.E.; Owens, P.; Chytil, A.; Aakre, M.; Wu, H.; Shyr, Y.; Moses, H.L. TGF-β Receptor II Loss Promotes Mammary Carcinoma Progression by Th17-Dependent Mechanisms. Cancer Discov. 2011, 1, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Consonni, F.M.; Morlacchi, S.; Sangaletti, S.; Bleve, A.; Totaro, M.G.; Larghi, P.; Rimoldi, M.; Tripodo, C.; Strauss, L.; et al. Tumor-Derived Prostaglandin E2 Promotes p50 NF-κB-Dependent Differentiation of Monocytic MDSCs. Cancer Res. 2020, 80, 2874–2888. [Google Scholar] [CrossRef]
- Condamine, T.; Ramachandran, I.; Youn, J.-I.; Gabrilovich, D.I. Regulation of Tumor Metastasis by Myeloid-Derived Suppressor Cells. Annu. Rev. Med. 2015, 66, 97–110. [Google Scholar] [CrossRef]
- Marvel, D.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J. Clin. Investig. 2015, 125, 3356–3364. [Google Scholar] [CrossRef]
- Ammons, D.T.; Harris, R.A.; Hopkins, L.S.; Kurihara, J.; Weishaar, K.; Dow, S. A single-cell RNA sequencing atlas of circulating leukocytes from healthy and osteosarcoma affected dogs. Front. Immunol. 2023, 14, 1162700. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Wang, H.; Yu, Z.; Zhu, Y.; Gao, Y. Specific inhibition of PI3Kδ/γ enhances the efficacy of anti-PD1 against osteosarcoma cancer. J. Bone Oncol. 2019, 16, 100206. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Su, S.; Shahriyari, L. Immune classification of osteosarcoma. Math. Biosci. Eng. 2021, 18, 1879–1897. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ru, J.; Liu, T.; Ma, C. Identification of a Novel Prognostic Gene Signature From the Immune Cell Infiltration Landscape of Osteosarcoma. Front. Cell Dev. Biol. 2021, 9, 718624. [Google Scholar] [CrossRef]
- Wei, J.; Fang, D.-L.; Huang, C.K.; Hua, S.-L.; Lu, X.-S. Screening a novel signature and predicting the immune landscape of metastatic osteosarcoma in children via immune-related lncRNAs. Transl. Pediatr. 2021, 10, 1851–1866. [Google Scholar] [CrossRef]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex But Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Yao, N.; Wang, G.; Tian, L.; Ma, J.; Shi, X.; Zhang, L.; Zhang, J.; Zhou, X.; Zhou, G.; et al. Vasculogenic mimicry: A new prognostic sign of human osteosarcoma. Hum. Pathol. 2014, 45, 2120–2129. [Google Scholar] [CrossRef]
- Ren, K.; Zhang, J.; Gu, X.; Wu, S.; Shi, X.; Ni, Y.; Chen, Y.; Lu, J.; Gao, Z.; Wang, C.; et al. Migration-inducing gene-7 independently predicts poor prognosis of human osteosarcoma and is associated with vasculogenic mimicry. Exp. Cell Res. 2018, 369, 80–89. [Google Scholar] [CrossRef]
- Li, Y.; Lin, S.; Xie, X.; Zhu, H.; Fan, T.; Wang, S. Highly enriched exosomal lncRNA OIP5-AS1 regulates osteosarcoma tumor angiogenesis and autophagy through miR-153 and ATG5. Am. J. Transl. Res. 2021, 13, 4211–4223. [Google Scholar]
- Assi, T.; Watson, S.; Samra, B.; Rassy, E.; Le Cesne, A.; Italiano, A.; Mir, O. Targeting the VEGF Pathway in Osteosarcoma. Cells 2021, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Espinosa-Cotton, M.; Guo, H.F.; Monette, S.; Cheung, N.V. Targeting tumor vasculature to improve antitumor activity of T cells armed ex vivo with T cell engaging bispecific antibody. J. Immunother. Cancer 2023, 11, e006680. [Google Scholar] [CrossRef]
- Yin, J.; Pan, S.; Guo, X.; Gao, Y.; Zhu, D.; Yang, Q.; Gao, J.; Zhang, C.; Chen, Y. Nb2C MXene-Functionalized Scaffolds Enables Osteosarcoma Phototherapy and Angiogenesis/Osteogenesis of Bone Defects. Nanomicro Lett. 2021, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, G.N.; Giatagana, E.M.; Berdiaki, A.; Spyridaki, I.; Hida, K.; Neagu, M.; Tsatsakis, A.M.; Nikitovic, D. The Role of IGF/IGF-IR-Signaling and Extracellular Matrix Effectors in Bone Sarcoma Pathogenesis. Cancers 2021, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Gomez-Salazar, M.; Tower, R.J.; Chang, L.; Morris, C.D.; McCarthy, E.F.; Ting, K.; Zhang, X.; James, A.W. NELL1 Regulates the Matrisome to Promote Osteosarcoma Progression. Cancer Res. 2022, 82, 2734–2747. [Google Scholar] [CrossRef]
- Tian, H.; Wu, R.; Feng, N.; Zhang, J.; Zuo, J. Recent advances in hydrogels-based osteosarcoma therapy. Front. Bioeng. Biotechnol. 2022, 10, 1042625. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lazar, A.; Ingram, D.; Wang, W.-L.; Zhang, W.; Jia, Z.; Ragoonanan, D.; Wang, J.; Xia, X.; Mahadeo, K.; et al. Abstract 4102: attIL12-T cell therapy destructs cancer-associated fibroblasts and extracellular matrix in heterogenous osteosarcoma xenograft models. Cancer Res. 2023, 83, 4102. [Google Scholar] [CrossRef]
- Loi, G.; Stucchi, G.; Scocozza, F.; Cansolino, L.; Cadamuro, F.; Delgrosso, E.; Riva, F.; Ferrari, C.; Russo, L.; Conti, M. Characterization of a Bioink Combining Extracellular Matrix-like Hydrogel with Osteosarcoma Cells: Preliminary Results. Gels 2023, 9, 129. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, H.J.; Lee, M.R.; Han, I. EMMPRIN expression is associated with metastatic progression in osteosarcoma. BMC Cancer 2021, 21, 1059. [Google Scholar] [CrossRef]
- Luong, N.; Lenz, J.A.; Modiano, J.F.; Olson, J.K. Extracellular Vesicles Secreted by Tumor Cells Promote the Generation of Suppressive Monocytes. Immunohorizons 2021, 5, 647–658. [Google Scholar] [CrossRef]
- Cambier, L.; Stachelek, K.; Triska, M.; Jubran, R.; Huang, M.; Li, W.; Zhang, J.; Li, J.; Cobrinik, D. Extracellular vesicle-associated repetitive element DNAs as candidate osteosarcoma biomarkers. Sci. Rep. 2021, 11, 94. [Google Scholar] [CrossRef]
- Mazumdar, A.; Urdinez, J.; Boro, A.; Arlt, M.J.E.; Egli, F.E.; Niederost, B.; Jaeger, P.K.; Moschini, G.; Muff, R.; Fuchs, B.; et al. Exploring the Role of Osteosarcoma-Derived Extracellular Vesicles in Pre-Metastatic Niche Formation and Metastasis in the 143-B Xenograft Mouse Osteosarcoma Model. Cancers 2020, 12, 3457. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lyu, P.; Andreev, D.; Jia, Y.; Zhang, F.; Bozec, A. Hypoxia-immune-related microenvironment prognostic signature for osteosarcoma. Front. Cell Dev. Biol. 2022, 10, 974851. [Google Scholar] [CrossRef]
- Fu, Y.; Bao, Q.; Liu, Z.; He, G.; Wen, J.; Liu, Q.; Xu, Y.; Jin, Z.; Zhang, W. Development and Validation of a Hypoxia-Associated Prognostic Signature Related to Osteosarcoma Metastasis and Immune Infiltration. Front. Cell Dev. Biol. 2021, 9, 633607. [Google Scholar] [CrossRef]
- Chen, H.; Fu, Y.; Feng, K.; Zhou, Y.; Wang, X.; Huang, H.; Chen, Y.; Wang, W.; Xu, Y.; Tian, H.; et al. Polydopamine-coated UiO-66 nanoparticles loaded with perfluorotributylamine/tirapazamine for hypoxia-activated osteosarcoma therapy. J. Nanobiotechnology 2021, 19, 298. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Nie, J.J.; Liu, X.; Ding, Z.; Luo, P.; Liu, Y.; Zhang, B.W.; Wang, R.; Liu, X.; Hai, Y.; et al. Zinc oxide nanoparticles inhibit osteosarcoma metastasis by downregulating β-catenin via HIF-1α/BNIP3/LC3B-mediated mitophagy pathway. Bioact. Mater. 2023, 19, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.-T.; Zhou, T.-J.; Zhang, C.; Liu, Y.-X.; Wang, W.-J.; Li, C.; Xing, L.; Jiang, H.-L. Hypoxia Inhibitor Combined with Chemotherapeutic Agents for Antitumor and Antimetastatic Efficacy against Osteosarcoma. Mol. Pharm. 2023, 20, 2612–2623. [Google Scholar] [CrossRef]
- Han, T.; Wu, Z.; Zhang, Z.; Liang, J.; Xia, C.; Yan, H. Comprehensive analysis of hypoxia-related genes for prognosis value, immune status, and therapy in osteosarcoma patients. Front. Pharmacol. 2022, 13, 1088732. [Google Scholar] [CrossRef]
- Shen, S.; Xu, Y.; Gong, Z.; Yao, T.; Qiao, D.; Huang, Y.; Zhang, Z.; Gao, J.; Ni, H.; Jin, Z.; et al. Positive Feedback Regulation of Circular RNA Hsa_circ_0000566 and HIF-1α promotes Osteosarcoma Progression and Glycolysis Metabolism. Aging Dis. 2023, 14, 529–547. [Google Scholar] [CrossRef]
- Lu, D.; Liu, R.; Zhou, Y.; Zhang, Z.; Jiang, X.; Xu, J.; Su, A.; Hu, Z. FOXO3a-dependent up-regulation of HSP90 alleviates cisplatin-induced apoptosis by activating FUNDC1-mediated mitophagy in hypoxic osteosarcoma cells. Cell. Signal. 2023, 101, 110500. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, R.; Zheng, S.; Fang, H.; Xu, M.; Zhong, L. The knockdown of lncRNA DLGAP1-AS2 suppresses osteosarcoma progression by inhibiting aerobic glycolysis via the miR-451a/HK2 axis. Cancer Sci. 2023. early view. [Google Scholar] [CrossRef]
- Subasinghe, S.A.A.S.; Ortiz, C.J.; Romero, J.; Ward, C.L.; Sertage, A.G.; Kurenbekova, L.; Yustein, J.T.; Pautler, R.G.; Allen, M.J. Toward quantification of hypoxia using fluorinated EuII/III-containing ratiometric probes. Proc. Natl. Acad. Sci. USA 2023, 120, e2220891120. [Google Scholar] [CrossRef] [PubMed]
- Hinton, K.; Kirk, A.; Paul, P.; Persad, S. Regulation of the Epithelial to Mesenchymal Transition in Osteosarcoma. Biomolecules 2023, 13, 398. [Google Scholar] [CrossRef]
- Gong, H.; Tao, Y.; Xiao, S.; Li, X.; Fang, K.; Wen, J.; Zeng, M.; Liu, Y.; Chen, Y. Identification of an EMT-related gene-based prognostic signature in osteosarcoma. Cancer Med. 2023, 12, 12912–12928. [Google Scholar] [CrossRef]
- Yiqi, Z.; Ziyun, L.; Qin, F.; Xingli, W.; Liyu, Y. Identification of 9-Gene Epithelial-Mesenchymal Transition Related Signature of Osteosarcoma by Integrating Multi Cohorts. Technol. Cancer Res. Treat. 2020, 19, 1533033820980769. [Google Scholar] [CrossRef]
- Peng, Y.X.; Yu, B.; Qin, H.; Xue, L.; Liang, Y.J.; Quan, Z.X. EMT-related gene expression is positively correlated with immunity and may be derived from stromal cells in osteosarcoma. PeerJ 2020, 8, e8489. [Google Scholar] [CrossRef]
- Yu, X.; Yustein, J.T.; Xu, J. Research models and mesenchymal/epithelial plasticity of osteosarcoma. Cell Biosci. 2021, 11, 94. [Google Scholar] [CrossRef]
- Wushou, A.; Hou, J.; Zhao, Y.J.; Shao, Z.M. Twist-1 up-regulation in carcinoma correlates to poor survival. Int. J. Mol. Sci. 2014, 15, 21621–21630. [Google Scholar] [CrossRef]
- Jin, C.; Feng, Y.; Ni, Y.; Shan, Z. MicroRNA-610 suppresses osteosarcoma oncogenicity via targeting TWIST1 expression. Oncotarget 2017, 8, 56174–56184. [Google Scholar] [CrossRef][Green Version]
- Ye, F.; Tian, L.; Zhou, Q.; Feng, D. LncRNA FER1L4 induces apoptosis and suppresses EMT and the activation of PI3K/AKT pathway in osteosarcoma cells via inhibiting miR-18a-5p to promote SOCS5. Gene 2019, 721, 144093. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.T.; Wang, X.; Wang, J.Y.; Xi, G.H.; Liu, Y. Downregulation of miR-22 Contributes to Epithelial-Mesenchymal Transition in Osteosarcoma by Targeting Twist1. Front. Oncol. 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, L.; Xiao, Z.; Wen, S.; Chen, L.; Yang, P. CircRAB3IP upregulates twist family BHLH transcription factor (TWIST1) to promote osteosarcoma progression by sponging miR-580-3p. Bioengineered 2021, 12, 3385–3397. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Yin, P.; Han, G.; Xu, M.; Wang, W.; Bi, W. MicroRNA-300 decreases cell viability, inhibits migration and promotes apoptosis of osteosarcoma cells via downregulation of Twist1. Mol. Med. Rep. 2017, 16, 3613–3618. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Z.; Wu, S.; Zang, X.; Liu, M.; Shi, J. miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST. J. Exp. Clin. Cancer Res. 2014, 33, 12. [Google Scholar] [CrossRef]
- Chen, J.; Xia, J.; Yu, Y.L.; Wang, S.Q.; Wei, Y.B.; Chen, F.Y.; Huang, G.Y.; Shi, J.S. HDAC5 promotes osteosarcoma progression by upregulation of Twist 1 expression. Tumour Biol. 2014, 35, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Ruh, M.; Stemmler, M.P.; Frisch, I.; Fuchs, K.; van Roey, R.; Kleemann, J.; Roas, M.; Schuhwerk, H.; Eccles, R.L.; Agaimy, A.; et al. The EMT transcription factor ZEB1 blocks osteoblastic differentiation in bone development and osteosarcoma. J. Pathol. 2021, 254, 199–211. [Google Scholar] [CrossRef]
- Shen, A.; Zhang, Y.; Yang, H.; Xu, R.; Huang, G. Overexpression of ZEB1 relates to metastasis and invasion in osteosarcoma. J. Surg. Oncol. 2012, 105, 830–834. [Google Scholar] [CrossRef]
- Yao, H.; Hou, G.; Wang, Q.Y.; Xu, W.B.; Zhao, H.Q.; Xu, Y.C. LncRNA SPRY4-IT1 promotes progression of osteosarcoma by regulating ZEB1 and ZEB2 expression through sponging of miR-101 activity. Int. J. Oncol. 2020, 56, 85–100. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Zhou, Z.; Jiang, X.; Shen, A. Transcription factor Snai1-1 induces osteosarcoma invasion and metastasis by inhibiting E-cadherin expression. Oncol. Lett. 2014, 8, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, R.; Hou, X.; Gao, Z.; Liu, X.; Zhang, W. SIRT2 promotes the viability, invasion and metastasis of osteosarcoma cells by inhibiting the degradation of Snail. Cell Death Dis. 2022, 13, 935. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, Z.; Song, C.; Deng, H.; Yang, R.; Zhou, L.; Sun, Y.; Zhang, Q. Glaucocalyxin A reverses EMT and TGF-β1-induced EMT by inhibiting TGF-β1/Smad2/3 signaling pathway in osteosarcoma. Chem.-Biol. Interact. 2019, 307, 158–166. [Google Scholar] [CrossRef]
- Ma, L.; Xue, W.; Ma, X. GATA3 is downregulated in osteosarcoma and facilitates EMT as well as migration through regulation of slug. OncoTargets Ther. 2018, 11, 7579–7589. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Dachert, J.; Ehrenfeld, V.; Habermann, K.; Dolgikh, N.; Fulda, S. Targeting ferroptosis in rhabdomyosarcoma cells. Int. J. Cancer 2020, 146, 510–520. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Ma, X.; Zhang, B.; Feng, H. Targeting ferroptosis in osteosarcoma. J. Bone Oncol. 2021, 30, 100380. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol. Int. 2019, 43, 1245–1256. [Google Scholar] [CrossRef]
- Lei, T.; Qian, H.; Lei, P.; Hu, Y. Ferroptosis-related gene signature associates with immunity and predicts prognosis accurately in patients with osteosarcoma. Cancer Sci. 2021, 112, 4785–4798. [Google Scholar] [CrossRef]
- Li, G.; Lei, J.; Xu, D.; Yu, W.; Bai, J.; Wu, G. Integrative analyses of ferroptosis and immune related biomarkers and the osteosarcoma associated mechanisms. Sci. Rep. 2023, 13, 5770. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.G.; Song, W.S. How can survival be improved in localized osteosarcoma? Expert Rev. Anticancer Ther. 2010, 10, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.R.; Hughes, B.G.; Paul, S.; Steadman, P.; Sommerville, S.; Dickinson, I.C.; Walpole, E.T.; Thomson, D.B.; Mar Fan, H.G.; Joubert, W.L. Single institution retrospective review of perioperative chemotherapy in adult and adolescent patients with operable osteosarcoma. Asia Pac. J. Clin. Oncol. 2016, 12, e222–e228. [Google Scholar] [CrossRef]
- Gill, J.; Gorlick, R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 2021, 18, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tan, X.; Jiang, Z.; Wang, H.; Yuan, H. Immune checkpoint inhibitors in osteosarcoma: A hopeful and challenging future. Front. Pharmacol. 2022, 13, 1031527. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Cheung, N.-K.V. GD2 or HER2 targeting T cell engaging bispecific antibodies to treat osteosarcoma. J. Hematol. Oncol. 2020, 13, 172. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Z.; Luo, W. Chimeric Antigen Receptor T-Cell Therapy: The Light of Day for Osteosarcoma. Cancers 2021, 13, 4469. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Zhu, J.; Wang, J.; Xia, K.; Liang, C.; Tao, H. Anti-CD166/4-1BB chimeric antigen receptor T cell therapy for the treatment of osteosarcoma. J. Exp. Clin. Cancer Res. 2019, 38, 168. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Chen, T.; Zhou, H.; Zhang, W.; Lin, N.; Yu, X.; Lou, Y.; Li, B.; Yinwang, E.; et al. Human γδ T cells induce CD8(+) T cell antitumor responses via antigen-presenting effect through HSP90-MyD88-mediated activation of JNK. Cancer Immunol. Immunother. 2023, 72, 1803–1821. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Liu, G.; Li, D.; Gu, Z.; Zhang, L.; Pan, Y.; Cui, X.; Wang, L.; Liu, G.; et al. B7-H3 targeted CAR-T cells show highly efficient anti-tumor function against osteosarcoma both in vitro and in vivo. BMC Cancer 2022, 22, 1124. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Middlemiss, S.; Saletta, F.; Gottschalk, S.; McCowage, G.B.; Kramer, B. Chimeric Antigen Receptor-modified T cells targeting EphA2 for the immunotherapy of paediatric bone tumours. Cancer Gene Ther. 2021, 28, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Su, S.; Kirshtein, A.; Shahriyari, L. Data-Driven Mathematical Model of Osteosarcoma. Cancers 2021, 13, 2367. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mohammad Mirzaei, N.; Shahriyari, L. Bio-Mechanical Model of Osteosarcoma Tumor Microenvironment: A Porous Media Approach. Cancers 2022, 14, 6143. [Google Scholar] [CrossRef] [PubMed]
| Name of Compound | Target Pathway | Mechanism of Action | Phase | NCT Number | |
|---|---|---|---|---|---|
| 1. | Cytokine-induced killer (CIK) | Adoptive immunotherapy | Cytokine-induced killer | I | NCT03782363 |
| 2. | Multicomponent immune-based therapy (MKC1106-PP) | Immunotherapy | T-cell | I | NCT00423254 |
| 3. | Haploidentical transplant and donor natural killer cells | Immunotherapy | NK cell activation | II | NCT02100891 |
| 4. | GD2-targeted modified T-cells (GD2 CAR-T) | Immunotherapy | CAR-T | I | NCT02107963 |
| 5. | Activated T cells armed with GD2 bispecific antibody | Immunotherapy | CAR-T | I/II | NCT02173093 |
| 6. | Pembrolizumab | Immunotherapy | PD1 | II | NCT02301039 |
| 7. | Nivolumab plus ipilimumab | Immunotherapy | PD1 and CTLA-4 | I/II | NCT02304458 |
| 8. | Nivolumab plus ipilimumab | Immunotherapy | PD1 and CTLA-4 | II | NCT02500797 |
| 9. | Durvalumab plus tremelimumab | Immunotherapy | PD1 and CTLA-4 | II | NCT02815995 |
| 10. | Nivolumab plus ipilimumab | Immunotherapy | PD1 and CTLA-4 | II | NCT02982486 |
| 11. | Avelumab | Immunotherapy | PDL1 | II | NCT03006848 |
| 12. | Avelumab | Immunotherapy | PDL1 | II | NCT03006848 |
| 13. | Pembrolizumab | Immunotherapy | PD1 | II | NCT03013127 |
| 14. | Haploidentical donor NK cells and Hu14.18-IL2 | Immunotherapy | NK cell activation | I | NCT03209869 |
| 15. | NKTR-214 and nivolumab | Immunotherapy | PD1 and IL2 agonist | II | NCT03282344 |
| 16. | Sarcoma-specific CAR-T cells | Immunotherapy | CAR-T | I/II | NCT03356782 |
| 17. | CAB-AXL-ADC plus PD-1 inhibitor | Immunotherapy | PD1 | I/II | NCT03425279 |
| 18. | EGFR806 CAR-T cells | Immunotherapy | EGFR | I | NCT03618381 |
| 19. | C7R-GD2 CAR-T cells | Immunotherapy | CAR-T | I | NCT03635632 |
| 20. | Vigil | Engineered autologous tumor cell immunotherapy | GM-CSF, TGFβ-1, and TGFβ-2 | NCT03842865 | |
| 21. | Famitinib plus camrelizumab | RTK and checkpoint inhibition | VEGFR-2, -3 and FGFR-1, -2, -3, -4 and PD1/PDL1 | II | NCT04044378 |
| 22. | Camrelizumab (PD1) with neoadjuvant chemotherapy | Checkpoint inhibition | PD1 | II | NCT04294511 |
| 23. | CAR-T plus chemotherapy | Immunotherapy | CAR-T cells and sarcoma vaccines | I/II | NCT04433221 |
| 24. | B7H3 CAR-T cells | Immunotherapy | CAR-T, B7H3-specific receptor | I | NCT04483778 |
| 25. | GD2-targeted modified T-cells (GD2 CAR-T) | Immunotherapy | CAR-T | I | NCT04539366 |
| 26. | Oleclumab plus durvalumab | Immunotherapy | Anti-CD73 monoclonal antibody and PD-1 | II | NCT04668300 |
| 27. | B7H3 CAR-T cells | Immunotherapy | CSRT, B7H3-specific receptor | I | NCT04897321 |
| 28. | Atezolizumab and cabozantinib | Immunotherapy | Immune checkpoint inhibitor | II | NCT05019703 |
| 29. | Tislelizumab | Immunotherapy | PD1 | II | NCT05241132 |
| 30. | Nivolumab plus ipilimumab | Immunotherapy | PD1 and CTLA-4 | II | NCT05302921 |
| 31. | RNA-lipid particle vaccines | Cancer vaccine | Cancer vaccine | I/II | NCT05660408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nirala, B.K.; Yamamichi, T.; Petrescu, D.I.; Shafin, T.N.; Yustein, J.T. Decoding the Impact of Tumor Microenvironment in Osteosarcoma Progression and Metastasis. Cancers 2023, 15, 5108. https://doi.org/10.3390/cancers15205108
Nirala BK, Yamamichi T, Petrescu DI, Shafin TN, Yustein JT. Decoding the Impact of Tumor Microenvironment in Osteosarcoma Progression and Metastasis. Cancers. 2023; 15(20):5108. https://doi.org/10.3390/cancers15205108
Chicago/Turabian StyleNirala, Bikesh K., Taku Yamamichi, D. Isabel Petrescu, Tasnuva N. Shafin, and Jason T. Yustein. 2023. "Decoding the Impact of Tumor Microenvironment in Osteosarcoma Progression and Metastasis" Cancers 15, no. 20: 5108. https://doi.org/10.3390/cancers15205108
APA StyleNirala, B. K., Yamamichi, T., Petrescu, D. I., Shafin, T. N., & Yustein, J. T. (2023). Decoding the Impact of Tumor Microenvironment in Osteosarcoma Progression and Metastasis. Cancers, 15(20), 5108. https://doi.org/10.3390/cancers15205108






