Ultra-Hypofractionated Re-Irradiation with Anti-PD-1 Immunotherapy for Locoregionally Recurrent (after Radical Chemo-Radiotherapy) Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatment and Treatment Evaluation
2.2. Radiotherapy Details
2.3. Immunotherapy
2.4. Statistical Analysis
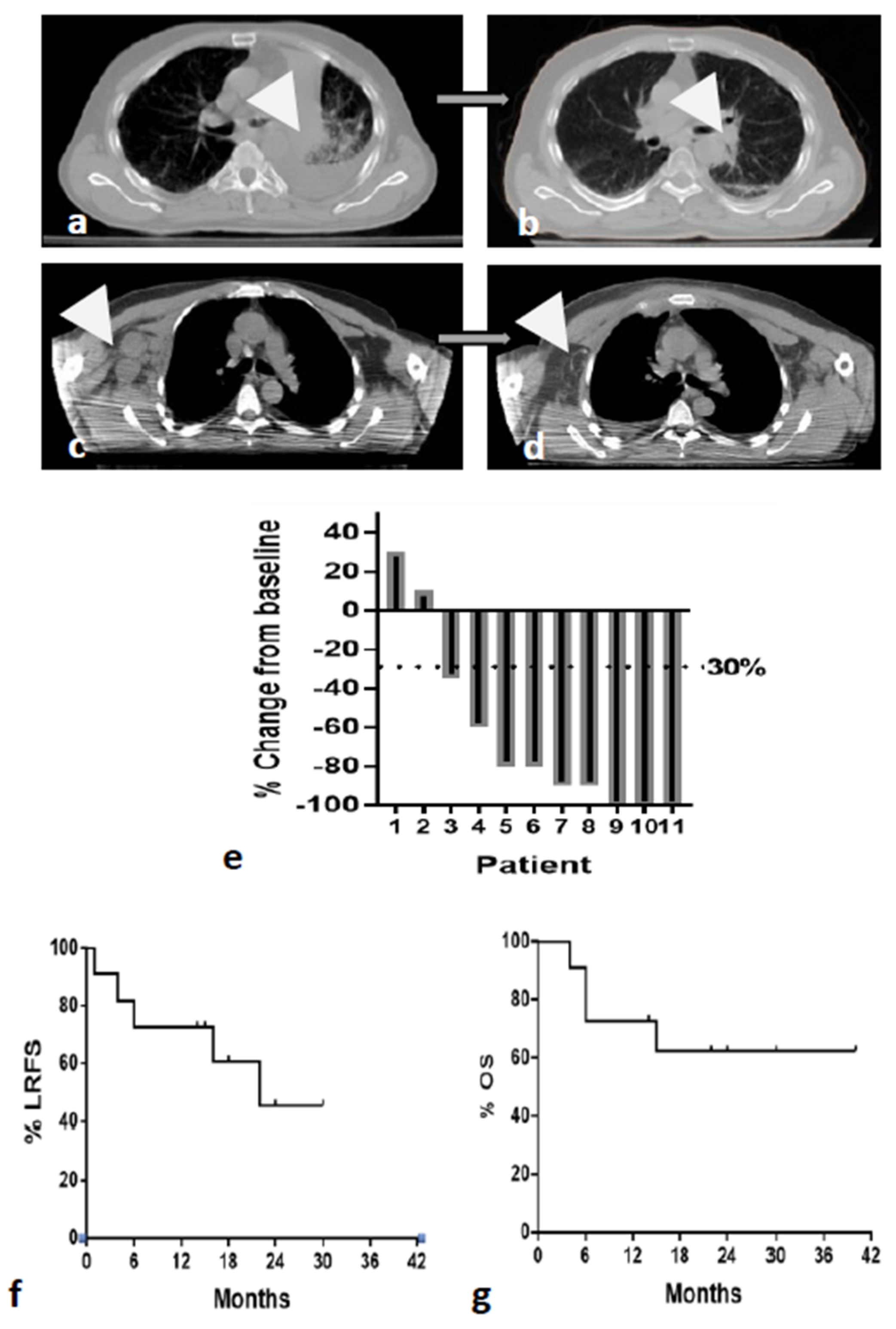
3. Results
3.1. Radiotherapy Toxicity
3.2. Immunotherapy Adverse Events
3.3. Tumor Response
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daly, M.E.; Singh, N.; Ismaila, N.; Antonoff, M.B.; Arenberg, D.A.; Bradley, J.; David, E.; Detterbeck, F.; Fruh, M.; Gubens, M.A.; et al. Management of Stage III Non-Small-Cell Lung Cancer: ASCO Guideline. J. Clin. Oncol. 2022, 40, 1356–1384. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes from the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Hunter, B.; Crockett, C.; Faivre-Finn, C.; Hiley, C.; Salem, A. Re-Irradiation of Recurrent Non-Small Cell Lung Cancer. Semin. Radiat. Oncol. 2021, 31, 124–132. [Google Scholar] [CrossRef]
- Tetar, S.; Dahele, M.; Griffioen, G.; Slotman, B.; Senan, S. High-dose conventional thoracic re-irradiation for lung cancer: Updated results. Lung Cancer 2015, 88, 235–236. [Google Scholar] [CrossRef]
- Okamoto, Y.; Murakami, M.; Yoden, E.; Sasaki, R.; Okuno, Y.; Nakajima, T.; Kuroda, Y. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 390–396. [Google Scholar] [CrossRef]
- Wu, K.L.; Jiang, G.L.; Qian, H.; Wang, L.J.; Yang, H.J.; Fu, X.L.; Zhao, S. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: A prospective phase I-II clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 1345–1350. [Google Scholar] [CrossRef]
- Sumodhee, S.; Bondiau, P.Y.; Poudenx, M.; Cohen, C.; Naghavi, A.O.; Padovani, B.; Maneval, D.; Gal, J.; Leysalle, A.; Ghalloussi, H.; et al. Long term efficacy and toxicity after stereotactic ablative reirradiation in locally relapsed stage III non-small cell lung cancer. BMC Cancer 2019, 19, 305. [Google Scholar] [CrossRef]
- Maranzano, E.; Draghini, L.; Anselmo, P.; Casale, M.; Arcidiacono, F.; Chirico, L.; Italiani, M.; Trippa, F. Lung reirradiation with stereotactic body radiotherapy. J. Radiosurg. SBRT 2016, 4, 61–68. [Google Scholar]
- Kilburn, J.M.; Kuremsky, J.G.; Blackstock, A.W.; Munley, M.T.; Kearns, W.T.; Hinson, W.H.; Lovato, J.F.; Miller, A.A.; Petty, W.J.; Urbanic, J.J. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother. Oncol. 2014, 110, 505–510. [Google Scholar] [CrossRef]
- Kelly, P.; Balter, P.A.; Rebueno, N.; Sharp, H.J.; Liao, Z.; Komaki, R.; Chang, J.Y. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1387–1393. [Google Scholar] [CrossRef]
- Horne, Z.D.; Dohopolski, M.J.; Clump, D.A.; Burton, S.A.; Heron, D.E. Thoracic reirradiation with SBRT for residual/recurrent and new primary NSCLC within or immediately adjacent to a prior high-dose radiation field. Pract. Radiat. Oncol. 2018, 8, e117–e123. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhao, Y.; Du, H.; Guo, X. Current Status of Immune Checkpoint Inhibitor Immunotherapy for Lung Cancer. Front. Oncol. 2021, 11, 704336. [Google Scholar] [CrossRef]
- de Castro, G., Jr.; Kudaba, I.; Wu, Y.L.; Lopes, G.; Kowalski, D.M.; Turna, H.Z.; Caglevic, C.; Zhang, L.; Karaszewska, B.; Laktionov, K.K.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy as First-Line Therapy in Patients with Non-Small-Cell Lung Cancer and Programmed Death Ligand-1 Tumor Proportion Score >/= 1% in the KEYNOTE-042 Study. J. Clin. Oncol. 2023, 41, 1986–1991. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Herrera, F.G.; Bourhis, J.; Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 2017, 67, 65–85. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.E.; Vanpouille-Box, C.; Melero, I.; Formenti, S.C.; Demaria, S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018, 39, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Tsoutsou, P.G.; Abatzoglou, I. Computed tomography assessment of lung density in patients with lung cancer treated with accelerated hypofractionated radio-chemotherapy supported with amifostine. Am. J. Clin. Oncol. 2009, 32, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Tsoutsou, P.G.; Froudarakis, M.E.; Bouros, D.; Koukourakis, M.I. Hypofractionated/accelerated radiotherapy with cytoprotection (HypoARC) combined with vinorelbine and liposomal doxorubicin for locally advanced non-small cell lung cancer (NSCLC). Anticancer Res. 2008, 28, 1349–1354. [Google Scholar]
- Koukourakis, M.I.; Patlakas, G.; Froudarakis, M.E.; Kyrgias, G.; Skarlatos, J.; Abatzoglou, I.; Bougioukas, G.; Bouros, D. Hypofractionated accelerated radiochemotherapy with cytopro-tection (Chemo-HypoARC) for inoperable non-small cell lung carcinoma. Anticancer Res. 2007, 27, 3625–3631. [Google Scholar]
- Koukourakis, M.I.; Damilakis, J. LQ-based model for biological radiotherapy planning. Med. Dosim. 1994, 19, 269–277. [Google Scholar] [CrossRef]
- Mulkey, F.; Theoret, M.R.; Keegan, P.; Pazdur, R.; Sridhara, R. Comparison of iRECIST versus RECIST V.1.1 in patients treated with an anti-PD-1 or PD-L1 antibody: Pooled FDA analysis. J. Immunother. Cancer 2020, 8, e000146. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 27 August 2023).
- LENT SOMA tables. Radiother. Oncol. 1995, 35, 17–60. [CrossRef]
- Goedegebuure, R.S.; Kleibeuker, E.A.; Buffa, F.M.; Castricum, K.C.; Haider, S.; Schulkens, I.A.; Ten Kroode, L.; van den Berg, J.; Jacobs, M.A.; van Berkel, A.M.; et al. Interferon-and STING-independent induction of type I interferon stimulated genes during fractionated irradiation. J. Exp. Clin. Cancer Res. 2021, 40, 161. [Google Scholar] [CrossRef]
- Xanthopoulou, E.T.; Koukourakis, I.M.; Kakouratos, C.; Nanos, C.; Kalaitzis, C.; Giatromanolaki, A.; Koukourakis, M.I. Irradiation-induced IFN-type-I pathway activation in prostate cancer cell lines. Cytokine 2023, 169, 156252. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A. Tumor draining lymph nodes, immune response, and radiotherapy: Towards a revisal of therapeutic principles. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188704. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A. Tumor microenvironment, immune response and post-radiotherapy tumor clearance. Clin. Transl. Oncol. 2020, 22, 2196–2205. [Google Scholar] [CrossRef]
- Wang, C.L.; Ho, A.S.; Chang, C.C.; Sie, Z.L.; Peng, C.L.; Chang, J.; Cheng, C.C. Radiotherapy enhances CXCR3highCD8+ T cell activation through inducing IFNγ-mediated CXCL10 and ICAM-1 expression in lung cancer cells. Cancer Immunol. Immunother. 2023, 72, 1865–1880. [Google Scholar] [CrossRef]
- Guipaud, O.; Jaillet, C.; Clément-Colmou, K.; François, A.; Supiot, S.; Milliat, F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 2018, 91, 20170762. [Google Scholar] [CrossRef]
- Theelen, W.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.U.; Aerts, J.; Bahce, I.; Niemeijer, A.L.N.; Chang, J.Y.; de Groot, P.M.; et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir. Med. 2021, 9, 467–475. [Google Scholar] [CrossRef]
- Belluomini, L.; Dionisi, V.; Palmerio, S.; Vincenzi, S.; Avancini, A.; Casali, M.; Riva, S.T.; Menis, J.; Mazzarotto, R.; Pilotto, S.; et al. Study Design and Rationale for Espera Trial: A Multicentre, Randomized, Phase II Clinical Trial Evaluating the Potential Efficacy of Adding SBRT to Pembrolizumab-Pemetrexed Maintenance in Responsive or Stable Advanced Non-Squamous NSCLC After Chemo-Immunotherapy Induction. Clin. Lung Cancer 2022, 23, e269–e272. [Google Scholar] [CrossRef]
- Schild, S.E.; Wang, X.; Bestvina, C.M.; Williams, T.; Masters, G.; Singh, A.K.; Stinchcombe, T.E.; Salama, J.K.; Wolf, S.; Zemla, T.; et al. Alliance A082002-a randomized phase II/III trial of modern immunotherapy-based systemic therapy with or without SBRT for PD-L1-negative, advanced non-small cell lung cancer. Clin. Lung Cancer 2022, 23, e317–e320. [Google Scholar] [CrossRef]
- Chang, J.Y.; Lin, S.H.; Dong, W.; Liao, Z.; Gandhi, S.J.; Gay, C.M.; Zhang, J.; Chun, S.G.; Elamin, Y.Y.; Fossella, F.V.; et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet 2023, 402, 871–881. [Google Scholar] [CrossRef]
- Koukourakis, I.M.; Giakzidis, A.G.; Koukourakis, M.I. Anti-PD-1 immunotherapy with dose-adjusted ultra-hypofractionated re-irradiation in patients with locoregionally recurrent head and neck cancer. Clin. Transl. Oncol. 2023, 25, 3032–3041. [Google Scholar] [CrossRef]
- Grambozov, B.; Nussdorfer, E.; Kaiser, J.; Gerum, S.; Fastner, G.; Stana, M.; Gaisberger, C.; Wass, R.; Studnicka, M.; Sedlmayer, F.; et al. Re-Irradiation for Locally Recurrent Lung Cancer: A Single Center Retrospective Analysis. Curr. Oncol. 2021, 28, 1835–1846. [Google Scholar] [CrossRef]
- Yang, W.C.; Hsu, F.M.; Chen, Y.H.; Shih, J.Y.; Yu, C.J.; Lin, Z.Z.; Lu, S.H.; Yang, J.C.; Cheng, A.L.; Kuo, S.H. Clinical outcomes and toxicity predictors of thoracic re-irradiation for locoregionally recurrent lung cancer. Clin. Transl. Radiat. Oncol. 2020, 22, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Schlampp, I.; Rieber, J.; Adeberg, S.; Bozorgmehr, F.; Heussel, C.P.; Steins, M.; Kappes, J.; Hoffmann, H.; Welzel, T.; Debus, J.; et al. Re-irradiation in locally recurrent lung cancer patients. Strahlenther. Onkol. 2019, 195, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Kim, Y.S.; Lee, S.W.; Lee, S.J.; Kang, J.H.; Hong, S.H.; Hong, J.Y.; Cheon, G. High-Dose Thoracic Re-irradiation of Lung Cancer Using Highly Conformal Radiotherapy Is Effective with Acceptable Toxicity. Cancer Res. Treat. 2019, 51, 1156–1166. [Google Scholar] [CrossRef]
- Patel, N.R.; Lanciano, R.; Sura, K.; Yang, J.; Lamond, J.; Feng, J.; Good, M.; Gracely, E.J.; Komarnicky, L.; Brady, L. Stereotactic body radiotherapy for re-irradiation of lung cancer recurrence with lower biological effective doses. J. Radiat. Oncol. 2015, 4, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, D.Y.; Wu, H.G.; Lee, J.H.; Kim, H.J. Treatment outcomes of re-irradiation using stereotactic ablative radiotherapy to lung: A propensity score matching analysis. Radiat. Oncol. 2021, 16, 222. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients | 11 |
|---|---|
| Age | 44–78 (median 71) |
| Gender | |
| Male | 9 |
| Female | 2 |
| Histology | |
| Squamous | 9 |
| Adenocarcinoma | 1 |
| Large cell undifferentiated | 1 |
| PD-L1 expression (*) | |
| Negative | 5 |
| Positive | 6 |
| Previous therapy | |
| Chemo-radiotherapy | 11 (**) |
| Site of recurrence | |
| In RT field | 11 |
| Contralateral lung | 1 |
| Supraclavicular/axillary | 1 |
| Upper mediastinum | 1 |
| Number of Patients | |
|---|---|
| Radiotherapy (RT) to locoregional | |
| previously irradiated sites | 8 |
| 8 Gy × 1 fraction | 3 |
| 8 Gy × 2 fractions | |
| RT to non-previously | |
| irradiated sites | |
| 8 Gy × 3 fractions | 3 |
| Concurrent immunotherapy | |
| Nivolumab | 5 |
| Pembrolizumab | 6 |
| Number of Patients | Irradiated Site | RT Lung Toxicity | RT Other Toxicity | irAEs | No IO Cycles/IO Type |
|---|---|---|---|---|---|
| 1 | lung | (***) | none | none | 6/N |
| 2 | lung | none | none | skin rash | 12/P |
| 3 | lung | none | none | none | 28/P |
| 4 | lung | (***) | none | asthenia | 20/P |
| 5 | lung | none | none | hypothyroidism | 25/N |
| nodes (*) | none | none | none | ||
| 6 | lung | (***) | none | renal | 8/P |
| 7 | lung | none | none | asthenia | 15/N |
| 8 | lung | none | none | none | 19/P |
| metastasis (**) | none | none | none | ||
| 9 | lung | (***) | none | none | 6/N |
| 10 | lung | none | none | none | 28/N |
| 11 | lung | (***) | none | none | 15/P |
| mediastinum | none | none | none |
| Number of Patients | Age | Sex | Histo- logy | Grade | PD-L1 Status | Recurrence after CRT (Months) | Recurrence Site | Re-RT Dose (Gy/ Fractions) | IO | Response (% Change) | Local Control (Months) /Status | Survival (Months) /Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | f | S | 2 | − | 12 | lung | 8/1 | N | 10 | 4/r | 4/d |
| 2 | 67 | m | S | 3 | + | 18 | lung | 8/1 | P | −60 | 15 | 15/d |
| 3 | 75 | m | S | 3 | + | 60 | lung | 8/2 | P | −90 | 22 | 22 |
| 4 | 44 | m | S | 2 | + | 50 | lung | 8/1 | P | −80 | 18 | 30 |
| 5 | 62 | m | U | 3 | − | 12 | lung | 8/1 | N | −100 | 24 | 40 |
| nodes (*) | 8/3 | −100 | ||||||||||
| 6 | 73 | m | S | 3 | + | 6 | lung | 8/1 | P | −90 | 6/r | 6/d |
| 7 | 78 | m | A | 3 | − | 11 | lung | 8/2 | N | −100 | 30 | 30 |
| 8 | 70 | m | S | 3 | + | 18 | lung | 8/1 | P | 35 | 16/r | 30 |
| metastasis (**) | 8/3 | −100 | ||||||||||
| 9 | 64 | m | S | 3 | + | 6 | lung | 8/2 | N | −100 | 1/r | 6/d |
| 10 | 74 | m | S | 2 | − | 13 | lung | 8/1 | N | −35 | 22/r | 24 |
| 11 | 75 | f | S | 2 | − | 14 | lung | 8/1 | P | −80 | 14 | 14 |
| mediastinum | 8/3 | −100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippatos, K.; Koukourakis, I.M.; Anevlavis, S.; Giaktzidis, A.; Koukourakis, M.I. Ultra-Hypofractionated Re-Irradiation with Anti-PD-1 Immunotherapy for Locoregionally Recurrent (after Radical Chemo-Radiotherapy) Non-Small Cell Lung Cancer. Cancers 2023, 15, 5083. https://doi.org/10.3390/cancers15205083
Filippatos K, Koukourakis IM, Anevlavis S, Giaktzidis A, Koukourakis MI. Ultra-Hypofractionated Re-Irradiation with Anti-PD-1 Immunotherapy for Locoregionally Recurrent (after Radical Chemo-Radiotherapy) Non-Small Cell Lung Cancer. Cancers. 2023; 15(20):5083. https://doi.org/10.3390/cancers15205083
Chicago/Turabian StyleFilippatos, Konstantinos, Ioannis M. Koukourakis, Stavros Anevlavis, Axiotis Giaktzidis, and Michael I. Koukourakis. 2023. "Ultra-Hypofractionated Re-Irradiation with Anti-PD-1 Immunotherapy for Locoregionally Recurrent (after Radical Chemo-Radiotherapy) Non-Small Cell Lung Cancer" Cancers 15, no. 20: 5083. https://doi.org/10.3390/cancers15205083
APA StyleFilippatos, K., Koukourakis, I. M., Anevlavis, S., Giaktzidis, A., & Koukourakis, M. I. (2023). Ultra-Hypofractionated Re-Irradiation with Anti-PD-1 Immunotherapy for Locoregionally Recurrent (after Radical Chemo-Radiotherapy) Non-Small Cell Lung Cancer. Cancers, 15(20), 5083. https://doi.org/10.3390/cancers15205083







