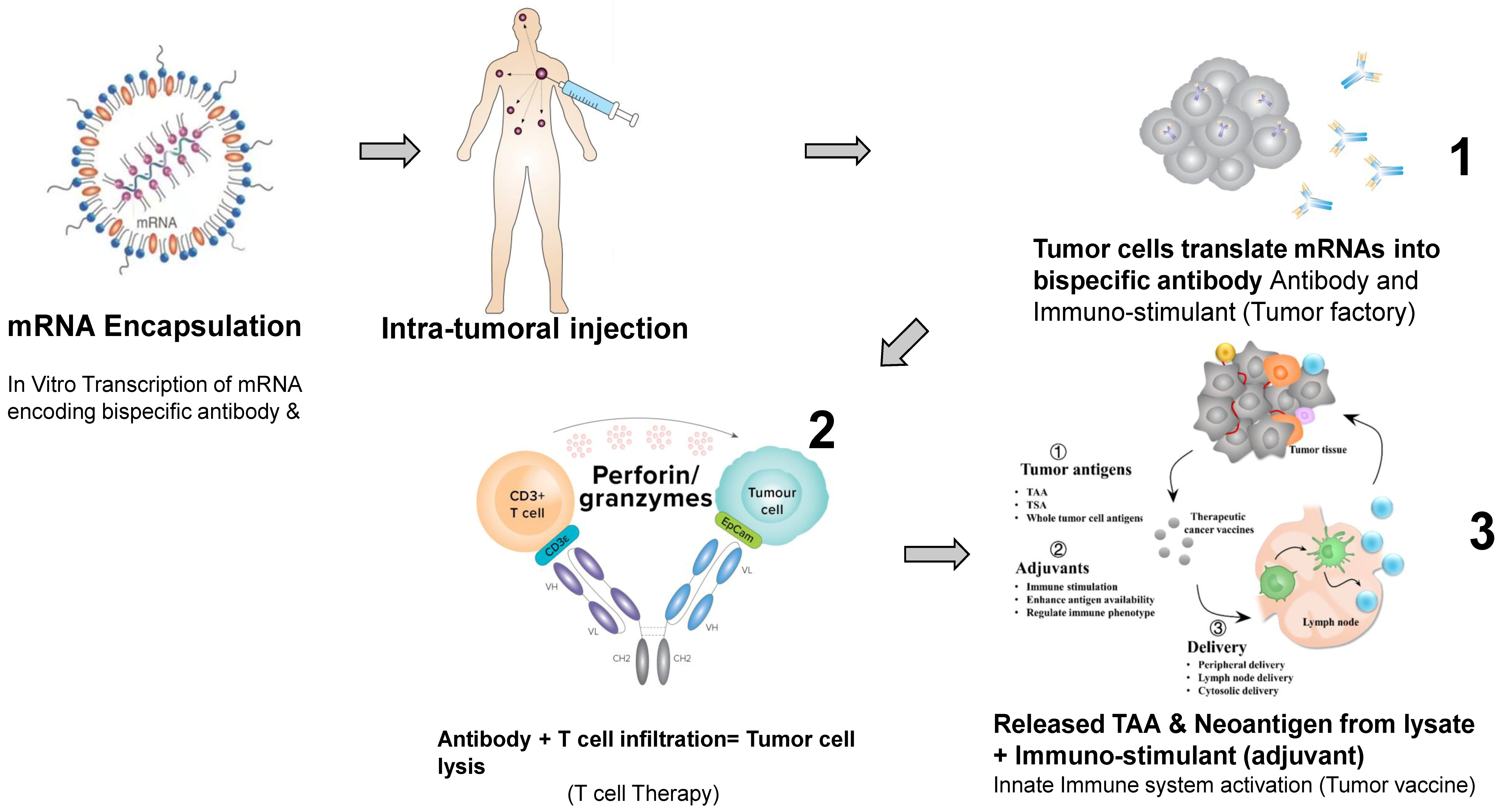
Editorial on “Cell Therapy, Bispecific Antibodies and Other Immunotherapies against Cancer”
Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Abrantes, R.; Duarte, H.O.; Gomes, C.; Walchli, S.; Reis, C.A. CAR-Ts: New perspectives in cancer therapy. FEBS Lett. 2022, 596, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.V.; June, C.H. Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clin. Cancer Res. 2016, 22, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Eshhar, Z. Therapeutic Potential of T Cell Chimeric Antigen Receptors (CARs) in Cancer Treatment: Counteracting Off-Tumor Toxicities for Safe CAR T Cell Therapy. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 59–83. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Cronk, R.J.; Zurko, J.; Shah, N.N. Bispecific Chimeric Antigen Receptor T Cell Therapy for B Cell Malignancies and Multiple Myeloma. Cancers 2020, 12, 2523. [Google Scholar] [CrossRef]
- Hegde, M.; Mukherjee, M.; Grada, Z.; Pignata, A.; Landi, D.; Navai, S.A.; Wakefield, A.; Fousek, K.; Bielamowicz, K.; Chow, K.K.; et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J. Clin. Investig. 2016, 126, 3036–3052. [Google Scholar] [CrossRef]
- Martyniszyn, A.; Krahl, A.C.; Andre, M.C.; Hombach, A.A.; Abken, H. CD20-CD19 Bispecific CAR T Cells for the Treatment of B-Cell Malignancies. Hum. Gene Ther. 2017, 28, 1147–1157. [Google Scholar] [CrossRef]
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; Krueger, W.; Worden, A.A.; Kadan, M.J.; Yim, S.; et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial. Nat. Med. 2020, 26, 1569–1575. [Google Scholar] [CrossRef]
- Zah, E.; Lin, M.Y.; Silva-Benedict, A.; Jensen, M.C.; Chen, Y.Y. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol. Res. 2016, 4, 498–508. [Google Scholar] [CrossRef]
- Zah, E.; Nam, E.; Bhuvan, V.; Tran, U.; Ji, B.Y.; Gosliner, S.B.; Wang, X.; Brown, C.E.; Chen, Y.Y. Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Nat. Commun. 2020, 11, 2283. [Google Scholar] [CrossRef]
- Rafiq, S.; Yeku, O.O.; Jackson, H.J.; Purdon, T.J.; van Leeuwen, D.G.; Drakes, D.J.; Song, M.; Miele, M.M.; Li, Z.; Wang, P.; et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018, 36, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Rupp, L.J.; Schumann, K.; Roybal, K.T.; Gate, R.E.; Ye, C.J.; Lim, W.A.; Marson, A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 2017, 7, 737. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, T.; Ansell, S.M. Immunomodulators in Lymphoma. Curr. Treat. Options Oncol. 2020, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef]
- Longo, V.; Brunetti, O.; Azzariti, A.; Galetta, D.; Nardulli, P.; Leonetti, F.; Silvestris, N. Strategies to Improve Cancer Immune Checkpoint Inhibitors Efficacy, Other Than Abscopal Effect: A Systematic Review. Cancers 2019, 11, 539. [Google Scholar] [CrossRef]
- Kilgour, M.K.; Bastin, D.J.; Lee, S.H.; Ardolino, M.; McComb, S.; Visram, A. Advancements in CAR-NK therapy: Lessons to be learned from CAR-T therapy. Front. Immunol. 2023, 14, 1166038. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Kerbauy, L.N.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Merino, A.; Maakaron, J.; Bachanova, V. Advances in NK cell therapy for hematologic malignancies: NK source, persistence and tumor targeting. Blood Rev. 2023, 60, 101073. [Google Scholar] [CrossRef]
- Nowak, J.; Bentele, M.; Kutle, I.; Zimmermann, K.; Luhmann, J.L.; Steinemann, D.; Kloess, S.; Koehl, U.; Roßberg, W.; Ahmed, A.; et al. CAR-NK Cells Targeting HER1 (EGFR) Show Efficient Anti-Tumor Activity against Head and Neck Squamous Cell Carcinoma (HNSCC). Cancers 2023, 15, 3169. [Google Scholar] [CrossRef]
- Romanski, A.; Uherek, C.; Bug, G.; Seifried, E.; Klingemann, H.; Wels, W.S.; Ottmann, O.G.; Tonn, T. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J. Cell. Mol. Med. 2016, 20, 1287–1294. [Google Scholar] [CrossRef]
- Wlodarczyk, M.; Pyrzynska, B. CAR-NK as a Rapidly Developed and Efficient Immunotherapeutic Strategy against Cancer. Cancers 2022, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.; Sienkiewicz, J.; Sun, J.; Zhang, S.; Huang, Y.; Zhou, H.; Harto, H.; Xu, S.; Berahovich, R.; Wu, L. CAR-NK Cells Generated with mRNA-LNPs Kill Tumor Target Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2023, 24, 13364. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, E.M.V.; Riise, R.; Saellstrom, S.; Karlsson, J.; Alsen, S.; Bucher, V.; Hemminki, A.E.; Bagge, R.O.; Ny, L.; Nilsson, L.M.; et al. Treatment with Anti-HER2 Chimeric Antigen Receptor Tumor-Infiltrating Lymphocytes (CAR-TILs) Is Safe and Associated with Antitumor Efficacy in Mice and Companion Dogs. Cancers 2023, 15, 648. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Yazdanifar, M.; Cocco, C.; Bertaina, A.; Airoldi, I. Engineering the Bridge between Innate and Adaptive Immunity for Cancer Immunotherapy: Focus on gammadelta T and NK Cells. Cells 2020, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Capsomidis, A.; Benthall, G.; Van Acker, H.H.; Fisher, J.; Kramer, A.M.; Abeln, Z.; Majani, Y.; Gileadi, T.; Wallace, R.; Gustafsson, K.; et al. Chimeric Antigen Receptor-Engineered Human Gamma Delta T Cells: Enhanced Cytotoxicity with Retention of Cross Presentation. Mol. Ther. 2018, 26, 354–365. [Google Scholar] [CrossRef]
- Du, S.H.; Li, Z.; Chen, C.; Tan, W.K.; Chi, Z.; Kwang, T.W.; Xu, X.H.; Wang, S. Co-Expansion of Cytokine-Induced Killer Cells and Vgamma9Vdelta2 T Cells for CAR T-Cell Therapy. PLoS ONE 2016, 11, e0161820. [Google Scholar] [CrossRef]
- Fleischer, L.C.; Becker, S.A.; Ryan, R.E.; Fedanov, A.; Doering, C.B.; Spencer, H.T. Non-signaling Chimeric Antigen Receptors Enhance Antigen-Directed Killing by gammadelta T Cells in Contrast to alphabeta T Cells. Mol. Ther. Oncolytics 2020, 18, 149–160. [Google Scholar] [CrossRef]
- Bumma, N.; Papadantonakis, N.; Advani, A.S. Structure, development, preclinical and clinical efficacy of blinatumomab in acute lymphoblastic leukemia. Future Oncol. 2015, 11, 1729–1739. [Google Scholar] [CrossRef]
- Nagorsen, D.; Kufer, P.; Baeuerle, P.A.; Bargou, R. Blinatumomab: A historical perspective. Pharmacol. Ther. 2012, 136, 334–342. [Google Scholar] [CrossRef]
- Nagorsen, D.; Bargou, R.; Ruttinger, D.; Kufer, P.; Baeuerle, P.A.; Zugmaier, G. Immunotherapy of lymphoma and leukemia with T-cell engaging BiTE antibody blinatumomab. Leuk. Lymphoma 2009, 50, 886–891. [Google Scholar] [CrossRef]
- d’Argouges, S.; Wissing, S.; Brandl, C.; Prang, N.; Lutterbuese, R.; Kozhich, A.; Suzich, J.; Locher, M.; Kiener, P.; Kufer, P.; et al. Combination of rituximab with blinatumomab (MT103/MEDI-538), a T cell-engaging CD19-/CD3-bispecific antibody, for highly efficient lysis of human B lymphoma cells. Leuk. Res. 2009, 33, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, V.; Seipel, K.; Banz, Y.; Wiedemann, G.; Porret, N.; Bacher, U.; Pabst, T. Glofitamab Treatment in Relapsed or Refractory DLBCL after CAR T-Cell Therapy. Cancers 2022, 14, 2516. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Schaefer, W.; Regula, J.T.; Dumontet, C.; Brinkmann, U.; Bacac, M.; Umana, P. Engineering therapeutic bispecific antibodies using CrossMab technology. Methods 2019, 154, 21–31. [Google Scholar] [CrossRef]
- Seckinger, A.; Delgado, J.A.; Moser, S.; Moreno, L.; Neuber, B.; Grab, A.; Lipp, S.; Merino, J.; Prosper, F.; Emde, M.; et al. Target Expression, Generation, Preclinical Activity, and Pharmacokinetics of the BCMA-T Cell Bispecific Antibody EM801 for Multiple Myeloma Treatment. Cancer Cell 2017, 31, 396–410. [Google Scholar] [CrossRef]
- Shanshal, M.; Caimi, P.F.; Adjei, A.A.; Ma, W.W. T-Cell Engagers in Solid Cancers-Current Landscape and Future Directions. Cancers 2023, 15, 2824. [Google Scholar] [CrossRef] [PubMed]
- Buchler, T.; Kovarova, L.; Musilova, R.; Bourkova, L.; Ocadlikova, D.; Bulikova, A.; Hanak, L.; Michalek, J.; Hajek, R. Generation of dendritic cells using cell culture bags—Description of a method and review of literature. Hematology 2004, 9, 199–205. [Google Scholar] [CrossRef]
- Dwivedi, R.; Pandey, R.; Chandra, S.; Mehrotra, D. Dendritic cell-based immunotherapy: A potential player in oral cancer therapeutics. Immunotherapy 2023, 15, 457–469. [Google Scholar] [CrossRef]
- Foley, R.; Tozer, R.; Wan, Y. Genetically modified dendritic cells in cancer therapy: Implications for transfusion medicine. Transfus. Med. Rev. 2001, 15, 292–304. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Batich, K.A.; Mohan, A.; Rahman, R.; Piantadosi, S.; Khasraw, M. Dendritic cell vaccine trials in gliomas: Untangling the lines. Neuro Oncol. 2023, 25, 1752–1762. [Google Scholar] [CrossRef]
- Lee, K.W.; Yam, J.W.P.; Mao, X. Dendritic Cell Vaccines: A Shift from Conventional Approach to New Generations. Cells 2023, 12, 2147. [Google Scholar] [CrossRef]
- Ma, X.; Shou, P.; Smith, C.; Chen, Y.; Du, H.; Sun, C.; Kren, N.P.; Michaud, D.; Ahn, S.; Vincent, B.; et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020, 38, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Yanmin Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616. [Google Scholar] [CrossRef] [PubMed]
- Marple, A.H.; Bonifant, C.L.; Shah, N.N. Improving CAR T-cells: The next generation. Semin. Hematol. 2020, 57, 115–121. [Google Scholar] [CrossRef]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Tureci, O.; Vormehr, M.; Kranz, L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Persano, F.; Persano, S. Advances in Lipid Nanoparticles for mRNA-Based Cancer Immunotherapy. Front. Chem. 2020, 8, 589959. [Google Scholar] [CrossRef]
- Ge, C.; Yang, X.; Xin, J.; Gong, X.; Wang, X.; Kong, L. Recent Advances in Antitumor Dendritic Cell Vaccines. Cancer Biother. Radiopharm. 2023, 38, 450–457. [Google Scholar] [CrossRef]
- Goutsouliak, K.; Veeraraghavan, J.; Sethunath, V.; De Angelis, C.; Osborne, C.K.; Rimawi, M.F.; Schiff, R. Towards personalized treatment for early stage HER2-positive breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 233–250. [Google Scholar] [CrossRef]
- Shin, E.C. Cancer immunotherapy: Special issue of BMB Reports in 2021. BMB Rep. 2021, 54, 1. [Google Scholar] [CrossRef]
- Vilgelm, A.E.; Johnson, D.B.; Richmond, A. Combinatorial approach to cancer immunotherapy: Strength in numbers. J. Leukoc. Biol. 2016, 100, 275–290. [Google Scholar] [CrossRef]
- Korman, A.J.; Peggs, K.S.; Allison, J.P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006, 90, 297–339. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubovskaya, V. Editorial on “Cell Therapy, Bispecific Antibodies and Other Immunotherapies against Cancer”. Cancers 2023, 15, 5053. https://doi.org/10.3390/cancers15205053
Golubovskaya V. Editorial on “Cell Therapy, Bispecific Antibodies and Other Immunotherapies against Cancer”. Cancers. 2023; 15(20):5053. https://doi.org/10.3390/cancers15205053
Chicago/Turabian StyleGolubovskaya, Vita. 2023. "Editorial on “Cell Therapy, Bispecific Antibodies and Other Immunotherapies against Cancer”" Cancers 15, no. 20: 5053. https://doi.org/10.3390/cancers15205053
APA StyleGolubovskaya, V. (2023). Editorial on “Cell Therapy, Bispecific Antibodies and Other Immunotherapies against Cancer”. Cancers, 15(20), 5053. https://doi.org/10.3390/cancers15205053



