The Diagnostic Performance of Multiparametric Ultrasound in the Qualitative Assessment of Inconclusive Cervical Lymph Nodes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Imaging Protocol
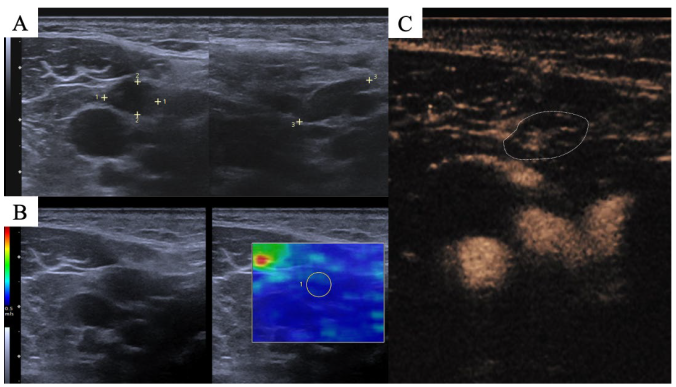
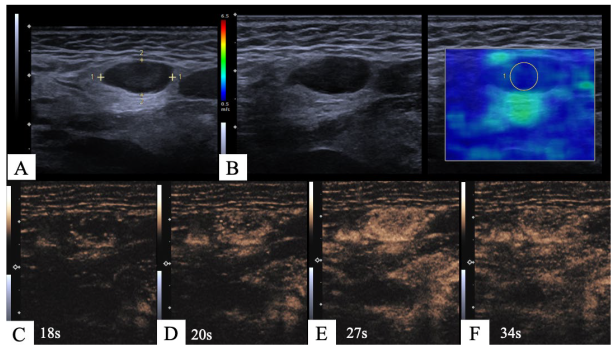
2.3. Image Interpretation
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Diagnostic Performance
3.2.1. Diagnostic Performance of Single Modalities
3.2.2. Diagnostic Performance of Combined Parameters
3.3. Perfusion Patterns, Homogeneity and Necrosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaddey, H.L.; Riegel, A.M. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Audet, N.; Beasley, N.J.; MacMillan, C.; Jackson, D.G.; Gullane, P.J.; Kamel-Reid, S. Lymphatic vessel density, nodal metastases, and prognosis in patients with head and neck cancer. Arch. Otolaryngol. Head. Neck Surg. 2005, 131, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Wunschel, M.; Neumeier, M.; Utpatel, K.; Reichert, T.E.; Ettl, T.; Spanier, G. Staging more important than grading? Evaluation of malignancy grading, depth of invasion, and resection margins in oral squamous cell carcinoma. Clin. Oral. Investig. 2021, 25, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Paleri, V.; Urbano, T.G.; Mehanna, H.; Repanos, C.; Lancaster, J.; Roques, T.; Patel, M.; Sen, M. Management of neck metastases in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S161–S169. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Grass, I.; Laudien, M.; Quetz, J.; Graefe, H.; Wollenberg, B.; Meyer, J.E. Comparison of Clinical Examination and Various Imaging Modalities in the Diagnosis of Head and Neck Cancer. Int. Arch. Otorhinolaryngol. 2021, 25, e179–e184. [Google Scholar] [CrossRef] [PubMed]
- Dudea, S.M.; Lenghel, M.; Botar-Jid, C.; Vasilescu, D.; Duma, M. Ultrasonography of superficial lymph nodes: Benign vs. malignant. Med. Ultrason. 2012, 14, 294–306. [Google Scholar]
- Liao, L.J.; Lo, W.C.; Hsu, W.L.; Wang, C.T.; Lai, M.S. Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalities. BMC Cancer 2012, 12, 236. [Google Scholar] [CrossRef]
- Lerchbaumer, M.H.; Wakonig, K.M.; Arens, P.; Dommerich, S.; Fischer, T. Quantitative Multiparametric Ultrasound (mpUS) in the Assessment of Inconclusive Cervical Lymph Nodes. Cancers 2022, 14, 1597. [Google Scholar] [CrossRef]
- Pehlivan, M.; Gurbuz, M.K.; Cingi, C.; Adapinar, B.; Degirmenci, A.N.; Acikalin, F.M.; Pinarbasli, M.O.; Colak, E. Diagnostic role of ultrasound elastography on lymph node metastases in patients with head and neck cancer. Braz. J. Otorhinolaryngol. 2019, 85, 297–302. [Google Scholar] [CrossRef]
- Dudau, C.; Hameed, S.; Gibson, D.; Muthu, S.; Sandison, A.; Eckersley, R.J.; Clarke, P.; Cosgrove, D.O.; Lim, A.K. Can contrast-enhanced ultrasound distinguish malignant from reactive lymph nodes in patients with head and neck cancers? Ultrasound Med. Biol. 2014, 40, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Lenon, M.S.L.; Storino Ramacciotti, L.; Medina, L.G.; Sayegh, A.S.; La Riva Rincon, A.; Perez, L.C.; Ghoreifi, A.; Lizana, M.; Jadvar, D.S.; et al. Multiparametric ultrasound of prostate: Role in prostate cancer diagnosis. Ther. Adv. Urol. 2022, 14, 17562872221145625. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S. Multiparametric Ultrasound (MPUS) Imaging: Terminology Describing the Many Aspects of Ultrasonography. Ultraschall Med. 2015, 36, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Zenk, J.; Bozzato, A.; Steinhart, H.; Greess, H.; Iro, H. Metastatic and inflammatory cervical lymph nodes as analyzed by contrast-enhanced color-coded Doppler ultrasonography: Quantitative dynamic perfusion patterns and histopathologic correlation. Ann. Otol. Rhinol. Laryngol. 2005, 114, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rao, M.; Ren, J.; Yang, X.; Wang, J.; Wu, Y.; Tao, X. Determination of Cervical Lymph Nodes Metastasis and Extra Nodal Extension Status by Quantitative Assessment of Border Irregularity and Apparent Diffusion Coefficient in Patients with Tongue Squamous Cell Carcinoma. J. Comput. Assist. Tomogr. 2021, 45, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, K.; Ji, Y.; Liu, H.; Fei, X.; Zhang, Y.; Li, J.; Luo, Y. Safety Analysis of Adverse Events of Ultrasound Contrast Agent Lumason/SonoVue in 49,100 Patients. Ultrasound Med. Biol. 2023, 49, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Rohan, K.; Ramesh, A.; Sureshkumar, S.; Vijayakumar, C.; Abdulbasith, K.M.; Krishnaraj, B. Evaluation of B-Mode and Color Doppler Ultrasound in the Diagnosis of Malignant Cervical Lymphadenopathy. Cureus 2020, 12, e9819. [Google Scholar] [CrossRef]
- Chammas, M.C.; Macedo, T.A.; Lo, V.W.; Gomes, A.C.; Juliano, A.; Cerri, G.G. Predicting malignant neck lymphadenopathy using color duplex sonography based on multivariate analysis. J. Clin. Ultrasound 2016, 44, 587–594. [Google Scholar] [CrossRef]
- Xiang, D.; Hong, Y.; Zhang, B.; Huang, P.; Li, G.; Wang, P.; Li, Z. Contrast-enhanced ultrasound (CEUS) facilitated US in detecting lateral neck lymph node metastasis of thyroid cancer patients: Diagnosis value and enhancement patterns of malignant lymph nodes. Eur. Radiol. 2014, 24, 2513–2519. [Google Scholar] [CrossRef]
- Spiesecke, P.; Neumann, K.; Wakonig, K.; Lerchbaumer, M.H. Contrast-enhanced ultrasound (CEUS) in characterization of inconclusive cervical lymph nodes: A meta-analysis and systematic review. Sci. Rep. 2022, 12, 7804. [Google Scholar] [CrossRef] [PubMed]
- Kunzel, J.; Brandenstein, M.; Zeman, F.; Symeou, L.; Platz Batista da Silva, N.; Jung, E.M. Multiparametric Ultrasound of Cervical Lymph Node Metastases in Head and Neck Cancer for Planning Non-Surgical Therapy. Diagnostics 2022, 12, 1842. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yang, D.; Hu, J.; Hao, X.; Gao, J.; Mao, Z. Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer. Res. 2008, 28, 1659–1666. [Google Scholar] [PubMed]
- Sharma, A.; Jaiswal, A.A.; Umredkar, G.; Barle, R.; Sharma, N.; Banerjee, P.K.; Garg, A.K.; Membally, R. Lymph Node Central Necrosis on the Computed Tomography as the Predictor of the Extra Capsular Spread in Metastatic Head and Neck Squamous Cell Carcinoma. Indian. J. Otolaryngol. Head. Neck Surg. 2017, 69, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Bredholt, G.; Mannelqvist, M.; Stefansson, I.M.; Birkeland, E.; Bo, T.H.; Oyan, A.M.; Trovik, J.; Kalland, K.H.; Jonassen, I.; Salvesen, H.B.; et al. Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget 2015, 6, 39676–39691. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.H.; Park, H.K.; Jang, K.T.; Hwang, J.A.; Kim, S. Pancreatic Ductal Adenocarcinoma: Rim Enhancement at MR Imaging Predicts Prognosis after Curative Resection. Radiology 2018, 288, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Yoon, D.Y.; Kim, S.S.; Rho, Y.S.; Chung, E.J.; Eom, J.S.; Lee, J.S. CT differentiation of abscess and non-infected fluid in the postoperative neck. Acta Radiol. 2013, 54, 48–53. [Google Scholar] [CrossRef]
- Ogura, I.; Oda, T.; Sue, M.; Sasaki, Y.; Hayama, K. Comparison between squamous cell carcinoma and inflammatory diseases of the oral and maxillofacial region using gallium-67 scintigraphy with computed tomography and magnetic resonance imaging. Pol. J. Radiol. 2018, 83, e452–e458. [Google Scholar] [CrossRef]
- Guo, Y.; Song, Q.; Pan, Q. Correlation analysis between rim enhancement features of contrast-enhanced ultrasound and lymph node metastasis in breast cancer. Am. J. Transl. Res. 2021, 13, 7193–7199. [Google Scholar]
- Tan, S.; Miao, L.Y.; Cui, L.G.; Sun, P.F.; Qian, L.X. Value of Shear Wave Elastography Versus Contrast-Enhanced Sonography for Differentiating Benign and Malignant Superficial Lymphadenopathy Unexplained by Conventional Sonography. J. Ultrasound Med. 2017, 36, 189–199. [Google Scholar] [CrossRef]
- Li, L.; Mori, S.; Sakamoto, M.; Takahashi, S.; Kodama, T. Mouse model of lymph node metastasis via afferent lymphatic vessels for development of imaging modalities. PLoS ONE 2013, 8, e55797. [Google Scholar] [CrossRef]
- Todsen, T.; Ewertsen, C.; Jenssen, C.; Evans, R.; Kuenzel, J. Head and Neck Ultrasound—EFSUMB Training Recommendations for the Practice of Medical Ultrasound in Europe. Ultrasound Int. Open 2022, 8, E29–E34. [Google Scholar] [CrossRef]
- Ahuja, A.T.; Ying, M. Sonographic evaluation of cervical lymph nodes. AJR Am. J. Roentgenol. 2005, 184, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
| Patients | 105 |
| Female | 37/105 (35.24%) |
| Male | 68/105 (64.76%) |
| Mean Age | 59.93 years (±16.59 years) |
| CLNs | 107 |
| Benign | 39/107 |
| Malignant | 68/107 |
| HNSCC | 30/68 (44.12%) |
| Lymphoma | 20/68 (29.41%) |
| Malignant melanoma | 9/68 (13.24%) |
| Adenocarcinoma (breast, salivary gland, and lung) | 5/68 (7.35%) |
| Other entities (prostate cancer, transitional cell carcinoma, atypical fibroxanthoma, and renal cell cancer) | 4/68 (5.88%) |
| Variable | Coef(B) | S.E. | Odds Ratio | 95% CI Lower–Upper | p-Value |
|---|---|---|---|---|---|
| B-mode US | 1.1726 | 0.481 | 5.619 | 2.190–14.420 | <0.001 |
| CCDS | 2.451 | 0.482 | 11.6 | 4.507–29.854 | <0.001 |
| SWE | 3.045 | 0.591 | 21 | 6.593–66.891 | <0.001 |
| CEUS | 4.238 | 0.643 | 69.3 | 19.67–244.149 | <0.001 |
| Sensitivity | Specificity | PPV | NPV | Accuracy | χ2 | p-Value | |
|---|---|---|---|---|---|---|---|
| Single Modalities | |||||||
| B-mode US | 87% | 46% | 74% | 67% | 72% | χ2 (1) = 14.236 | <0.001 |
| CCDS | 85% | 67% | 82% | 72% | 79% | χ2 (1) = 29.974 | <0.001 |
| SWE | 71% | 90% | 92% | 64% | 78% | χ2 (1) = 36.115 | <0.001 |
| CEUS | 93% | 85% | 91% | 87% | 90% | χ2 (1) = 13.219 | <0.001 |
| CEUS Characteristics | |||||||
| Perfusion pattern | 65% | 72% | 80% | 54% | 67% | χ2 (1) = 64.605 | <0.001 |
| Homogeneity | 75% | 74% | 84% | 63% | 75% | χ2 (1) = 24.638 | <0.001 |
| Necrosis | 60% | 82% | 85% | 54% | 68% | χ2 (1) = 17.967 | <0.001 |
| Combined Modalities | |||||||
| B-US + CCDS | 87% | 44% | 73% | 65% | 73% | χ2 (1) = 12.415 | <0.001 |
| SWE + CEUS | 91% | 77% | 87% | 83% | 90% | χ2 (1) = 51.485 | <0.001 |
| B-US + CCDS + SWE | 96% | 36% | 72% | 82% | 76% | χ2 (1) = 18.072 | <0.001 |
| B-US + CCDS + CEUS | 96% | 38% | 73% | 83% | 75% | χ2 (1) = 20.536 | <0.001 |
| mpUS | 97% | 36% | 73% | 88% | 83% | χ2 (1) = 21.168 | <0.001 |
| Variable | Coef(B) | S.E. | Odds Ratio | 95% CI Lower–Upper | p-Value |
|---|---|---|---|---|---|
| B-mode US | 0.039 | 0.0859 | 1.039 | 0.193–5.592 | >0.05 |
| CCDS | 0.331 | 0.938 | 1.393 | 0.221–8.762 | >0.05 |
| SWE | 1.367 | 0.778 | 3.925 | 0.854–18.045 | >0.05 |
| CEUS | 3.291 | 0.939 | 26.875 | 4.266–169.321 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakonig, K.M.; Dommerich, S.; Fischer, T.; Arens, P.; Hamm, B.; Olze, H.; Lerchbaumer, M.H. The Diagnostic Performance of Multiparametric Ultrasound in the Qualitative Assessment of Inconclusive Cervical Lymph Nodes. Cancers 2023, 15, 5035. https://doi.org/10.3390/cancers15205035
Wakonig KM, Dommerich S, Fischer T, Arens P, Hamm B, Olze H, Lerchbaumer MH. The Diagnostic Performance of Multiparametric Ultrasound in the Qualitative Assessment of Inconclusive Cervical Lymph Nodes. Cancers. 2023; 15(20):5035. https://doi.org/10.3390/cancers15205035
Chicago/Turabian StyleWakonig, Katharina Margherita, Steffen Dommerich, Thomas Fischer, Philipp Arens, Bernd Hamm, Heidi Olze, and Markus Herbert Lerchbaumer. 2023. "The Diagnostic Performance of Multiparametric Ultrasound in the Qualitative Assessment of Inconclusive Cervical Lymph Nodes" Cancers 15, no. 20: 5035. https://doi.org/10.3390/cancers15205035
APA StyleWakonig, K. M., Dommerich, S., Fischer, T., Arens, P., Hamm, B., Olze, H., & Lerchbaumer, M. H. (2023). The Diagnostic Performance of Multiparametric Ultrasound in the Qualitative Assessment of Inconclusive Cervical Lymph Nodes. Cancers, 15(20), 5035. https://doi.org/10.3390/cancers15205035









