Carbon Ion Radiotherapy: An Evidence-Based Review and Summary Recommendations of Clinical Outcomes for Skull-Base Chordomas and Chondrosarcomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Skull-Base Chordoma
3.2. Skull-Base Chondrosarcoma
3.3. Combined Outcomes
3.4. Re-Irradiation
3.5. Pediatrics
3.6. Cost Effectiveness
4. Discussion
4.1. Comparisons to Other Forms of Radiotherapy
4.2. Recommendations for CIRT
- Unresectable or subtotally removed chordoma or chondrosarcoma;
- High-volume residual disease (>25 mL);
- Recurrence following prior surgery;
- Recurrence following prior irradiation;
- Tumors not encroaching or directly abutting the brainstem or optic apparatus.
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chhabra, A.M.; Rice, S.R.; Holtzman, A.; Choi, J.I.; Hasan, S.; Press, R.H.; Chang, J.; Halasz, L.; Tsai, H.K.; Wang, C.J.; et al. Clinical outcomes and toxicities of 100 patients treated with proton therapy for chordoma on the proton collaborative group prospective registry. Radiother. Oncol. 2023, 183, 109551. [Google Scholar] [CrossRef]
- Mercado, C.E.; Holtzman, A.L.; Rotondo, R.; Rutenberg, M.S.; Mendenhall, W.M. Proton therapy for skull base tumors: A review of clinical outcomes for chordomas and chondrosarcomas. Head Neck 2019, 41, 536–541. [Google Scholar] [CrossRef]
- Rich, T.A.; Schiller, A.; Suit, H.D.; Mankin, H.J. Clinical and pathologic review of 48 cases of chordoma. Cancer 1985, 56, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Palm, R.F.; Oliver, D.E.; Yang, G.Q.; Abuodeh, Y.; Naghavi, A.O.; Johnstone, P.A.S. The role of dose escalation and proton therapy in perioperative or definitive treatment of chondrosarcoma and chordoma: An analysis of the National Cancer Data Base. Cancer 2019, 125, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lim, G.; Grosshans, D.; Mohan, R.; Cao, W. A biological effect-guided optimization approach using beam distal-edge avoidance for intensity-modulated proton therapy. Med. Phys. 2020, 47, 3816–3825. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, A.L.; Bates, J.E.; Morris, C.G.; Rutenberg, M.S.; Indelicato, D.J.; Tavanaiepour, D.; Mendenhall, W.M. Impact of Type of Treatment Center and Access to Care on Mortality and Survival for Skull Base Chordoma and Chondrosarcoma. J. Neurol. Surg. B Skull Base 2022, 83, 328–338. [Google Scholar] [CrossRef]
- Holtzman, A.L.; Dagan, R.; Mendenhall, W.M. Proton Radiotherapy for Skull-Base Malignancies: Imaging Considerations of Radiotherapy and Complications. Oral. Maxillofac. Surg. Clin. N. Am. 2023, 35, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Rutenberg, M.S.; Beltran, C. Future Perspective: Carbon Ion Radiotherapy for Head and Neck and Skull Base Malignancies. Oral. Maxillofac. Surg. Clin. N. Am. 2023, 35, 485–492. [Google Scholar] [CrossRef]
- Sahgal, A.; Chan, M.W.; Atenafu, E.G.; Masson-Cote, L.; Bahl, G.; Yu, E.; Millar, B.A.; Chung, C.; Catton, C.; O’Sullivan, B.; et al. Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: Preliminary outcomes. Neuro Oncol. 2015, 17, 889–894. [Google Scholar] [CrossRef]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front. Oncol. 2020, 10, 82. [Google Scholar] [CrossRef]
- Fujisawa, H.; Genik, P.C.; Kitamura, H.; Fujimori, A.; Uesaka, M.; Kato, T.A. Comparison of human chordoma cell-kill for 290 MeV/n carbon ions versus 70 MeV protons in vitro. Radiat. Oncol. 2013, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, F.; Hamdi, D.H.; Lepleux, C.; Temelie, M.; Nicol, A.; Austry, J.B.; Lesueur, P.; Vares, G.; Savu, D.; Nakajima, T.; et al. High LET Radiation Overcomes In Vitro Resistance to X-rays of Chondrosarcoma Cell Lines. Technol. Cancer Res. Treat. 2019, 18, 1533033819871309. [Google Scholar] [CrossRef] [PubMed]
- Rudmik, L.; Smith, T.L. Development of an evidence-based review with recommendations using an online iterative process. Int. Forum Allergy Rhinol. 2011, 1, 431–437. [Google Scholar] [CrossRef]
- Kuan, E.C.; Wang, E.W.; Adappa, N.D.; Beswick, D.M.; London, N.R., Jr.; Su, S.Y.; Wang, M.B.; Abuzeid, W.M.; Alexiev, B.; Alt, J.A.; et al. International consensus statement on allergy and rhinology: Sinonasal tumors. Int. Forum Allergy Rhinol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Karger, C.P.; Feuerhake, A.; Nikoghosyan, A.; Combs, S.E.; Jäkel, O.; Edler, L.; Scholz, M.; Debus, J. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Mizoe, J.E.; Hasegawa, A.; Takagi, R.; Bessho, H.; Onda, T.; Tsujii, H. Carbon ion radiotherapy for skull base chordoma. Skull Base 2009, 19, 219–224. [Google Scholar] [CrossRef]
- Uhl, M.; Mattke, M.; Welzel, T.; Roeder, F.; Oelmann, J.; Habl, G.; Jensen, A.; Ellerbrock, M.; Jäkel, O.; Haberer, T.; et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: First long-term results. Cancer 2014, 120, 3410–3417. [Google Scholar] [CrossRef]
- Mattke, M.; Ohlinger, M.; Bougatf, N.; Harrabi, S.; Wolf, R.; Seidensaal, K.; Welzel, T.; Röder, F.; Gerum, S.; Ellerbrock, M.; et al. Proton and carbon ion beam treatment with active raster scanning method in 147 patients with skull base chordoma at the Heidelberg Ion Beam Therapy Center—A single-center experience. Strahlenther. Onkol. 2023, 199, 160–168. [Google Scholar] [CrossRef]
- Takagi, M.; Demizu, Y.; Nagano, F.; Terashima, K.; Fujii, O.; Jin, D.; Mima, M.; Niwa, Y.; Katsui, K.; Suga, M.; et al. Treatment outcomes of proton or carbon ion therapy for skull base chordoma: A retrospective study. Radiat. Oncol. 2018, 13, 232. [Google Scholar] [CrossRef]
- Koto, M.; Ikawa, H.; Kaneko, T.; Hagiwara, Y.; Hayashi, K.; Tsuji, H. Long-term outcomes of skull base chordoma treated with high-dose carbon-ion radiotherapy. Head Neck 2020, 42, 2607–2613. [Google Scholar] [CrossRef]
- Iannalfi, A.; D’Ippolito, E.; Riva, G.; Molinelli, S.; Gandini, S.; Viselner, G.; Fiore, M.R.; Vischioni, B.; Vitolo, V.; Bonora, M.; et al. Corrigendum to: Proton and carbon ion radiotherapy in skull base chordomas: A prospective study based on a dual particle and a patient-customized treatment strategy. Neuro Oncol. 2022, 24, 1402–1403. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; O’Connor, K.P.; Mahajan, A.; Carlson, M.L.; Van Gompel, J.J. Carbon ion radiotherapy for skull base chordomas and chondrosarcomas: A systematic review and meta-analysis of local control, survival, and toxicity outcomes. J. Neurooncol 2020, 147, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Nikoghosyan, A.; Hof, H.; Didinger, B.; Combs, S.E.; Jäkel, O.; Karger, C.P.; Edler, L.; Debus, J. Carbon ion radiotherapy of skull base chondrosarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Mattke, M.; Welzel, T.; Oelmann, J.; Habl, G.; Jensen, A.D.; Ellerbrock, M.; Haberer, T.; Herfarth, K.K.; Debus, J. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: First report of long-term results. Cancer 2014, 120, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Mattke, M.; Vogt, K.; Bougatf, N.; Welzel, T.; Oelmann-Avendano, J.; Hauswald, H.; Jensen, A.; Ellerbrock, M.; Jäkel, O.; Haberer, T.; et al. High control rates of proton- and carbon-ion-beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg Ion Beam Therapy Center. Cancer 2018, 124, 2036–2044. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Haberer, T.; Jäkel, O.; Thilmann, C.; Krämer, M.; Enghardt, W.; Kraft, G.; Wannenmacher, M.; Debus, J. Radiotherapy for chordomas and low-grade chondrosarcomas of the skull base with carbon ions. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Nikoghosyan, A.; Thilmann, C.; Haberer, T.; Jäkel, O.; Karger, C.; Scholz, M.; Kraft, G.; Wannenmacher, M.; Debus, J. Carbon ion radiotherapy for chordomas and low-grade chondrosarcomas of the skull base. Results in 67 patients. Strahlenther. Onkol. 2003, 179, 598–605. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Nikoghosyan, A.; Didinger, B.; Debus, J. Carbon ion radiation therapy for chordomas and low grade chondrosarcomas—Current status of the clinical trials at GSI. Radiother. Oncol. 2004, 73 (Suppl. 2), S53–S56. [Google Scholar] [CrossRef]
- Combs, S.E.; Nikoghosyan, A.; Jaekel, O.; Karger, C.P.; Haberer, T.; Münter, M.W.; Huber, P.E.; Debus, J.; Schulz-Ertner, D. Carbon ion radiotherapy for pediatric patients and young adults treated for tumors of the skull base. Cancer 2009, 115, 1348–1355. [Google Scholar] [CrossRef]
- Koto, M.; Hasegawa, A.; Takagi, R.; Fujikawa, A.; Morikawa, T.; Kishimoto, R.; Jingu, K.; Tsujii, H.; Kamada, T. Risk factors for brain injury after carbon ion radiotherapy for skull base tumors. Radiother. Oncol. 2014, 111, 25–29. [Google Scholar] [CrossRef]
- Uhl, M.; Welzel, T.; Oelmann, J.; Habl, G.; Hauswald, H.; Jensen, A.; Ellerbrock, M.; Debus, J.; Herfarth, K. Active raster scanning with carbon ions: Reirradiation in patients with recurrent skull base chordomas and chondrosarcomas. Strahlenther. Onkol. 2014, 190, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Gao, J.; Hu, J.; Hu, W.; Yang, J.; Qiu, X.; Hu, C.; Kong, L.; Lu, J.J. The preliminary results of proton and carbon ion therapy for chordoma and chondrosarcoma of the skull base and cervical spine. Radiat. Oncol. 2019, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, P.; Cai, X.; Hong, Z.; Yu, Z.; Zhang, Q.; Fu, S. Carbon Ion Radiotherapy for Patients with Extracranial Chordoma or Chondrosarcoma—Initial Experience from Shanghai Proton and Heavy Ion Center. J. Cancer 2019, 10, 3315–3322. [Google Scholar] [CrossRef]
- Dong, M.; Liu, R.; Zhang, Q.; Luo, H.; Wang, D.; Wang, Y.; Chen, J.; Ou, Y.; Wang, X. Efficacy and safety of carbon ion radiotherapy for bone sarcomas: A systematic review and meta-analysis. Radiat. Oncol. 2022, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Nikoghosyan, A.; Thilmann, C.; Haberer, T.; Jäkel, O.; Karger, C.; Kraft, G.; Wannenmacher, M.; Debus, J. Results of carbon ion radiotherapy in 152 patients. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Nikoghosyan, A.; Ellerbrock, M.; Ecker, S.; Debus, J.; Münter, M.W. Re-irradiation with scanned charged particle beams in recurrent tumours of the head and neck: Acute toxicity and feasibility. Radiother. Oncol. 2011, 101, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Kalbe, A.; Nikoghosyan, A.; Ackermann, B.; Jäkel, O.; Haberer, T.; Debus, J. Carbon ion radiotherapy performed as re-irradiation using active beam delivery in patients with tumors of the brain, skull base and sacral region. Radiother. Oncol. 2011, 98, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, O.; Land, B.; Combs, S.E.; Schulz-Ertner, D.; Debus, J. On the cost-effectiveness of Carbon ion radiation therapy for skull base chordoma. Radiother. Oncol. 2007, 83, 133–138. [Google Scholar] [CrossRef]
- Sprave, T.; Verma, V.; Sterzing, F.; Bruckner, T.; Hees, K.; Land, B.; Jäkel, O.; Herfarth, K.; Debus, J.; Uhl, M. Cost-Effectiveness of Carbon Ion Radiation Therapy for Skull Base Chordoma Utilizing Long-Term (10-Year) Outcome Data. Anticancer Res. 2018, 38, 4853–4858. [Google Scholar] [CrossRef]
- Napieralska, A.; Blamek, S. Intracranial chordoma: Radiosurgery, hypofractionated stereotactic radiotherapy and treatment outcomes. Rep. Pract. Oncol. Radiother. 2021, 26, 764–772. [Google Scholar] [CrossRef]
- Martin, J.J.; Niranjan, A.; Kondziolka, D.; Flickinger, J.C.; Lozanne, K.A.; Lunsford, L.D. Radiosurgery for chordomas and chondrosarcomas of the skull base. J. Neurosurg. 2007, 107, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Wang, Z.C.; Sun, S.B.; Wang, M.H.; Luo, B.; Liu, P. Gamma knife radiosurgery for residual skull base chordomas. Neurol. Res. 2008, 30, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Niranjan, A.; Lunsford, L.D. Radiosurgery for Chordoma and Chondrosarcoma. Prog. Neurol. Surg. 2019, 34, 207–214. [Google Scholar] [CrossRef]
- Förander, P.; Bartek, J., Jr.; Fagerlund, M.; Benmaklouf, H.; Dodoo, E.; Shamikh, A.; Stjärne, P.; Mathiesen, T. Multidisciplinary management of clival chordomas; long-term clinical outcome in a single-institution consecutive series. Acta Neurochir. 2017, 159, 1857–1868. [Google Scholar] [CrossRef]
- Evans, L.T.; DeMonte, F.; Grosshans, D.R.; Ghia, A.J.; Habib, A.; Raza, S.M. Salvage Therapy for Local Progression following Definitive Therapy for Skull Base Chordomas: Is There a Role of Stereotactic Radiosurgery? J. Neurol. Surg. B Skull Base 2020, 81, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gaito, S.; Aznar, M.C.; Burnet, N.G.; Crellin, A.; France, A.; Indelicato, D.; Kirkby, K.J.; Pan, S.; Whitfield, G.; Smith, E. Assessing Equity of Access to Proton Beam Therapy: A Literature Review. Clin. Oncol. (R. Coll. Radiol.) 2023, 35, e528–e536. [Google Scholar] [CrossRef] [PubMed]
- Gaito, S.; Hwang, E.J.; France, A.; Aznar, M.C.; Burnet, N.; Crellin, A.; Holtzman, A.L.; Indelicato, D.J.; Timmerman, B.; Whitfield, G.A.; et al. Outcomes of Patients Treated in the UK Proton Overseas Programme: Central Nervous System Group. Clin. Oncol. (R. Coll. Radiol.) 2023, 35, 283–291. [Google Scholar] [CrossRef]
- Xiang, M.; Chang, D.T.; Pollom, E.L. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 2020, 126, 3560–3568. [Google Scholar] [CrossRef]
- De Leo, A.N.; Holtzman, A.L.; Ho, M.W.; Morris, C.G.; Rutenberg, M.S.; Rotondo, R.L.; Bates, J.E.; Indelicato, D.J.; Rao, D.; Asa Hasan, M.; et al. Vision loss following high-dose proton-based radiotherapy for skull-base chordoma and chondrosarcoma. Radiother. Oncol. 2021, 158, 125–130. [Google Scholar] [CrossRef]
- Holtzman, A.L.; Rutenberg, M.S.; De Leo, A.N.; Rao, D.; Patel, J.; Morris, C.G.; Indelicato, D.J.; Mendenhall, W.M. The incidence of brainstem toxicity following high-dose conformal proton therapy for adult skull-base malignancies. Acta Oncol. 2022, 61, 1026–1031. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Rotondo, R.L.; Mailhot Vega, R.B.; Holtzman, A.L.; Looi, W.S.; Morris, C.G.; Sandler, E.S.; Aldana, P.R.; Bradley, J.A. Local Control After Proton Therapy for Pediatric Chordoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Cavallo, I.; Gandini, S.; Ingargiola, R.; Pecorilla, M.; Imparato, S.; Rossi, E.; Mirandola, A.; Ciocca, M.; Orlandi, E.; et al. Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy. Cancers 2021, 13, 4423. [Google Scholar] [CrossRef] [PubMed]

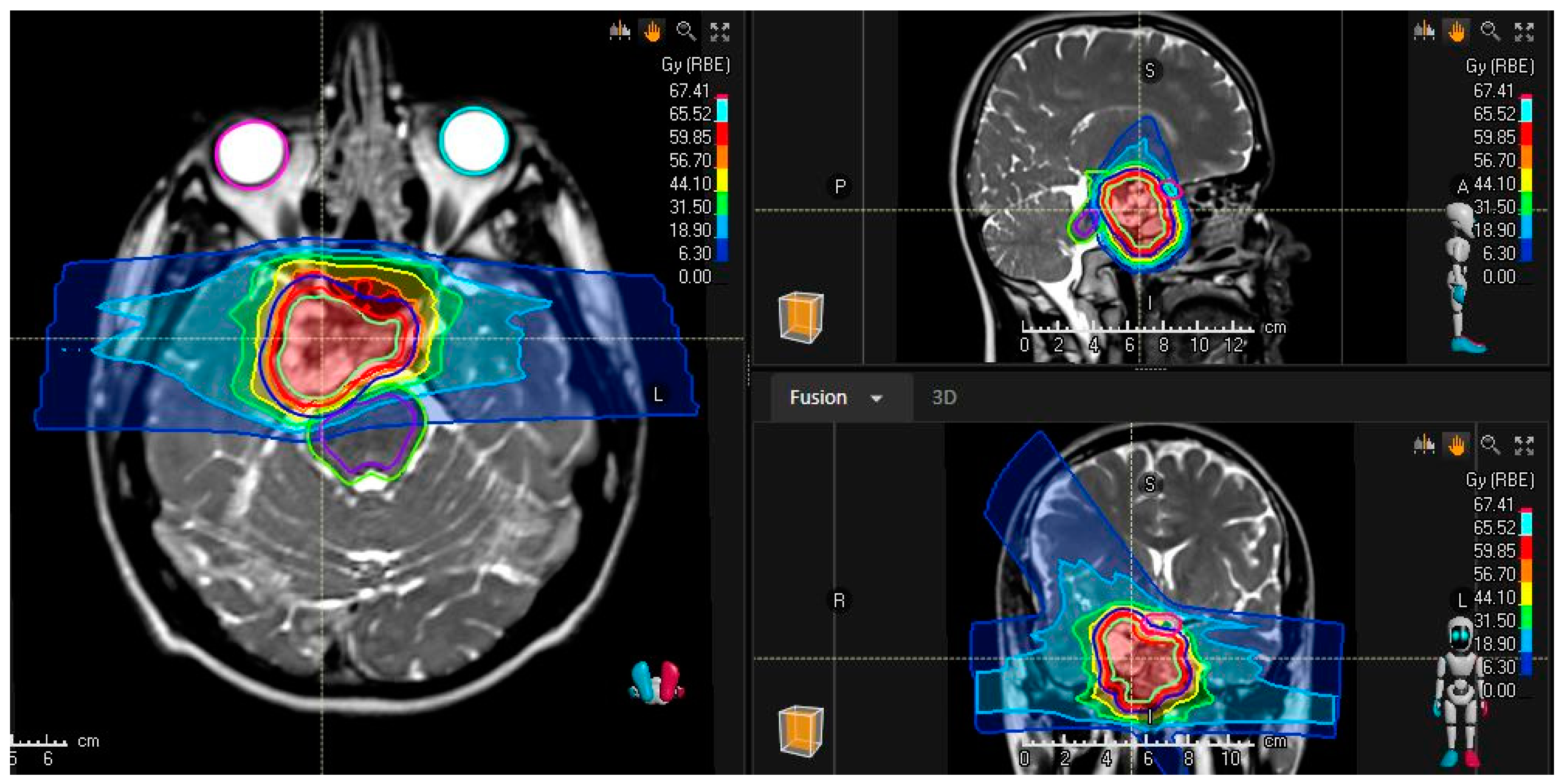
| Items | Explanation |
|---|---|
| Aggregate Grade of Evidence | Chordoma: C (Level 2: 1 study; Level 3: 3 studies; Level 4: 6 studies) Chondrosarcoma: C (Level 2: 1 study; Level 3: 1 study; Level 4: 2 studies) Combined series: (Level 2: 1 study; Level 3: 2 studies; Level 4: 6 studies) |
| Benefit | CIRT provides LC and OS benefits for skull-base chordoma and chondrosarcoma when used as monotherapy or adjuvantly following surgery. |
| Harm | CIRT morbidity is related to the extent and site of the tumor and toxicities are in line with reported literature compared to other highly conformal radiation delivery techniques. |
| Cost | Because of the increased tumor control in published models for chordoma, CIRT is reported to be highly cost-effective. There are no such analyses available for chondrosarcoma. |
| Benefit-Harm Assessment | Preponderance of benefits over harms. |
| Value Judgments | CIRT should be considered for radioresistant histologies with gross residual disease at the time of treatment or following treatment recurrence. |
| Policy Level | Option |
| Intervention | CIRT should be considered for improving LC and OS when weighed for patient-specific and tumor features and is optional when available. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtzman, A.L.; Seidensaal, K.; Iannalfi, A.; Kim, K.H.; Koto, M.; Yang, W.-C.; Shiau, C.-Y.; Mahajan, A.; Ahmed, S.K.; Trifiletti, D.M.; et al. Carbon Ion Radiotherapy: An Evidence-Based Review and Summary Recommendations of Clinical Outcomes for Skull-Base Chordomas and Chondrosarcomas. Cancers 2023, 15, 5021. https://doi.org/10.3390/cancers15205021
Holtzman AL, Seidensaal K, Iannalfi A, Kim KH, Koto M, Yang W-C, Shiau C-Y, Mahajan A, Ahmed SK, Trifiletti DM, et al. Carbon Ion Radiotherapy: An Evidence-Based Review and Summary Recommendations of Clinical Outcomes for Skull-Base Chordomas and Chondrosarcomas. Cancers. 2023; 15(20):5021. https://doi.org/10.3390/cancers15205021
Chicago/Turabian StyleHoltzman, Adam L., Katharina Seidensaal, Alberto Iannalfi, Kyung Hwan Kim, Masashi Koto, Wan-Chin Yang, Cheng-Ying Shiau, Anita Mahajan, Safia K. Ahmed, Daniel M. Trifiletti, and et al. 2023. "Carbon Ion Radiotherapy: An Evidence-Based Review and Summary Recommendations of Clinical Outcomes for Skull-Base Chordomas and Chondrosarcomas" Cancers 15, no. 20: 5021. https://doi.org/10.3390/cancers15205021
APA StyleHoltzman, A. L., Seidensaal, K., Iannalfi, A., Kim, K. H., Koto, M., Yang, W.-C., Shiau, C.-Y., Mahajan, A., Ahmed, S. K., Trifiletti, D. M., Peterson, J. L., Koffler, D. M., Vallow, L. A., Hoppe, B. S., & Rutenberg, M. S. (2023). Carbon Ion Radiotherapy: An Evidence-Based Review and Summary Recommendations of Clinical Outcomes for Skull-Base Chordomas and Chondrosarcomas. Cancers, 15(20), 5021. https://doi.org/10.3390/cancers15205021





