1. Introduction
Oral squamous cell carcinoma (OSCC) accounts for the majority of head and neck cancers and ranks among the most prevalent cancers worldwide [
1]. Lymphatic metastasis, which is associated with poor patient prognosis, is common in advanced tumor stages [
2].
Tumor cell dissemination requires the activation of the epithelial-to-mesenchymal transition (EMT) process. This promotes the loss of basal–apical polarity, breaks down tight and adhesive junctions, and allows the gain of motility in a mesenchymal spindle-like morphology. Physiologically, EMT occurs during development and wound healing [
3]. However, in tumor cells, it is associated with invasiveness, metastasis, and enhanced tumor cell plasticity [
4]. The EMT process provides tumor cells with a high degree of motility, enabling them to navigate the extracellular matrix (ECM), invade lymphatic or blood vessels, and spread to regional lymph nodes or distant tissues [
5]. Some of the disseminated tumor cells (DTCs) colonize a distant organ, where they are likely to remain dormant [
6].
Metastasis initiates when DTCs counteract quiescent signals in the local microenvironment, such as TGFβ2, BMP4, and BMP7 [
7]. They then revert to the epithelial phenotype through the mesenchymal-to-epithelial transition (MET) program. This stimulates proliferation, leads to macrometastasis formation, and can result in cancer recurrence [
5,
8]. Throughout this process, metastatic cells exhibit plasticity, exhibiting varying phenotypes at different stages of this process.
Connexins (Cxs) are transmembrane proteins essential for gap junction (GJ) formation. They enable the direct passage of small molecules, such as ions, second messengers, metabolites, and microRNAs, mediating intercellular communication (GJIC) [
9]. Additionally, they mediate the transport of cytosolic molecules to the extracellular milieu, playing a pivotal role in cellular homeostasis, as well as cell growth and development [
9]. Among them, Cx43 is the most recognized human Cx protein [
10], and our research indicates it serves as an independent prognostic factor in OSCC [
11]. Cx43 expression can change during tumor progression. During EMT, tumor cells decrease their membrane-bound Cx43 expression, facilitating cell detachment and increasing cell motility [
12]. However, during implantation and MET, they increase it to support tumor cell contacts via GJs [
13], interacting with the endothelial barrier and surrounding cells in the TME [
14,
15]. Cxs and GJIC exhibit both pro- and antiproliferative effects, depending on the cell type and microenvironment. This is partly due to the exchange of molecules such as ATP, cAMP, or specific miRNAs between tumor cells and TME cells [
16]. These functions may vary based on tumor type, tumor stage, interacting cell types, Cx molecule subtype, and their expression levels [
17]. Cx43 within cells is also linked to microtubules, migration regulation, and apoptosis inhibition [
18,
19,
20]. Overexpression of Cx43 in MDA-MB-231 breast cancer cells increases the expression of the epithelial markers E-cadherin and ZO-1, whereas Cx43 knockdown leads to the appearance of the mesenchymal protein N-cadherin [
21], establishing a connection between Cx43 expression and MET. However, the exact role of Cx43 in EMT and MET has not been conclusively determined [
22].
EMMPRIN/CD147 is a multifunctional transmembrane glycoprotein that mediates the interaction between tumor and stromal cells [
23]. Overexpressed in over 70% of human tumors, its expression is associated with higher tumor grade and stage, metastasis, and poor prognosis [
24]. EMMPRIN is primarily recognized for its proangiogenic role, triggering VEGF and MMPs through homophilic interactions. We have identified an epitope in its extracellular domain I that is responsible for both activities [
25]. Additionally, EMMPRIN acts as a chaperone for lactate transporters MCT-1 and MCT-4, facilitating lactate efflux, which is vital for tumor cells primarily dependent on glycolysis. Increased extracellular lactate levels have been shown to upregulate mesenchymal markers such as vimentin and N-cadherin [
26], suggesting an indirect association of EMMPRIN with EMT. Furthermore, EMMPRIN regulates hyaluronan synthesis and can bind to its receptor CD44, a recognized cellular stem cell biomarker that contributes to tumor cell invasiveness and chemoresistance [
27]. In summary, these properties suggest that EMMPRIN plays roles in tumor cell metabolism, survival, proliferation, invasiveness, metastasis, and angiogenesis, and likely promotes EMT [
28].
In this study, we mapped the metastatic process from healthy oral mucosa (OM) to solid lymph node metastasis (LNM) in OSCC. Using immunohistochemistry, we examined the expression profiles of Cx43 and EMMPRIN alongside the known EMT markers E-cadherin and vimentin and assessed the prognostic significance of all marker proteins and the combined marker system.
2. Materials and Methods
2.1. Patients
The sample size was determined using StatMate software (version 2, GraphPad Software, Boston, MA, USA). With a sample size of 24, we achieved 95% power to detect a difference between means of 56.31 with a significance level (alpha) of 0.05 (two-tailed). We utilized tumor tissue samples from 24 OSCC patients, primarily treated surgically between 2016 and 2019, for immunohistochemical evaluation. For assessment of baseline clinical characteristics, tumor stages T1 and T2 were grouped together, as were stages T3 and T4. In addition, American Joint Commission on Cancer (AJCC) clinical stages I and II were combined, as were stages III and IV. Regarding nodal status analysis, patients were categorized into lymph node positive and lymph node negative groups. The primary clinical endpoints were disease-free survival (DFS) and overall survival (OS). Before enrollment, patients provided written informed consent. The study adhered to the principles of the Declaration of Helsinki and received approval from a clinical ethics committee (approval no. 07/06/09, updated April 2018).
2.2. Tissue Sample Processing and Semiautomated Semiquantitative Immunohistochemical Analysis
Tumor tissue samples from patients were promptly obtained post-surgical resection, preserved in neutral buffered 4% formalin, and then embedded in paraffin. Immunohistochemical staining was performed on 2 µm sections using a fully automated slide stainer (Agilent Technologies, Santa Clara, CA, USA), as specified in
Table 1.
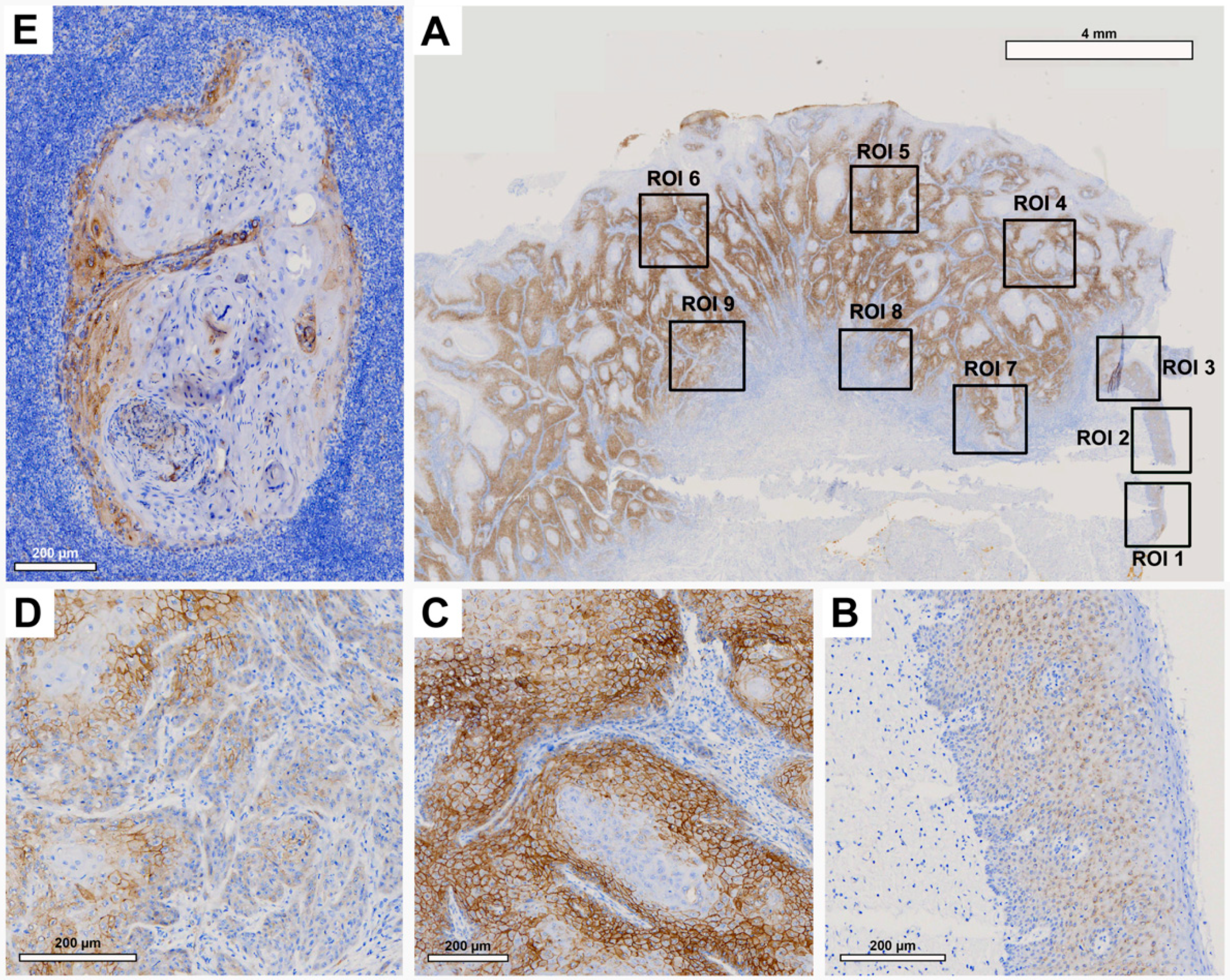
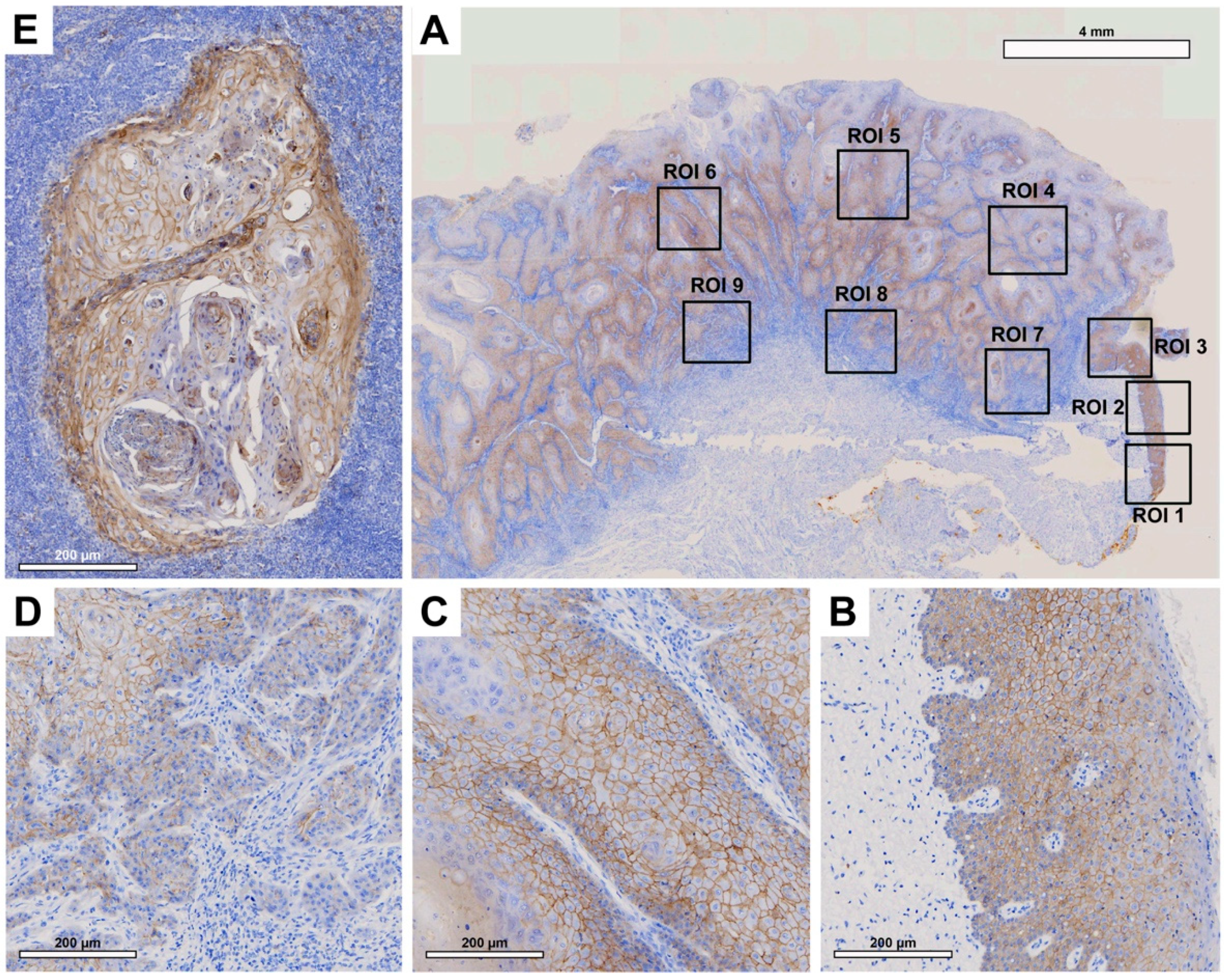
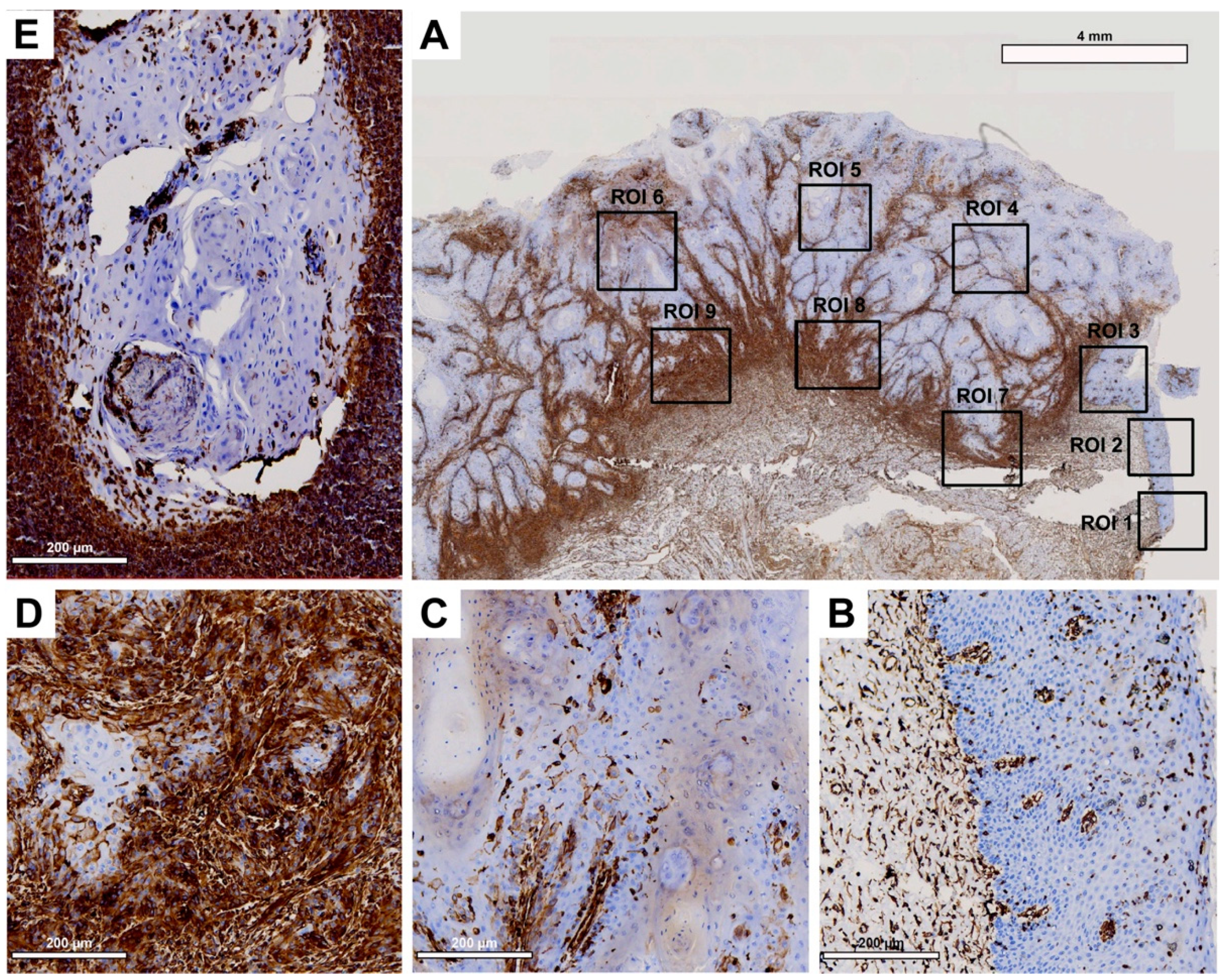
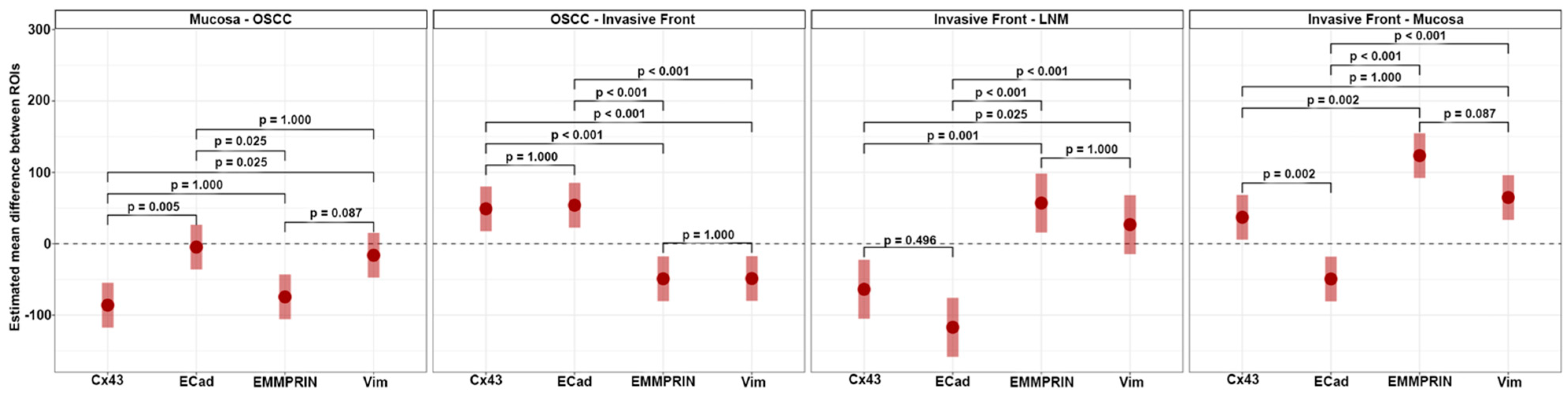
Tissue slides were digitized at 20× magnification with a resolution of 0.5 μm/pixel using a Motic EasyScan One slide scanner (Motic, Hong Kong, China). For semiautomated semiquantitative immunohistochemical assessment, we employed the open-source image analysis software quPath [
29]. To comprehensively map the metastatic process (
Figure 1), we analyzed tissue samples from primary tumors (along with adherent healthy oral mucosa) and corresponding local cervical lymph node metastases (LNM). Different regions of interest (ROIs) were digitally defined, as illustrated in
Figure 2,
Figure 3,
Figure 4 and
Figure 5. For OSCC tissue samples, we defined three ROIs in the adjacent healthy mucosa, three ROIs in the center of the primary OSCC, and another three at the invasive front (IF). In LNM, three ROIs were distributed over the entire metastasis. Each ROI was defined to be approximately 1 cm
2 in size.
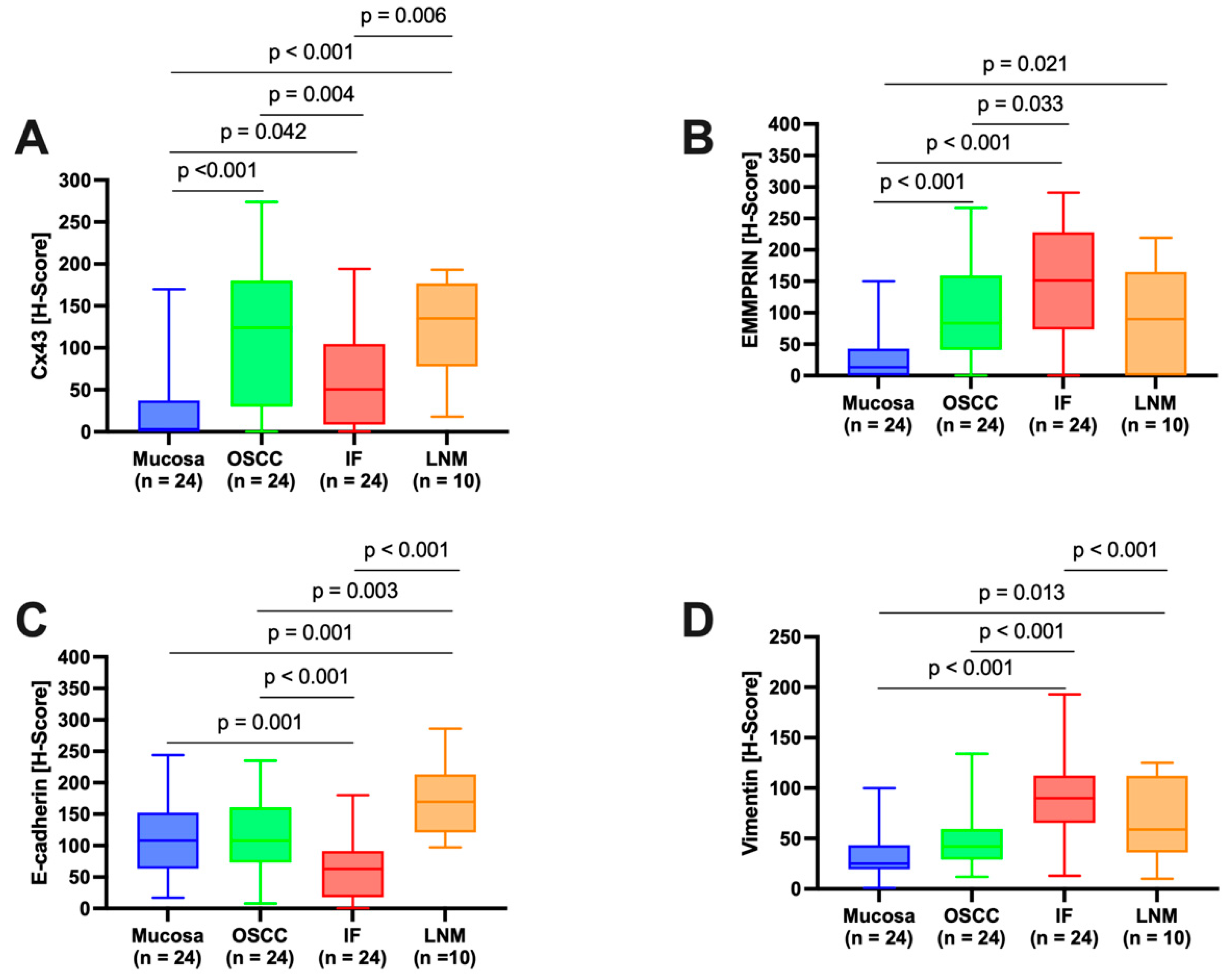
The quPath cell detection algorithm was performed within all ROIs. The software was calibrated to differentiate between tumor and stromal cells. To enhance accuracy, this training was performed three times utilizing an artificial intelligence (AI) function. Using the software’s default settings, the immunohistochemically labeled marker proteins (Cx43, EMMPRIN, E-cadherin, and vimentin) were semiautomatically scored based on the percentage of tumor cells showing positive staining and signal intensity. The histoscore (H-score) was calculated by adding 3× the percentage of tumor cells with strong staining, 2× the percentage with moderate staining, and 1× the percentage of weak staining. This method yielded scores that ranged from 0 (all tumor cells negative) to 300 (all tumor cells strongly positive).
2.3. Statistical Analysis
To gauge the dynamics of protein expression across various ROIs, expression differences (Δ) were computed. For each protein, all pairwise expression differences between ROIs were determined. These differences were then assessed using pairwise contrast tests grounded on linear mixed-effects models, which modeled expression by ROI. The resulting estimates are reported with 95% confidence intervals and p-values adjusted using Tukey’s test. A full linear mixed-effect model for protein abundance was fitted with protein, tissue, and their interactions as predictors. Drawing from this model, we estimated the expected marginal means for the differences between ‘neighboring’ ROIs. To contrast each of these differences among all protein pairs, contrast tests were employed. The results, accompanied by Holm adjusted p-values, are presented alongside the expected marginal effects with their 95% confidence intervals. To assess potential associations between both clinical and protein expression data with DFS and OS, univariable Cox proportional hazards regression models were employed. Each model’s fit was evaluated against the null model using likelihood ratio tests. All protein variables with a p-value smaller than 0.1 from these likelihood ratio tests were rescreened for association with DFS and OS adjusted for AJCC stage and age. The limited number of patients and events limits the complexity of the models that can be fitted to the data. Given that the variables adjuvant therapy, pT, pN, and AJCC stage were highly correlated, the AJCC stage was selected to represent this group of variables. The output model coefficients were reported as hazard ratios (HR) accompanied by a 95% confidence interval and their corresponding p-value. For both prognostic endpoints (DFS and OS), a multivariable Cox regression model was fitted using only protein expression data, according to the following equations:
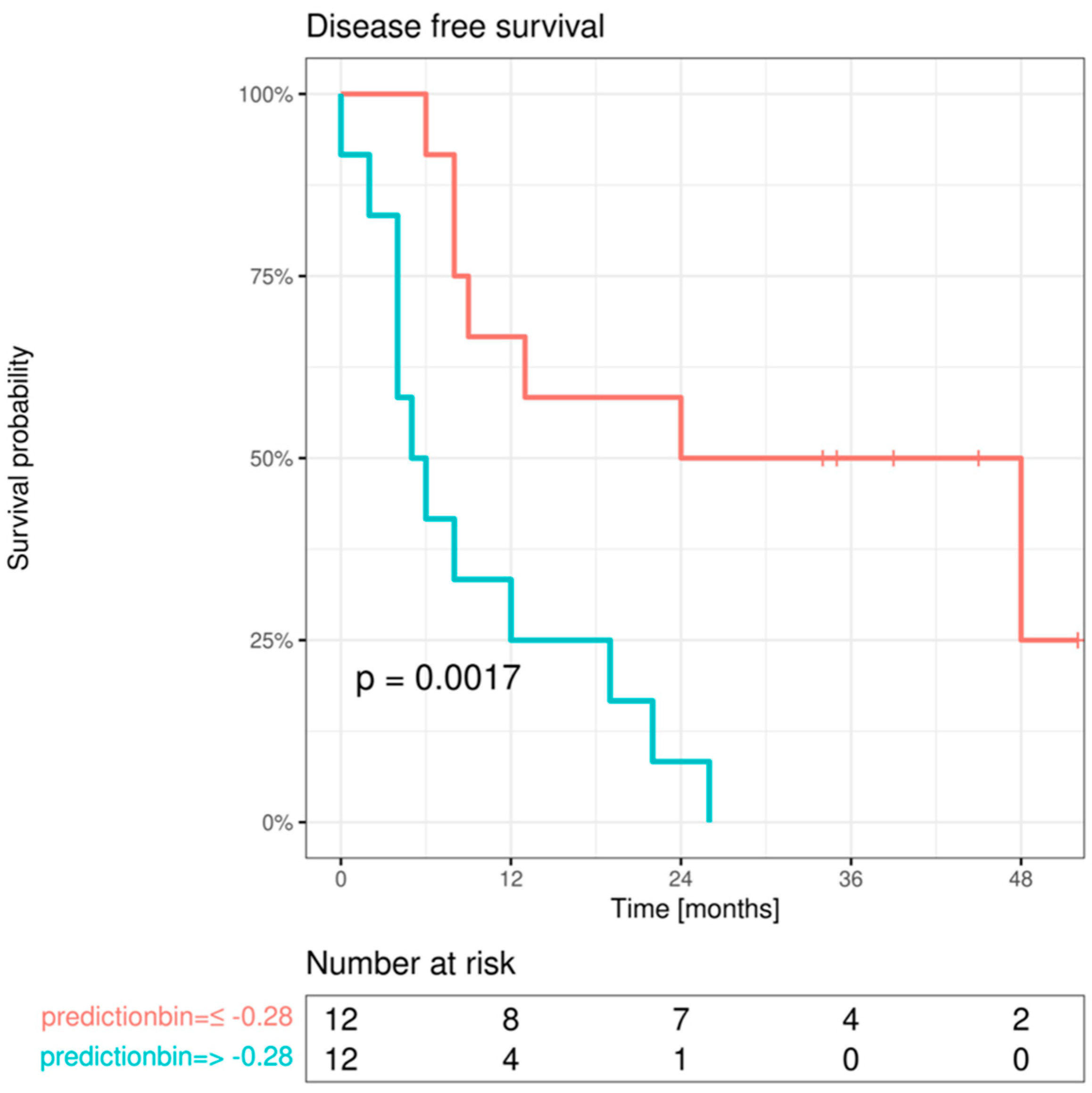
For every protein, the ROI/ROI difference showing the most significant univariate association with survival (as determined by the
p-value from the likelihood ratio tests) was selected. Given the limited sample size, only the top three markers were selected. The resulting model coefficients were reported as hazard ratios (HR) accompanied by a 95% confidence interval and their respective
p-value. For visualization of the effect, model predictions were binarized at the median and maximally selected rank statistic. Subsequently, Kaplan–Meier curves were plotted in the resulting subgroups and compared using log-rank tests. All statistical tests were conducted with a significance level set at α = 5%. All analyses were performed with the statistical software R (version 4.1.2; R Core Team 2021) [
30] using the R-package lme4 (version 1.1.28) [
31] for the mixed effect regression models and emmeans (version 1.7.2) [
32] for computing the expected marginal effects and performing contrast tests. The full dataset is available online at
https://doi.org/10.25625/FNR4EX (accessed on 27 September 2023).
4. Discussion
Metastasis represents a milestone in OSCC progression [
33], and it is associated with poor patient prognosis and decreased health-related quality of life [
2]. In this study, we mapped the metastatic process from healthy oral mucosa to cervical lymph node metastasis in tissue samples from 24 OSCC patients. We evaluated the expression profiles of both transmembrane proteins Cx43 and EMMPRIN, along with the known EMT markers E-cadherin and vimentin, using immunohistochemistry. Moreover, we investigated the prognostic impact of each protein and tested whether a combined biomarker system could significantly predict the clinical endpoints DFS and OS.
Cx43 expression was detected in all analyzed tissue types, including oral mucosa, center of primary OSCC, invasive front, and lymph node metastases. Expression was at its lowest in oral mucosa and exhibited a significant increase as it progressed toward the center of the primary OSCC. Cx43 expression showed a decline toward the invasive front, followed by a subsequent increase in lymph node metastases. The expression profile of Cx43 paralleled that of the established epithelial marker E-cadherin [
34]. Both Cx43 and E-cadherin are transmembrane proteins that are important for the formation of intercellular junctions, gap junction channels, and the maintenance of intercellular communication [
15]. This may explain the increase in Cx43 toward the center of the primary OSCC, where cells proliferate and interact with each other. Toward the invasive front, where tumor cells undergo EMT to gain the ability to migrate and metastasize [
35], intercellular junctions must be disrupted and Cx43 and E-cadherin expression are reduced [
11]. In lymph node metastasis, where tumor cells communicate with each other again, both protein expression profiles (Cx43 and E-cadherin) are restored. This underscores the connection between Cx43 expression and mesenchymal-to-epithelial transition (MET) features. While our prior studies showed the independent prognostic significance of Cx43 in OSCC [
11], we were unable to validate this effect in the present cohort using multivariable Cox regression analysis, possibly attributed to the limited number of cases in this study.
In this study, we demonstrated that EMMPRIN is highly expressed in both physiological oral mucosa and primary OSCCs. Similar results have been reported previously by Rajshri et al. [
36] and Min et al. [
37]. However, we demonstrated for the first time that EMMPRIN expression significantly increases toward the invasive front of OSCC and decreases in lymph node metastasis. The EMMPRIN expression profile corresponds to that of the well-known mesenchymal marker vimentin [
38]. These findings imply that high EMMPRIN expression in migrating OSCC tumor cells plays a crucial role in facilitating invasion into the surrounding tissue and metastasis. This feature is not required for lymph node metastases, where the focus is on cellular cluster growth, and consequently, EMMPRIN expression is reduced, thereby associating EMMPRIN with EMT features. Furthermore, among all the markers examined, EMMPRIN expression had the strongest prognostic impact on clinical endpoints, inducing DFS and OS, as measured through multivariable Cox regression analysis. We have recently shown that EMMPRIN plays a role in preventing tumor cells from entering the dormant state, and that knocking down EMMPRIN pushes cells toward dormancy [
39]. Therefore, considering the potential of targeting EMMPRIN expression in metastatic OSCC tumor cells, this approach may hold promise as a novel antimetastatic therapy.
Furthermore, we provided evidence that the combined biomarker system of Cx43, EMMPRIN, E-cadherin, and vimentin possesses a significant predictive capacity for both DFS and OS in patients with metastatic OSCC. We suggest this combined system warrants comprehensive evaluation in a larger number of patients before considering its potential implementation in routine clinical practice.







_Amit_Rahat.jpg)


