1. Introduction
Breast cancer is the most common malignancy diagnosed among women of reproductive age [
1]. The treatment of young breast cancer patients is challenging, since it is associated with additional possible age-related issues [
2]. Among the potential negative consequences of anticancer therapies, the risk of developing premature ovarian insufficiency (POI) represents one of the main sources of distress in this patient population [
3]. POI might result in long-term sequelae with an impaired quality of life (QoL).
Indeed, several studies have demonstrated the risks and mechanisms of chemotherapy-induced ovarian damage. Chemotherapy agents can induce both structural and functional damage [
4]; there is evidence of follicle destruction, characterized by the apoptotic death of oocyte and somatic cells [
5,
6], as well as vascular damage [
7,
8] and the premature activation and atresia of primordial follicles [
9]. However, limited data are currently available on the use of other newer anticancer therapies including targeted agents.
CDK4/6 inhibitors combined with endocrine therapy are the standard of care for the treatment of patients with HR+/HER2− advanced breast cancer [
10]. Four randomized clinical trials have also investigated the role of adjuvant CDK4/6 inhibitors in addition to endocrine therapy in patients with intermediate and high-risk HR+/HER2− early breast cancer. These studies differed for the type of CDK4/6 inhibitors used and for the duration of treatment. Two trials failed to demonstrate improved outcomes for patients receiving adjuvant palbociclib in the PALLAS and penelopeB trials [
11,
12]. On the other hand, the MonarchE study showed a significant improvement in invasive disease-free survival (iDFS) and distant relapse-free survival (DRFS) at 4 years with the addition of 2 years of adjuvant abemaciclib to endocrine therapy [
13]. At a median follow-up of 42 months, the addition of abemaciclib to adjuvant endocrine therapy led to a 6.4% and 5.9% absolute improvement in the 4-year iDFS (hazard ratio (HR) 0.664; 95% confidence interval (CI) 0.578–0.762) and DRFS (HR 0.659; 95% CI 0.567–0.767), respectively [
13]. More recently, the NATALEE trial investigating 3 years of adjuvant ribociclib in addition to endocrine therapy reported positive results, albeit after a short follow-up [
14]. At a median follow-up of 27.7 months, the addition of ribociclib to adjuvant endocrine therapy led to 3.3% and 2.2% absolute improvements in the 3-year iDFS (HR 0.748; 95% CI 0.618–0.906) and DRFS (HR 0.739; 95% CI 0.603–0.905), respectively [
14]. As of now, the only CDK 4/6 inhibitor currently approved for clinical use in the early setting is abemaciclib for patients with high-risk HR+/HER2− early breast cancer.
Investigating the potential gonadotoxicity of CDK 4/6 inhibitors is particularly relevant, considering that almost all patient candidates for this treatment in the curative setting have received prior chemotherapy, with its known impact on ovarian function and reserve. Nevertheless, among the adjuvant trials investigating the addition of a CDK 4/6 inhibitor to endocrine therapy, the phase III penelopeB trial (using palbociclib) is the only one thus far to report the potential gonadotoxicity of this agent [
12,
15]. The results from the biomarker analysis of this study showed that palbociclib did not significantly affect estradiol and follicle-stimulating hormone (FSH) levels when added to endocrine therapy after chemotherapy. Due to these limited data and the lack of evidence on abemaciclib, fully informed treatment decision making by premenopausal patients concerned about the potential development of POI and infertility and candidates selected to receive this treatment following chemotherapy remains difficult. This review aims to summarize the available evidence on the physiological role of cyclin D and CDK4/6 in the ovarian cell cycle and follicle maturation, as well as data on cell lines and experimental animal models regarding the effect of CDK 4/6 inhibition on ovarian physiological processes.
2. Cyclin D-CDK4/6 Complexes
Cell cycle progression from a state of quiescence (G0) to the G1 phase is mediated by cyclin-dependent kinases (CDKs). CDKs are serine/threonine protein kinases that are activated by binding to cyclins and by blocking their inhibitor proteins [
16]. CDK4 and CDK6 are considered as promoters of G1 cell cycle progression.
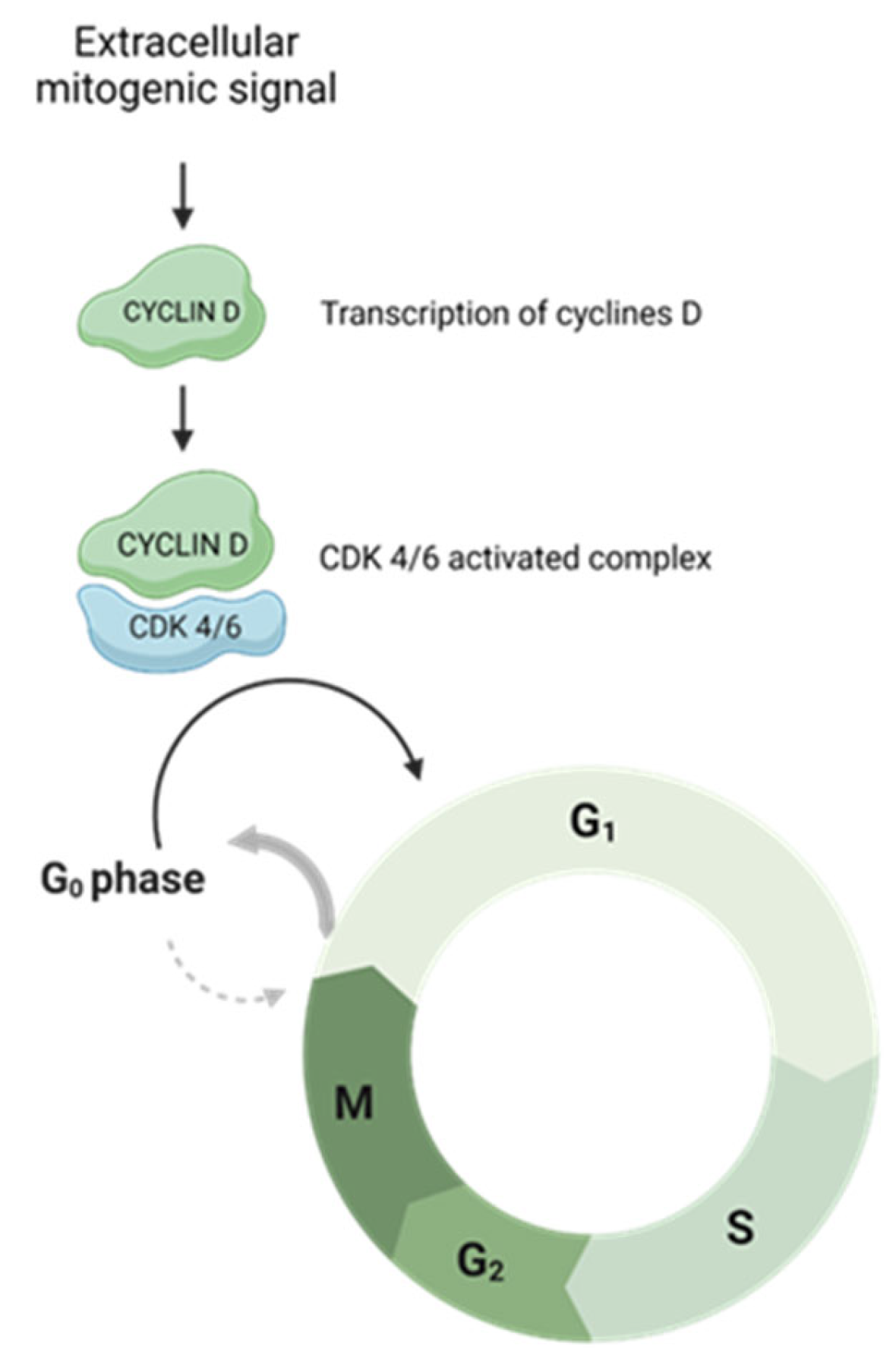
The activation of specific cyclin–CDK complexes leads to exit from a state of quiescence (G0) and entry into the cell cycle in phase G1 and then to continue through the subsequent steps. Complexes responsible for G0–G1 transition are constituted by the D type cyclins (D1, D2 and D3), CDK4 and CDK6. Once a mitogen signal activates these complexes, the cells are enabled to exit from the state of quiescence and proceed through the G1 phase [
17,
18]. The cell cycle progression through CDK4 and CDK6 activation is illustrated in
Figure 1.
Upon an extracellular mitogenic signal, the cyclin D-CDK4/6 complex is active. This drives the cell cycle towards G0–G1 transition.
D-type cyclins differ from other cyclins in their activation mechanism. While all cyclins are transcriptionally induced by other cyclins during cell cycle progression, the D-type is controlled by an extracellular mitogenic environment. Therefore, they are considered ‘connectors’ between the extracellular microenvironment and the cell cycle machinery [
19].
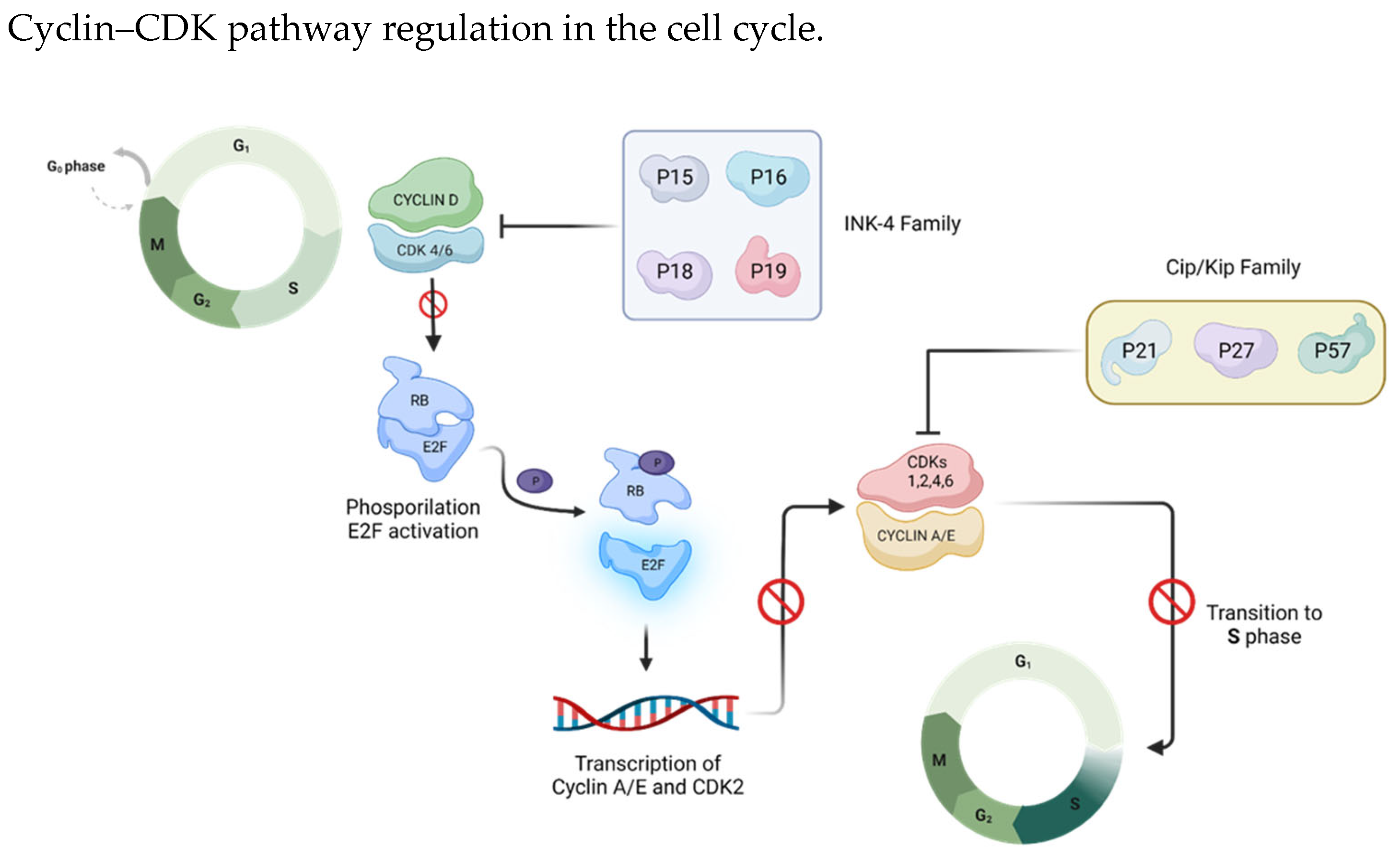
The cyclin D-CDK4/6 complex plays a pivotal role in governing the G1/S transition through the retinoblastoma (Rb)-E2F pathway. During the G1 phase, this complex phosphorylates and inactivates Rb. Subsequently, cyclin E-CDK2 drives the phosphorylation process to completion, activating E2F transcription factors to enable the transcription of pro-proliferative genes [
20] (
Figure 2).
Cyclin D-CDK4/6 complex phosphorylation on RB (retinoblastoma factor)/E2F (transcription factor) is the key event for cell cycle progression: RB phosphorylation enables E2F release and the transcription of pro-proliferative genes.
Given their important role in the cell cycle, CDK–cyclin complexes’ interaction is modulated by members of two groups of CDK inhibitors: the CIP/KIP-type family (p21, p27, and p57), which binds to both the cyclin and CDK subunits to inhibit CDK1, CDK2, CDK4 and CDK6 [
21], and the INK-type family (p16
INK4a, p15
INK4b, p18
INK4c and p19
INK4d), which specifically binds to CDK4 and CDK6 monomers, weakening CDK interaction with cyclin D [
22] (
Figure 3). In the quiescent state G0, CDK4 and CDK6 are inactive, being bound to the inhibitor INK4, and the expression of cyclin D is absent. As a consequence, Rb is active and represses the transcription factor E2F [
18]. After the removal of the repressive proteins INK4, the cells immediately enter the G1 phase and proceed through it through the action of cyclin D, leading to transcriptional activation [
23] (
Figure 3).
The INK4 family (p16INK4a, p15INK4b, p18INK4c and p19INK4d) and the Cip/Kip family (p21, p27, and p57) are two types of cell cycle endogenous inhibitors. The INK 4 family specifically binds and inhibits cyclin D/CDK 4/6 complexes, whereas the Cip/Kip family can bind both cyclin and CDK subunits to inhibit CDK1, CDK2, CDK4 and CDK6.
3. Cyclin D-CDK 4/6 in Ovarian Development
CDK4 and CDK6, as well the three D-type cyclins, are essential for proliferation in several cell types [
24]. Mouse embryos defective for either CDK4 or CDK6 display normal organogenesis [
25] but die during the late stages of embryonic development due to severe anemia [
26]. This suggests that, at least in mice, each of these CDKs can compensate for the ablation of the other and thus be compatible with life, whereas the loss of both kinases primarily affects the proliferation of erythroid progenitors.
This behavior may be explained by the fact that embryo cells have a short G1 phase and do not stop at the G0 stage, and they do not require all the cellular mechanisms involved in the signaling of cell cycle re-entry from G0 [
27].
On the other hand, in adult differentiated tissues, studies reported ovarian failure related to CDK4 deficiency. CDK4-deficient mice have small ovaries, defects in corpus luteum formation and a reduced ovulation efficiency, as well as embryo implantation failures [
28]. CDK4-deficient mice were shown to be sterile; they are characterized by extended estrus cycles and reduced serum progesterone levels [
29,
30]. A subsequent investigation revealed that administering daily progesterone injections successfully reinstated implantation and fertility in CDK4-deficient mice [
31]. These findings strongly suggest that infertility in mice lacking CDK4 is likely associated with diminished serum progesterone levels. However, the complete mechanism underlying this infertility is not yet fully understood.
The limited manifestations of CDK4 deficiency could be explained by the ability of CDK6 to replace the function of CDK4 [
19]. On the other hand, CDK6 deficient mice are not sterile [
25].
These results indicate that cyclin-dependent kinases such as D-type cyclin-dependent kinases are not essential for cell cycle entry and suggest the existence of alternative mechanisms to initiate cell proliferation upon mitogenic stimulation. In addition, these findings indicate that CDK4 and CDK6 could have overlapping functions.
4. Cyclin D in Granulosa Cells
In the ovary, granulosa cells are the somatic cells responsible for ovarian function. Granulosa cell cycle progression is crucial for follicular maturation, ovulation, and the genesis of the corpus luteum [
24,
26].
Inside primordial follicles, granulosa cells are suspended in the G0 phase. However, upon follicular activation, these cells re-enter the cell cycle. In rodents, this event leads to the substantial development of preovulatory follicles within 3 days [
27] (p. 20).
In rats, sheep [
32] and humans [
33], the proliferation rates of granulosa cells change with the stage of maturation of the follicle and its size: cell cycle progression is slow when follicles are recruited from the primary to secondary stage of development, while the granulosa cells of the preantral follicles initiate an increase in the rate of cycle progression.
In this context, FSH is crucial for promoting granulosa cell proliferation through the up-regulation of cyclin D2, while luteinizing hormone (LH), via MAPK1/3 activation, causes cell cycle arrest by activating CDK endogenous inhibitors [
34].
The functions of cyclin D1, D2, and D3 appear to be specific to different organs. Cyclin D2 plays a particularly predominant role in the development and function of the gonads [
35], and it is specifically expressed in granulosa cells. Its expression increases under FSH, estradiol and insulin stimuli [
36]. FSH stimulation, in fact, results in the formation and subsequent activation of the cyclin D2/CDK4 complex, responsible for the initiation of DNA synthesis [
37]. On the other hand, LH, via MAPK1/3 activation, causes cell cycle arrest by activating CDK endogenous inhibitors [
38].
In the cultured granulosa cells of rats, it has been shown that the expression of cyclin D2 is FSH-dependent and that this molecule has a crucial role in the growth and maturation of follicles [
32,
33].
Consistent with this, female mice deficient in cyclin D2 display a mutant-like ovarian phenotype, with reduced granulosa cell proliferation, anovulation and infertility [
27,
32].
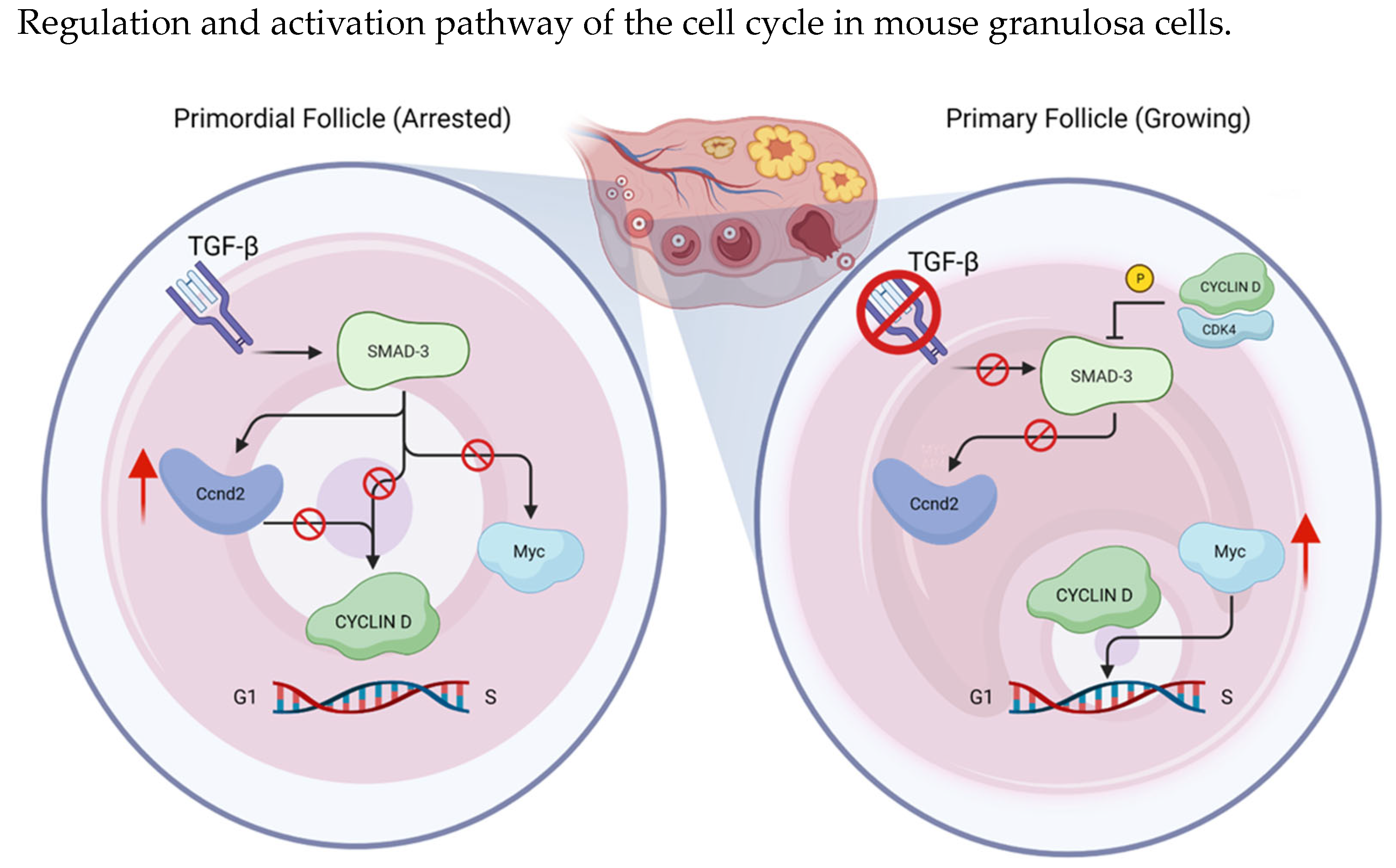
Another regulatory factor of cyclin D2 is SMAD3, whose transcription is activated by Transforming Growth Factor Beta (TGFβ); it binds the cyclin D2 promoter, maintaining inhibited transcription [
30]. TGFβ-mediated SMAD2/3 transcription factors are specifically expressed in primordial granulosa cell nuclei, and TGFβ signaling is known to be important for maintaining growth arrest in various cell types. Evidence suggests that cyclin D-CDK4 complex phosphorylates and inhibits SMAD3 [
19]. Furthermore, it seems to directly bind the Ccnd2 and Myc genes, regulating their transcription. In arrested primordial follicles, SMAD3 promotes Ccnd2 expression and represses Myc, ensuring that granulosa cells do not progress in the cell cycle. The eventual loss of SMAD3 leads to the transcription of Myc and progression in growing follicles [
30] (
Figure 4).
SMAD-3 phosphorylation and its resulting inhibition by the cyclin D/CDK4 complex is crucial to G1-S transition in cell cycle progression; SMAD3 activation by TGFβ promotes Ccnd2 (Cyclin D2) expression and represses Myc, ensuring that granulosa cells do not progress in the cell cycle, keeping the follicle in a primordial condition.
5. Cyclin D-CDK4/6 Complexes in the Oocytes
To reach the state of full competence, oocytes undergo several morphological and molecular modifications. Around birth, oocytes are arrested in the meiotic prophase I, and they remain in this stage until puberty, when the preovulatory surge of LH causes ovulation. After the LH surge, oocytes pass through the G2/M transition, enter anaphase, and then complete the first meiosis. Meiotic oocytes achieve their maturation in the metaphase of meiosis II without entering the S phase and are arrested in this stage until fertilization.
Several molecules are involved in regulating meiosis resumption in oocytes. Prophase I arrest results from the maintenance of low activity of the complex cyclin B1-CDK1, known as maturation promoting factor (MPF), through the phosphorylation-mediated inactivation of CDK1 and degradation of cyclin B1. The LH surge activates CDK1, which both phosphorylates different meiotic phosphoproteins and protects them from dephosphorylation by inhibiting the activities of CDK1-antagonizing protein phosphatases [
39,
40]. Although there is no S phase in meiosis, the cell cycle regulators of G1/S transition during mitosis may be involved in meiotic progression. Regarding CDK4/6, the cyclin D-CDK4/6 complex and p27—the CDK4 inhibitor—were found to be expressed in mouse oocytes [
41]. The dynamics of their amounts, structure and localization strongly suggest their involvement in the regulation of oocyte development, but whether the cyclin D-CDK4/6 complex regulates meiotic cell cycle progression is not known. More recently, Dong et al. [
42] observed different roles of the cyclin D-CDK4/6 complex in oocyte meiosis.
CDK4/6 inhibition can lead to the dysfunction of the main phases of meiosis. In particular, it can interfere with spindle assembly checkpoint (SAC) activity, leading to aberration in chromosome segregation; this results in an incorrect number of chromosomes (aneuploidy) in the oocyte. The inactivation of SAC causes a premature loss of cyclin B1 via ubiquitin-mediated degradation; moreover, there is a decrease in MPF activity in meiotic oocytes.
Therefore, the cyclin D-CDK4/6 complex is required for regulating meiotic progression in mouse oocytes and mediating control of the SAC to prevent aneuploidy in female meiosis I.
6. Role of Exogenous CDK Inhibitors (CDKi) in the Ovary
Limited data have been published about the role of exogenous CDKi in the ovary and their possible gonadotoxicity.
The specific CDK4/6 inhibitor palbociclib has a role not only in CDK4/6 activity but also in activation through the phosphorylation of the bulk of cyclin D-CDK4 complexes. A study conducted on Chinese hamster ovarian cells (CHO) showed that, in absence of the inhibitor p21, palbociclib treatment increased the stability and activation of cyclin D3-CDK4/6 complexes [
43]. This effect was not observed in the presence of p21 co-expression, suggesting that palbociclib and p21 competed for the stabilization of cyclin D3-CDK4 complexes. Furthermore, recent studies [
44,
45] showed a potential “protective” role of palbociclib in granulosa cells in regard to chemerin, which is an adipokine secreted mainly from adipose tissue that causes reactive oxygen species (ROS) accumulation and triggers apoptosis in mouse granulosa cells. Evidence suggests that palbociclib has an antiapoptotic effect on immortalized human granulosa-lutein (hGL) cells treated with chemerin. These results can be explained by the fact that palbociclib can diminish ROS generation in hGL cells and suppresses chemerin-induced apoptotic protein expression.
Nevertheless, another study showed that CDK4/6 inhibitor activity causes incomplete DNA replication, leading to cell damage. This drives the cell to exit the cell cycle in a p53-dependent manner [
46]. Indeed, if cells fail to withdraw from the cell cycle following DNA replication problems, they enter mitosis with fragmented chromosomes and excessive DNA damage, which further limits their proliferative potential, leading to senescence [
46].
In fact, the data obtained in this study using a retinal epithelial cell (RPE1) line demonstrated that G1 arrest becomes problematic in long-term treatment [
46].
A comparison between immortalized RPE1 and a type of breast cancer cell (MCF7) was performed in order to reveal potential differences in cellular response to CDK4/6 inhibition. Following treatment with palbociclib, both breast cancer and RPE1 cells underwent DNA damage, but its consequences were worse in healthy cells. Indeed, 3 days of exposure to palbociclib treatment were needed to reduce the long-term proliferation of RPE1 cells to a level comparable to that reached after 7 days of therapy in breast cancer cells. Hence, the study links CDK4/6 inhibitors to genotoxic stress, which can occur in healthy cells, such as RPE1 and granulosa cells [
46]. CDK4/6 inhibitors induce cell cycle arrest in tumor cells, leading to apoptosis [
47,
48]. As recently demonstrated in the 2022 study of Crozier et al., this apoptotic effect can also be seen in healthy cells (RPE1).
Overall, the data on palbociclib are conflicting and cannot yet confirm a potential damaging role of CDK4/6 exogenous inhibition in the ovary and specifically in granulosa cells.
To date, there are no consistent data on abemaciclib and ribociclib ovarian toxicity.
7. Conclusions
Cyclins and their associated CDKs are key regulators of cell cycles, contributing to the transition from the G1 to S phase [
49]. Considering their crucial role in controlling proliferation, senescence, migration, apoptosis and angiogenesis, the dysregulation of cyclin–CDK represents a hallmark of cancer.
In the ovary and, more specifically, in the granulosa cells of primordial follicles, the consecutive activation of different CDKs leads the cell to move from a quiescent state in the G0 phase to the G1 phase, back into the cell cycle. The transition into these different phases is regulated by the activation of specific cyclin–CDK complexes. Following a mitogenic signal, the D type of cyclins and CDK 4 and 6 are the first complexes to be activated. Once this checkpoint is bypassed, the cell proceeds through the G1 phase and commits to the completion of the mitotic cycle and divide.
The impact of the currently available CDK4/6 inhibitors ribociclib, abemaciclib and palbociclib on healthy ovarian cells has not yet been fully investigated. In this regard, the currently available data are limited and controversial. On the one hand, research has shown a possible protective role of palbociclib in ovarian cells; on the other hand, it appears that this agent could lead healthy cells to a state of replication stress due to cell cycle interruption resulting in excessive DNA damage, which further limits their proliferative potential.
The growing use of CDK4/6 inhibitors in the clinical setting and the paucity of data on their potential gonadotoxicity highlights the need to pursue dedicated research efforts in this area to further improve treatment decision making for premenopausal women who are candidates for this targeted treatment.
Author Contributions
G.S. and S.O. designed the review and conducted the literature review. G.S. and S.O. wrote the first draft of the paper. L.A. provided resources for the creation of figures within the paper. M.L. assisted in writing the paper. All authors made substantial inputs into all the drafts of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
“Associazione Italiana per la Ricerca sul Cancro” (AIRC), grant number MFAG 2020 ID 24698.
Conflicts of Interest
Matteo Lambertini reports his advisory role for Roche, Lilly, Novartis, Astrazeneca, Pfizer, Seagen, Gilead, MSD and Exact Sciences; speaker honoraria from Roche, Lilly, Novartis, Pfizer, Sandoz, Libbs, Daiichi Sankyo and Takeda; travel grants from Gilead and Daiichi Sankyo; and research support (to the Institution) from Gilead outside the submitted work. Eva Blondeaux reports research support (to the Institution) from Gilead outside the submitted work. All the other authors declare no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Paluch-Shimon, S.; Cardoso, F.; Partridge, A.H.; Abulkhair, O.; Azim, H.A.; Bianchi-Micheli, G.; Cardoso, M.J.; Curigliano, G.; Gelmon, K.A.; Gentilini, O.; et al. ESO-ESMO Fifth International Consensus Guidelines for Breast Cancer in Young Women (BCY5). Ann. Oncol. 2022, 33, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.-B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility Preservation and Post-Treatment Pregnancies in Post-Pubertal Cancer Patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Bildik, G.; Benlioglu, C.; Turan, V.; Dilege, E.; Ozel, M.; Kim, S.; Oktem, O. Breast Cancer Treatment and Ovarian Function. Reprod. BioMed. Online 2023, 46, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Oktem, O.; Oktay, K. A Novel Ovarian Xenografting Model to Characterize the Impact of Chemotherapy Agents on Human Primordial Follicle Reserve. Cancer Res. 2007, 67, 10159–10162. [Google Scholar] [CrossRef] [PubMed]
- Plowchalk, D.R.; Mattison, D.R. Phosphoramide Mustard Is Responsible for the Ovarian Toxicity of Cyclophosphamide. Toxicol. Appl. Pharmacol. 1991, 107, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Bildik, G.; Akin, N.; Senbabaoglu, F.; Sahin, G.N.; Karahuseyinoglu, S.; Ince, U.; Taskiran, C.; Selek, U.; Yakin, K.; Guzel, Y.; et al. GnRH Agonist Leuprolide Acetate Does Not Confer Any Protection against Ovarian Damage Induced by Chemotherapy and Radiation In Vitro. Hum. Reprod. 2015, 30, 2912–2925. [Google Scholar] [CrossRef]
- Meirow, D.; Dor, J.; Kaufman, B.; Shrim, A.; Rabinovici, J.; Schiff, E.; Raanani, H.; Levron, J.; Fridman, E. Cortical Fibrosis and Blood-Vessels Damage in Human Ovaries Exposed to Chemotherapy. Potential Mech. Ovarian Inj. Hum. Reprod. 2007, 22, 1626–1633. [Google Scholar] [CrossRef]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide Triggers Follicle Activation and “Burnout”; AS101 Prevents Follicle Loss and Preserves Fertility. Sci. Transl. Med. 2013, 5, 185ra62. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Mayer, E.L.; Fesl, C.; Hlauschek, D.; Garcia-Estevez, L.; Burstein, H.J.; Zdenkowski, N.; Wette, V.; Miller, K.D.; Balic, M.; Mayer, I.A.; et al. Treatment Exposure and Discontinuation in the PALbociclib CoLlaborative Adjuvant Study of Palbociclib With Adjuvant Endocrine Therapy for Hormone Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Early Breast Cancer (PALLAS/AFT-05/ABCSG-42/BIG-14-03). J. Clin. Oncol. 2022, 40, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Marmé, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.-B.; Bear, H.; McCarthy, N.; Melé Olivé, M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer-The Penelope-B Trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Toi, M.; O’Shaughnessy, J.; Rastogi, P.; Campone, M.; Neven, P.; Huang, C.-S.; Huober, J.; Jaliffe, G.G.; Cicin, I.; et al. Abemaciclib plus Endocrine Therapy for Hormone Receptor-Positive, HER2-Negative, Node-Positive, High-Risk Early Breast Cancer (monarchE): Results from a Preplanned Interim Analysis of a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2023, 24, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.-S.; Fasching, P.A.; Crown, J.; Bardia, A.; Chia, S.; Im, S.-A.; Martin, M.; et al. Ribociclib and Endocrine Therapy as Adjuvant Treatment in Patients with HR+/HER2- Early Breast Cancer: Primary Results from the Phase III NATALEE Trial. J. Clin. Oncol. 2023, 41, LBA500. [Google Scholar] [CrossRef]
- Furlanetto, J.; Marmé, F.; Thode, C.; Nekljudova, V.; Liu, Y.; Martin Jimenez, M.; Reimer, T.; Knudsen, E.; Denkert, C.; Bassy, M.; et al. 60MO Ovarian Function in Young Patients (Pts) Treated with Postneoadjuvant Palbociclib (PAL) and Endocrine Therapy (ET) for Hormone Receptor (HR)-Positive, HER2-Negative Early Breast Cancer (BC): Explorative Analysis in Penelope-B. Ann. Oncol. 2022, 33, S149–S150. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. To Cycle or Not to Cycle: A Critical Decision in Cancer. Nat. Rev. Cancer 2001, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Nurse, P. A Long Twentieth Century of the Cell Cycle and Beyond. Cell 2000, 100, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, A.; Bartoletti, M.; Masoudi-Khoram, N.; Sorio, R.; Puglisi, F.; Belletti, B.; Baldassarre, G. Inhibition of CDK4/6 as Therapeutic Approach for Ovarian Cancer Patients: Current Evidences and Future Perspectives. Cancers 2021, 13, 3035. [Google Scholar] [CrossRef]
- Kozar, K.; Sicinski, P. Cell Cycle Progression without Cyclin D-CDK4 and Cyclin D-CDK6 Complexes. Cell Cycle 2005, 4, 388–391. [Google Scholar] [CrossRef]
- Rubin, S.M.; Sage, J.; Skotheim, J.M. Integrating Old and New Paradigms of G1/S Control. Mol. Cell 2020, 80, 183–192. [Google Scholar] [CrossRef]
- Russo, A.A.; Jeffrey, P.D.; Patten, A.K.; Massagué, J.; Pavletich, N.P. Crystal Structure of the p27Kip1 Cyclin-Dependent-Kinase Inibitor Bound to the Cyclin A–Cdk2 Complex. Nature 1996, 382, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Hallett, S.T.; Pastok, M.W.; Morgan, R.M.L.; Wittner, A.; Blundell, K.L.I.M.; Felletar, I.; Wedge, S.R.; Prodromou, C.; Noble, M.E.M.; Pearl, L.H.; et al. Differential Regulation of G1 CDK Complexes by the Hsp90-Cdc37 Chaperone System. Cell Rep. 2017, 21, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. CDK Inhibitors: Positive and Negative Regulators of G1-Phase Progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sakamoto, K.; Wagner, K.-U. D-Type Cyclins Are Important Downstream Effectors of Cytokine Signaling That Regulate the Proliferation of Normal and Neoplastic Mammary Epithelial Cells. Mol. Cell. Endocrinol. 2014, 382, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Sotillo, R.; Santamaría, D.; Galán, J.; Cerezo, A.; Ortega, S.; Dubus, P.; Barbacid, M. Mammalian Cells Cycle without the D-Type Cyclin-Dependent Kinases Cdk4 and Cdk6. Cell 2004, 118, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Mammalian Cyclin-Dependent Kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Bockstaele, L.; Coulonval, K.; Kooken, H.; Paternot, S.; Roger, P.P. Regulation of CDK4. Cell Div. 2006, 1, 25. [Google Scholar] [CrossRef]
- Pagano, M.; Jackson, P.K. Wagging the Dogma: Tissue-Specific Cell Cycle Control in the Mouse Embryo. Cell 2004, 118, 535–538. [Google Scholar] [CrossRef]
- Robker, R.L.; Richards, J.S. Hormone-Induced Proliferation and Differentiation of Granulosa Cells: A Coordinated Balance of the Cell Cycle Regulators Cyclin D2 and p27Kip1. Mol. Endocrinol. 1998, 12, 924–940. [Google Scholar] [CrossRef]
- Granados-Aparici, S.; Hardy, K.; Franks, S.; Sharum, I.B.; Waite, S.L.; Fenwick, M.A. SMAD3 Directly Regulates Cell Cycle Genes to Maintain Arrest in Granulosa Cells of Mouse Primordial Follicles. Sci. Rep. 2019, 9, 6513. [Google Scholar] [CrossRef]
- Moons, D.S.; Jirawatnotai, S.; Tsutsui, T.; Franks, R.; Parlow, A.F.; Hales, D.B.; Gibori, G.; Fazleabas, A.T.; Kiyokawa, H. Intact Follicular Maturation and Defective Luteal Function in Mice Deficient for Cyclin- Dependent Kinase-4. Endocrinology 2002, 143, 647–654. [Google Scholar] [CrossRef][Green Version]
- Cannon, J.D.; Cherian-Shaw, M.; Lovekamp-Swan, T.; Chaffin, C.L. Granulosa Cell Expression of G1/S Phase Cyclins and Cyclin-Dependent Kinases in PMSG-Induced Follicle Growth. Mol. Cell. Endocrinol. 2007, 264, 6–15. [Google Scholar] [CrossRef]
- Gougeon, A. Ovarian Follicular Growth in Humans: Ovarian Ageing and Population of Growing Follicles. Maturitas 1998, 30, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, H.; Kineman, R.D.; Manova-Todorova, K.O.; Soares, V.C.; Hoffman, E.S.; Ono, M.; Khanam, D.; Hayday, A.C.; Frohman, L.A.; Koff, A. Enhanced Growth of Mice Lacking the Cyclin-Dependent Kinase Inhibitor Function of P27(Kip1). Cell 1996, 85, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Carthon, B.C.; Neumann, C.A.; Das, M.; Pawlyk, B.; Li, T.; Geng, Y.; Sicinski, P. Genetic Replacement of Cyclin D1 Function in Mouse Development by Cyclin D2. Mol. Cell. Biol. 2005, 25, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Kayampilly, P.P.; Menon, K.M.J. Dihydrotestosterone Inhibits Insulin-Stimulated Cyclin D2 Messenger Ribonucleic Acid Expression in Rat Ovarian Granulosa Cells by Reducing the Phosphorylation of Insulin Receptor Substrate-1. Endocrinology 2006, 147, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Roy, S.K. Follicle Stimulating Hormone-Induced DNA Synthesis in the Granulosa Cells of Hamster Preantral Follicles Involves Activation of Cyclin-Dependent Kinase-4 Rather than Cyclin D2 Synthesis. Biol. Reprod. 2004, 70, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Chatterjee, R.; McGarrigle, H.H.; Ahmed, F.; Thomas, N.S. P107 Is Active in the Nucleolus in Non-Dividing Human Granulosa Lutein Cells. J. Mol. Endocrinol. 2000, 25, 275–286. [Google Scholar] [CrossRef]
- Filatov, M.; Khramova, Y.; Semenova, M. Molecular Mechanisms of Prophase I Meiotic Arrest Maintenance and Meiotic Resumption in Mammalian Oocytes. Reprod. Sci. 2019, 26, 1519–1537. [Google Scholar] [CrossRef]
- Adhikari, D.; Liu, K. The Regulation of Maturation Promoting Factor during Prophase I Arrest and Meiotic Entry in Mammalian Oocytes. Mol. Cell. Endocrinol. 2014, 382, 480–487. [Google Scholar] [CrossRef]
- Kohoutek, J.; Dvorák, P.; Hampl, A. Temporal Distribution of CDK4, CDK6, D-Type Cyclins, and P27 in Developing Mouse Oocytes. Biol. Reprod. 2004, 70, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Meng, T.-G.; Li, J.; Wang, F.; Li, Y.; Ouyang, Y.-C.; Hou, Y.; Wang, Z.-B.; Schatten, H.; Sun, Q.-Y. Inhibition of CDK4/6 Kinases Causes Production of Aneuploid Oocytes by Inactivating the Spindle Assembly Checkpoint and Accelerating First Meiotic Progression. Biochim. Biophys. Acta BBA Mol. Cell Res. 2021, 1868, 119044. [Google Scholar] [CrossRef] [PubMed]
- Paternot, S.; Colleoni, B.; Bisteau, X.; Roger, P.P. The CDK4/CDK6 Inhibitor PD0332991 Paradoxically Stabilizes Activated Cyclin D3-CDK4/6 Complexes. Cell Cycle 2014, 13, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, Z.; Fu, Y.; Wu, R.; Wang, Y.; Liu, C.; Yang, L.; Zhang, H. Involvement of Obesity-Associated Upregulation of Chemerin/Chemokine-like Receptor 1 in Oxidative Stress and Apoptosis in Ovaries and Granulosa Cells. Biochem. Biophys. Res. Commun. 2019, 510, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Shan, Y.; Jia, C.; Xu, Y. CDK4/6 Inhibitor Protects Chemerin-Induced Human Granulosa-Lutein Cells from Apoptosis by Inhibiting the P53/P21 (Waf) Pathway. Mol. Reprod. Dev. 2019, 86, 1561–1568. [Google Scholar] [CrossRef]
- Crozier, L.; Foy, R.; Mouery, B.L.; Whitaker, R.H.; Corno, A.; Spanos, C.; Ly, T.; Cook, J.G.; Saurin, A.T. CDK4/6 Inhibitors Induce Replication Stress to Cause Long-Term Cell Cycle Withdrawal. EMBO J. 2022, 41, e108599. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Litchfield, L.M.; Webster, Y.; Chio, L.-C.; Wong, S.S.; Stewart, T.R.; Dowless, M.; Dempsey, J.; Zeng, Y.; Torres, R.; et al. Genomic Aberrations That Activate D-Type Cyclins Are Associated with Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib. Cancer Cell 2017, 32, 761–776.e6. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernández, M.; Malumbres, M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell 2020, 37, 514–529. [Google Scholar] [CrossRef]
- Gao, X.; Leone, G.W.; Wang, H. Cyclin D-CDK4/6 Functions in Cancer. Adv. Cancer Res. 2020, 148, 147–169. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).