Oncotherapeutic Strategies in Early Onset Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
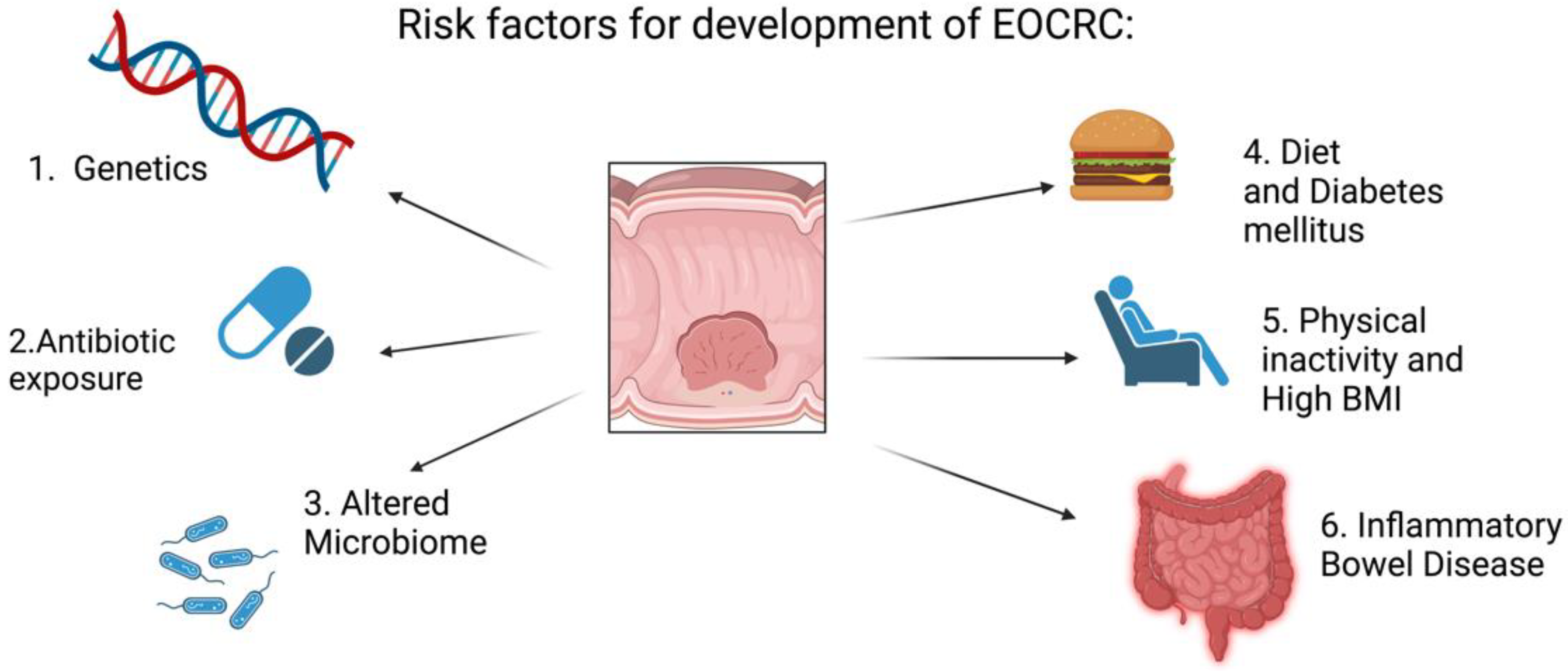
2. Risk Factors for the Development of Colorectal Cancer
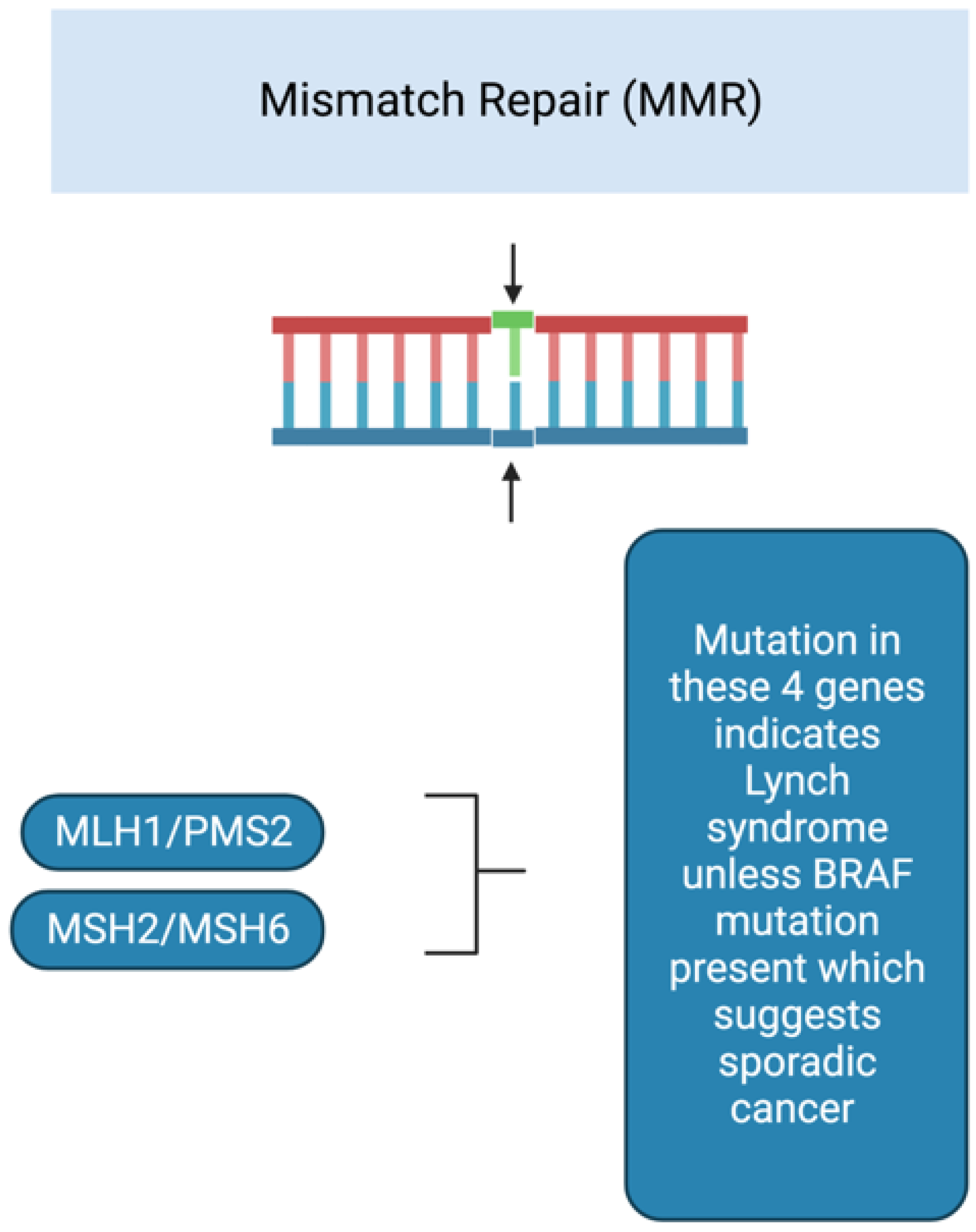
3. Cancer Predisposition Syndromes
4. Diagnostic Delays
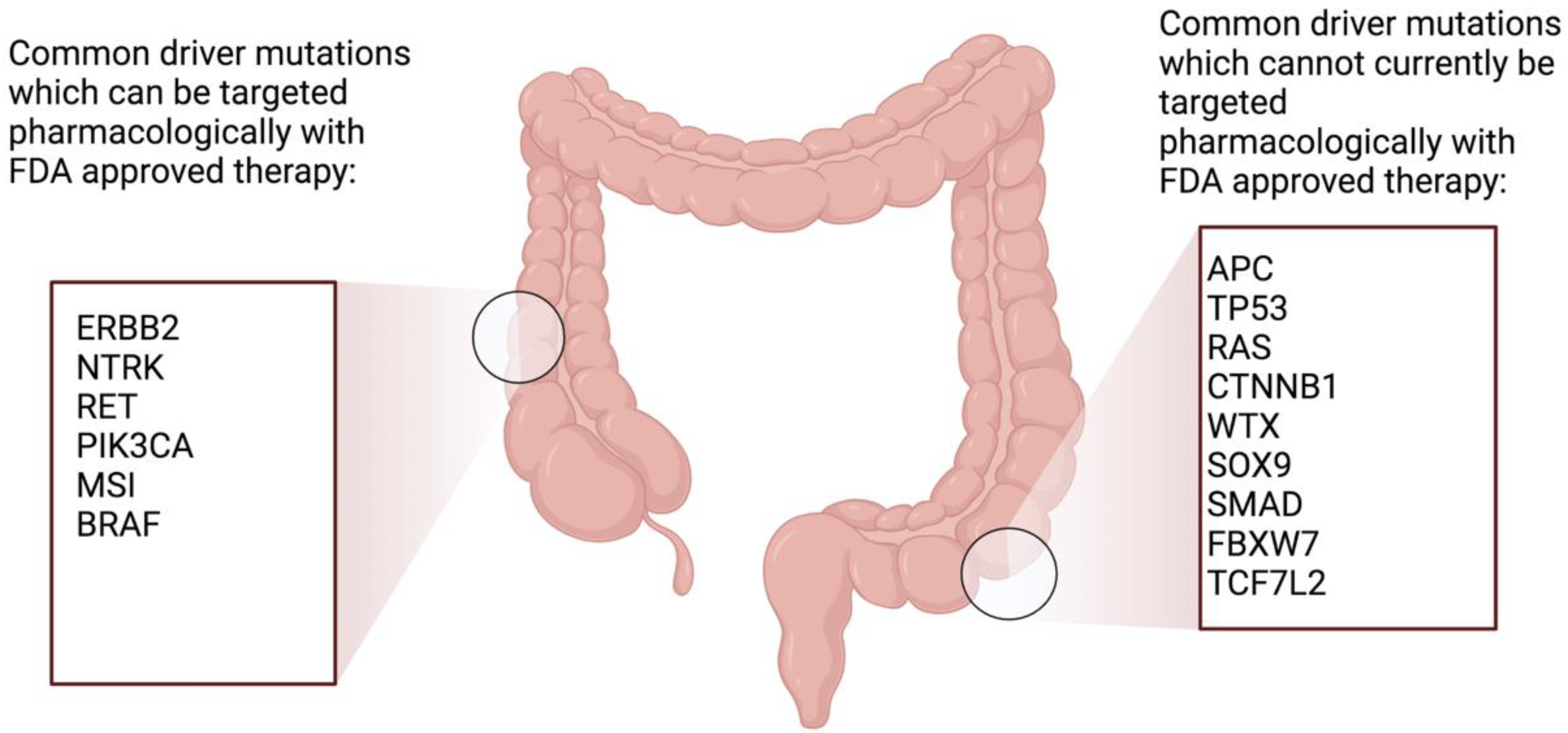
5. Treatment Strategies
6. Chemotherapy Regimens Used in EOCRC
7. Treatment of Metastatic EOCRC
8. Additional Targeted Agents in Metastatic EOCRC
9. Manipulation of The Immune System to Treat EOCRC
10. Treatment of Early-Stage EOCRC
11. Oligometastatic Disease
12. Future Perspectives for the Treatment of Early Onset Colorectal Cancer
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Woodhouse, M.; Gupta, S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: A SEER-based analysis with comparison to other young-onset cancers. J. Investig. Med. 2017, 65, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Ireland, N.C.R. Cancer in Ireland 1994-2019: Annual Report of the National Cancer Registry; National Cancer Registry Ireland: Cork, Ireland, 2021. [Google Scholar]
- Pan, H.; Zhao, Z.; Deng, Y.; Zheng, Z.; Huang, Y.; Huang, S.; Chi, P. The global, regional, and national early-onset colorectal cancer burden and trends from 1990 to 2019: Results from the Global Burden of Disease Study 2019. BMC Public Health 2022, 22, 1896. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.G.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef]
- Hnatyszyn, A.; Hryhorowicz, S.; Kaczmarek-Ryś, M.; Lis, E.; Słomski, R.; Scott, R.J.; Pławski, A. Colorectal carcinoma in the course of inflammatory bowel diseases. Hered. Cancer Clin. Pract. 2019, 17, 18. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef]
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-de-Mesquita, B.; Skeie, G.; Olsen, A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; et al. Consumption of dairy products and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS ONE 2013, 8, e72715. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Viennois, E.; Merlin, D.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifier-Induced Low-Grade Inflammation Promotes Colon Carcinogenesis. Cancer Res. 2017, 77, 27–40. [Google Scholar] [CrossRef]
- Chang, D.T.; Pai, R.K.; Rybicki, L.A.; Dimaio, M.A.; Limaye, M.; Jayachandran, P.; Koong, A.C.; Kunz, P.A.; Fisher, G.A.; Ford, J.M.; et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod. Pathol. 2012, 25, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Lynch, J.F.; Attard, T.A. Diagnosis and management of hereditary colorectal cancer syndromes: Lynch syndrome as a model. Cmaj 2009, 181, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, A.M.; Abdile, A.; Adamina, M.; Aigner, F.; d’Allens, L.; Allmer, C.; Álvarez, A.; Anula, R.; Andric, M.; Atallah, S.; et al. Characteristics of Early-Onset vs Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021, 156, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, A.M.; Winter, D.C.; Lynch, L. The therapeutic and prognostic implications of immunobiology in colorectal cancer: A review. Br. J. Cancer 2021, 125, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, Y.M.; de Jong, A.E.; Morreau, H.; Tops, C.M.; Vasen, H.F.; Wijnen, J.T.; Breuning, M.H.; Bröcker-Vriends, A.H. Diagnostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): A guide for clinicians. CA Cancer J. Clin. 2006, 56, 213–225. [Google Scholar] [CrossRef]
- Adar, T.; Rodgers, L.H.; Shannon, K.M.; Yoshida, M.; Ma, T.; Mattia, A.; Lauwers, G.Y.; Iafrate, A.J.; Chung, D.C. A tailored approach to BRAF and MLH1 methylation testing in a universal screening program for Lynch syndrome. Mod. Pathol. 2017, 30, 440–447. [Google Scholar] [CrossRef]
- Capper, D.; Voigt, A.; Bozukova, G.; Ahadova, A.; Kickingereder, P.; von Deimling, A.; von Knebel Doeberitz, M.; Kloor, M. BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int. J. Cancer 2013, 133, 1624–1630. [Google Scholar] [CrossRef]
- REACCT Collaborative. Impact of microsatellite status in early-onset colonic cancer. Br. J. Surg. 2022, 109, 632–636. [Google Scholar] [CrossRef]
- Lynch, H.T.; de la Chapelle, A. Hereditary colorectal cancer. N. Engl. J. Med. 2003, 348, 919–932. [Google Scholar] [CrossRef]
- Sieber, O.M.; Lipton, L.; Crabtree, M.; Heinimann, K.; Fidalgo, P.; Phillips, R.K.; Bisgaard, M.L.; Orntoft, T.F.; Aaltonen, L.A.; Hodgson, S.V.; et al. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N. Engl. J. Med. 2003, 348, 791–799. [Google Scholar] [CrossRef]
- Bisgaard, M.L.; Ripa, R.; Knudsen, A.L.; Bülow, S. Familial adenomatous polyposis patients without an identified APC germline mutation have a severe phenotype. Gut 2004, 53, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Riaz, R.; Masood, N.; Benish, A. Red flag symptoms: Detailed account of clinicopathological features in young-onset colorectal cancer. Intest. Res. 2017, 15, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.W.; Sundaram, V.; Chew, T.A.; Ladabaum, U. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated With Longer Duration of Symptoms or Time to Diagnosis. Clin. Gastroenterol. Hepatol. 2017, 15, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.D.; Ferrari, A.; Ries, L.; Whelan, J.; Bleyer, W.A. Cancer in adolescents and young adults: A narrative review of the current status and a view of the future. JAMA Pediatr. 2016, 170, 495–501. [Google Scholar] [CrossRef]
- Indini, A.; Bisogno, G.; Cecchetto, G.; Vitellaro, M.; Signoroni, S.; Massimino, M.; Riccipetitoni, G.; Zecca, M.; Dall’Igna, P.; De Pasquale, M.D.; et al. Gastrointestinal tract carcinoma in pediatric and adolescent age: The Italian TREP project experience. Pediatr. Blood Cancer 2017, 64, e26658. [Google Scholar] [CrossRef]
- Khan, S.A.; Morris, M.; Idrees, K.; Gimbel, M.I.; Rosenberg, S.; Zeng, Z.; Li, F.; Gan, G.; Shia, J.; LaQuaglia, M.P.; et al. Colorectal cancer in the very young: A comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J. Pediatr. Surg. 2016, 51, 1812–1817. [Google Scholar] [CrossRef]
- O’Sullivan, D.E.; Cheung, W.Y.; Boyne, D.J.; Jarada, T.N.; Tang, P.A.; Gill, S.; Hilsden, R.J.; Brenner, D.R. Treatment patterns and survival outcomes of early-onset colorectal cancer patients in Alberta, Canada: A population-based study. Cancer Treat. Res. Commun. 2022, 32, 100585. [Google Scholar] [CrossRef]
- Blanke, C.D.; Bot, B.M.; Thomas, D.M.; Bleyer, A.; Kohne, C.H.; Seymour, M.T.; de Gramont, A.; Goldberg, R.M.; Sargent, D.J. Impact of young age on treatment efficacy and safety in advanced colorectal cancer: A pooled analysis of patients from nine first-line phase III chemotherapy trials. J. Clin. Oncol. 2011, 29, 2781–2786. [Google Scholar] [CrossRef]
- Soto, J.; Alonso, N.G.; Beranek, M.B.; Villa, J.L.C.; de la Tabla, G.O.; Jiménez, C.L.; Bianchi, M.A.; Rodríguez, R.J.; Lozano, R.M.; Valles, M.A.; et al. Analysis of survival trends, clinical, and molecular characteristics of patients with early-onset colorectal cancer (EOCRC). J. Clin. Oncol. 2022, 40, 59. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, E.R.; Hong, S.N.; Chang, D.K.; Kim, Y.H. Long-Term Outcome and Prognostic Factors of Sporadic Colorectal Cancer in Young Patients: A Large Institutional-Based Retrospective Study. Med. Baltim. 2016, 95, e3641. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Okamoto, K.; Kasi, P.M.; Kawakami, H. Molecular Biomarkers in the Personalized Treatment of Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2016, 14, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Lawler, W.E.; Shum, M.K.; Dakhil, S.R.; Spira, A.I.; Barlesi, F.; Reck, M.; Garassino, M.C.; Spigel, D.R.; Alvarez, D.; et al. KRYSTAL-12: A randomized phase 3 study of adagrasib (MRTX849) versus docetaxel in patients (pts) with previously treated non-small-cell lung cancer (NSCLC) with KRASG12C mutation. J. Clin. Oncol. 2021, 39, TPS9129. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, F.; Goeman, F.; Pallocca, M.; Sperati, F.; Pizzuti, L.; Melucci, E.; Casini, B.; Amoreo, C.A.; Gallo, E.; Diodoro, M.G.; et al. Deep sequencing and pathway-focused analysis revealed multigene oncodriver signatures predicting survival outcomes in advanced colorectal cancer. Oncogenesis 2018, 7, 55. [Google Scholar] [CrossRef]
- Yuan, Y.; Bao, J.; Chen, Z.; Villanueva, A.D.; Wen, W.; Wang, F.; Zhao, D.; Fu, X.; Cai, Q.; Long, J.; et al. Multi-omics analysis to identify susceptibility genes for colorectal cancer. Hum. Mol. Genet. 2021, 30, 321–330. [Google Scholar] [CrossRef]
- Xu, X.; Gong, C.; Wang, Y.; Hu, Y.; Liu, H.; Fang, Z. Multi-omics analysis to identify driving factors in colorectal cancer. Epigenomics 2020, 12, 1633–1650. [Google Scholar] [CrossRef]
- Li, J.; Dittmar, R.L.; Xia, S.; Zhang, H.; Du, M.; Huang, C.C.; Druliner, B.R.; Boardman, L.; Wang, L. Cell-free DNA copy number variations in plasma from colorectal cancer patients. Mol. Oncol. 2017, 11, 1099–1111. [Google Scholar] [CrossRef]
- Lee, K.T.; Vider, J.; Tang, J.C.; Gopalan, V.; Lam, A.K. GAEC1 drives colon cancer progression. Mol. Carcinog. 2019, 58, 1145–1154. [Google Scholar] [CrossRef]
- Wang, Y.; He, S.; Zhu, X.; Qiao, W.; Zhang, J. High copy number of mitochondrial DNA predicts poor prognosis in patients with advanced stage colon cancer. Int. J. Biol. Markers 2016, 31, e382–e388. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Okita, A.; Takahashi, S.; Ouchi, K.; Inoue, M.; Watanabe, M.; Endo, M.; Honda, H.; Yamada, Y.; Ishioka, C. Consensus molecular subtypes classification of colorectal cancer as a predictive factor for chemotherapeutic efficacy against metastatic colorectal cancer. Oncotarget 2018, 9, 18698–18711. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.; Wilson, A.J.; Shi, Q.; Corner, G.A.; Arañes, M.J.; Nicholas, C.; Lesser, M.; Mariadason, J.M.; Augenlicht, L.H. Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells. Br. J. Cancer 2004, 91, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.P.; Duarte, G.S.; Sterrantino, C.; Pais, H.L.; Quintela, A.; Martins, A.P.; Costa, J. Triplet (FOLFOXIRI) versus doublet (FOLFOX or FOLFIRI) backbone chemotherapy as first-line treatment of metastatic colorectal cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2017, 118, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef]
- Falcone, A.; Ricci, S.; Brunetti, I.; Pfanner, E.; Allegrini, G.; Barbara, C.; Crinò, L.; Benedetti, G.; Evangelista, W.; Fanchini, L.; et al. Phase III Trial of Infusional Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan (FOLFOXIRI) Compared With Infusional Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) As First-Line Treatment for Metastatic Colorectal Cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007, 25, 1670–1676. [Google Scholar] [CrossRef]
- Cejas, P.; López-Gómez, M.; Aguayo, C.; Madero, R.; de Castro Carpeño, J.; Belda-Iniesta, C.; Barriuso, J.; Moreno García, V.; Larrauri, J.; López, R.; et al. KRAS mutations in primary colorectal cancer tumors and related metastases: A potential role in prediction of lung metastasis. PLoS ONE 2009, 4, e8199. [Google Scholar] [CrossRef]
- Botrel, T.E.A.; Clark, L.G.O.; Paladini, L.; Clark, O.A.C. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: A systematic review and meta-analysis. BMC Cancer 2016, 16, 677. [Google Scholar] [CrossRef]
- Welch, S.; Spithoff, K.; Rumble, R.B.; Maroun, J. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: A systematic review. Ann. Oncol. 2010, 21, 1152–1162. [Google Scholar] [CrossRef]
- Amado, R.G.; Wolf, M.; Peeters, M.; Van Cutsem, E.; Siena, S.; Freeman, D.J.; Juan, T.; Sikorski, R.; Suggs, S.; Radinsky, R.; et al. Wild-Type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 1626–1634. [Google Scholar] [CrossRef]
- Shaib, W.; Mahajan, R.; El-Rayes, B. Markers of resistance to anti-EGFR therapy in colorectal cancer. J. Gastrointest. Oncol. 2013, 4, 308–318. [Google Scholar] [PubMed]
- Venook, A.P.; Ou, F.-S.; Lenz, H.-J.; Kabbarah, O.; Qu, X.; Niedzwiecki, D.; Zemla, T.; Goldberg, R.M.; Hochster, H.S.; O’Neil, B.H.; et al. Primary (1°) tumor location as an independent prognostic marker from molecular features for overall survival (OS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB / SWOG 80405 (Alliance). J. Clin. Oncol. 2017, 35, 3503. [Google Scholar] [CrossRef]
- Kather, J.N.; Halama, N.; Jaeger, D. Genomics and emerging biomarkers for immunotherapy of colorectal cancer. Semin. Cancer Biol. 2018, 52, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Koopman, M.; Kortman, G.A.; Mekenkamp, L.; Ligtenberg, M.J.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.; van Krieken, J.H. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 2009, 100, 266–273. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Lonardi, S.; Zagonel, V.; Cutsem, E.V.; Limon, M.L.; Wong, M.; Hendlisz, A.; Aglietta, M.; Garcia-Alfonso, P.; Neyns, B.; et al. Nivolumab (NIVO) + low-dose ipilimumab (IPI) as first-line (1L) therapy in microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): Two-year clinical update. J. Clin. Oncol. 2020, 38, 4040. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef]
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus guidelines for the detection of immunogenic cell death. OncoImmunology 2014, 3, e955691. [Google Scholar] [CrossRef]
- Rayner, E.; van Gool, I.C.; Palles, C.; Kearsey, S.E.; Bosse, T.; Tomlinson, I.; Church, D.N. A panoply of errors: Polymerase proofreading domain mutations in cancer. Nat. Rev. Cancer 2016, 16, 71–81. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Bellone, S.; Buza, N.; Choi, J.; Schwartz, P.E.; Schlessinger, J.; Lifton, R.P. Regression of Chemotherapy-Resistant Polymerase ε (POLE) Ultra-Mutated and MSH6 Hyper-Mutated Endometrial Tumors with Nivolumab. Clin. Cancer Res. 2016, 22, 5682–5687. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Freeman-Mills, L.; Rayner, E.; Glaire, M.; Briggs, S.; Vermeulen, L.; Fessler, E.; Medema, J.P.; Boot, A.; Morreau, H.; et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: A retrospective, pooled biomarker study. Lancet Gastroenterol. Hepatol. 2016, 1, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Temko, D.; Van Gool, I.C.; Rayner, E.; Glaire, M.; Makino, S.; Brown, M.; Chegwidden, L.; Palles, C.; Depreeuw, J.; Beggs, A.; et al. Somatic POLE exonuclease domain mutations are early events in sporadic endometrial and colorectal carcinogenesis, determining driver mutational landscape, clonal neoantigen burden and immune response. J. Pathol. 2018, 245, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Seymour, M.T.; Morton, D. FOxTROT: An international randomised controlled trial in 1052 patients (pts) evaluating neoadjuvant chemotherapy (NAC) for colon cancer. J. Clin. Oncol. 2019, 37, 3504. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Compton, C.C.; Fielding, L.P.; Burgart, L.J.; Conley, B.; Cooper, H.S.; Hamilton, S.R.; Hammond, M.E.; Henson, D.E.; Hutter, R.V.; Nagle, R.B.; et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch. Pathol. Lab. Med. 2000, 124, 979–994. [Google Scholar] [CrossRef]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef]
- van Oostendorp, S.E.; Smits, L.J.H.; Vroom, Y.; Detering, R.; Heymans, M.W.; Moons, L.M.G.; Tanis, P.J.; de Graaf, E.J.R.; Cunningham, C.; Denost, Q.; et al. Local recurrence after local excision of early rectal cancer: A meta-analysis of completion TME, adjuvant (chemo)radiation, or no additional treatment. Br. J. Surg. 2020, 107, 1719–1730. [Google Scholar] [CrossRef]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2020, 3, e2030097. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Trevisan, F.; Cabiddu, M.; Sgroi, G.; Bruschieri, L.; Rausa, E.; Ghidini, M.; Turati, L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann. Surg. 2020, 271, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Nordlinger, B.; Adam, R.; Köhne, C.H.; Pozzo, C.; Poston, G.; Ychou, M.; Rougier, P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer 2006, 42, 2212–2221. [Google Scholar] [CrossRef]
- Lee, W.S.; Yun, S.H.; Chun, H.K.; Lee, W.Y.; Yun, H.R.; Kim, J.; Kim, K.; Shim, Y.M. Pulmonary resection for metastases from colorectal cancer: Prognostic factors and survival. Int. J. Colorectal Dis. 2007, 22, 699–704. [Google Scholar] [CrossRef]
- Kemeny, N. Management of liver metastases from colorectal cancer. Oncology (Williston Park) 2006, 20, 1161–1176, 1179. [Google Scholar]
- Aloia, T.A.; Vauthey, J.N.; Loyer, E.M.; Ribero, D.; Pawlik, T.M.; Wei, S.H.; Curley, S.A.; Zorzi, D.; Abdalla, E.K. Solitary colorectal liver metastasis: Resection determines outcome. Arch. Surg. 2006, 141, 460–466. [Google Scholar] [CrossRef] [PubMed]
- van der Pool, A.E.; Damhuis, R.A.; Ijzermans, J.N.; de Wilt, J.H.; Eggermont, A.M.; Kranse, R.; Verhoef, C. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: A population-based series. Colorectal Dis. 2012, 14, 56–61. [Google Scholar] [CrossRef]
- HQIP. Trends, Characteristics and Outcomes for Patients Diagnosed under 50 Years Old with Metastatic Colon Cancer in England; HQIP: London, UK, 2019. [Google Scholar]
- Boselli, C.; Cirocchi, R.; Gemini, A.; Grassi, V.; Avenia, S.; Polistena, A.; Sanguinetti, A.; Burattini, M.F.; Pironi, D.; Santoro, A.; et al. Surgery for colorectal cancer in elderly: A comparative analysis of risk factor in elective and urgency surgery. Aging Clin. Exp. Res. 2017, 29, 65–71. [Google Scholar] [CrossRef]
- Parisi, A.; Trastulli, S.; Ricci, F.; Regina, R.; Cirocchi, R.; Grassi, V.; Gemini, A.; Pironi, D.; D’Andrea, V.; Santoro, A.; et al. Analysis of long-term results after liver surgery for metastases from colorectal and non-colorectal tumors: A retrospective cohort study. Int. J. Surg. 2016, 30, 25–30. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.; Raab, H.R.; Weitz, J.; Lordick, F.; Hartmann, J.T.; Stoehlmacher-Williams, J.; Lang, H.; Trarbach, T.; et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann. Oncol. 2014, 25, 1018–1025. [Google Scholar] [CrossRef]
- Ye, L.C.; Liu, T.S.; Ren, L.; Wei, Y.; Zhu, D.X.; Zai, S.Y.; Ye, Q.H.; Yu, Y.; Xu, B.; Qin, X.Y.; et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J. Clin. Oncol. 2013, 31, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Moretto, R.; Rossini, D.; Zucchelli, G.; Lonardi, S.; Bergamo, F.; Santini, D.; Cupini, S.; Tomasello, G.; Caponnetto, S.; Zaniboni, A.; et al. Oligometastatic colorectal cancer: Prognosis, role of locoregional treatments and impact of first-line chemotherapy-a pooled analysis of TRIBE and TRIBE2 studies by Gruppo Oncologico del Nord Ovest. Eur. J. Cancer 2020, 139, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Baimas-George, M.; Baker, E.; Kamionek, M.; Salmon, J.S.; Sastry, A.; Levi, D.; Vrochides, D. A Complete Pathological Response to Pembrolizumab following ex vivo Liver Resection in a Patient with Colorectal Liver Metastases. Chemotherapy 2018, 63, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Onaitis, M.W.; Petersen, R.P.; Haney, J.C.; Saltz, L.; Park, B.; Flores, R.; Rizk, N.; Bains, M.S.; Dycoco, J.; D’Amico, T.A.; et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann. Thorac. Surg. 2009, 87, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, A.J.; Jayakrishnan, T.T.; Rajeev, R.; Rilling, W.S.; Thomas, J.P.; George, B.; Johnston, F.M.; Gamblin, T.C.; Turaga, K.K. Comparative Effectiveness of Hepatic Artery Based Therapies for Unresectable Colorectal Liver Metastases: A Meta-Analysis. PLoS ONE 2015, 10, e0139940. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Paweletz, C.P.; Kuang, Y.; Mach, S.L.; O’Connell, A.; Messineo, M.M.; Luke, J.J.; Butaney, M.; Kirschmeier, P.; Jackman, D.M.; et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin. Cancer Res. 2014, 20, 1698–1705. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Fehm, T.N.; Grimm-Glang, D.; Rody, A.; Krawczyk, N. Liquid Biopsy in Metastatic Breast Cancer: Current Role of Circulating Tumor Cells and Circulating Tumor DNA. Oncol. Res. Treat. 2022, 45, 4–11. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef]
- Pozzi, V.; Campagna, R.; Sartini, D.; Emanuelli, M. Nicotinamide N-Methyltransferase as Promising Tool for Management of Gastrointestinal Neoplasms. Biomolecules 2022, 12, 1173. [Google Scholar] [CrossRef]
- Gao, Y.; van Haren, M.J.; Buijs, N.; Innocenti, P.; Zhang, Y.; Sartini, D.; Campagna, R.; Emanuelli, M.; Parsons, R.B.; Jespers, W.; et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021, 64, 12938–12963. [Google Scholar] [CrossRef]
- van Haren, M.J.; Gao, Y.; Buijs, N.; Campagna, R.; Sartini, D.; Emanuelli, M.; Mateuszuk, L.; Kij, A.; Chlopicki, S.; de Castilla, P.E.M.; et al. Esterase-Sensitive Prodrugs of a Potent Bisubstrate Inhibitor of Nicotinamide N-Methyltransferase (NNMT) Display Cellular Activity. Biomolecules 2021, 11, 1357. [Google Scholar] [CrossRef]
- van Haren, M.J.; Zhang, Y.; Thijssen, V.; Buijs, N.; Gao, Y.; Mateuszuk, L.; Fedak, F.A.; Kij, A.; Campagna, R.; Sartini, D.; et al. Macrocyclic peptides as allosteric inhibitors of nicotinamide N-methyltransferase (NNMT). RSC Chem. Biol. 2021, 2, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Xing, J.; Jiang, Z.; Zhang, Z.; Zhang, H.; Wang, D.; Tang, D. Effects of Long Non-Coding RNAs Induced by the Gut Microbiome on Regulating the Development of Colorectal Cancer. Cancers 2022, 14, 5813. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Yu, Q.; Pu, Y.; Xing, C. Upregulation of Long Noncoding RNA MALAT1 in Colorectal Cancer Promotes Radioresistance and Aggressive Malignance. Int. J. Gen. Med. 2022, 15, 8365–8380. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Reilly, M.; Linehan, A.; Krstic, A.; Kolch, W.; Sheahan, K.; Winter, D.C.; Mc Dermott, R. Oncotherapeutic Strategies in Early Onset Colorectal Cancer. Cancers 2023, 15, 552. https://doi.org/10.3390/cancers15020552
O’Reilly M, Linehan A, Krstic A, Kolch W, Sheahan K, Winter DC, Mc Dermott R. Oncotherapeutic Strategies in Early Onset Colorectal Cancer. Cancers. 2023; 15(2):552. https://doi.org/10.3390/cancers15020552
Chicago/Turabian StyleO’Reilly, Mary, Anna Linehan, Aleksandar Krstic, Walter Kolch, Kieran Sheahan, Des C. Winter, and Ray Mc Dermott. 2023. "Oncotherapeutic Strategies in Early Onset Colorectal Cancer" Cancers 15, no. 2: 552. https://doi.org/10.3390/cancers15020552
APA StyleO’Reilly, M., Linehan, A., Krstic, A., Kolch, W., Sheahan, K., Winter, D. C., & Mc Dermott, R. (2023). Oncotherapeutic Strategies in Early Onset Colorectal Cancer. Cancers, 15(2), 552. https://doi.org/10.3390/cancers15020552






