Real World Data for Pancreatic Adenocarcinoma from a Population-Based Study in France
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
2.3. Ethics Approval
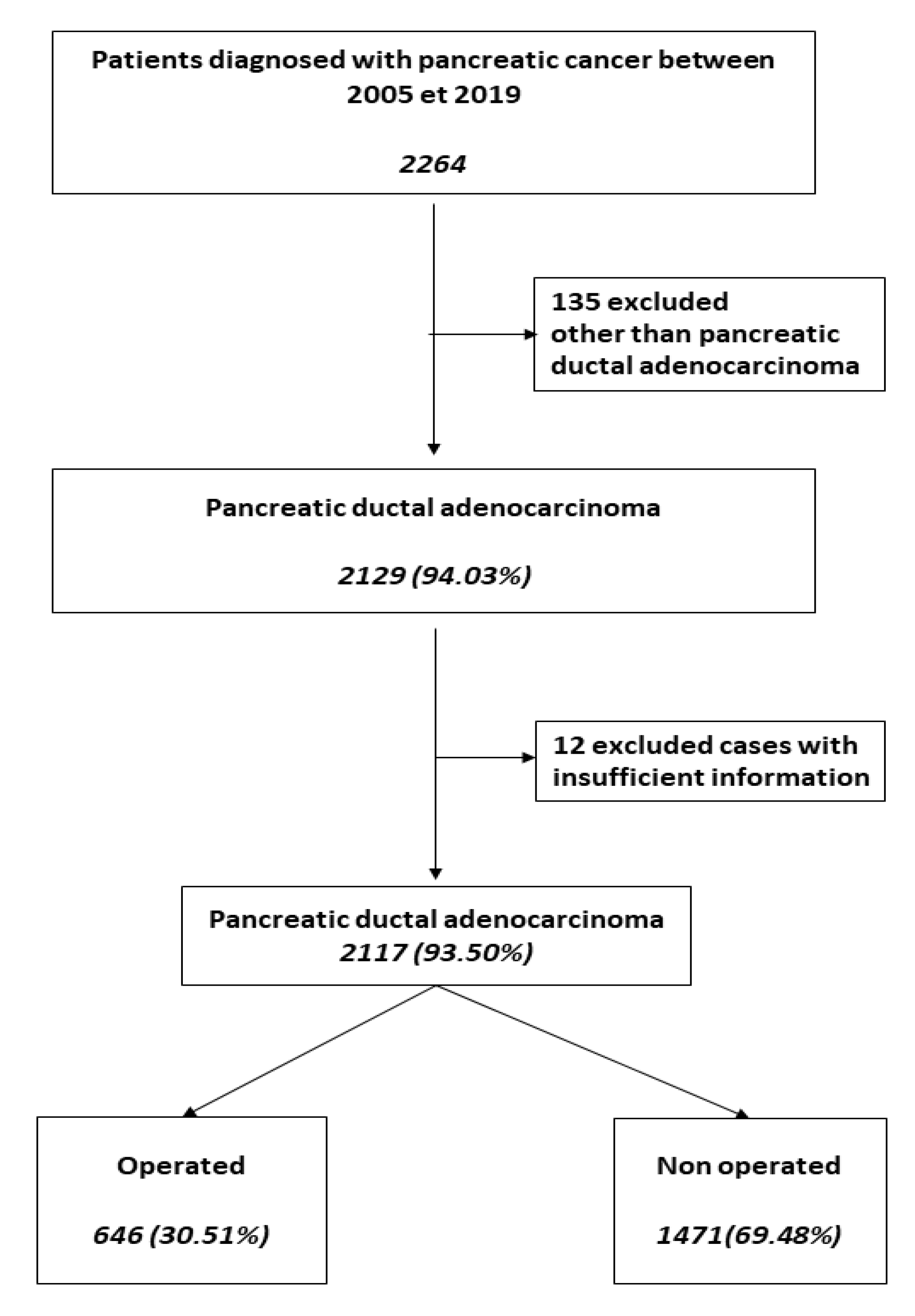
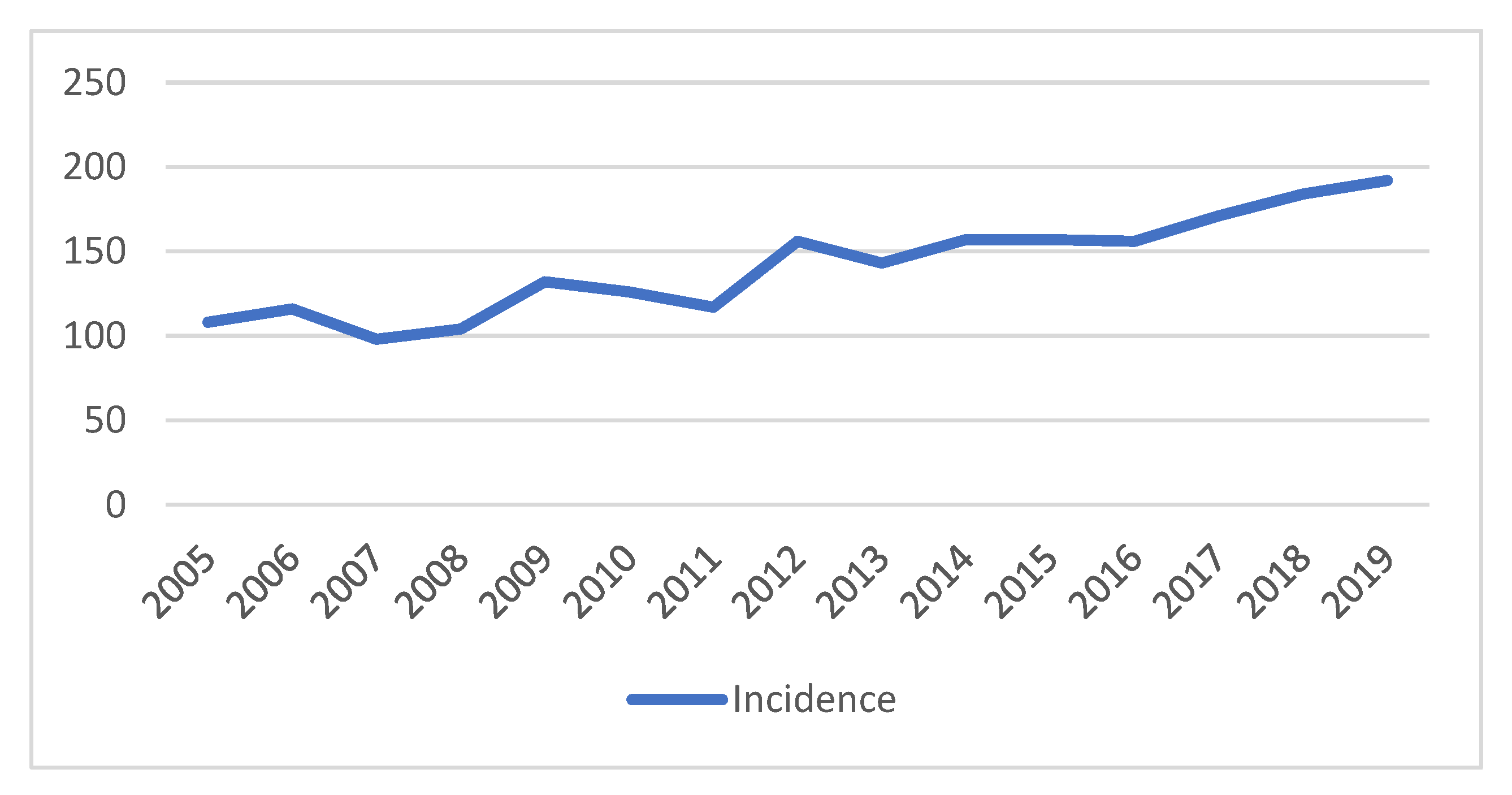
3. Results
3.1. Patient Characteristics
3.2. Treatment
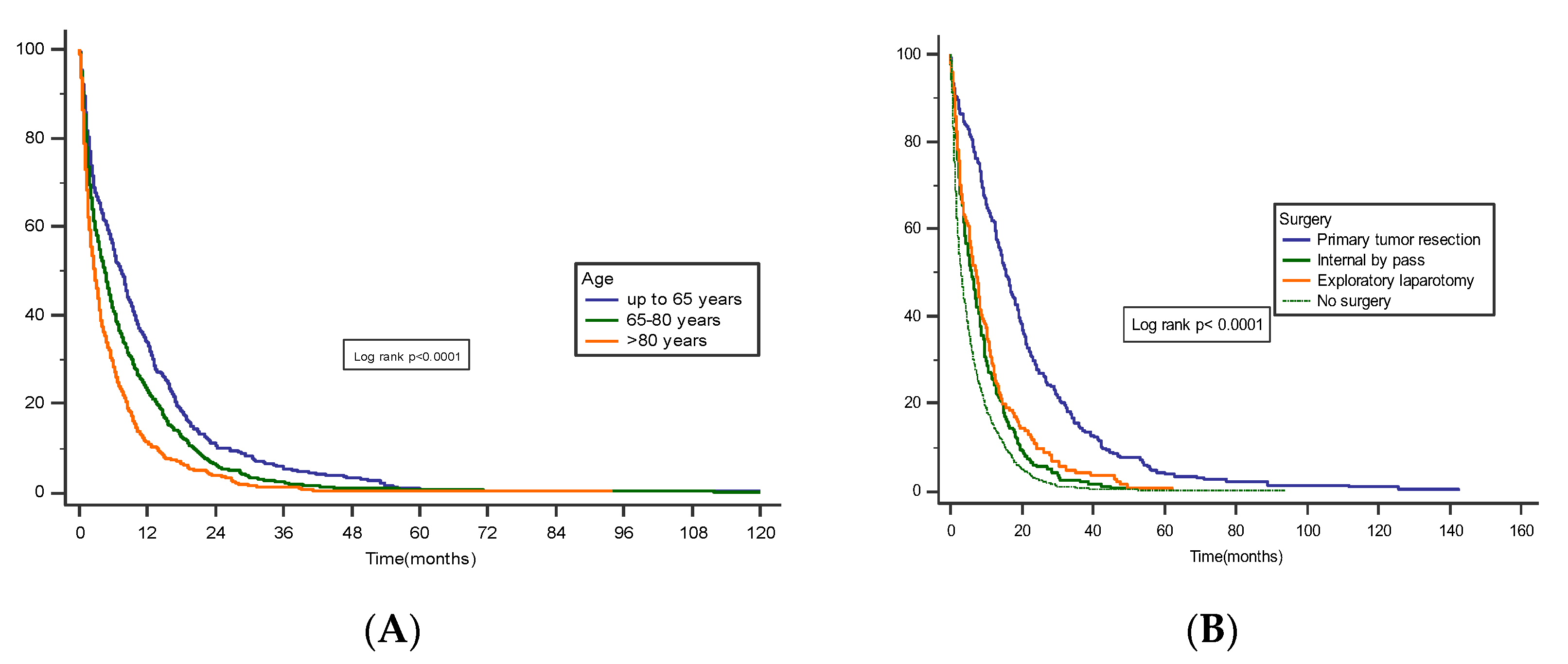
3.3. Survival and Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types Of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Azar, I.; Virk, G.; Esfandiarifard, S.; Wazir, A.; Mehdi, S. Treatment and Survival Rates of Stage IV Pancreatic Cancer at VA Hospitals: A Nation-Wide Study. J. Gastrointest. Oncol. 2019, 10, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Partensky, C.; Bray, F. More Deaths from Pancreatic Cancer than Breast Cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.M.; Mills, K.; Mendonça, S.C.; Abel, G.A.; Basu, B.; Carroll, N.; Ballard, S.; Lancaster, J.; Hamilton, W.; Rubin, G.P.; et al. Symptoms and Patient Factors Associated with Diagnostic Intervals for Pancreatic Cancer (SYMPTOM Pancreatic Study): A Prospective Cohort Study. Lancet Gastroenterol. Hepatol. 2016, 1, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the Outcomes of Pancreatic Cancer Surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Marchegiani, G.; Andrianello, S.; Malleo, G.; De Gregorio, L.; Scarpa, A.; Mino-Kenudson, M.; Maggino, L.; Ferrone, C.R.; Lillemoe, K.D.; Bassi, C.; et al. Does Size Matter in Pancreatic Cancer? Ann. Surg. 2017, 266, 142–148. [Google Scholar] [CrossRef]
- Chandrasegaram, M.D.; Goldstein, D.; Simes, J.; Gebski, V.; Kench, J.G.; Gill, A.J.; Samra, J.S.; Merrett, N.D.; Richardson, A.J.; Barbour, A.P. Meta-Analysis of Radical Resection Rates and Margin Assessment in Pancreatic Cancer. Br. J. Surg. 2015, 102, 1459–1472. [Google Scholar] [CrossRef]
- Hartwig, W.; Werner, J.; Jäger, D.; Debus, J.; Büchler, M.W. Improvement of Surgical Results for Pancreatic Cancer. Lancet Oncol. 2013, 14, e476–e485. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Heh, V.; Pawlik, T.M.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Williams, T.; Abushahin, L.; Bridges, J.F.P.; Santry, H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1129. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C. The Global Burden of Pancreatic Cancer. Arch. Med. Sci. 2020, 16, 820–824. [Google Scholar] [CrossRef]
- Krejs, G.J. Pancreatic Cancer: Epidemiology and Risk Factors. Dig. Dis. 2010, 28, 355–358. [Google Scholar] [CrossRef]
- Lepage, C.; Capocaccia, R.; Hackl, M.; Lemmens, V.; Molina, E.; Pierannunzio, D.; Sant, M.; Trama, A.; Faivre, J.; Hackl, M.; et al. Survival in Patients with Primary Liver Cancer, Gallbladder and Extrahepatic Biliary Tract Cancer and Pancreatic Cancer in Europe 1999–2007: Results of EUROCARE-5. Eur. J. Cancer 2015, 51, 2169–2178. [Google Scholar] [CrossRef]
- Lee, M.; Kwon, W.; Kim, H.; Byun, Y.; Han, Y.; Kang, J.S.; Choi, Y.J.; Jang, J.-Y. The Role of Location of Tumor in the Prognosis of the Pancreatic Cancer. Cancers 2020, 12, 2036. [Google Scholar] [CrossRef]
- Defining the Molecular Pathology of Pancreatic Body and Tail Adenocarcinoma. Br. J. Surg. 2018, 105, e183–e191. [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; Quinn, M.C.; et al. Genomic Analyses Identify Molecular Subtypes of Pancreatic Cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Petermann, D.; Demartines, N.; Schäfer, M. Is Tumour Size an Underestimated Feature in the Current TNM System for Malignancies of the Pancreatic Head? Hpb 2013, 15, 872–881. [Google Scholar] [CrossRef]
- Garcea, G.; Dennison, A.; Pattenden, C.; Neal, C.; Sutton, C.; Berry, D. Survival Following Curative Resection for Pancreatic Ductal Adenocarcinoma. A Systematic Review of the Literature. JOP J. Pancreas 2008, 9, 99–132. [Google Scholar]
- Scheufele, F.; Friess, H. Palliative Surgery in Advanced Pancreatic Cancer. In Pancreatic Cancer; Neoptolemos, J.P., Urrutia, R., Abbruzzese, J.L., Büchler, M.W., Eds.; Springer: New York, NY, USA, 2018; pp. 857–873. ISBN 978-1-4939-7193-0. [Google Scholar]
- Poruk, K.E.; Wolfgang, C.L. Palliative Management of Unresectable Pancreas Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 327–337. [Google Scholar] [CrossRef]
- Cheung, S.L.H.; Teoh, A.Y.B. Optimal Management of Gastric Outlet Obstruction in Unresectable Malignancies. Gut Liver 2022, 16, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y. The Role of Radiotherapy for Pancreatic Malignancies: A Population-Based Analysis of the SEER Database. Clin. Transl. Oncol. 2022, 24, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Yoon, Y.B.; Kim, Y.-T.; Ryu, J.K.; Yoon, W.J.; Lee, S.H. Survival and Prognostic Factors of Unresectable Pancreatic Cancer. J. Clin. Gastroenterol. 2008, 42, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Wolony-Rokicka, E.; Sutkowski, K.; Gradziel, A.; Dorsz, Ż.; Tukiendorf, A.; Lipinski, J.; Wydmancki, J. Tolerance and Efficacy of Palliative Radiotherapy for Advanced Pancreatic Cancer: A Retrospective Analysis of Single-Institutional Experiences. Mol. Clin. Oncol. 2016, 4, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of Adjuvant Gemcitabine and Capecitabine with Gemcitabine Monotherapy in Patients with Resected Pancreatic Cancer (ESPAC-4): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Jooste, V.; Bengrine-Lefevre, L.; Manfredi, S.; Quipourt, V.; Grosclaude, P.; Facy, O.; Lepage, C.; Ghiringhelli, F.; Bouvier, A.-M. Management and Outcomes of Pancreatic Cancer in French Real-World Clinical Practice. Cancers 2022, 14, 1675. [Google Scholar] [CrossRef]
- Schizas, D.; Charalampakis, N.; Kole, C.; Economopoulou, P.; Koustas, E.; Gkotsis, E.; Ziogas, D.; Psyrri, A.; Karamouzis, M.V. Immunotherapy for Pancreatic Cancer: A 2020 Update. Cancer Treat. Rev. 2020, 86, 102016. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Bange, E.M.; Doucette, A.; Gabriel, P.E.; Porterfield, F.; Harrigan, J.J.; Wang, R.; Wojcieszynski, A.P.; Boursi, B.; Mooney, B.I.; Reiss, K.A.; et al. Opportunity Costs of Receiving Palliative Chemotherapy for Metastatic Pancreatic Ductal Adenocarcinoma. JCO Oncol. Pract. 2020, 16, e678–e687. [Google Scholar] [CrossRef]
- Badic, B.; Oguer, M.; Cariou, M.; Kermarrec, T.; Bouzeloc, S.; Nousbaum, J.-B.; Robaszkiewicz, M.; Quénéhervé, L. Ostomy Prevalence and Survival in Elderly Patients with Stage III and IV Rectal Cancer. Geriatr. Gerontol. Int. 2021, 21, 670–675. [Google Scholar] [CrossRef]
| Characteristics | Total n = 2117 | Pancreatic Cancer Resection n = 293 (13.8%) | Palliative Treatment n = 1824 (86.2%) | p-Value |
|---|---|---|---|---|
| Gender | 0.98 | |||
| Male | 1081 (51.1) | 149 (50.85) | 932 (51.09) | |
| Female | 1036 (48.9) | 144 (49.15) | 892 (48.91) | |
| Diagnostic Age (years) | 73 (IQR * 65.0–81.0) | 69 (IQR 62.0–75.0) | 75 (IQR 66.0–82.0) | <0.0001 |
| Primary tumor site | <0.0001 | |||
| Head | 1120 (52.9) | 208 (70.99) | 912 (50.00) | |
| Neck | 281 (13.3) | 24 (8.19) | 257 (14.09) | |
| Body | 301 (14.2) | 30 (10.24) | 271 (14.86) | |
| Tail | 53 (2.5) | 6 (2.05) | 47 (2.58) | |
| Diffuse lesions | 362 (17.1) | 25 (8.53) | 337 (18.47) | |
| Clinical manifestations | ||||
| Jaundice | 707 (33.39) | 151 (51.53) | 556 (30.48) | <0.0001 |
| Weight loss | 949 (44.8) | 69 (23.54) | 880 (48.24) | <0.0001 |
| Abdominal pain | 1016 (47.99) | 113 (38.56) | 903 (49.50) | <0.001 |
| Unknown | 195 (9.2) | 40 (13.65) | 155 (8.49) |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR 1 | 95% CI 2 | p | HR 1 | 95% CI 2 | p | |
| Age | 1.01 | 1.01 to 1.01 | <0.0001 | 1.01 | 1.01 to 1.02 | <0.0001 |
| Tumor location (tail) | 1.3 | 1.14 to 1.49 | 0.0001 | |||
| Surgical resection * | 0.36 | 0.31 to 0.42 | <0.0001 | 0.38 | 0.33 to 0.44 | <0.0001 |
| Tumor size | 1.01 | 1.01 to 1.02 | <0.0001 | |||
| Well differentiated | 0.67 | 0.57 to 0.77 | <0.0001 | |||
| Moderately differentiated | 0.83 | 0.73 to 0.93 | 0.003 | |||
| Lymphatic tumor emboli | 1.2 | 1.09 to 1.32 | 0.0001 | |||
| Positive lymph nodes | 1.24 | 1.12 to 1.38 | 0.0001 | 1.24 | 1.08 to 1.35 | <0.001 |
| Absence of metastases ** | 0.65 | 0.59 to 0.72 | <0.0001 | 0.66 | 0.59 to 0.75 | <0.0001 |
| Postoperative chemotherapy | 0.36 | 0.22 to 0.60 | 0.0001 | 0.36 | 0.20 to 0.63 | <0.001 |
| Palliative chemotherapy | 0.52 | 0.35 to 0.76 | 0.0009 | 0.63 | 0.42 to 0.93 | 0.02 |
| Palliative radiotherapy | 0.69 | 0.56 to 0.85 | <0.0001 | 0.73 | 0.57 to 0.91 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badic, B.; Morvan, M.; Quénéhervé, L.; Bouzeloc, S.; Kermarrec, T.; Nousbaum, J.-B.; Reboux, N. Real World Data for Pancreatic Adenocarcinoma from a Population-Based Study in France. Cancers 2023, 15, 525. https://doi.org/10.3390/cancers15020525
Badic B, Morvan M, Quénéhervé L, Bouzeloc S, Kermarrec T, Nousbaum J-B, Reboux N. Real World Data for Pancreatic Adenocarcinoma from a Population-Based Study in France. Cancers. 2023; 15(2):525. https://doi.org/10.3390/cancers15020525
Chicago/Turabian StyleBadic, Bogdan, Marie Morvan, Lucille Quénéhervé, Servane Bouzeloc, Tiphaine Kermarrec, Jean-Baptiste Nousbaum, and Noémi Reboux. 2023. "Real World Data for Pancreatic Adenocarcinoma from a Population-Based Study in France" Cancers 15, no. 2: 525. https://doi.org/10.3390/cancers15020525
APA StyleBadic, B., Morvan, M., Quénéhervé, L., Bouzeloc, S., Kermarrec, T., Nousbaum, J.-B., & Reboux, N. (2023). Real World Data for Pancreatic Adenocarcinoma from a Population-Based Study in France. Cancers, 15(2), 525. https://doi.org/10.3390/cancers15020525





