1. Introduction
An abnormal cell growth that occurs in the colon or rectum is simply recognized as colorectal cancer (CRC). A noncancerous cell growth, known as a polyp, can develop in the mucosal layer of the colon or rectum, and may develop into CRC. Less than 10% of polyps have a high potential to progress into invasive cancer over 10–20 years. CRC occurs predominantly in adults aged 50 and older [
1]. In 2020, CRC was recognized as the third most common cancer type worldwide and the second most common cause of cancer death. Geographically, the occurrence of CRC is highest in Asian regions. The International Agency for Research on Cancer (IARC) estimated that the global burden of CRC will increase by 56%, and CRC related mortality will increase by 69% between 2020 and 2040 [
2]. Therefore, a strong research focus on the discovery of novel anticancer therapeutics is urgently required.
Many scientists have focused their attention on the development of innovative drugs derived from natural sources such as plants, lichens, and micro-organisms over a few decades. A lichen is a symbiotic living form associated with a fungus (mycobiont) and a cyanobacterium or green alga (the photobiont), or both [
2]. Some endolichenic fungi (ELF) reside inside the lichen thalli and produce bioactive secondary metabolites with medicinal and economic potential. These secondary metabolites show cytotoxic, antioxidant, antifungal, and antibacterial bioactivities, which are crucial in drug development in the pharmaceutical industry [
3,
4]. Therefore, the ability of ELF to produce unique secondary metabolites with anti-cancer properties provides a novel opportunity to identify effective cancer therapeutics [
5,
6,
7]. Libertellenone T (
B) is a novel cytotoxic compound isolated from secondary metabolites extracted from the endolichenic fungus, EL000327, from
Pseudoplectania sp. found in the lichen
Graphis, collected from Hallasan in Jeju Island, South Korea in 2009.
Many chemotherapeutics exert cytotoxicity and trigger cancer cell death via inducing apoptosis in cells. Apoptosis, or programmed cell death, is a cell suicide process that activates when their continuous survival is blocked [
8]. Morphological and biochemical changes such as chromatin condensation, nuclear fragmentation, cell shrinkage, membrane blebbing, DNA, protein breakdown, and caspase activation can be observed in apoptotic cells. Cancer cells evade natural cell death as a result of genetic mutations acquired during transformation from normal cells to malignant cells. Disruption of the balance between pro-apoptotic and anti-apoptotic proteins, reduction in caspase function, and impairment of death receptor signaling all cause apoptosis resistance in cancer cells [
9]. Therefore, restoration of these functions by therapeutics can successfully prevent cancer progression. Furthermore, alterations that occur in cell cycle regulators at check points cause abnormal cell proliferation in cancers [
10]. Thus, the induction of G2/M phase arrest is another target of many anti-cancer agents. Controlling cell progression through the cell cycle by regulating related proteins or disrupting tubulin organization eventually leads cells to G2/M phase arrest and subsequent cell death [
11,
12].
Autophagy (macroautophagy) mainly involves the formation of an autophagosome by engulfing damaged organelles, fusion with lysosome, and degradation of cellular debris by lysosomal hydrolase [
13,
14]. Autophagy plays a paradoxical role in cancer progression. In the early stage of many cancers, autophagy acts as a tumor suppressor by protecting cell homeostasis. Conversely, autophagy promotes tumor growth in more advanced stages of cancer by increasing stress tolerance [
15]. Many anticancer therapeutics are known to activate autophagy concurrently with apoptosis. This activation of autophagy can either promote or suppress cancer cell survival. Therefore, the role of autophagy is crucial in anticancer drug development [
16]. Furthermore, the relationship between autophagy and apoptosis is unclear and yet to be investigated.
Reactive oxygen species (ROS) are highly reactive and short-lived small molecules in the form of free radicals [
17]. ROS support many physiological functions in cells under optimum conditions. However, the over production of ROS can have a deleterious effect on cells and trigger oxidative stress. The generation of ROS can be triggered by both endogenous and exogenous sources such as mitochondrial transport chain leakage, high metabolic rate, environmental pollutants, radiation, and drugs [
18]. ROS are responsible for activating many cellular signaling pathways that lead to cell survival or cell death including autophagy, apoptosis, and necrosis. c-Jun N-terminal kinase (JNK) signaling is activated in response to ER stress or activation of the mitochondrial pathway of apoptosis by ROS [
19].
In this study, we investigated the possible mechanisms underlying the impact of
B on CRC cells. We selected four CRC cell lines, HCT116, DLD1, HT29, and Caco2, harboring different genetic mutations and status of microsatellite instability, to evaluate the effect of
B on them. HCT116 is a microsatellite instable (MSI) cell line with KRAS and PIK3CA mutations. DLD1 is the MSI cell line, harboring KRAS, PIK3CA, and TP53 mutations. HT29 is microsatellite stable (MSS), and BRAF and PIK3CA are muted cell lines, while Caco2 is MSS and the wild type of KRAS PIK3CA, TP53 [
20]. Furthermore, we demonstrated that
B induced mitotic arrest, apoptosis, and autophagy via activating the ROS/JNK signaling pathway. In addition, we found that
B-induced autophagy promotes cell survival. Furthermore,
B exhibited synergy not only with the known anticancer drug
5-Fluorouracil (5-FU), but also with a novel compound D. Collectively, our data suggest that
B is a potential candidate as an anticancer therapeutic with clinical application.
2. Materials and Methods
2.1. Fungal Strain
Lichen specimens of
Graphis were collected from Hallasan in Jeju Island, South Korea in 2009. Voucher specimen was deposited in the Korean Lichen Research Institute, Sunchon National University, Korea. The endolichenic fungus EL000327 was isolated with the surface sterilization method [
21].
2.2. ITS Sequencing
EL000327 was cultured for 2–3 weeks on potato dextrose agar (PDA) medium at 25 °C. The total DNA was extracted following the manufacturer’s instructions from EL000327 using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Universal primers ITS1F (5’-CTTGGTCATTTAGAGGAAGTAA-3’) [
22] and LR5 (5’-ATCCTGAGGGAAACTTC-3’) [
23] were amplified with the internal transcribed spacer (ITS) region of the rDNA gene of EL000327 and ITS sequencing was performed as described [
5] (
Table S1).
2.3. Preparation of Secondary Metabolite Extract of EL000327
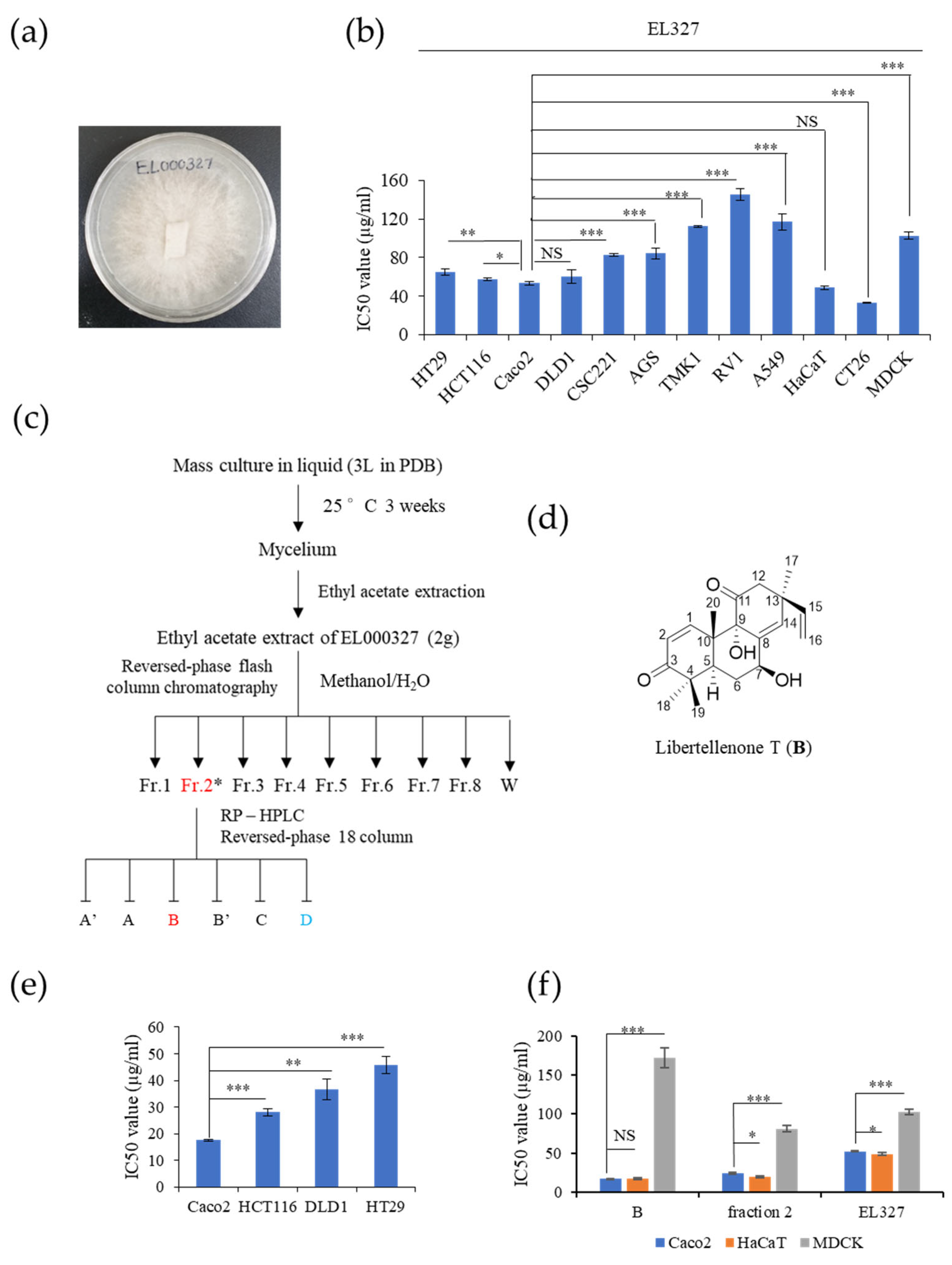
EL000327 was cultured on potato dextrose agar (PDA) medium at 25 °C for approximately 2–3 weeks until visible colonies were evident. ELF mycelia grown on agar were cut and inoculated into 200 mL potato dextrose broth (PDB) in 500 mL Erlenmeyer flasks (3 L) and incubated at 25 °C in a shaking incubator at 150 rpm for approximately 3–4 weeks. Then, 200 mL ethyl acetate (EA) was added to each flask, and the flask was shaken for approximately 2 h. Each culture was then filtered to separate the filtrate and mycelia. The filtrate was separated into water- and EA-soluble layers by allowing the filtrate to stand in a separating funnel. A total of 5.8 g of crude extracts of EL000327 was obtained by evaporating EA to dryness under a vacuum using a rotary evaporator. The crude extract was dissolved in 100% DMSO for use in experiments.
2.4. Isolation, Purification, and Identification of Chemical Structure of Compound B
The crude extract (5.8 g) was subjected to open column chromatography purification on a RP C18 flash column by the step gradient elution of methanol/H
2O from 20% to 100% of methanol, subsequently, to afford eight fractions (labeled 327-F1 ~ 327-F8). Fraction 327-F2 (360 mg) (H
2O:MeOH = 60:40) was purified by reversed-phase HPLC (Phenomenex Luna C-18 (2), 250 × 100 mm, 2.0 mL/min, 5 μm, 100 Å, UV = 254 nm) (
Figure S8) using an isocratic solvent system with 47% acetonitrile in water to yield 7β-9α-dihydroxy-1,8(14),15-pimaratrien-3,11-dione (B, 95 mg, purity: 96.7%) as pink oil.
1H NMR (400 MHz, CD
3OD)
δ: 7.25 (d,
J = 10.4 Hz, 1H), 6.02 (d,
J = 2.2 Hz, 1H), 5.93 (d,
J = 10.4 Hz, 1H), 5.74 (dd,
J = 17.2, 10.4 Hz, 1H), 4.99 (dd,
J = 17.3, 0.9 Hz, 1H), 4.95 (dd,
J = 10.4, 0.9 Hz, 1H), 4.40 (m, 1H), 1.29 (s, 3H), 1.20 (s, 3H), 1.18 (s, 3H);
13C NMR (100 MHz, CD
3OD)
δ: 212.1, 206.3, 159.5, 145.4, 141.5, 130.9, 128.8, 69.6, 54.0, 45.7, 45.3, 43.2, 42.9, 33.2, 28.5, 28.1, 22.8, 20.8. HR-FAB-MS
m/
z [M+H]
+ 331.1904 (calcd. for C
20H
27O
4, 331.1909) (
Figures S6 and S7).
2.5. Cell Culture
Human CRC cell lines HT29, HCT116, DLD1, Caco2, colon stemness cancer cell line; CSC221, human gastric cancer cell lines; AGS, TMK1, human prostate cancer cell line; RV1, human lung cancer cell line; A549, mouse colon cancer cell line; CT26 and canine kidney epithelial cell line; and MDCK were purchased from the Korean Cell Line Bank (Seoul, Korea). Cells were cultured in DMEM or RPMI culture medium (GenDEPOT, Katy, TX, USA) supplemented with 10% fetal bovine serum (FBS) (GenDEPOT, Katy, TX, USA) and 1% penicillin–streptomycin solution, and incubated in a humidified atmosphere at 37 °C in 5% CO2.
2.6. Cell Viability Assay
The viability of the cells was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay (Sigma-Aldrich, St. Louis, MO, USA). The 2 × 104–4 × 104 cells/mL were seeded in 96-well plates. After the attachment, cells were treated with different concentrations of B, EL000327, D, and 5-FU for 48 h in the presence or absence of various inhibitors (Z-VAD-FMK (10 µM) (R&D System, Inc, McKinley Place N.E, MN, USA), 3-MA (1mM), CQ (10 µM), NAC (5 mM) (Sigma-Aldrich, St. Louis, MO, USA), and SP600125 (10 µM) (Cell Signaling Technology, MA, USA). Cells treated with 0.01% of DMSO were used as the control. Next, 15 µL of the MTT reagent was added to each well and incubated for 4 h at 37 °C. The medium was aspirated completely and 150 µL of DMSO (Sigma-Aldrich, St. Louis, MO, USA) was added to the cells before the absorbance was measured at 540 nm by a microplate reader (Bio Tek Instruments, Winooskim, VT, USA) using Gen 5 (2.03.1) software. SPSS statistical software 23 was used for the IC50 calculation. Synergic effects of B with D or 5-FU were assessed by compuSyn software.
2.7. Cell Cycle Analysis by Flow Cytometry
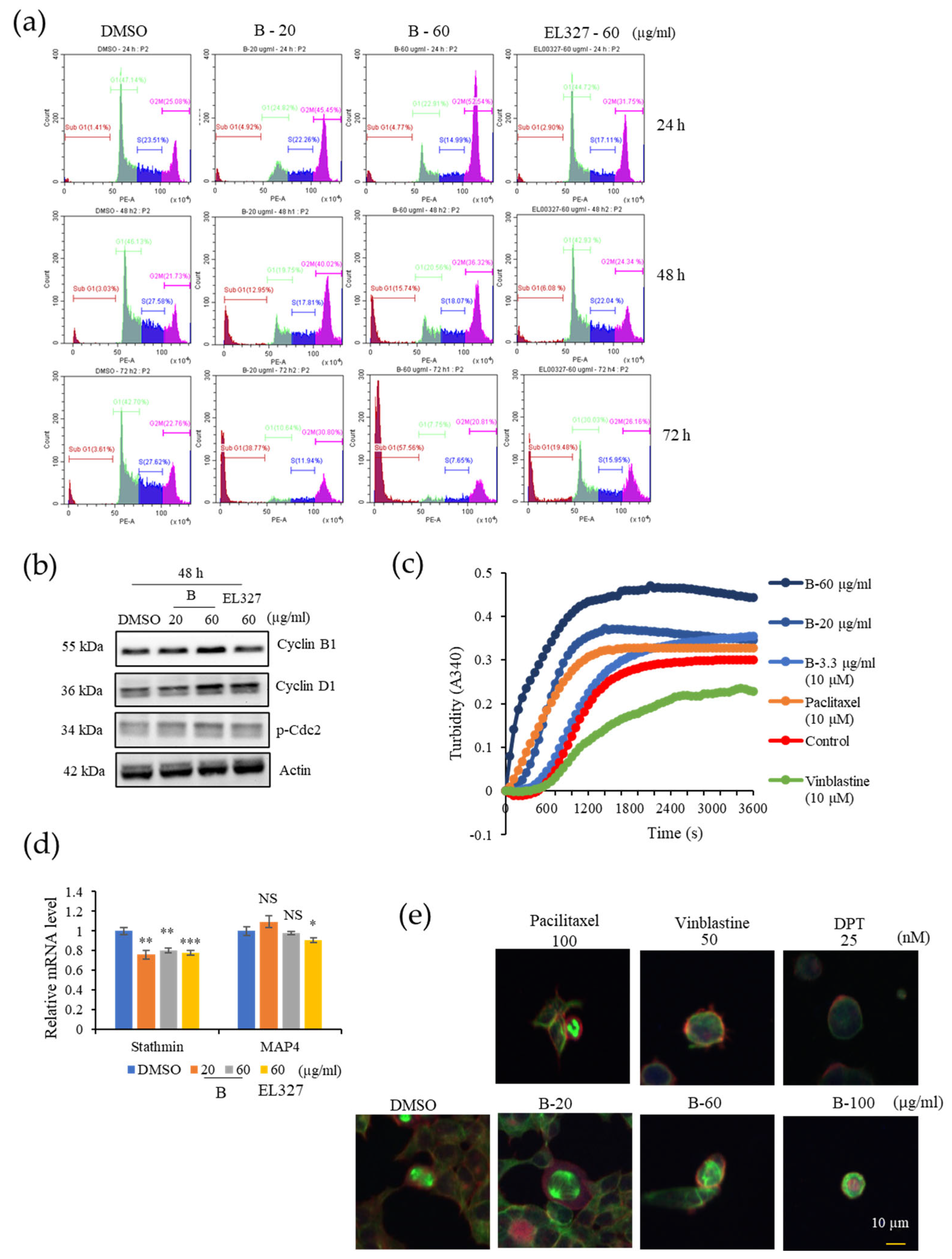
Caco2, HCT116, DLD1, and HT29 cells were seeded in 6-well plates at the density of 1.5–2 × 105 cells/well, incubated overnight, and treated with 0.01% of DMSO, various concentrations of B, and 60 µg/mL of EL000327 for 24 h, 48 h, or 72 h. Cells were harvested and washed with FACS washing buffer, incubated with trypsin solution, followed by RNase A for 10 min at room temperature. Cells were centrifuged and pellets were collected and stained with 100 mL of 4 mg/mL PI (Sigma-Aldrich, St. Louis, MO, USA) for 2 h in the dark at 4 °C. Cell cycle analysis was performed on a CytoFLEX instrument (Beckman Coulter Life Sciences, Indianapolis, IN, USA).
2.8. Western Blotting
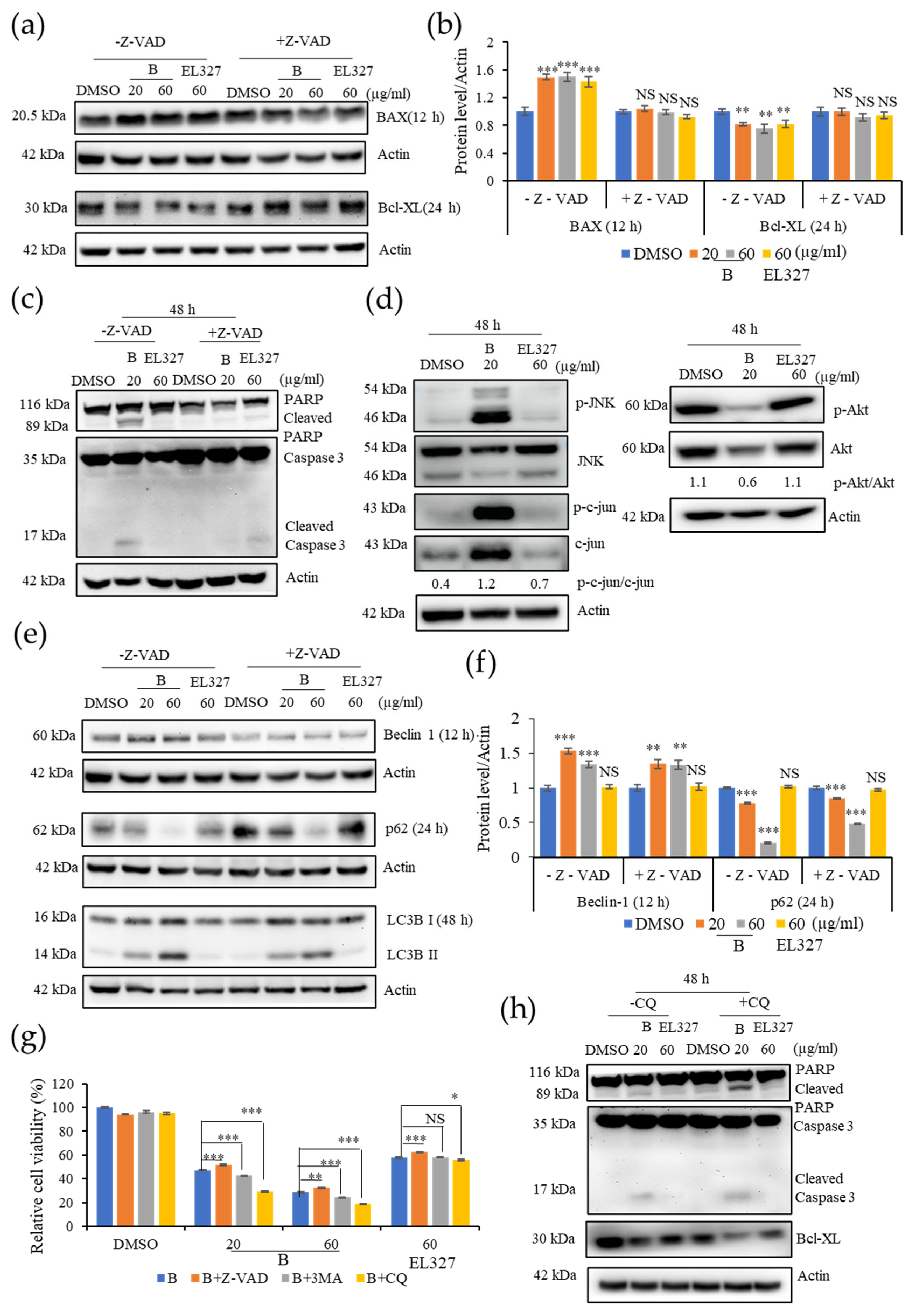
Caco2 cells were cultured in 6-well plates at the density of 2 × 105 cells/well overnight and treated with 0.01% of DMSO, different concentrations of B, and 60 µg/mL of EL000327 for 24 h or 48 h in the presence or absence of various inhibitors (Z-VAD-FMK (10 µM), 3-MA (1 mM), CQ (10 µM), NAC (5 mM), and SP600125 (10 µM). Cells were harvested and lysed, and the protein concentrations were determined by the BCA protein assay following the manufacturer’s instructions. Then, 25 or 50 µg of the total extract was separated by SDS-PAGE (12%) and transferred to a blotting membrane at 1.2 A for 6 h. Membranes were blocked with 5% of skim milk for 1 h followed by incubation with various primary antibodies (Cyclin B1, D1, p-Cdc2, BAX, Bcl-XL, PARP, caspase-3, Beclin 1, P-62, LC3B I/II, p-JNK, JNK, p-Akt, Akt, NF-κB, Actin purchased from Cell Signaling Technology, MA, USA) for 2 h at room temperature (RT). Blots were washed and incubated with horseradish peroxidase-conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, MA, USA) for 30 to 60 min at RT. Specific antibody binding was detected under chemiluminescence imaging (iBright FL1000 Imaging System, Thermo Fisher Sciences, biomolecular imager, Amersham ImageQuantTM 800 Western Blot Imaging System) and measured by Multi Gauge 3.0. software. Relative density was calculated against the density of the actin bands.
2.9. Tubulin Polymerization Assay
The effect of B on tubulin organization was detected using the Tubulin Polymerization Assay Kit (Cytoskeleton, Inc., Denver, CO, USA) according to the manufacturer’s instructions. In brief, tubulin proteins (>99% pure) were suspended at a final concentration of 3.0 mg/mL in ice-cold TP, and the tubulin solution was incubated at 37 °C with a general tubulin buffer with or without B (3.3, 20, 60 µg/mL). Paclitaxel and vinblastine (10 µM) were used as positive controls for the stabilization or destabilization of the microtubules, respectively. Tubulin polymerization was measured by continuously monitoring the change in turbidity at 340 nm by a microplate reader using Gen 5 (2.03.1) software.
2.10. Quantitative Real-Time PCR
Total RNA of 0.01% of DMSO, B (20, 60 µg/mL), and EL000327 (60 µg/mL) treated Caco2 cells were extracted using RNAiso Plus (TaKaRa, Kusatsu, Shiga, Japan) according to the manufacturer’s instructions. cDNA was reverse transcribed from 3 µg of total RNA of each treated group using the M-MLV Reverse Transcriptase Kit (Invitrogen, Carlsbad, CA, USA). mRNA levels of stathmin and MAP4 was measured using stathmin (forward) 5-GGTGGCGGCAGGACTTTCCTTATCCCAGTTGATT-3 and (reverse) 5- TTCTCGTGCTCTCGTTTCTCAGCCAGCTGCTTC-3; MAP4 (forward) 5-CCCTTTCTGAGGTAGCGTGCCTTGTGGAGGT -3 and (reverse) 5-CTGGCTCCCTCATGTTCTTGGCACAGCAGA-3 primers and SYBR green (Enzynomics). qRT-PCR reaction and analysis were performed using CFX (Bio-Rad, Hercules, CA, USA).
2.11. Immunofluorescence (IF) Imaging
Caco2 cells were cultured on cover slips at the density of 1 × 105 in a 12-well plate. After the adherence, cells were treated with 0.01% of DMSO, different concentrations of B, paclitaxel (100 nM), vinblastine (50 nM), and deoxyphodophyllotoxin (DPT) (25 nM) for 24 h. Cells were washed with phosphate-buffered saline (PBS) three times, followed by fixation with 4% paraformaldehyde in PBS for 10 min, permeabilization with 0.1% Triton™ X-100 for 10 min at RT, and blocking with 1% BSA in PBS for 1 h at RT. The cells were labeled with alpha tubulin (B-5-1-2) Alexa Fluor 488 Mouse Monoclonal Antibody, at 2 µg/mL in 0.1% BSA, and incubated for 3 h at RT. Cells were washed three times with PBS for 5 min after every step. Then, the cells were blocked again with blocking solution containing 1% BSA for 30–45 min at RT. Cells were stained again with fluorescent phalloidin staining solution and incubated for 30–60 min at RT. After washing with PBS, cover slips were mounted on glass slides with prolong gold with DAPI and left overnight at RT. Images were taken using a K1-Fluo Confocal Laser Scanning Microscope (Nanoscope Systems, Daejeon, Republic of Korea).
2.12. Hoechst Staining
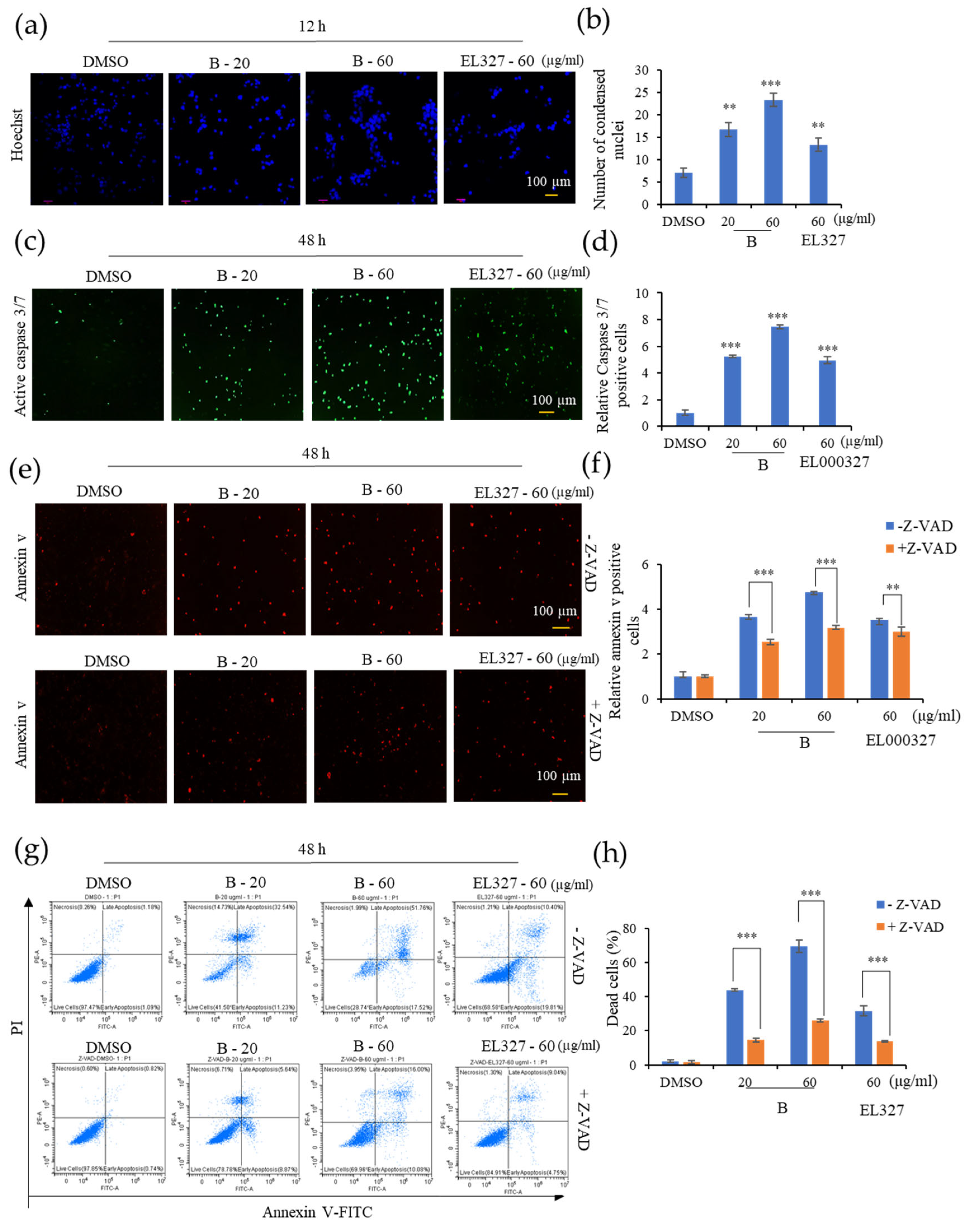
Caco2, HCT116, DLD1, and HT29 cells were seeded in a 12-well plate containing cover slips at a density of 1 × 105 cells/well. After overnight incubation, cells were treated with 0.01% of DMSO, B (20, 60 µg/mL), and EL000327 (60 µg/mL) for 12 or 24 h. Cells were washed with PBS, followed by fixation with 4% paraformaldehyde for 15 min. After washing again with PBS, cells were permeabilized in 0.1% Triton X-100 (Sigma-Aldrich) for 30 min, and stained with Hoechst 33258 (Sigma-Aldrich) for 1 h in the dark at room temperature. Cells were assessed by Nikon Eclipse 400 fluorescence microscope (Nikon Instech Co. Ltd., Kawasaki, Japan) to identify the morphological changers in the nuclei.
2.13. IncuCyte™ Caspase-3/7 and Annexin v Apoptosis Assay
Caco2 cells were seeded in a 96-well plate at a density of 2.5 × 103 cells/well. Cells were grown overnight to 25–30% confluence at the start of the assay. Media were supplemented with 5 μM of Caspase-3/7 (green) reagent (4440, Essen Bioscience, Morgan Rd, Ann Arbor, MI, USA) or Annexin V (red) reagent (4641, Essen Bioscience, Morgan Rd, Ann Arbor, MI, USA) diluted to 1:200 and added to the cells treated with 0.01% of DMSO, different concentrations of B, and EL000327 for 48 h in the presence or absence of Z-VAD-FMK (10 µM). The apoptosis of cells was determined by fluorescence scanning performed every 2 h for 48 h by the IncuCyte Zoom® instrument with a 10× objective and analyzed with the Standard Scan Type.
2.14. Apoptosis Analysis by Flow Cytometry
Caco2, HCT116, DLD1, and HT29 cells were cultured in a 6-well plate at the density of 2 × 105 cells/well until adherence. Cells were treated with 0.01% of DMSO, different concentrations of B, and EL000327 for 48 h in the presence or absence of Z-VAD-FMK (10 µM). Cells were harvested and washed with PBS, resuspended in 100 µL of 1x binding buffer followed by staining with 5 µL of 50 µg/mL propidium iodide (PI; BD Biosciences, San Jose, CA, USA) and 3 µL of Annexin V–FITC (BD, Biosciences, San Jose, CA, USA), for 30 min in the dark. Death cells were detected by flow cytometry on a CytoFLEX instrument (Beckman Coulter Life Sciences, Indianapolis, IN, USA).
2.15. Measurement of ROS Generation
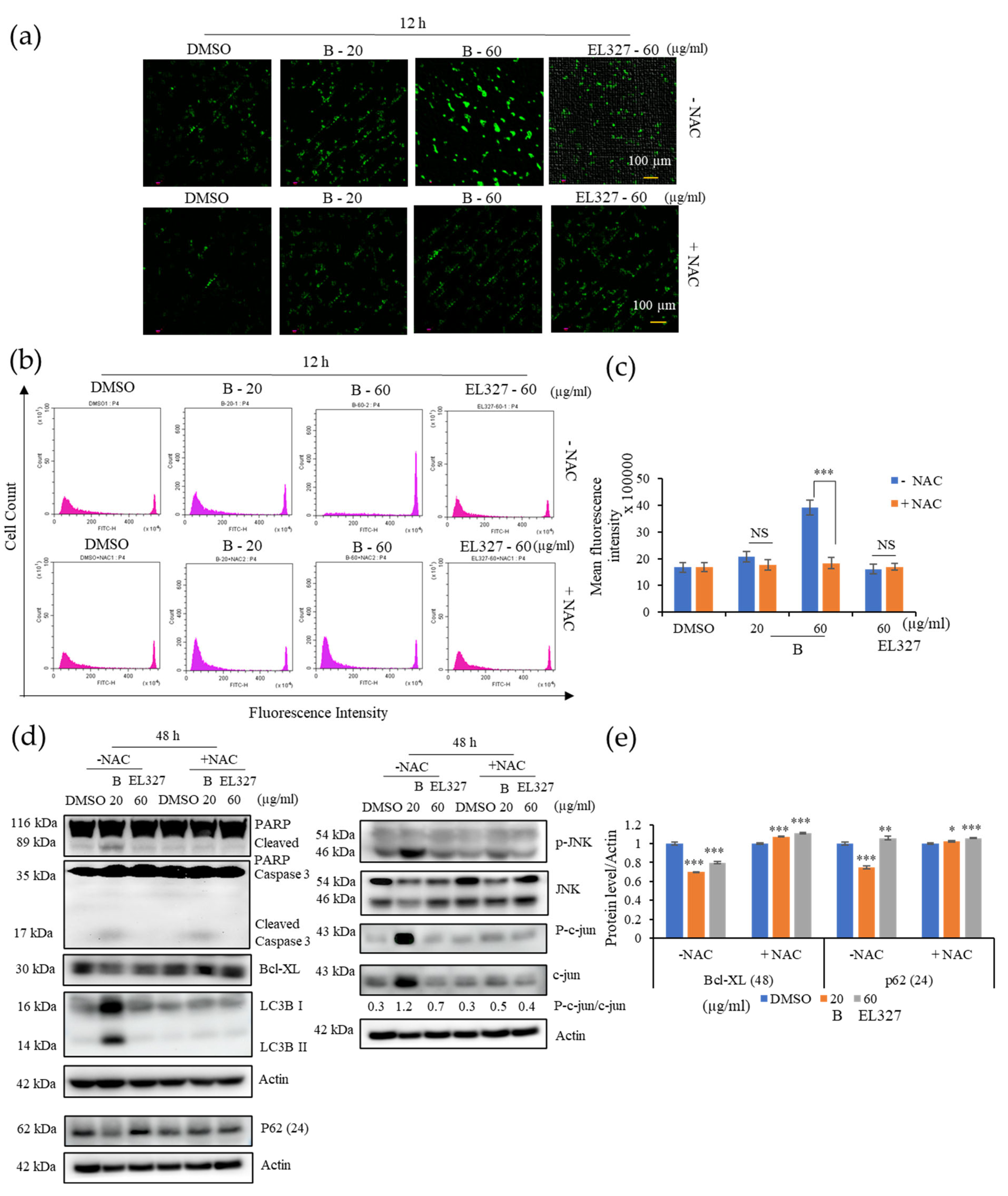
Caco2 cells were seeded in a 6-well plate at the density of 2 × 105 cells/well overnight and treated with 0.01% of DMSO, different concentrations of B, and EL000327 for 12 h in the presence or absence of NAC (5 mM). Cells were incubated with DCFH-DA (10 µM) in DMEM medium without FBS for 30 min at 37 °C and washed three times with DMEM. ROS generation was determined by fluorescence microscopy (K1-Fluo Confocal Laser Scanning Microscope, Nanoscope Systems, Daejeon, Republic of Korea) and flow cytometry (CytoFLEX; Beckman Coulter Life Sciences, Indianapolis, IN, USA) using peroxide-sensitive fluorescence probe DCFH-DA.
2.16. Statistical Analysis
All experiments were performed at least three times. Data are expressed as means ± standard deviation (SD). All statistical analyses were performed using Sigma Plot version 12.5. The Student’s t-test was used to compare the statistical significance between two groups. Unless indicated otherwise, a p-value < 0.05 was considered significant.
4. Discussion
Natural products have been highly recognized as rich reservoirs of bioactive compounds with the potential to lead to the discovery of novel anticancer therapeutics. As a result of the tremendous efforts by scientists, many naturally derived anticancer agents have been discovered and successfully developed into drugs within the last 30 years. Such compounds account for approximately 25% of newly approved anticancer drugs [
25]. Lichen substances and the secondary metabolites of endolichenic fungi have also been proven to have a wide range of anticancer activities against various types of cancers [
5,
26,
27,
28,
29,
30,
31]. Therefore, it is essential to carry out a thorough investigation of bioactive compounds derived from the rich bioresources of lichens. Libertellenone T
(B) is a novel cytotoxic compound, isolated as a pink oil from a secondary metabolite extract of the endolichenic fungi EL000327. Its molecular formula was determined to be C
20H
26O
4, based on HR-FAB-MS, coupled with the analysis of the NMR data. As the crude extract of EL000327 exerted comparatively high cytotoxicity on CRC, which is considered to be the second most lethal cancer type in the world [
32], the cytotoxicity of
B on CRC was assessed. Interestingly, the effect of
B on the CRC cells was much stronger than EL000327 and the Caco2 cells were highly sensitive to the treatment of
B compared to the HCT116, DLD1, and HT29 cells. Furthermore, the effect of
B on the non-cancerous cell line Madin–Darby canine kidney epithelial cells (MDCK) was very low and the human non-cancerous cell line HaCaT was similar to the Caco2 cells. In the current study, the mechanisms underlying
B-induced cell death were comprehensively investigated. We found that
B induced mitotic arrest in CRC cells by stabilizing microtubules and preventing their depolymerization. Moreover,
B activated apoptosis and autophagy via the ROS/JNK signaling pathway.
B-induced autophagy had a protective effect on Caco2 cells. Most importantly,
B showed synergy with the well-known chemotherapeutic 5-FU and another novel compound D isolated from the same crude extract of EL000327. Here, we mainly used Caco2 cells to study the mechanism of action of
B, but our results revealed that
B has an effect on other CRC cells such as HCT116, DLD1, and HT29. The induction of apoptosis in these cells upon the treatment of
B was confirmed by the results of Hoechst staining and flow cytometry.
Check points prevent cells with damaged DNA from entering the next phase of the cell cycle. This phenomenon is highly regulated by a series of proteins, and the G2/M transition is mainly regulated by the Cyclin B/Cdc2 (Cdk1) complex. Phosphorylation of Cdc2 negatively regulates cell cycle progression from the G2 phase to M phase [
11]. Furthermore, the Cyclin B/Cdk1 complex is highly activated in the metaphase as it supports the assembly of the mitotic apparatus and chromosome alignment. Once chromosomes are properly attached to spindles, the APC/C is activated and promotes progression to anaphase.
Degradation of cyclin B by the activation of the APC/C complex leads to Cdk1 inactivation [
33]. Flow cytometric analysis of cell cycle progression in our study indicated a clear accumulation of Caco2 cells in the G2/M phase than other CRC cell lines HCT116, DLD1, and HT29 after treatment with
B for 24 h. However, increasing G2/M cell populations compared to the control indicated that HCT116, DLD1, and HT29 cells require longer treatment to induce G2/M phase arrest. Furthermore, treatment with
B resulted in the upregulation of Cyclin B1 and p-Cdc-2 expression. Therefore, we continued our studies to investigate whether
B affected the microtubule dynamics in cells. According to the results of our in vitro tubulin polymerization assay,
B stabilized microtubules in a manner similar to the clinically approved microtubule stabilizer, paclitaxel. These results were further confirmed by the immunofluorescence staining of cells treated with
B and paclitaxel. Microtubule targeting agents in anticancer therapy can be classified into two groups based on their mode of action. Microtubule destabilizers prevent microtubule formation by inhibiting tubulin dimerization. In contrast, microtubule stabilizers promote tubulin dimerization and stabilize microtubules [
34]. Both the stabilization and destabilization of microtubules lead to mitotic catastrophe followed by cell death due to failure to form the spindle required for chromosome segregation in the M phase of the cell cycle. Similarly, our compound,
B, induced cell death after prolonged mitotic arrest, as shown by the appearance of a sub G1 population in cell cycle analysis.
Apoptosis is the process of programmed cell death and the most popular target of many anticancer therapies. Dysregulation of apoptosis signals in cancers promotes abnormal cell growth and tumorigenesis [
9]. Restoring the lost apoptotic function in cancer cells is the main objective of much of the research into cancer treatments. The initiation of apoptosis in cells can be identified by morphological changes such as nuclear fragmentation, chromatin condensation, cell shrinkage, and membrane blebbing [
35,
36]. Hoechst, Caspase 3/7, and Annexin V staining demonstrated the initiation of apoptosis in CRC cells upon treatment with
B. Apoptosis can be classed as either caspase-dependent or caspase-independent. Many anticancer agents mainly activate the caspase-dependent mitochondrial pathway. In addition, the death receptor mediated, and endoplasmic reticulum pathways also activate caspases at the final phase of apoptosis. Disruption of the mitochondrial membrane potential followed by translocation of AIF and endonuclease G to the nucleus induces caspase-independent apoptosis [
37]. Data from the IncuCyte apoptosis assay and flow cytometric analysis of apoptosis demonstrated that treatment with the caspase inhibitor Z-VAD-FMK significantly reduced the extent to which
B induced apoptosis in cells, indicating that apoptosis induced by
B is caspase-dependent. Furthermore, Western blot analysis showed the activation of the proapoptotic protein BAX, the anti-apoptotic protein Bcl-xL, and eventually, Caspase-3and PARP cleavage in response to
B treatment. Caspase-3 is the executioner caspase in all caspase dependent apoptosis pathways. Caspase-3 can be cleaved and activated by the upstream caspase, caspase-8, in the extrinsic pathway as well as by caspase-9, an initiating caspase, in the intrinsic pathway [
38]. Cleavage of PARP by caspases is considered as a hallmark of apoptosis and an indicator that caspase dependent apoptosis has been accomplished [
39]. Here, as predicted, Z-VAD-FMK treatment significantly impaired the expression of apoptotic markers in cells treated with
B. Interestingly, the expression of the autophagic proteins Beclin 1 and LC3BI/II slightly decreased while p62 expression slightly increased upon treatment with
B in the presence of Z-VAD-FMK. However, this result is not sufficient to assess the action of
B toward autophagy in the presence of Z-VAD-FMK.
Autophagy plays a dual role in cancer treatment by either supporting or preventing cancer cell survival. In the current study,
B induced autophagy in the Caco2 cells. Moreover, treatment with the autophagy inhibitors 3MA and CQ significantly increased
B-induced cell death, suggesting that the activation of autophagy promotes CRC cell survival. Western blot analysis further confirmed that the inhibition of autophagy enhanced the activation of apoptosis, as demonstrated by elevated cleaved caspase-3 and PARP levels in the presence of CQ. Beclin 1, P62, and LC3BI/II are key regulators of the autophagic process. Beclin 1 regulates autophagosome formation at the beginning of autophagy. During autophagosome formation, cytosolic LC3B-I is converted to the membrane-bound LC3B-II form. Binding of LC3B to the adapter protein p62/SQSTM1 facilitates autophagic degradation [
40]. In the present study, we observed increased the expressions of Beclin 1 and, LC3B-II and decreased p62 upon treatment with
B. While elevated expression of Beclin 1 and, LC3B is indicative of the activation of autophagy in CRC cells, generally, activation of autophagy results in reduced expression of p62. However, under some circumstances, p62 expression can be elevated by upregulation of p62 transcription during starvation of cells, regardless of the effect of autophagy. Under prolonged starvation, p62 expression is restored to basal levels by transcriptional regulation, even, when its expression has been decreased by autophagic activities at earlier time points [
41,
42]. Furthermore, p62 transcription is modulated by oxidative stress (Nrf2), the Ras/MAPK pathway, and the JNK/c-Jun pathway as well as some chemicals, including autophagy inducers [
43]. Following treatment with
B, cell viability was lower in the presence of CQ than 3MA. Furthermore, the level of LC3B-II was significantly increased upon treatment with CQ in western blot analysis. CQ inhibits autophagy by inhibiting fusion of the autophagosome and lysosome, and by degradation of the autophagolysosome [
44]. These results suggest that
B may induce autophagy by promoting late phase autophagy, fusion, and degradation. Moreover, the expression of autophagy related proteins was decreased when apoptosis was suppressed by Z-VAD-FMK. In contrast, expression of apoptotic markers was increased when autophagy was blocked by CQ. This leads to the hypothesis that
B predominantly induces apoptosis in CRC cells, and that autophagy is activated as a counter mechanism to protect the cells from apoptosis. However, this potential interconnection between activation of apoptosis and autophagy by
B needs further, detailed investigation.
Our study revealed that ROS generation is highly induced upon the treatment with B. Excess levels of ROS can be deleterious to cells due to the induction of oxidative stress within cells. Blocking ROS generation using the antioxidant NAC decreased expression of both apoptotic and autophagy markers in response to treatment with B, as demonstrated by western blot analysis. Furthermore, NAC significantly decreased the phosphorylation of JNK and expression of c-JUN. Given that ROS generation began after 12 h of treatment with B, while apoptotic markers were detected after 48 h, it seems likely that B induced apoptosis is initiated by ROS. ROS activate many signaling pathways (PI3K/Akt, MAPK, Nrf2) and transcription factors (NF-κB, p53) that eventually induce apoptosis, autophagy, or necrosis. Oxidants like OH•, ONOO−, and H2O induce apoptosis and/or necrosis, while O2•− and H2O2 induce autophagy and mostly trigger cell survival.
Cell viability upon treatment with
B was markedly increased in the presence of the NAC and JNK inhibitor, SP600125, while apoptosis was significantly decreased, according to flow cytometric and western blot analysis. Furthermore, levels of LC3B were also reduced in the presence of SP600125, indicating that autophagy was inhibited. Taken together, this suggests that JNK plays a significant role in the apoptosis and autophagy activated by treatment with
B. Induction of c-jun/JNK signaling by ROS blocks the antiapoptotic protein, Bcl-2, and activates the proapoptotic proteins in the Bcl-2 family, which are critical for the release of cytochrome c to the cytosol. Activation of caspase-9 and the effector caspase-3 by cytochrome c eventually leads to cell death via the mitochondrial apoptotic pathway. ROS can also activate the extrinsic apoptotic pathway by directly causing damage to DNA [
45]. Taken together, the above results suggest that
B induces apoptosis and autophagy in CRC via activation of ROS/JNK signaling (
Figure 8).
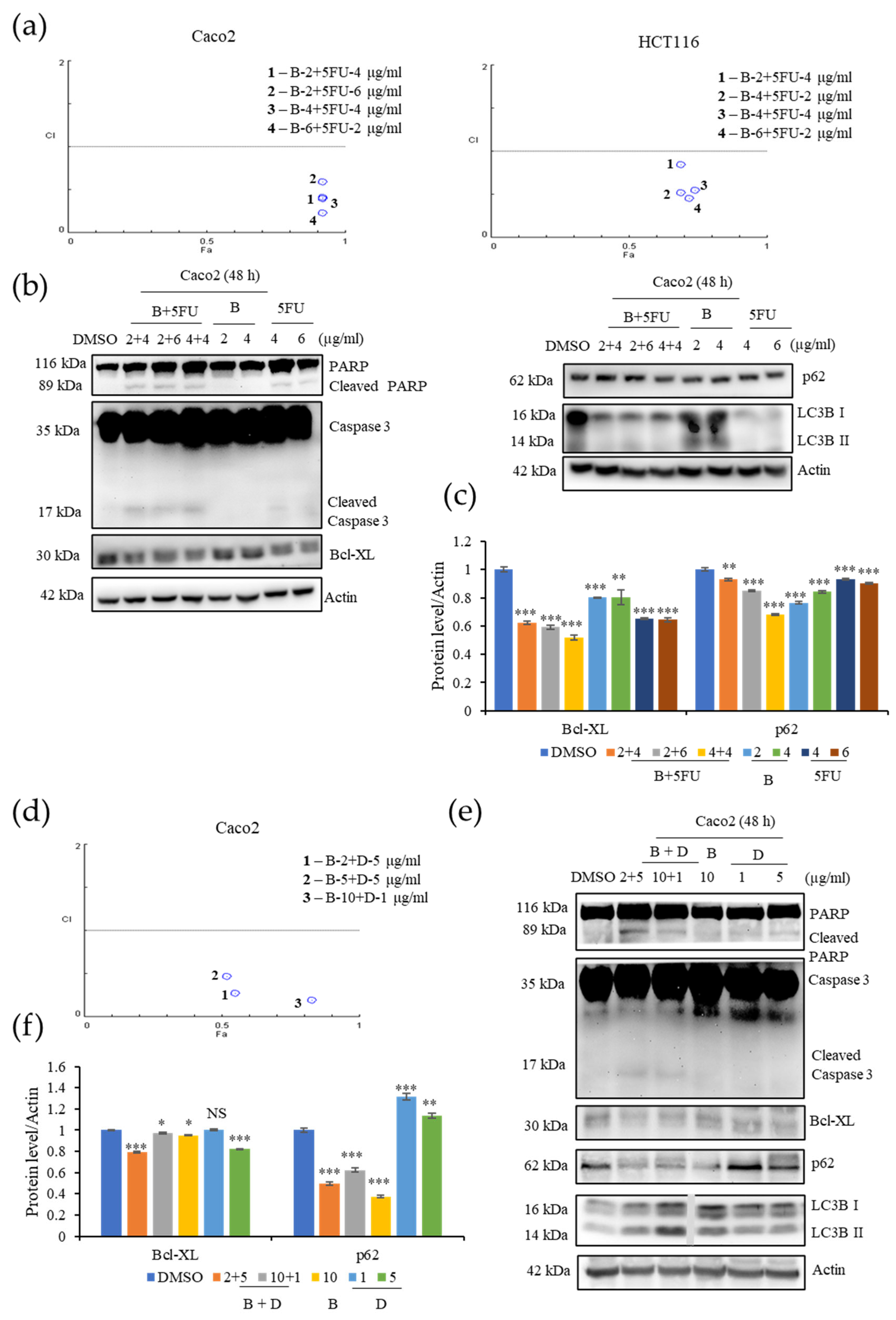
EL000327 is the crude extract from which B was isolated. This crude extract contained six compounds, including B and another highly cytotoxic compound, D. In our study, we compared the effect of our isolated compound on CRC cells with that of the crude extract, EL000327. Initiation of apoptosis upon treatment with EL000327 was detected by Hoechst staining, the incucyte apoptosis assay, and flow cytometric analysis of apoptosis. However, apoptotic markers were not clearly detected by western blot analysis at the same time points as B. Induction of cell cycle arrest, activation of autophagy, or the ROS/JNK pathway related proteins in Caco2 cells were also not observed upon treatment with EL000327. These results suggest that interactions between different compounds in the crude extract may alter the activity of EL000327. Alternatively, the effects of EL000327 and B may have been detected at different time points because EL000327 was either faster or slower to act than B.
5-Fluorouracil (
5-FU) is a first-line treatment for many cancers, including CRC. 5-FU leads cells to death by preventing DNA replication and RNA synthesis through inhibition of cellular thymidylate synthase (TS) [
46,
47]. Combination treatments in which 5-FU is combined with different anticancer agents, enhance the anticancer effect and response rate of these treatments. Similarly, combination of
B with 5-FU enhanced the cytotoxicity of
B toward Caco2 and HCT116 cells at significantly lower treatment concentrations [
48,
49]. Combination of
B and 5-FU induced apoptosis in Caco2 cells by enhancing the expression of cleaved caspase-3and PARP. Further investigations are required to understand the mechanism by which cytotoxicity is increased in the synergy between
B and 5-FU. Furthermore,
B exhibited excellent synergic effects with compound D. A combination of
B and D induced apoptosis and autophagy in the Caco2 cells at comparatively low concentrations. These results provide evidence that
B may act synergistically with a range of different anticancer agents to enhance their cytotoxic effect.
B is a novel naturally-derived compound, and this is the first study of the activity of B on CRC cells. We thoroughly investigated the mechanisms by which B induces apoptotic cell death and have laid the groundwork for a detailed analysis of the clinical usefulness of B. Moreover, we have demonstrated that the combination of B with known and novel anticancer agents may provide effective new treatment options for patients with CRC, which warrants further investigation.