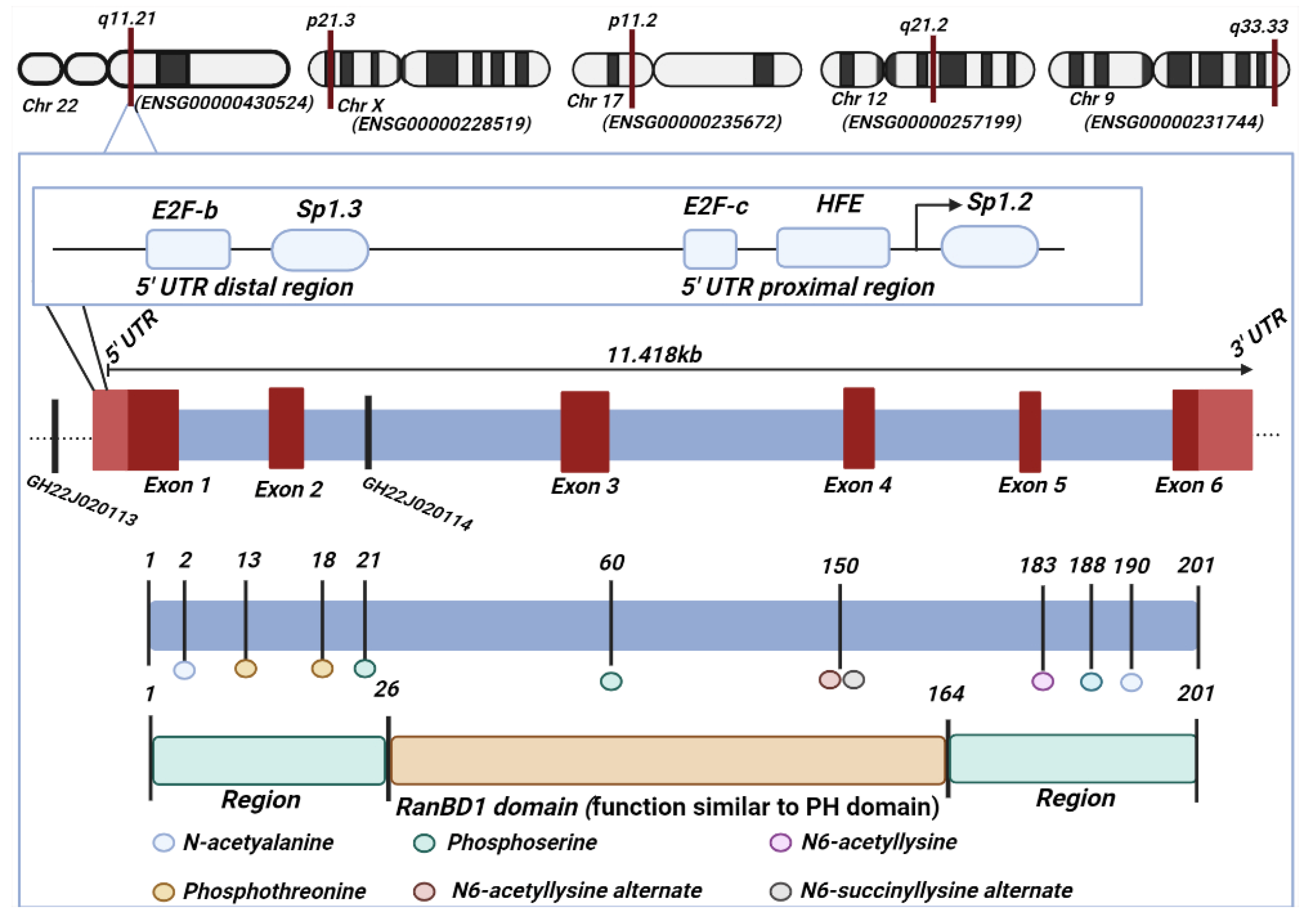
RANBP1 (RAN Binding Protein 1): The Missing Genetic Piece in Cancer Pathophysiology and Other Complex Diseases
Abstract
Simple Summary
Abstract
1. Introduction
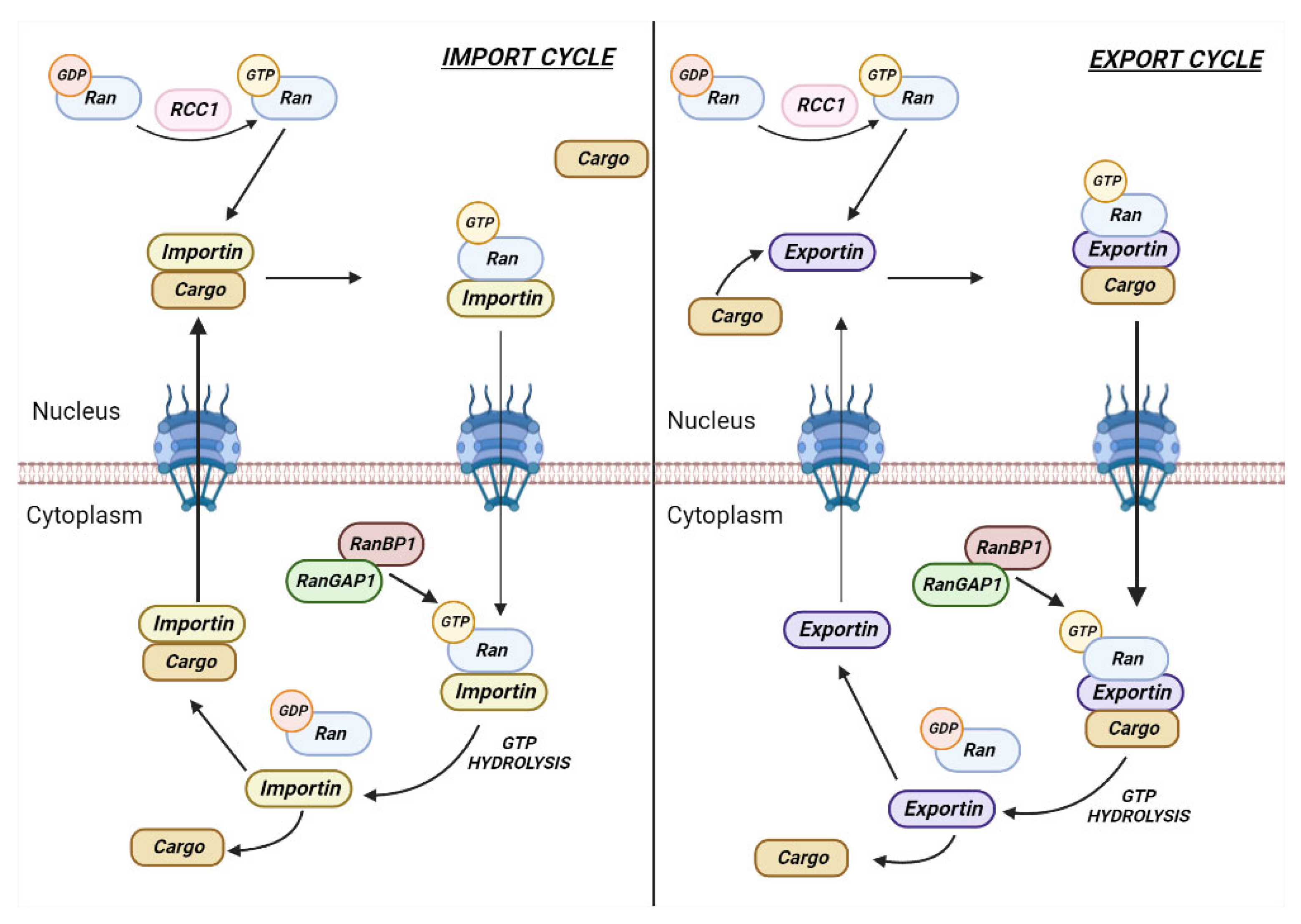
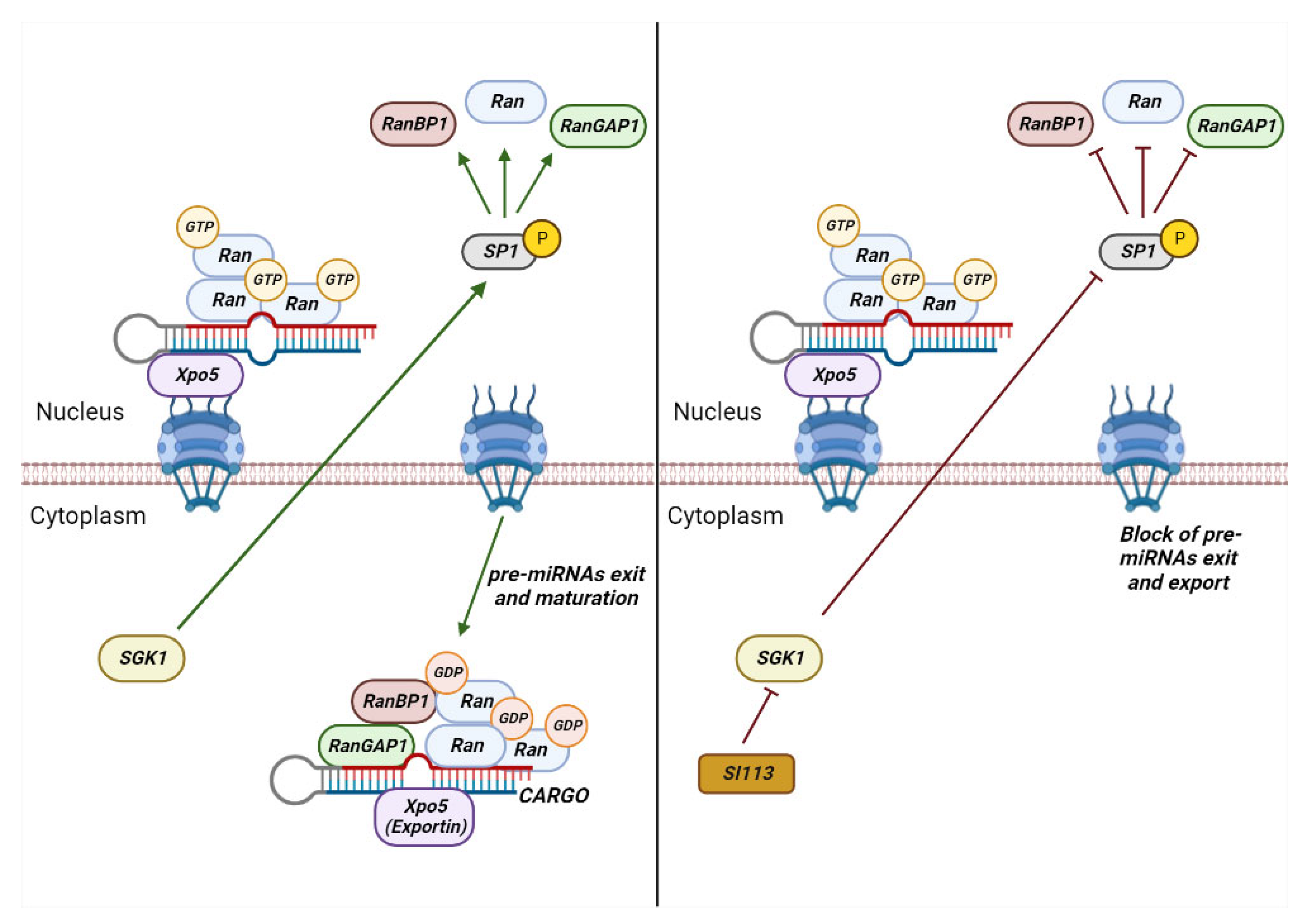
2. Role of RANBP1 in Nuclear Transport
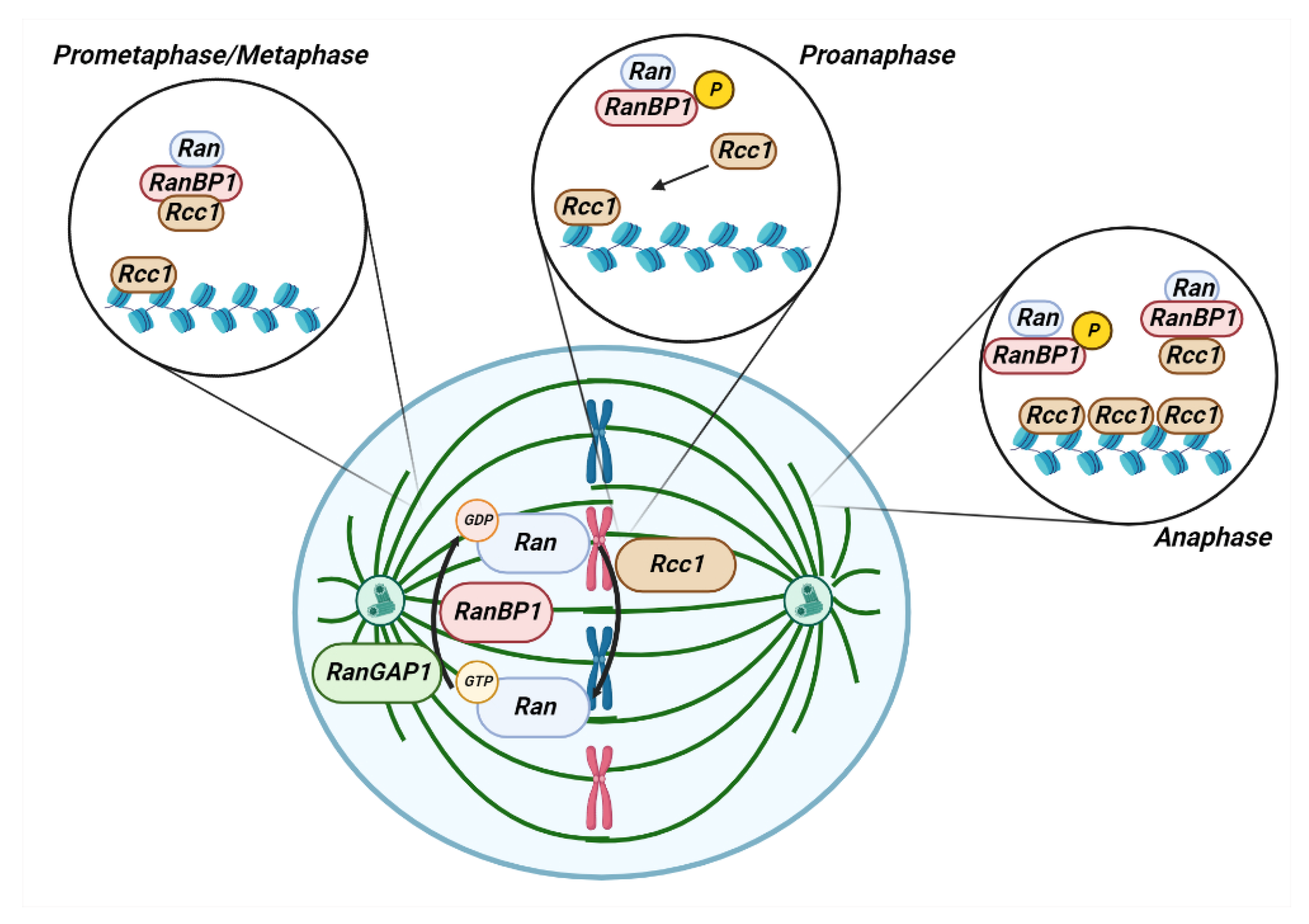
3. Role of RANBP1 in Mitotic Stability
4. Diseases Overview
4.1. RANBP1 and Neoplastic Diseases
4.2. RANBP1 and Central Nervous System Diseases
4.3. RANBP1 in Viral and Bacterial Lifecycle
4.4. RANBP1 in Teratogenesis and Infertility
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gene: RANBP1 (ENSG00000099901)-Summary-Homo_sapiens-GRCh37 Archive Browser 108. Available online: https://grch37.ensembl.org/Homo_sapiens/Gene/Summary?db=core;g=ENSG00000099901;r=22:20103461-20114878 (accessed on 9 November 2022).
- RANBP1 Gene-GeneCards | RANG Protein | RANG Antibody. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=RANBP1&keywords=RANBP1 (accessed on 9 November 2022).
- RANBP1-Ran-Specific GTPase-Activating Protein-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P43487/entry#P43487-1 (accessed on 9 November 2022).
- RANBP1 RAN Binding Protein 1 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/5902 (accessed on 8 November 2022).
- Plafker, K.; Macara, I.G. Facilitated Nucleocytoplasmic Shuttling of the Ran Binding Protein RanBP1. Mol. Cell. Biol. 2000, 20, 3510–3521. [Google Scholar] [CrossRef]
- Koyama, M.; Matsuura, Y. An Allosteric Mechanism to Displace Nuclear Export Cargo from CRM1 and RanGTP by RanBP1. EMBO J. 2010, 29, 2002–2013. [Google Scholar] [CrossRef]
- Bischoff, F.R.; Görlich, D. RanBP1 Is Crucial for the Release of RanGTP from Importin β-Related Nuclear Transport Factors. FEBS Lett. 1997, 419, 249–254. [Google Scholar] [CrossRef]
- Li, H.Y.; Ng, W.P.; Wong, C.H.; Iglesias, P.A.; Zheng, Y. Coordination of Chromosome Alignment and Mitotic Progression by the Chromosome-Based Ran Signal. Cell Cycle 2007, 6, 1886–1895. [Google Scholar] [CrossRef]
- Hayashi, N.; Yokoyama, N.; Seki, T.; Azuma, Y.; Ohba, T.; Nishimoto, T. RanBP1, a Ras-like Nuclear G Protein Binding to Ran/TC4, Inhibits RCC1 via Ran/TC4. Mol. Gen. Genet. 1995, 247, 661–669. [Google Scholar] [CrossRef]
- Bischoff, F.R.; Krebber, H.; Smirnova, E.; Dong, W.; Ponstingl, H. Co-Activation of RanGTPase and Inhibition of GTP Dissociation by Ran-GTP Binding Protein RanBP1. EMBO J. 1995, 14, 705–715. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Thul, P.J.; Akesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A Subcellular Map of the Human Proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An Atlas of the Protein-Coding Genes in the Human, Pig, and Mouse Brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A Single-Cell Type Transcriptomics Map of Human Tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef]
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A Genome-Wide Transcriptomic Analysis of Protein-Coding Genes in Human Blood Cells. Science 2019, 366, eaax9198. [Google Scholar] [CrossRef]
- Uhlén, M.; Karlsson, M.J.; Hober, A.; Svensson, A.S.; Scheffel, J.; Kotol, D.; Zhong, W.; Tebani, A.; Strandberg, L.; Edfors, F.; et al. The Human Secretome. Sci. Signal. 2019, 12, eaaz0274. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Min, S.; Zhang, Y.; Zhang, Y.; Zhou, Q.; Shen, X.; Jia, D.; Han, J.; Sun, Q. Distinct RanBP1 Nuclear Export and Cargo Dissociation Mechanisms between Fungi and Animals. Elife 2019, 8, e41331. [Google Scholar] [CrossRef]
- Lavia, P.; Macleod, D.; Bird, A. Coincident Start Sites for Divergent Transcripts at a Randomly Selected CpG-Rich Island of Mouse. EMBO J. 1987, 6, 2773–2779. [Google Scholar] [CrossRef]
- Ren, M.; Villamarin, A.; Shih, A.; Coutavas, E.; Moore, M.S.; Locurcio, M.; Clarke, V.; Oppenheim, J.D.; D’eustachio, P.; Rush, M.G. Separate Domains of the Ran GTPase Interact with Different Factors To Regulate Nuclear Protein Import and RNA Processing. Mol. Cell. Biol. 1995, 15, 2117–2124. [Google Scholar] [CrossRef]
- Gorlich, D.; Kostkat, S.; Kraftt, R.; Dingwall, C.; Laskey, R.A.; Hartmannt, E.; Prehn, S. Two Different Subunits of Importin Cooperate to Recognize Nuclear Localization Signals and Bind Them to the Nuclear Envelope. Curr. Biol. 1995, 5, 383–392. [Google Scholar] [CrossRef]
- Chi, N.C.; Adam, E.J.H.; Visser, G.D.; Adam, S.A. RanBP1 Stabilizes the Interaction of Ran with P97 in Nuclear Protein Import. J. Cell Biol. 1996, 135, 559–569. [Google Scholar] [CrossRef]
- Richards, S.A.; Lounsbury, K.M.; Carey, K.L.; Macara, I.G. A Nuclear Export Signal Is Essential for the Cytosolic Localization of the Ran Binding Protein, RanBP1. J. Cell Biol. 1996, 134, 1157–1168. [Google Scholar] [CrossRef]
- Melchior, F.; Gerace, L. Mechanisms of Nuclear Protein Import. Curr. Opin. Cell Biol. 1995, 7, 310–318. [Google Scholar] [CrossRef]
- Moroianu, J.; Blobel, G. Protein Export from the Nucleus Requires the GTPase Ran and GTP Hydrolysis. Proc. Natl. Acad. Sci. USA 1995, 92, 4318–4322. [Google Scholar] [CrossRef]
- Hieda, M.; Tachibana, T.; Yokoya, F.; Kose, S.; Imamoto, N.; Yoneda, Y. A Monoclonal Antibody to the COOH-Terminal Acidic Portion of Ran Inhibits Both the Recycling of Ran and Nuclear Protein Import in Living Cells. J. Cell Biol. 1999, 144, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Kuersten, S.; Ohno, M.; Mattaj, I.W. Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol. 2001, 11, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Clouse, K.N.; Luo, M.-J.; Zhou, Z.; Reed, R. A Ran-Independent Pathway for Export of Spliced MRNA. Nat. Cell. Biol. 2001, 3, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, V.; D’Antona, L.; Talarico, C.; Capula, M.; Catalogna, G.; Iuliano, R.; Schenone, S.; Roperto, S.; Bianco, C.; Perrotti, N.; et al. SGK1 Affects RAN/RANBP1/RANGAP1 via SP1 to Play a Critical Role in Pre-MiRNA Nuclear Export: A New Route of Epigenomic Regulation. Sci. Rep. 2017, 7, 45361. [Google Scholar] [CrossRef] [PubMed]
- Ozugergin, I.; Piekny, A. Complementary Functions for the Ran Gradient during Division. Small GTPases 2021, 12, 177–187. [Google Scholar] [CrossRef] [PubMed]
- di Matteo, G.; Fuschi, P.; Zerfass, K.; Moretti, S.; Ricordy, R.; Cenciarelli, C.; Tripodi, M.; Jansen-Durr, P.; Lavia, P.; Sapienza, L.; et al. Transcriptional Control of the Htf9-A/RanBP-1 Gene during the Cell Cycle. Cell Growth Differ. 1995, 6, 1213–1224. [Google Scholar]
- Battistoni, A.; Guarguaglini, G.; Degrassi, F.; Pittoggi, C.; Palena, A.; di Matteo, G.; Pisano, C.; Cundari, E.; Lavia, P. Deregulated Expression of the RanBP1 Gene Alters Cell Cycle Progression in Murine Fibroblasts. J. Cell Sci. 1997, 110, 2345–2357. [Google Scholar] [CrossRef]
- Guarguaglini, G.; Battistoni, A.; Pittoggi, C.; di Matteo, G.; di Fiore, B.; Lavia, P. Expression of the Murine RanBP1 and Htf9 Genes Is Regulated from a Shared Bidirectional Promoter during Cell Cycle Progression. Biochem. J. 1997, 325, 277–286. [Google Scholar] [CrossRef]
- di Fiore, B.; Guarguaglini, G.; Palena, A.; Kerkhoven, R.M.; Bernards, R.; Lavia, P. Two E2F Sites Control Growth-Regulated and Cell Cycle-Regulated Transcription of the Htf9-a/RanBP1 Gene through Functionally Distinct Mechanisms. J. Biol. Chem. 1999, 274, 10339–10348. [Google Scholar] [CrossRef]
- Yau, K.C.; Arnaoutov, A.; Aksenova, V.; Kaufhold, R.; Chen, S.; Dasso, M. RanBP1 Controls the Ran Pathway in Mammalian Cells through Regulation of Mitotic RCC1 Dynamics. Cell Cycle 2020, 19, 1899–1916. [Google Scholar] [CrossRef]
- Kalab, P.; Pu, R.T.; Dasso, M. The Ran GTPase Regulates Mitotic Spindle Assembly. Curr. Biol. 1999, 9, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Guarguaglini, G.; Renzi, L.; D’Ottavio, F.; Di Fiore, B.; Casenghi, M.; Cundari, E.; Lavia, P. Regulated Ran-Binding Protein 1 Activity Is Required for Organization and Function of the Mitotic Spindle in Mammalian Cells In Vivo. Cell Growth Differ. 2000, 11, 455–465. [Google Scholar] [PubMed]
- Arnaoutov, A.; Dasso, M. The Ran GTPase Regulates Kinetochore Function. Dev. Cell 2003, 5, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Hieda, M.; Katahira, J.; Yoneda, Y. Phosphorylation of RanGAP1 Stabilizes Its Interaction with Ran and RanBP1. Cell Struct. Funct. 2005, 30, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Ciciarello, M.; Mangiacasale, R.; Roscioli, E.; Rensen, W.M.; Lavia, P. RANBP1 Localizes a Subset of Mitotic Regulatory Factors on Spindle Microtubules and Regulates Chromosome Segregation in Human Cells. J. Cell Sci. 2007, 120, 3748–3761. [Google Scholar] [CrossRef]
- Ciciarello, M.; Roscioli, E.; di Fiore, B.; di Francesco, L.; Sobrero, F.; Bernard, D.; Mangiacasale, R.; Harel, A.; Schininà, M.E.; Lavia, P. Nuclear Reformation after Mitosis Requires Downregulation of the Ran GTPase Effector RanBP1 in Mammalian Cells. Chromosoma 2010, 119, 651–668. [Google Scholar] [CrossRef]
- Hwang, H.I.; Ji, J.H.; Jang, Y.J. Phosphorylation of Ran-Binding Protein-1 by Polo-like Kinase-1 Is Required for Interaction with Ran and Early Mitotic Progression. J. Biol. Chem. 2011, 286, 33012–33020. [Google Scholar] [CrossRef]
- Zhang, M.S.; Arnaoutov, A.; Dasso, M. RanBP1 Governs Spindle Assembly by Defining Mitotic Ran-GTP Production. Dev. Cell 2014, 31, 393–404. [Google Scholar] [CrossRef]
- Pujol, G.; Söderqvist, H.; Radu, A. Age-Associated Reduction of Nuclear Protein Import in Human Fibroblasts. Biochem. Biophys. Res. Commun. 2002, 294, 354–358. [Google Scholar] [CrossRef]
- di Fiore, B.; Ciciarello, M.; Mangiacasale, R.; Palena, A.; Tassin, A.-M.; Cundari, E.; Lavia, P. Mammalian RanBP1 Regulates Centrosome Cohesion during Mitosis. J. Cell Sci. 2003, 116, 3399–3411. [Google Scholar] [CrossRef]
- Wang, W.; Budhu, A.; Forgues, M.; Wang, X.W. Temporal and Spatial Control of Nucleophosmin by the Ran-Crm1 Complex in Centrosome Duplication. Nat. Cell Biol. 2005, 7, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Culjkovic-Kraljacic, B.; Baguet, A.; Volpon, L.; Amri, A.; Borden, K.L.B. The Oncogene EIF4E Reprograms the Nuclear Pore Complex to Promote MRNA Export and Oncogenic Transformation. Cell Rep. 2012, 2, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Etchin, J.; Sun, Q.; Kentsis, A.; Farmer, A.; Zhang, Z.C.; Sanda, T.; Mansour, M.R.; Barcelo, C.; McCauley, D.; Kauffman, M.; et al. Antileukemic Activity of Nuclear Export Inhibitors That Spare Normal Hematopoietic Cells. Leukemia 2013, 27, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Duan, X.; Li, Q.; Li, Q.; Wang, Y. High Expression of Ran Binding Protein 1 Predicts Poor Outcomes in Hepatocellular Carcinoma Patients: A Cancer Genome Atlas Database Analysis. J. Gastrointest. Oncol. 2021, 12, 2966–2984. [Google Scholar] [CrossRef] [PubMed]
- Pathria, G.; Wagner, C.; Wagner, S.N. Inhibition of CRM1-Mediated Nucleocytoplasmic Transport: Triggering Human Melanoma Cell Apoptosis by Perturbing Multiple Cellular Pathways. J. Investig. Dermatol. 2012, 132, 2780–2790. [Google Scholar] [CrossRef]
- Cáceres-Gorriti, K.Y.; Carmona, E.; Barrès, V.; Rahimi, K.; Létourneau, I.J.; Tonin, P.N.; Provencher, D.; Mes-Masson, A.M. RAN Nucleo-Cytoplasmic Transport and Mitotic Spindle Assembly Partners XPO7 and TPX2 Are New Prognostic Biomarkers in Serous Epithelial Ovarian Cancer. PLoS ONE 2014, 9, e91000. [Google Scholar] [CrossRef] [PubMed]
- Talarico, C.; Dattilo, V.; D’Antona, L.; Menniti, M.; Bianco, C.; Ortuso, F.; Alcaro, S.; Schenone, S.; Perrotti, N.; Amato, R. SGK1, the New Player in the Game of Resistance: Chemo-Radio Molecular Target and Strategy for Inhibition. Cell. Physiol. Biochem. 2016, 39, 1863–1876. [Google Scholar] [CrossRef]
- Amato, R.; Menniti, M.; Agosti, V.; Boito, R.; Costa, N.; Bond, H.M.; Barbieri, V.; Tagliaferri, P.; Venuta, S.; Perrotti, N. IL-2 Signals through Sgk1 and Inhibits Proliferation and Apoptosis in Kidney Cancer Cells. J. Mol. Med. 2007, 85, 707–721. [Google Scholar] [CrossRef]
- Amato, R.; D’Antona, L.; Porciatti, G.; Agosti, V.; Menniti, M.; Rinaldo, C.; Costa, N.; Bellacchio, E.; Mattarocci, S.; Fuiano, G.; et al. Sgk1 Activates MDM2-Dependent P53 Degradation and Affects Cell Proliferation, Survival, and Differentiation. J. Mol. Med. 2009, 87, 1221–1239. [Google Scholar] [CrossRef]
- Amato, R.; Scumaci, D.; D’Antona, L.; Iuliano, R.; Menniti, M.; di Sanzo, M.; Faniello, M.C.; Colao, E.; Malatesta, P.; Zingone, A.; et al. Sgk1 Enhances RANBP1 Transcript Levels and Decreases Taxol Sensitivity in RKO Colon Carcinoma Cells. Oncogene 2013, 32, 4572–4578. [Google Scholar] [CrossRef]
- Talarico, C.; D’Antona, L.; Scumaci, D.; Barone, A.; Gigliotti, F.; Fiumara, C.V.; Dattilo, V.; Gallo, E.; Visca, P.; Ortuso, F.; et al. Preclinical Model in HCC: The SGK1 Kinase Inhibitor SI113 Blocks Tumor Progression in Vitro and in Vivo and Synergizes with Radiotherapy. Oncotarget 2015, 6, 37511. [Google Scholar] [CrossRef]
- D’Antona, L.; Amato, R.; Talarico, C.; Ortuso, F.; Menniti, M.; Dattilo, V.; Iuliano, R.; Gigliotti, F.; Artese, A.; Costa, G.; et al. SI113, a Specific Inhibitor of the Sgk1 Kinase Activity That Counteracts Cancer Cell Proliferation. Cell. Physiol. Biochem. 2015, 35, 2006–2018. [Google Scholar] [CrossRef] [PubMed]
- Talarico, C.; Dattilo, V.; D’Antona, L.; Barone, A.; Amodio, N.; Belviso, S.; Musumeci, F.; Abbruzzese, C.; Bianco, C.; Trapasso, F.; et al. SI113, a SGK1 Inhibitor, Potentiates the Effects of Radiotherapy, Modulates the Response to Oxidative Stress and Induces Cytotoxic Autophagy in Human Glioblastoma Multiforme Cells. Oncotarget 2016, 7, 15868. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, C.; Matteoni, S.; Persico, M.; Ascione, B.; Schenone, S.; Musumeci, F.; Amato, R.; Perrotti, N.; Matarrese, P.; Paggi, M.G. The Small Molecule SI113 Hinders Epithelial-to-mesenchymal Transition and Subverts Cytoskeletal Organization in Human Cancer Cells. J. Cell. Physiol. 2019, 234, 22529–22542. [Google Scholar] [CrossRef] [PubMed]
- Conza, D.; Mirra, P.; Calì, G.; Tortora, T.; Insabato, L.; Fiory, F.; Schenone, S.; Amato, R.; Beguinot, F.; Perrotti, N.; et al. The SGK1 Inhibitor SI113 Induces Autophagy, Apoptosis, and Endoplasmic Reticulum Stress in Endometrial Cancer Cells. J. Cell. Physiol. 2017, 232, 3735–3743. [Google Scholar] [CrossRef]
- Catalogna, G.; Talarico, C.; Dattilo, V.; Gangemi, V.; Calabria, F.; D’Antona, L.; Schenone, S.; Musumeci, F.; Bianco, C.; Perrotti, N.; et al. The SGK1 Kinase Inhibitor SI113 Sensitizes Theranostic Effects of the 64CuCl2 in Human Glioblastoma Multiforme Cells. Cell. Physiol. Biochem. 2017, 43, 108–119. [Google Scholar] [CrossRef]
- D’Antona, L.; Dattilo, V.; Catalogna, G.; Scumaci, D.; Fiumara, C.V.; Musumeci, F.; Perrotti, G.; Schenone, S.; Tallerico, R.; Spoleti, C.B.; et al. In Preclinical Model of Ovarian Cancer, the SGK1 Inhibitor SI113 Counteracts the Development of Paclitaxel Resistance and Restores Drug Sensitivity. Transl. Oncol. 2019, 12, 1045–1055. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Catalogna, G.; Gallo, E.; di Martino, S.; Mileo, A.M.; Carosi, M.; Dattilo, V.; Schenone, S.; Musumeci, F.; Lavia, P.; et al. The Small Molecule SI113 Synergizes with Mitotic Spindle Poisons in Arresting the Growth of Human Glioblastoma Multiforme. Oncotarget 2017, 8, 110743–110755. [Google Scholar] [CrossRef]
- Zheng, D.; Cao, M.; Zuo, S.; Xia, X.; Zhi, C.; Lin, Y.; Deng, S.; Yuan, X. Correction: RANBP1 Promotes Colorectal Cancer Progression by Regulating Pre-MiRNA Nuclear Export via a Positive Feedback Loop with YAP. Oncogene 2022, 41, 930–942. [Google Scholar] [CrossRef]
- Grbčić, P.; Fučkar Čupić, D.; Gamberi, T.; Kraljević Pavelić, S.; Sedić, M. Proteomic Profiling of BRAFV600E Mutant Colon Cancer Cells Reveals the Involvement of Nucleophosmin/c-Myc Axis in Modulating the Response and Resistance to BRAF Inhibition by Vemurafenib. Int. J. Mol. Sci. 2021, 22, 6174. [Google Scholar] [CrossRef]
- Hilliard, M.; Frohnert, C.; Spillner, C.; Marcone, S.; Nath, A.; Lampe, T.; Fitzgerald, D.J.; Kehlenbach, R.H. The Anti-Inflammatory Prostaglandin 15-Deoxy-Δ12,14- PGJ2 Inhibits CRM1-Dependent Nuclear Protein Export. J. Biol. Chem. 2010, 285, 22202–22210. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chong, Y.; Liu, H.; Han, Y.; Niu, M. Novel Reversible Selective Inhibitor of CRM1 for Targeted Therapy in Ovarian Cancer. J. Ovarian Res. 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rensen, W.M.; Roscioli, E.; Tedeschi, A.; Mangiacasale, R.; Ciciarello, M.; di Gioia, S.A.; Lavia, P. RanBP1 Downregulation Sensitizes Cancer Cells to Taxol in a Caspase-3-Dependent Manner. Oncogene 2009, 28, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Nan, G.; Zhao, S.H.; Wang, T.; Chao, D.; Tian, R.F.; Wang, W.J.; Fu, X.; Lin, P.; Guo, T.; Wang, B.; et al. CD147 Supports Paclitaxel Resistance via Interacting with RanBP1. Oncogene 2022, 41, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Thangavelu, P.U.; Duijf, P.H.G. Overexpression of Ran GTPase Components Regulating Nuclear Export, but Not Mitotic Spindle Assembly, Marks Chromosome Instability and Poor Prognosis in Breast Cancer. Target. Oncol. 2016, 11, 677–686. [Google Scholar] [CrossRef]
- Mencarelli, C.; Nitarska, J.; Kroecher, T.; Ferraro, F.; Massey, K.; Riccio, A.; Pichaud, F. RanBP1 Couples Nuclear Export and Golgi Regulation through LKB1 to Promote Cortical Neuron Polarity. Cell Rep. 2018, 24, 2529–2539.e4. [Google Scholar] [CrossRef]
- Barriga, E.H.; Alasaadi, D.N.; Mencarelli, C.; Mayor, R.; Pichaud, F. RanBP1 Plays an Essential Role in Directed Migration of Neural Crest Cells during Development. Dev. Biol. 2022, 492, 79–86. [Google Scholar] [CrossRef]
- Maynard, T.M.; Haskell, G.T.; Bhasin, N.; Lee, J.M.; Gassman, A.A.; Lieberman, J.A.; LaMantia, A.-S. RanBP1, a Velocardiofacial/DiGeorge Syndrome Candidate Gene, Is Expressed at Sites of Mesenchymal/Epithelial Induction. Mech. Dev. 2002, 111, 177–180. [Google Scholar] [CrossRef]
- Orphanet. 22q11.2 Deletion Syndrome. Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=it&Expert=567 (accessed on 17 October 2022).
- Butcher, N.J.; Marras, C.; Pondal, M.; Rusjan, P.; Boot, E.; Christopher, L.; Repetto, G.M.; Fritsch, R.; Chow, E.W.C.; Masellis, M.; et al. Neuroimaging and Clinical Features in Adults with a 22q11.2 Deletion at Risk of Parkinson’s Disease. Brain 2017, 140, 1371–1383. [Google Scholar] [CrossRef]
- Yudin, D.; Fainzilber, M. Ran on Tracks-Cytoplasmic Roles for a Nuclear Regulator. J. Cell Sci. 2009, 122, 587–593. [Google Scholar] [CrossRef]
- Cheong, H.S.; Park, B.L.; Kim, E.M.; Park, C.S.; Sohn, J.W.; Kim, B.J.; Kim, J.W.; Kim, K.H.; Shin, T.M.; Choi, I.G.; et al. Association of RANBP1 Haplotype with Smooth Pursuit Eye Movement Abnormality. Am. J. Med.Genet. Part B Neuropsychiatr. Genet. 2011, 156, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Paronett, E.M.; Meechan, D.W.; Karpinski, B.A.; LaMantia, A.S.; Maynard, T.M. Ranbp1, Deleted in DiGeorge/22q11.2 Deletion Syndrome, Is a Microcephaly Gene That Selectively Disrupts Layer 2/3 Cortical Projection Neuron Generation. Cereb. Cortex 2015, 25, 3977–3993. [Google Scholar] [CrossRef] [PubMed]
- Wenger, T.L.; Kao, C.; McDonald-Mcginn, D.M.; Zackai, E.H.; Bailey, A.; Schultz, R.T.; Morrow, B.E.; Emanuel, B.S.; Hakonarson, H. The Role of MGluR Copy Number Variation in Genetic and Environmental Forms of Syndromic Autism Spectrum Disorder. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zolotukhin, A.S.; Felber, B.K. Mutations in the Nuclear Export Signal of Human Ran-Binding Protein RanBP1 Block the Rev-Mediated Posttranscriptional Regulation of Human Immunodeficiency Virus Type 1. J. Biol. Chem. 1997, 272, 11356–11360. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.W.; Tong, J.G.; Tom, S.K.; Szabo, P.A.; Cavanagh, C.; Dikeakos, J.D.; Barr, S.D. Interferon-Induced HERC5 Is Evolving under Positive Selection and Inhibits HIV-1 Particle Production by a Novel Mechanism Targeting Rev/RRE-Dependent RNA Nuclear Export. Retrovirology 2014, 11, 27. [Google Scholar] [CrossRef]
- Floer, M.; Blobel, G. Putative Reaction Intermediates in Crm1-Mediated Nuclear Protein Export. J. Biol. Chem. 1999, 274, 16279–16286. [Google Scholar] [CrossRef]
- Rothmeier, E.; Pfaffinger, G.; Hoffmann, C.; Harrison, C.F.; Grabmayr, H.; Repnik, U.; Hannemann, M.; Wölke, S.; Bausch, A.; Griffiths, G.; et al. Activation of Ran GTPase by a Legionella Effector Promotes Microtubule Polymerization, Pathogen Vacuole Motility and Infection. PLoS Pathog. 2013, 9, e1003598. [Google Scholar] [CrossRef]
- Hilbi, H.; Rothmeier, E.; Hoffmann, C.; Harrison, C.F. Beyond Rab GTPases Legionella Activates the Small GTPase Ran to Promote Microtubule Polymerization, Pathogen Vacuole Motility, and Infection. Small GTPases 2014, 5, e972859. [Google Scholar] [CrossRef]
- Mangos, S.; Vanderbeld, B.; Krawetz, R.; Sudol, K.; Kelly, G.M. Ran Binding Protein RanBP1 in Zebrafish Embryonic Development. Mol. Reprod. Dev. 2001, 59, 235–248. [Google Scholar] [CrossRef]
- Nagai, M.; Moriyama, T.; Mehmood, R.; Tokuhiro, K.; Ikawa, M.; Okabe, M.; Tanaka, H.; Yoneda, Y. Mice Lacking Ran Binding Protein 1 Are Viable and Show Male Infertility. FEBS Lett. 2011, 585, 791–796. [Google Scholar] [CrossRef]
- Davalieva, K.; Rusevski, A.; Velkov, M.; Noveski, P.; Kubelka-Sabit, K.; Filipovski, V.; Plaseski, T.; Dimovski, A.; Plaseska-Karanfilska, D. Comparative Proteomics Analysis of Human FFPE Testicular Tissues Reveals New Candidate Biomarkers for Distinction among Azoospermia Types and Subtypes. J. Proteom. 2022, 267, 104686. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audia, S.; Brescia, C.; Dattilo, V.; D’Antona, L.; Calvano, P.; Iuliano, R.; Trapasso, F.; Perrotti, N.; Amato, R. RANBP1 (RAN Binding Protein 1): The Missing Genetic Piece in Cancer Pathophysiology and Other Complex Diseases. Cancers 2023, 15, 486. https://doi.org/10.3390/cancers15020486
Audia S, Brescia C, Dattilo V, D’Antona L, Calvano P, Iuliano R, Trapasso F, Perrotti N, Amato R. RANBP1 (RAN Binding Protein 1): The Missing Genetic Piece in Cancer Pathophysiology and Other Complex Diseases. Cancers. 2023; 15(2):486. https://doi.org/10.3390/cancers15020486
Chicago/Turabian StyleAudia, Salvatore, Carolina Brescia, Vincenzo Dattilo, Lucia D’Antona, Pierluigi Calvano, Rodolfo Iuliano, Francesco Trapasso, Nicola Perrotti, and Rosario Amato. 2023. "RANBP1 (RAN Binding Protein 1): The Missing Genetic Piece in Cancer Pathophysiology and Other Complex Diseases" Cancers 15, no. 2: 486. https://doi.org/10.3390/cancers15020486
APA StyleAudia, S., Brescia, C., Dattilo, V., D’Antona, L., Calvano, P., Iuliano, R., Trapasso, F., Perrotti, N., & Amato, R. (2023). RANBP1 (RAN Binding Protein 1): The Missing Genetic Piece in Cancer Pathophysiology and Other Complex Diseases. Cancers, 15(2), 486. https://doi.org/10.3390/cancers15020486







