The Role of Pharmacotherapy in Treatment of Meningioma: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
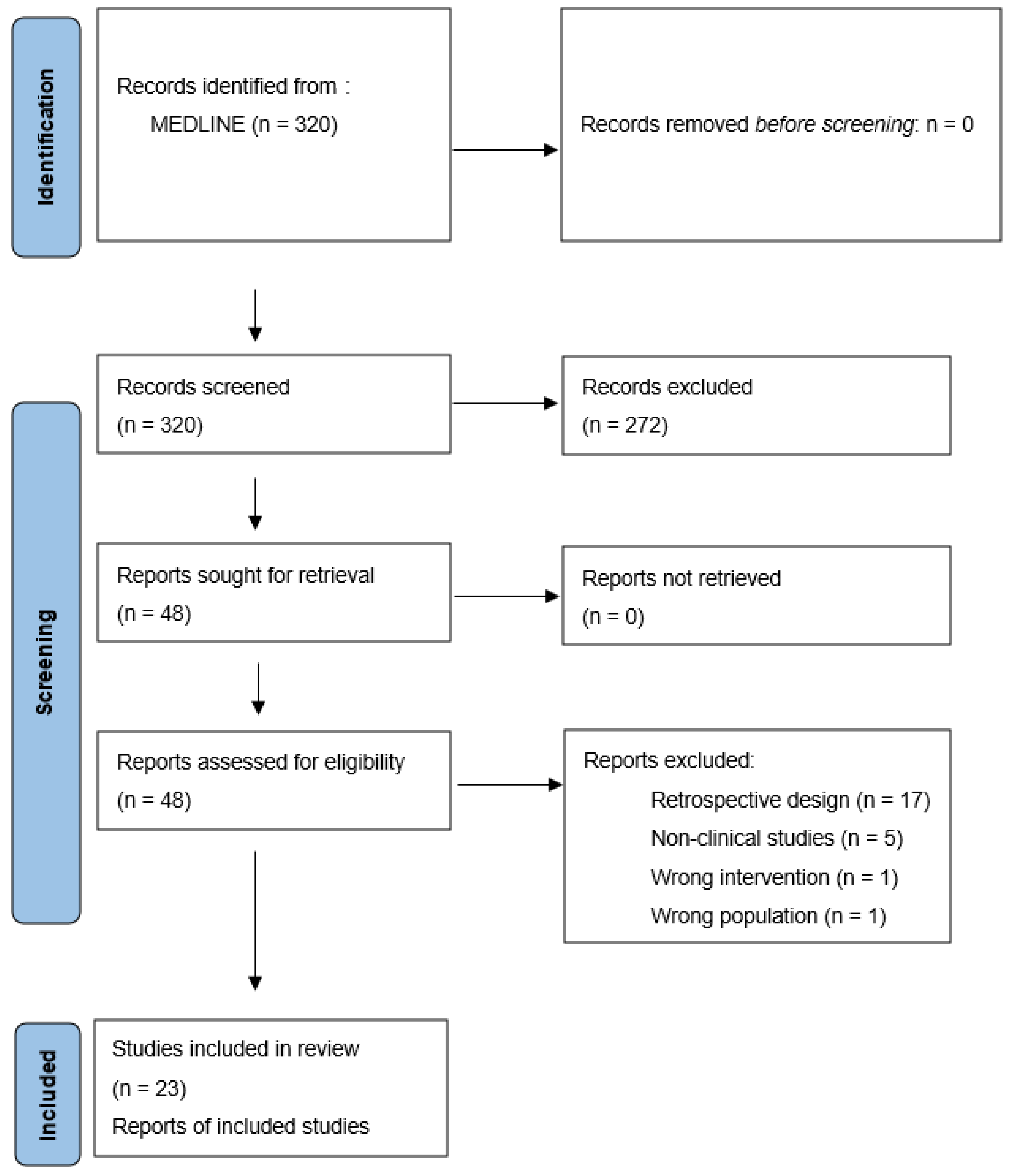
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
3. Results
3.1. Completed Clinical Trials
3.1.1. Study Characteristics
3.1.2. Outcomes
3.2. Ongoing Clinical Trials
3.2.1. Status and Coordination
3.2.2. Trial Design
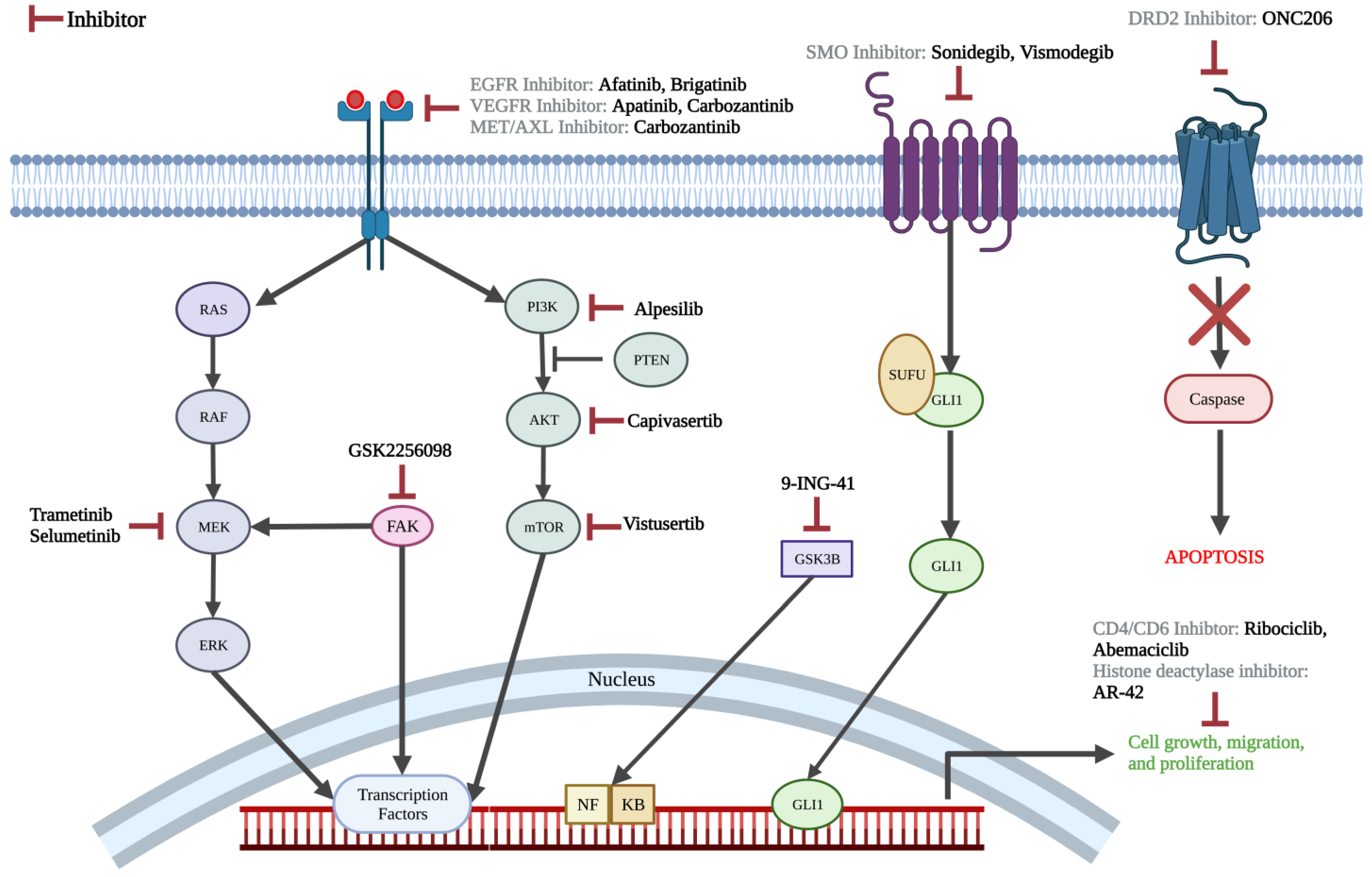
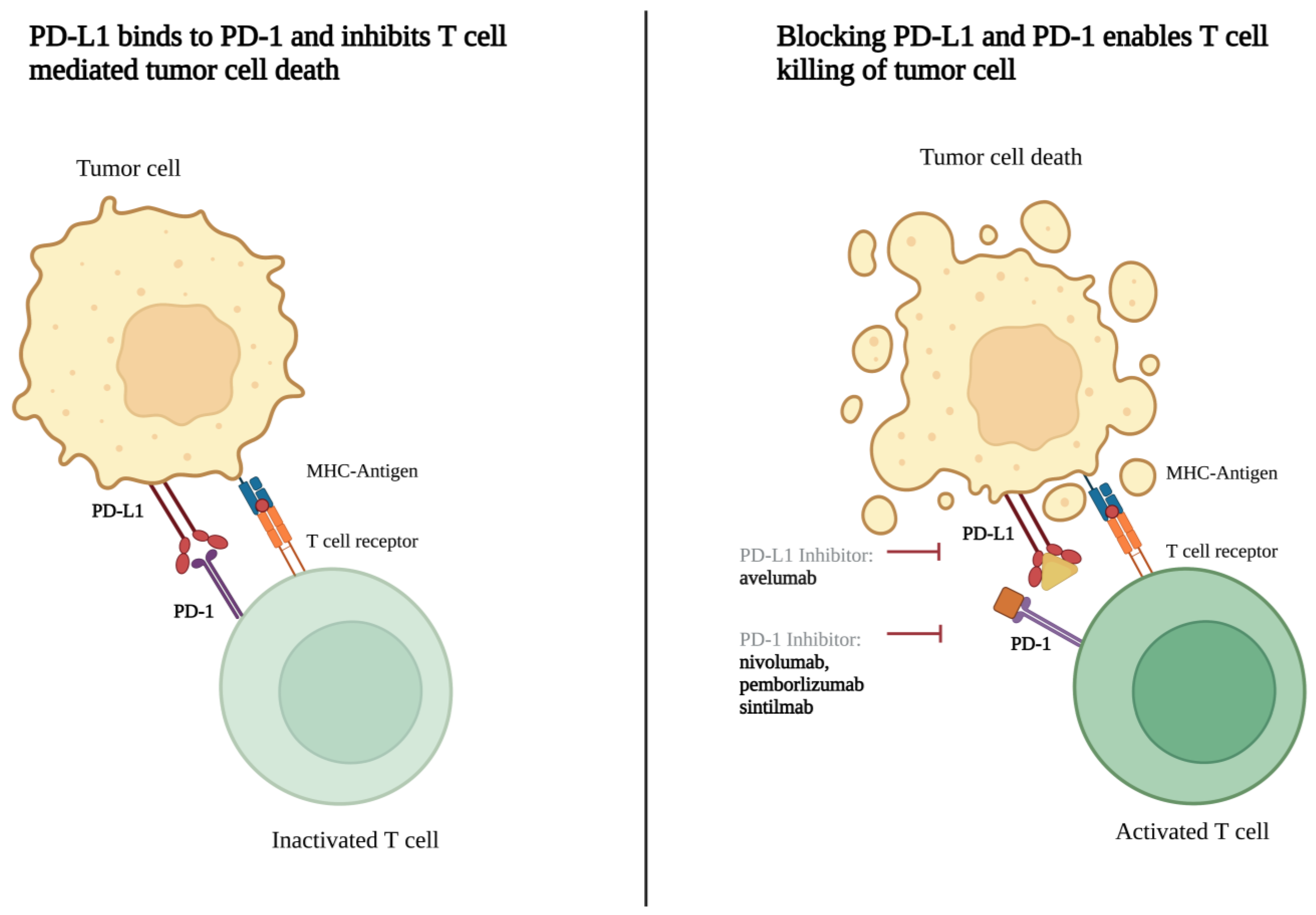
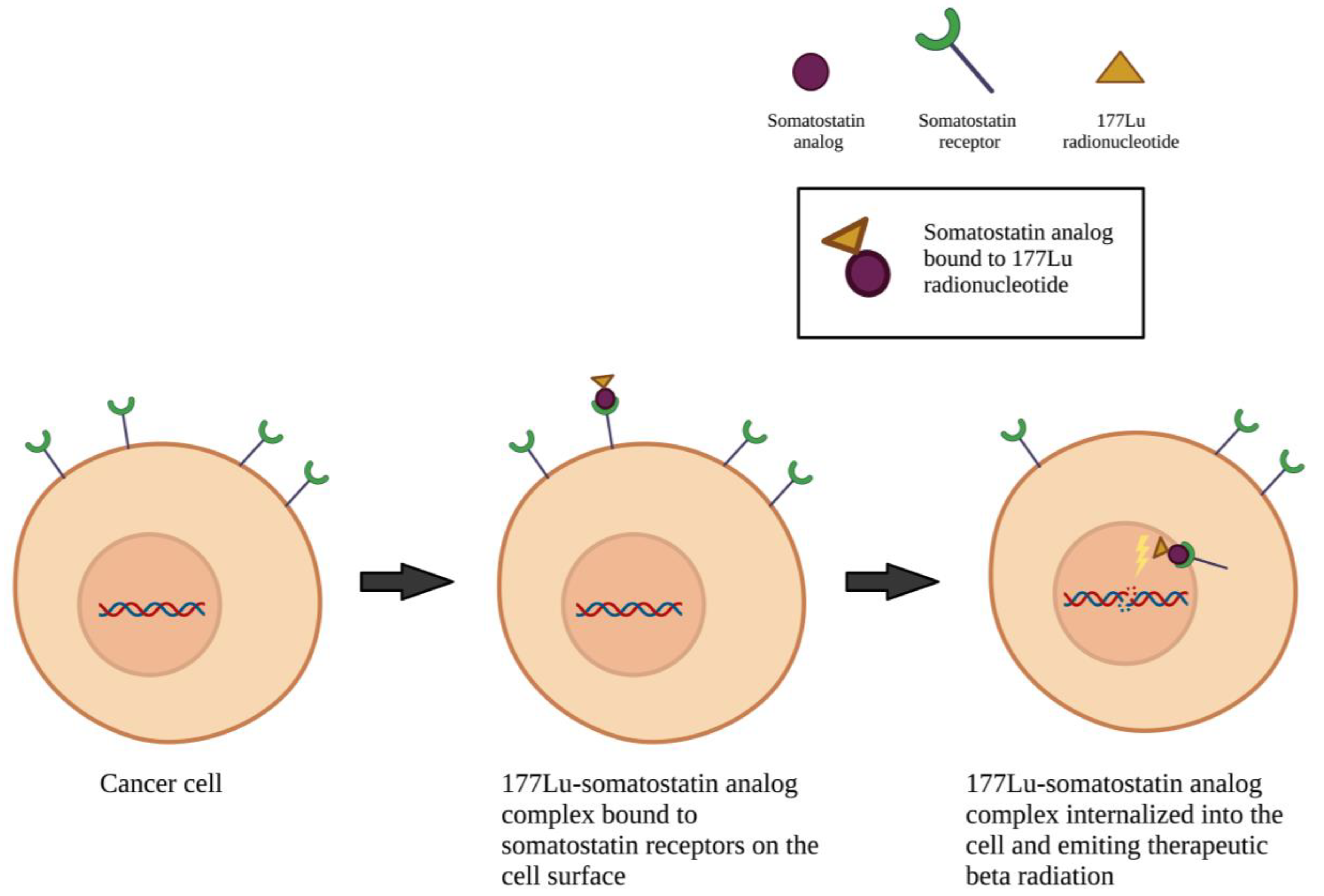
3.2.3. Pharmacotherapy Targets
4. Discussion
4.1. Completed Clinical Trials
4.2. On-Going Clinical Trials
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge Base, Treatment Outcomes, and Uncertainties. A RANO Review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO Guidelines for the Diagnosis and Treatment of Meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A Clinically Applicable Integrative Molecular Classification of Meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Stichel, D.; Hielscher, T.; Sievers, P.; Berghoff, A.S.; Schrimpf, D.; Sill, M.; Euskirchen, P.; Blume, C.; Patel, A.; et al. Integrated Molecular-Morphologic Meningioma Classification: A Multicenter Retrospective Analysis, Retrospectively and Prospectively Validated. J. Clin. Oncol. 2021, 39, 3839–3852. [Google Scholar] [CrossRef]
- Bayley, J.C.; Hadley, C.C.; Harmanci, A.O.; Harmanci, A.S.; Klisch, T.J.; Patel, A.J. Multiple Approaches Converge on Three Biological Subtypes of Meningioma and Extract New Insights from Published Studies. Sci. Adv. 2022, 8, eabm6247. [Google Scholar] [CrossRef]
- Patel, A.J.; Wan, Y.-W.; Al-Ouran, R.; Revelli, J.-P.; Cardenas, M.F.; Oneissi, M.; Xi, L.; Jalali, A.; Magnotti, J.F.; Muzny, D.M.; et al. Molecular Profiling Predicts Meningioma Recurrence and Reveals Loss of DREAM Complex Repression in Aggressive Tumors. Proc. Natl. Acad. Sci. USA 2019, 116, 21715–21726. [Google Scholar] [CrossRef]
- Magill, S.T.; Vasudevan, H.N.; Seo, K.; Villanueva-Meyer, J.E.; Choudhury, A.; John Liu, S.; Pekmezci, M.; Findakly, S.; Hilz, S.; Lastella, S.; et al. Multiplatform Genomic Profiling and Magnetic Resonance Imaging Identify Mechanisms Underlying Intratumor Heterogeneity in Meningioma. Nat. Commun. 2020, 11, 4803. [Google Scholar] [CrossRef]
- Choudhury, A.; Magill, S.T.; Eaton, C.D.; Prager, B.C.; Chen, W.C.; Cady, M.A.; Seo, K.; Lucas, C.-H.G.; Casey-Clyde, T.J.; Vasudevan, H.N.; et al. Meningioma DNA Methylation Groups Identify Biological Drivers and Therapeutic Vulnerabilities. Nat. Genet. 2022, 54, 649–659. [Google Scholar] [CrossRef]
- Euskirchen, P.; Peyre, M. Management of Meningioma. Presse Med. 2018, 47, e245–e252. [Google Scholar] [CrossRef]
- Apra, C.; Peyre, M.; Kalamarides, M. Current Treatment Options for Meningioma. Expert Rev. Neurother. 2018, 18, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Osswald, M. Meningiomas: Overview and New Directions in Therapy. Semin. Neurol. 2018, 38, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Messerer, M.; Richoz, B.; Cossu, G.; Dhermain, F.; Hottinger, A.F.; Parker, F.; Levivier, M.; Daniel, R.T. Recent Advances in the Management of Atypical Meningiomas. Neurochirurgie 2016, 62, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Schiff, D. Multimodality Therapy of Patients with Refractory Meningiomas. Curr Treat. Options Oncol. 2019, 20, 50. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.J.; Abrey, L.E.; Lassman, A.B.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Yung, W.K.A.; Gilbert, M.R.; Aldape, K.D.; Wen, P.Y. A Phase I Trial of Erlotinib in Patients with Nonprogressive Glioblastoma Multiforme Postradiation Therapy, and Recurrent Malignant Gliomas and Meningiomas. Neuro Oncol 2010, 12, 87–94. [Google Scholar] [CrossRef]
- Markwalder, T.M.; Seiler, R.W.; Zava, D.T. Antiestrogenic Therapy of Meningiomas--a Pilot Study. Surg. Neurol. 1985, 24, 245–249. [Google Scholar] [CrossRef]
- Jääskeläinen, J.; Laasonen, E.; Kärkkäinen, J.; Haltia, M.; Troupp, H. Hormone Treatment of Meningiomas: Lack of Response to Medroxyprogesterone Acetate (MPA). A Pilot Study of Five Cases. Acta Neurochir. 1986, 80, 35–41. [Google Scholar] [CrossRef]
- Grunberg, S.M.; Weiss, M.H. Lack of Efficacy of Megestrol Acetate in the Treatment of Unresectable Meningioma. J. Neurooncol. 1990, 8, 61–65. [Google Scholar] [CrossRef]
- Grunberg, S.M.; Weiss, M.H.; Spitz, I.M.; Ahmadi, J.; Sadun, A.; Russell, C.A.; Lucci, L.; Stevenson, L.L. Treatment of Unresectable Meningiomas with the Antiprogesterone Agent Mifepristone. J. Neurosurg. 1991, 74, 861–866. [Google Scholar] [CrossRef]
- Goodwin, J.W.; Crowley, J.; Eyre, H.J.; Stafford, B.; Jaeckle, K.A.; Townsend, J.J. A Phase II Evaluation of Tamoxifen in Unresectable or Refractory Meningiomas: A Southwest Oncology Group Study. J. Neurooncol. 1993, 15, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Adjuvant Combined Modality Therapy for Malignant Meningiomas. J. Neurosurg. 1996, 84, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Schrell, U.M.; Rittig, M.G.; Anders, M.; Koch, U.H.; Marschalek, R.; Kiesewetter, F.; Fahlbusch, R. Hydroxyurea for Treatment of Unresectable and Recurrent Meningiomas. II. Decrease in the Size of Meningiomas in Patients Treated with Hydroxyurea. J. Neurosurg. 1997, 86, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Newton, H.B.; Slivka, M.A.; Stevens, C. Hydroxyurea Chemotherapy for Unresectable or Residual Meningioma. J. Neurooncol. 2000, 49, 165–170. [Google Scholar] [CrossRef]
- Muhr, C.; Gudjonsson, O.; Lilja, A.; Hartman, M.; Zhang, Z.J.; Långström, B. Meningioma Treated with Interferon-Alpha, Evaluated with [(11)C]-L-Methionine Positron Emission Tomography. Clin. Cancer Res. 2001, 7, 2269–2276. [Google Scholar]
- Mason, W.P.; Gentili, F.; Macdonald, D.R.; Hariharan, S.; Cruz, C.R.; Abrey, L.E. Stabilization of Disease Progression by Hydroxyurea in Patients with Recurrent or Unresectable Meningioma. J. Neurosurg. 2002, 97, 341–346. [Google Scholar] [CrossRef]
- Rosenthal, M.A.; Ashley, D.L.; Cher, L. Treatment of High Risk or Recurrent Meningiomas with Hydroxyurea. J. Clin. Neurosci. 2002, 9, 156–158. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Tsao-Wei, D.D.; Groshen, S. Temozolomide for Treatment-Resistant Recurrent Meningioma. Neurology 2004, 62, 1210–1212. [Google Scholar] [CrossRef]
- Loven, D.; Hardoff, R.; Sever, Z.B.; Steinmetz, A.P.; Gornish, M.; Rappaport, Z.H.; Fenig, E.; Ram, Z.; Sulkes, A. Non-Resectable Slow-Growing Meningiomas Treated by Hydroxyurea. J. Neurooncol. 2004, 67, 221–226. [Google Scholar] [CrossRef]
- Newton, H.B.; Scott, S.R.; Volpi, C. Hydroxyurea Chemotherapy for Meningiomas: Enlarged Cohort with Extended Follow-Up. Br. J. Neurosurg. 2004, 18, 495–499. [Google Scholar] [CrossRef]
- Hahn, B.M.; Schrell, U.M.H.; Sauer, R.; Fahlbusch, R.; Ganslandt, O.; Grabenbauer, G.G. Prolonged Oral Hydroxyurea and Concurrent 3d-Conformal Radiation in Patients with Progressive or Recurrent Meningioma: Results of a Pilot Study. J. Neurooncol. 2005, 74, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Tsao-Wei, D.D.; Groshen, S. Salvage Chemotherapy with CPT-11 for Recurrent Meningioma. J. Neurooncol. 2006, 78, 271–276. [Google Scholar] [CrossRef]
- Grunberg, S.M.; Weiss, M.H.; Russell, C.A.; Spitz, I.M.; Ahmadi, J.; Sadun, A.; Sitruk-Ware, R. Long-Term Administration of Mifepristone (RU486): Clinical Tolerance during Extended Treatment of Meningioma. Cancer Investig. 2006, 24, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Weston, G.J.; Martin, A.J.; Mufti, G.J.; Strong, A.J.; Gleeson, M.J. Hydroxyurea Treatment of Meningiomas: A Pilot Study. Skull Base 2006, 16, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Glantz, M.J. Interferon-Alpha for Recurrent World Health Organization Grade 1 Intracranial Meningiomas. Cancer 2008, 113, 2146–2151. [Google Scholar] [CrossRef]
- Wen, P.Y.; Yung, W.K.A.; Lamborn, K.R.; Norden, A.D.; Cloughesy, T.F.; Abrey, L.E.; Fine, H.A.; Chang, S.M.; Robins, H.I.; Fink, K.; et al. Phase II Study of Imatinib Mesylate for Recurrent Meningiomas (North American Brain Tumor Consortium Study 01-08). Neuro-Oncology 2009, 11, 853–860. [Google Scholar] [CrossRef]
- Norden, A.D.; Raizer, J.J.; Abrey, L.E.; Lamborn, K.R.; Lassman, A.B.; Chang, S.M.; Yung, W.K.A.; Gilbert, M.R.; Fine, H.A.; Mehta, M.; et al. Phase II Trials of Erlotinib or Gefitinib in Patients with Recurrent Meningioma. J. Neurooncol. 2010, 96, 211–217. [Google Scholar] [CrossRef]
- Johnson, D.R.; Kimmel, D.W.; Burch, P.A.; Cascino, T.L.; Giannini, C.; Wu, W.; Buckner, J.C. Phase II Study of Subcutaneous Octreotide in Adults with Recurrent or Progressive Meningioma and Meningeal Hemangiopericytoma. Neuro-Oncology 2011, 13, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Norden, A.D.; Desjardins, A.; Vredenburgh, J.J.; Herndon, J.E.; Coan, A.; Sampson, J.H.; Gururangan, S.; Peters, K.B.; McLendon, R.E.; et al. Phase II Study of Gleevec® plus Hydroxyurea (HU) in Adults with Progressive or Recurrent Meningioma. J. Neurooncol. 2012, 106, 409–415. [Google Scholar] [CrossRef]
- Raizer, J.J.; Grimm, S.A.; Rademaker, A.; Chandler, J.P.; Muro, K.; Helenowski, I.; Rice, L.; McCarthy, K.; Johnston, S.K.; Mrugala, M.M.; et al. A Phase II Trial of PTK787/ZK 222584 in Recurrent or Progressive Radiation and Surgery Refractory Meningiomas. J. Neurooncol. 2014, 117, 93–101. [Google Scholar] [CrossRef]
- Simó, M.; Argyriou, A.A.; Macià, M.; Plans, G.; Majós, C.; Vidal, N.; Gil, M.; Bruna, J. Recurrent High-Grade Meningioma: A Phase II Trial with Somatostatin Analogue Therapy. Cancer Chemother. Pharmacol. 2014, 73, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Rankin, C.; Grunberg, S.; Sherrod, A.E.; Ahmadi, J.; Townsend, J.J.; Feun, L.G.; Fredericks, R.K.; Russell, C.A.; Kabbinavar, F.F.; et al. Double-Blind Phase III Randomized Trial of the Antiprogestin Agent Mifepristone in the Treatment of Unresectable Meningioma: SWOG S9005. J. Clin. Oncol. 2015, 33, 4093–4098. [Google Scholar] [CrossRef]
- Kaley, T.J.; Wen, P.; Schiff, D.; Ligon, K.; Haidar, S.; Karimi, S.; Lassman, A.B.; Nolan, C.P.; DeAngelis, L.M.; Gavrilovic, I.; et al. Phase II Trial of Sunitinib for Recurrent and Progressive Atypical and Anaplastic Meningioma. Neuro-Oncology 2015, 17, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Marincek, N.; Radojewski, P.; Dumont, R.A.; Brunner, P.; Müller-Brand, J.; Maecke, H.R.; Briel, M.; Walter, M.A. Somatostatin Receptor-Targeted Radiopeptide Therapy with 90Y-DOTATOC and 177Lu-DOTATOC in Progressive Meningioma: Long-Term Results of a Phase II Clinical Trial. J. Nucl. Med. 2015, 56, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Ligon, K.L.; Hammond, S.N.; Muzikansky, A.; Reardon, D.A.; Kaley, T.J.; Batchelor, T.T.; Plotkin, S.R.; Raizer, J.J.; Wong, E.T.; et al. Phase II Study of Monthly Pasireotide LAR (SOM230C) for Recurrent or Progressive Meningioma. Neurology 2015, 84, 280–286. [Google Scholar] [CrossRef]
- Mazza, E.; Brandes, A.; Zanon, S.; Eoli, M.; Lombardi, G.; Faedi, M.; Franceschi, E.; Reni, M. Hydroxyurea with or without Imatinib in the Treatment of Recurrent or Progressive Meningiomas: A Randomized Phase II Trial by Gruppo Italiano Cooperativo Di Neuro-Oncologia (GICNO). Cancer Chemother. Pharmacol. 2016, 77, 115–120. [Google Scholar] [CrossRef]
- Karsy, M.; Hoang, N.; Barth, T.; Burt, L.; Dunson, W.; Gillespie, D.L.; Jensen, R.L. Combined Hydroxyurea and Verapamil in the Clinical Treatment of Refractory Meningioma: Human and Orthotopic Xenograft Studies. World Neurosurg. 2016, 86, 210–219. [Google Scholar] [CrossRef]
- Shih, K.C.; Chowdhary, S.; Rosenblatt, P.; Weir, A.B.; Shepard, G.C.; Williams, J.T.; Shastry, M.; Burris, H.A.; Hainsworth, J.D. A Phase II Trial of Bevacizumab and Everolimus as Treatment for Patients with Refractory, Progressive Intracranial Meningioma. J. Neurooncol. 2016, 129, 281–288. [Google Scholar] [CrossRef]
- Graillon, T.; Sanson, M.; Campello, C.; Idbaih, A.; Peyre, M.; Peyrière, H.; Basset, N.; Autran, D.; Roche, C.; Kalamarides, M.; et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clin. Cancer Res. 2020, 26, 552–557. [Google Scholar] [CrossRef]
- Karajannis, M.A.; Mauguen, A.; Maloku, E.; Xu, Q.; Dunbar, E.M.; Plotkin, S.R.; Yaffee, A.; Wang, S.; Roland, J.T.; Sen, C.; et al. Phase 0 Clinical Trial of Everolimus in Patients with Vestibular Schwannoma or Meningioma. Mol. Cancer Ther. 2021, 20, 1584–1591. [Google Scholar] [CrossRef]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C.; Cairncross, J.G. Response Criteria for Phase II Studies of Supratentorial Malignant Glioma. J. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescandolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic Sequencing of Meningiomas Identifies Oncogenic SMO and AKT1 Mutations. Nat. Genet. 2013, 45, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Nakasu, S.; Nakasu, Y.; Nakajima, M.; Matsuda, M.; Handa, J. Preoperative Identification of Meningiomas That Are Highly Likely to Recur. J. Neurosurg. 1999, 90, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Abedalthagafi, M.; Bi, W.L.; Aizer, A.A.; Merrill, P.H.; Brewster, R.; Agarwalla, P.K.; Listewnik, M.L.; Dias-Santagata, D.; Thorner, A.R.; Van Hummelen, P.; et al. Oncogenic PI3K Mutations Are as Common as AKT1 and SMO Mutations in Meningioma. Neuro Oncol 2016, 18, 649–655. [Google Scholar] [CrossRef]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avşar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic Analysis of Non-NF2 Meningiomas Reveals Mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef]
- Reuss, D.E.; Piro, R.M.; Jones, D.T.W.; Simon, M.; Ketter, R.; Kool, M.; Becker, A.; Sahm, F.; Pusch, S.; Meyer, J.; et al. Secretory Meningiomas Are Defined by Combined KLF4 K409Q and TRAF7 Mutations. Acta Neuropathol. 2013, 125, 351–358. [Google Scholar] [CrossRef]
- Sahm, F.; Bissel, J.; Koelsche, C.; Schweizer, L.; Capper, D.; Reuss, D.; Böhmer, K.; Lass, U.; Göck, T.; Kalis, K.; et al. AKT1E17K Mutations Cluster with Meningothelial and Transitional Meningiomas and Can Be Detected by SFRP1 Immunohistochemistry. Acta Neuropathol. 2013, 126, 757–762. [Google Scholar] [CrossRef]
- Ruttledge, M.H.; Sarrazin, J.; Rangaratnam, S.; Phelan, C.M.; Twist, E.; Merel, P.; Delattre, O.; Thomas, G.; Nordenskjöld, M.; Collins, V.P. Evidence for the Complete Inactivation of the NF2 Gene in the Majority of Sporadic Meningiomas. Nat. Genet. 1994, 6, 180–184. [Google Scholar] [CrossRef]
- Harmancı, A.S.; Youngblood, M.W.; Clark, V.E.; Coşkun, S.; Henegariu, O.; Duran, D.; Erson-Omay, E.Z.; Kaulen, L.D.; Lee, T.I.; Abraham, B.J.; et al. Integrated Genomic Analyses of de Novo Pathways Underlying Atypical Meningiomas. Nat. Commun. 2017, 8, 14433. [Google Scholar] [CrossRef]
- Bi, W.L.; Greenwald, N.F.; Abedalthagafi, M.; Wala, J.; Gibson, W.J.; Agarwalla, P.K.; Horowitz, P.; Schumacher, S.E.; Esaulova, E.; Mei, Y.; et al. Genomic Landscape of High-Grade Meningiomas. NPJ Genom. Med. 2017, 2, 15. [Google Scholar] [CrossRef]
- Preusser, M.; Brastianos, P.K.; Mawrin, C. Advances in Meningioma Genetics: Novel Therapeutic Opportunities. Nat. Rev. Neurol. 2018, 14, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Birzu, C.; Peyre, M.; Sahm, F. Molecular Alterations in Meningioma: Prognostic and Therapeutic Perspectives. Curr. Opin. Oncol. 2020, 32, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Nigim, F.; Wakimoto, H.; Kasper, E.M.; Ackermans, L.; Temel, Y. Emerging Medical Treatments for Meningioma in the Molecular Era. Biomedicines 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, A.A.; Wagle, N.; Zada, G. Recent Developments in Chemotherapy for Meningiomas: A Review. Neurosurg. Focus 2013, 35, E18. [Google Scholar] [CrossRef]
- Houshmandi, S.S.; Emnett, R.J.; Giovannini, M.; Gutmann, D.H. The Neurofibromatosis 2 Protein, Merlin, Regulates Glial Cell Growth in an ErbB2- and Src-Dependent Manner. Mol. Cell. Biol. 2009, 29, 1472–1486. [Google Scholar] [CrossRef]
- Shapiro, I.M.; Kolev, V.N.; Vidal, C.M.; Kadariya, Y.; Ring, J.E.; Wright, Q.; Weaver, D.T.; Menges, C.; Padval, M.; McClatchey, A.I.; et al. Merlin Deficiency Predicts for FAK Inhibitor Sensitivity: A Synthetic Lethal Relationship. Sci. Transl. Med. 2014, 6, 237ra68. [Google Scholar] [CrossRef]
- Karsy, M.; Guan, J.; Cohen, A.; Colman, H.; Jensen, R.L. Medical Management of Meningiomas: Current Status, Failed Treatments, and Promising Horizons. Neurosurg. Clin. N. Am. 2016, 27, 249–260. [Google Scholar] [CrossRef]
- Dasanu, C.A.; Samara, Y.; Codreanu, I.; Limonadi, F.M.; Hamid, O.; Alvarez-Argote, J. Systemic Therapy for Relapsed/Refractory Meningioma: Is There Potential for Antiangiogenic Agents? J. Oncol. Pharm. Pract. 2019, 25, 638–647. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Hilton, D.A.; Shivane, A.; Kirk, L.; Bassiri, K.; Enki, D.G.; Hanemann, C.O. Activation of Multiple Growth Factor Signalling Pathways Is Frequent in Meningiomas. Neuropathology 2016, 36, 250–261. [Google Scholar] [CrossRef]
- Yun, S.; Koh, J.M.; Lee, K.S.; Seo, A.N.; Nam, K.H.; Choe, G. Expression of C-MET in Invasive Meningioma. J. Pathol. Transl. Med. 2015, 49, 44–51. [Google Scholar] [CrossRef]
- Gupta, S.; Bi, W.L.; Dunn, I.F. Medical Management of Meningioma in the Era of Precision Medicine. Neurosurg. Focus 2018, 44, E3. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, L.; Du, Y.; Huang, L.F.; Braun, F.K.; Kogiso, M.; Zhao, Y.; Li, C.; Lindsay, H.; Zhao, S.; et al. Patient-Derived Orthotopic Xenograft (PDOX) Mouse Models of Primary and Recurrent Meningioma. Cancers 2020, 12, 1478. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-KB in Development and Progression of Human Cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Cavalcante, L.; Bastos, B.R.; Powell, S.F.; Ma, W.W.; Sahebjam, S.; Harvey, D.; De Souza, A.L.; Dhawan, M.S.; Safran, H.; et al. Phase I Study of 9-Ing-41, a Small Molecule Selective Glycogen Synthase Kinase-3 Beta (GSK-3β) Inhibitor, as a Single Agent and Combined with Chemotherapy, in Patients with Refractory Tumors. JCO 2020, 38, 3507. [Google Scholar] [CrossRef]
- Theeler, B.J.; Jung, J.; Burton, E.; Leeper, H.; Wu, J.; Zaghloul, K.; Ray-Chaudhury, A.; Quezado, M.; Raffeld, M.; Yuan, Y.; et al. First-in-Human Dose Escalation and Food Effect Study of Oral ONC206 in Adults with Recurrent Primary CNS Neoplasms. JCO 2021, 39, TPS2072. [Google Scholar] [CrossRef]
- Arasanz, H.; Gato-Cañas, M.; Zuazo, M.; Ibañez-Vea, M.; Breckpot, K.; Kochan, G.; Escors, D. PD1 Signal Transduction Pathways in T Cells. Oncotarget 2017, 8, 51936–51945. [Google Scholar] [CrossRef]
- Han, S.J.; Reis, G.; Kohanbash, G.; Shrivastav, S.; Magill, S.T.; Molinaro, A.M.; McDermott, M.W.; Theodosopoulos, P.V.; Aghi, M.K.; Berger, M.S.; et al. Expression and Prognostic Impact of Immune Modulatory Molecule PD-L1 in Meningioma. J. Neurooncol. 2016, 130, 543–552. [Google Scholar] [CrossRef]
- Du, Z.; Abedalthagafi, M.; Aizer, A.A.; McHenry, A.R.; Sun, H.H.; Bray, M.-A.; Viramontes, O.; Machaidze, R.; Brastianos, P.K.; Reardon, D.A.; et al. Increased Expression of the Immune Modulatory Molecule PD-L1 (CD274) in Anaplastic Meningioma. Oncotarget 2015, 6, 4704–4716. [Google Scholar] [CrossRef]
- Wild, D.; Fani, M.; Fischer, R.; Del Pozzo, L.; Kaul, F.; Krebs, S.; Fischer, R.; Rivier, J.E.F.; Reubi, J.C.; Maecke, H.R.; et al. Comparison of Somatostatin Receptor Agonist and Antagonist for Peptide Receptor Radionuclide Therapy: A Pilot Study. J. Nucl. Med. 2014, 55, 1248–1252. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Barnholtz-Sloan, J.S. Medical Treatment of Recurrent Meningiomas. Expert Rev. Neurother. 2011, 11, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Region | Participants | Meningioma Grade * | Age (mean) | KPS (Mean, Range) | Intervention |
|---|---|---|---|---|---|---|
| Markwalder et al., 1985 [17] | Switzerland | 6 (5F/1M) | N/A | 68.5y | N/A | Tamoxifen, 30 mg T.D.S. |
| Jääskeläinen et al., 1986 [18] | Finland | 5 (5F) | I (n = 4) III (n = 1) | N/A | N/A | Medroxyprogestrone acetate, 1000 mg I.M. daily for 5 d and 1000 mg I.M. weekly thereafter |
| Grunberg et al., 1990 [19] | USA | 9 (5F/4M) | N/A | 41.55 y | Median: 70% (90–100) | Megestrol acetate, 160–320 mg Q.I.D. |
| Grunberg et al., 1991 [20] | USA | 14 (8F/6M) | N/A | 54.07 y | Median: 90% (60–100) | Mifepristone, 200 mg P.O. daily for min of 1 y |
| Goodwin et al., 1993 [21] | USA | 19 (13F/6M) | N/A | Median: 58 y | N/A | Tamoxifen, 40 mg B.I.D. for 4 d and 10 mg B.I.D. thereafter |
| Chamberlain et al., 1996 [22] | USA | 14 (6F/8M) | N/A | Median: 51 y | Median: 90% (70–100) | 3 or 6 1-month cycles of cyclophosphamide (500 mg/m2/d, I.V. days 1–3), doxorubicin (15 mg/m2/d, days 1–3), and vincristine (1.4 mg/m2/d any day between days 10–14) |
| Schrell et al., 1997 [23] | Germany | 4 (2F/2M) | N/A | 48.25 y | All >70% | Hydroxyurea, 1000–1500 mg/day (approx. 20 mg/kg/d), daily P.O., for min of 2 y |
| Newton et al., 2000 [24] | USA | 17 (13F/4M) | N/A | 57.2 y | N/A | Hydroxyurea, 20 mg/kg/d (1250–1500 mg) P.O. |
| Muhr et al., 2001 [25] | Sweden | 12 (8F/4M) | I (n = 6) II (n = 1) III (n = 3) | 56 y | N/A | Interferon a, 1,500,000–5,000,000 IU S.C. daily |
| Mason et al., 2002 [26] | USA/Canada | 20 (11F/9M) | I (n = 16) II (n = 3) III (n = 1) | Median: 59 y | 80% (50–100) | Hydroxyurea, 1000–1500 mg/d (20–30 mg/kg/day) until clinical or imaging evidence of progression or 2 y |
| Rosenthal et al., 2002 [27] | Australia | 15 (13F/2M) | I (n = 10) II (n = 5) | Median: 39 y | N/A | Hydroxyurea, 20 mg/kg/d daily P.O. |
| Chamberlain et al., 2004 [28] | USA | 16 (11F/5M) | I (n = 16) | Median: 62.5 y | Median: 80% (60–100) | Temozolomide, 50–75 mg/m2/d P.O. daily for 42 days, 28 d drug holiday thereafter |
| Loven et al., 2004 [29] | Israel | 12 (7F/5M) | I (n = 8) II (n = 4) | N/A | ECOG: grade I (n = 6), grade II (n = 4), grade III (n = 2) | Hydroxyurea, 20 mg/kg/d daily P.O. for 24 mo. |
| Newton et al., 2004 [30] | USA | 21 (17F/4M) | I (n = 16) II (n = 1) | Median: 59 y | >60% | Hydroxyurea, 20 mg/kg/d P.O. |
| Hahn et al., 2005 [31] | Germany | 21 (14F/7M) | I (n = 13) II (n = 2) III (n = 2) | Median: 60 y | N/A | Hydroxyurea, 1000–1500 mg (20 mg/kg/d) P.O. |
| Chamberlain et al., 2006 [32] | USA | 16 (11F/5M) | N/A | Median: 60.5 y | Median: 80% (60–100) | Irinotecan, 350–600 mg/m2/d I.V. every 3 w for 9 w |
| Grunberg et al., 2006 [33] | USA | 28 (19F/9M) | N/A | Median: 56 y | N/A | Mifepristone, 200 mg P.O. daily |
| Weston et al., 2006 [34] | UK | 6 (F) | I (n = 5) | 46 y | N/A | Hydroxyurea: starting at 15 mg/kg/d P.O. for 1 y |
| Chamberlain et al., 2008 [35] | USA | 35 (29F/6M) | I (n = 35) | median: 61 y | Median: 80% (60–100) | Interferon-a, 10,000,000 U/m2 S.C. every other day |
| Wen et al., 2009 [36] | USA | 23 (13F/10M) | I (n = 13) II (n = 5) III (n = 5) | Median: 58 y | Median: 80% (60–100) | Imatinib mesylate, 600–800 mg/d daily P.O. for 4 w cycles |
| Norden et al., 2009 [37] | USA | 25 (13F/12M) | I (n = 8) II (n = 9) III (n = 8) | Median: 57 y | Median: 90% (60–100) | Gefitinib 500–1000 mg/d daily P.O. OR erlotinib 150 mg/d, daily P.O. in 4 w cycles |
| Johnson et al., 2011 [38] | USA | 12 (3F/9M) | I (n = 3) II (n = 3) III (n = 6) | 48.91y | All ECOG<3 | Octreotide, 500 mcg S.C. T.D.S |
| Reardon et al., 2012 [39] | USA | 21 (12F/9M) | I (n = 8) II (n = 9) III (n = 4) | Median: 51 y | Median: 80% (70–100) | Imatinib, 400–500 mg/d daily, hydroxyurea 1000 mg/day B. I. D. |
| Raizer et al., 2014 [40] | USA | 25 (10F/15M) | I (n = 2) II (n = 14) III (n = 8) | Median: 59 y | Median: 80% (60–100) | Vatalanib, 500–1000 mg/d B. I. D. P.O. in 4 w cycles |
| Simo et al., 2014 [41] | Spain | 9 (1F/8M) | II (n = 5) III (n = 4) | Median: 65 y | Median: 80% (60–100) | Octreotide LAR: 30–40 mg I.M. every 28 d |
| Ji et al., 2015 [42] | USA | 164 (116F/48M) Intervention: 80 (57F/23M), comparator: 84 (59F/25M) | N/A | Median (intervention): 60.6 y Median (comparator): 53.2 y | All ECOG<3 | Intervention: mifepristone, 200 mg P.O. daily for 2 y or disease progression Comparator: placebo |
| Kaley et al., 2015 [43] | USA | 36 (22F/14M) EC: 13 (8F/5M) | I (n = 4) II (n = 30) III (n = 6) | Median: 61 y Median (EC): 48 y | Median: 80% (60–100) Median (EC): 90% (60–100) | Sunitinib malate, 50 mg/d, for days 1–28 of 42 d cycles |
| Marincek et al., 2015 [44] | Switzerland | 34 (25F/9M) | N/A | Median: 61.3 y | N/A | 90Y-DOTATOC and 177Lu-DOTATOC for 3 d every 6 (or more) w |
| Norden et al., 2015 [45] | USA | 34 (17F/17M) | I (n = 16) II (n = 12) III (n = 6) | Median: 54 y | Median: 85% (60–100) | Octreotide LAR, 60 mg I.M. every 4 w |
| Mazza et al., 2016 [46] | Italy | 15 (8F/7M) arm A, combinatorial intervention (n = 7) arm B, hydroxyurea alone (n = 8) | Arm A: I (n = 1) II (n = 4) III (n = 1) Arm B: I (n = 1) II (n = 5) | Median: Arm A = 68 y Arm B = 68.5y | Median ECOG: Arm A = 1 (0–2) Arm B = 1 (0–2) | Arm A: hydroxyurea, 1000 mg/d B. I. D. and imatinib, 400–600 mg/d daily Arm B: hydroxyurea, 1000 mg/d B. I. D. |
| Karsy et al., 2016 [47] | USA | 7 (6F/1M) | I (n = 2) II (n = 5) | Median: 56 y | 90–100% | Hydroxyurea, 20 mg/kg/d (1000–1500 mg/d) B. I. D. P.O. and verapamil, 120–480 mg/d B. I. D. P.O. |
| Shih et al., 2016 [48] | USA | 17 (9F/8M) | I (n = 4) II (n = 7) III (n = 5) | Median: 59 y | Median ECOG: 1 (0–3) | Bevacizumab, 10 mg/kg I.V. on days 1/15 of 28 d cycles Everolumus, 10 mg P.O. on days 1/15 of 28 d cycles |
| Graillon et al., 2020 [49] | France | 20 (11F/9M) | I (n = 2) II (n = 10) III (n = 8) | Median: 55 y | all 50% and higher | Octreotide LAR, 30 mg I.M. monthly for 1–3 y Everolimus, 10 mg P.O. daily for 1–3 y |
| Karajannis et al., 2021 [50] | USA | 8 (5F/3M) | I (n = 8) | 43.12 y | all 60% and higher | Everolimus, 10 mg P.O. daily for 10 d preoperatively |
| Author | Partial/Complete Radiographic Response | Stable Radiographic Response | Time to Tumor Progression (Median, Range) | Overall Survival (Median, Range) | 6-Month Progression-Free Survival | Grade III/IV/V Toxicities * |
|---|---|---|---|---|---|---|
| Markwalder et al., 1985 [17] | 16.66% | 50% | 16 mo. (8–24) | 24 mo. | 100% | N/A |
| Jääskeläinen et al., 1986 [18] | 0 | N/A | Grade I: 21–45 mo. Grade III: 8 w | N/A | 80% | N/A |
| Grunberg et al., 1990 [19] | 0 | N/A | N/A | N/A | N/A | N/A |
| Grunberg et al., 1991 [20] | 30.76% | 38.46% | 5.33 mo. (2–8) | N/A | N/A | N/A |
| Goodwin et al., 1993 [21] | 5% | 32% | 15.1 mo. | N/A | N/A | N/A |
| Chamberlain et al., 1996 [22] | 21% | 79% | 4.6 y (2.2–7.1) | 5.3 y (2.6–7.6) | N/A | N/A |
| Schrell et al., 1997 [23] | N/A | N/A | N/A | N/A | N/A | N/A |
| Newton et al., 2000 [24] | 0 | 88% | 80 w (20 -> 144) | N/A | N/A | Hematological (n = 5) |
| Muhr et al., 2001 [25] | N/A | N/A | N/A | N/A | N/A | N/A |
| Mason et al., 2002 [26] | N/A | N/A | Grade I: 54 w (41–66) Grade II: 25.33 w (12–45) Grade III: 24 w | N/A | N/A | Hematological (n = 3) |
| Rosenthal et al., 2002 [27] | 0 | 85% | N/A | N/A | N/A | Hematological (n = 1), dermatological (n = 1) |
| Chamberlain et al., 2004 [28] | 0 | 81.25% | 5 mo. (2.5–5) | 7.5 mo. (95%CI 7–8) | N/A | Hematological (n = 22), constitutional (n = 3), neurological (n = 1) |
| Loven et al., 2004 [29] | 10% | 0 | 13 mo. (4–24) | N/A | N/A | Hematological (n = 4), dermatological (n = 1) |
| Newton et al., 2004 [30] | 0 | 90% | Mean: 176 w (20 -> 328) | N/A | N/A | Hematological (n = 6) |
| Hahn et al., 2005 [31] | 0 | 71.5% | 59 w (10–175) | N/A | N/A | N/A |
| Chamberlain et al., 2006 [32] | 0 | 81% | 4.5 mo. (2.25–10.5) | 7 mo. (95% CI: 7–8) | 6% | GI and hematological (n = 12), neutropenic fever (n = 1) |
| Grunberg et al., 2006 [33] | 17.85% | N/A | N/A | N/A | N/A | N/A |
| Weston et al., 2006 [34] | 0 | 50% | N/A | N/A | N/A | N/A |
| Chamberlain et al., 2008 [35] | 0 | 74.28% | 7 mo. (2–24) | 8 mo. (3–28) | 54% | fatigue (n = 6), hematological (n = 7), GI (n = 1) |
| Wen et al., 2009 [36] | 0 | 47.36% | 2 mo. (0.7–34) | N/A | 29.4% | Hematological (n = 4), fluid and electrolyte (n = 3), other (n = 3) |
| Norden et al., 2009 [37] | 0 | 32% | 10 w (95% CI 8–20) | 23 mo. | 28% | 22 |
| Johnson et al., 2011 [38] | 0 | 75% | 17 w (3 -> 957) | 2.7 y (22 d–9.7 y) | 33.33% | N/A |
| Reardon et al., 2012 [39] | 0 | 66.66% | 7.0 mo. (95% CI 3.8–9.2) | 66.0 mo. (95% CI 20.7, 66.0) | 61.9% (95% CI 38.1–78.8) | Hematological (n = 3), other (n = 3) |
| Raizer et al., 2014 [40] | N/A | N/A | grade II: 6.5 mo. grade 3: 3.6 mo. | Grade II: 26.0 mo. Grade 3: 23 mo. | Grade II: 64.3% Grade III: 37.5% | Hepatic (n = 5), constitutional (n = 4) |
| Simo et al., 2014 [41] | 0 | 33.33% | 4.23 mo. (1–9.4) | 18.7 mo. (2.7–39.9) | 44.4% | 0 |
| Ji et al., 2015 [42] | Intervention: 1.4% Comparator: 1% | Intervention: 55% Comparator: 52% | Intervention: 10 mo. (95% CI 7–13) Comparator: 11 mo. (95% CI 6–18) | Intervention: 8 y Comparator: 12 y | N/A | Intervention: n = 37 Comparator: n = 25 |
| Kaley et al., 2015 [43] | 5.55% | 69.44% | 5.2 mo. (95% CI: 2.8–8.3) | 24.6 mo. (95% CI: 16.5–38.4) | 42% | CNS hemorrhage (n = 3), thrombotic microangiopathy (n = 2), GI (n = 1) |
| Marincek et al., 2015 [44] | 0 | 65.6% | N/A | Mean: 8.6 y | N/A | Hematological (n = 3), renal (n = 1) |
| Norden et al., 2015 [45] | 0 | 75% | Grade I: 26 w (12–43) Grade II/III: 15 w (8–20) | Grade I: N/A Grade II/III: 104 w (77–158) | 32% | Metabolic (n = 10), fatigue (n = 2), other (n = 4) |
| Mazza et al., 2016 [46] | 0 | Arm A: 57.14% Arm B: 100% | Arm A: 4 mo. Arm B: 19 mo. | Arm A: 6 mo. Arm B: 27.5 mo. | N/A | Neurological (n = 2), GI (n = 1), hematological (n = 1) |
| Karsy et al., 2016 [47] | 0 | N/A | 8.0 mo. (95% CI 6.1–9.9) | 30.0 mo. (95% CI 22.8–37.2) | 85% (95% CI 5.5–97.0%) | Hematological (n = 5), other (n = 1) |
| Shih et al., 2016 [48] | 0 | 88% | 22 mo. (95% CI 4.5–26.8) | 23.85 mo. (95% CI 9–33.1) | 69% | Hematological (n = 1), metabolic (n = 2), renal (n = 2), GI (n = 2), other (n = 3) |
| Graillon et al., 2020 [49] | 0 | N/A | 6.6 mo. (95% CI 2.7–15) | N/A | 55% (95% CI 31.3–73.5) | Stomatitis (n = 3), other (n = 5) |
| Karajannis et al., 2021 [50] | N/A | N/A | N/A | N/A | N/A | 0 |
| Investigator | NCT Number | Region | Funding | Intervention | Trial Sites | Phase | Target Enrollment | Meningioma Only | Study Length | Recruitment status | Primary Outcome of Interest |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy | |||||||||||
| Scott R. Plotkin | 03071874 | USA | Both | AZD2014 (vistusertib), PO BID on days 2 and 7; 28-day cycles | 3 | 2 | 25 | Yes | 2017–2024 | Active, not recruiting | PFS |
| Thomas Graillon | 03631953 | France | Public | Trametinib (1.5 mg/d daily), Alpelisib (120–200 mg/d daily) | 1 | 1 | 25 | Yes | 2019–2022 | Recruiting | Toxicity |
| Jun-ping Zhang | 04501705 | China | Private | Apatinib Mesylate, 500 mg PO daily, until progressive disease; 28-day cycles | 1 | N/A | 29 | Yes | 2020–2025 | Recruiting | PFS |
| Nader Sanai | 02933736 | USA | Private | Ribociclib (LEE011), 900 mg PO QD; total of 5 doses before surgery | 1 | 1 | 48 | No | 2016–2022 | Recruiting | Pharmacokinetics, Toxicity |
| Rupesh Kotecha | 05425004 | USA | Private | Cabozantinib 60 mg QD for 28 days | 1 | 2 | 24 | Yes | 2022–2024 | Recruiting | PFS |
| NA | 05130866 | NA | Private | AR-42 (OSU-HDAC42) 30–60 mg 3 times a wk followed by 1 wk off | NA | 2/3 | 89 | Yes | 2021–2027 | Not yet recruiting | PFS |
| Ludimila Cavalcante | 04239092 | USA | Private | 9-ING-41 (9.3 mg/kg IV twice per week), w. or w/o irinotecan (50 mg/m2/d IV days 1–5 of 21 d cycles) | 8 | 1 | 48 | No | 2020–2023 | Recruiting | Toxicity |
| Scott Plotkin | 04374305 | USA | Private | Brigatinib, PO daily | 4 | 2 | 80 | No | 2020–2029 | Recruiting | PFS |
| Trent Hummel | 03095248 | USA | Private | Selumetinib, 75 mg/d PO BID; 28 d cycles. up to 2 y | 1 | 2 | 34 | No | 2017–2023 | Recruiting | PFS |
| Mark Gilbert | 04541082 | USA | Both | ONC206 (imipridone class of anti-cancer small molecules) | 1 | 1 | 102 | No | 2020–2024 | Recruiting | Toxicity |
| Giles W. Robinson | 03434262 | USA | Private | Gemcitabine (IV), ribociclib (PO), sonidegib (PO), trametinib (PO), G-CSF (SC) | 1 | 1 | 108 | No | 2018–2025 | Recruiting | Pharmacokinetics, Toxicity |
| Priscilla Brastianos | 02523014 | USA | Both | A: Vismodegib PO Q.D.; 28 d cycles B: GSK2256098 PO QD; 28 d cycles C: Capivasertib PO BID; days 1–4 of 7 D: Abemaciclib PO BID; 28 d cycles | 705 | 2 | 124 | Yes | 2015–2024 | Recruiting | PFS |
| Thomas Kaley | 03220646 | USA | Private | Abemaciclib, 200 mg PO BID; 28 d cycles | 8 | 2 | 78 | No | 2017–2023 | Recruiting | PFS, RRR |
| Santosh Kesari | 02423525 | USA | Private | Afatinib, 80–280 mg, PO every 4 d or 7 d | 1 | 1 | 24 | No | 2016–2021 | Active, not recruiting | Toxicity |
| Hormone Therapy | |||||||||||
| Dominik Cordier | 04997317 | Switzerland | Public | A: IV 4.5 GBq 177Lu-DOTA-JR11 (300–1300 ug) once; 2nd cycle of 200 ug B: IV 4.5 GBq 177Lu-DOTA-JR11 (300–1300 ug) once; 2nd cycle of 200 ug | 1 | 1 | 18 | Yes | 2021–2025 | Recruiting | Toxicity |
| Kenneth Merrell | 04082520 | USA | Public | IV Ga 68-DOTATE and then IV 177Lu-DOTA over 30–40 min. Cycles repeat every 8 wk for up to 6 mo. | 1 | 2 | 41 | Yes | 2019–2024 | Recruiting | PFS |
| Ralph Salloum | 05278208 | USA | Public | IV 177Lu-DOTATE (200 mCi) once every 8 wk for 8 mo. | 9 | 1/2 | 65 | No | 2022–2026 | Not yet recruiting | Toxicity PFS |
| Erik Sulman | 03971461 | USA | Private | IV 177Lu-DOTATE every 8 wk for 4 doses | 2 | 2 | 32 | Yes | 2019–2023 | Recruiting | PFS |
| Biological Therapy | |||||||||||
| David Reardon | 02648997 | USA | Private | A. nivolumab, 240 mg q2w. B. EBRT followed by 4 cycles of nivolumab (2mg/kg q3w) + ipilimumab (1 mg/kg every 3 weeks) followed by nivolumab (480 mg q4w) | 1 | 2 | 50 | Yes | 2016–2023 | Recruiting | PFS |
| Priscilla Brastianos | 03279692 | USA | Private | IV pembrolizumab q3w | 2 | 2 | 26 | Yes | 2017–2021 | Active, not recruiting | PFS |
| Feng Chen | 04728568 | China | Public | IV sintilimab q3w | 1 | NA | 15 | Yes | 2020–2023 | Recruiting | PFS |
| Jiayi Huang | 03267836 | USA | Private | IV avelumab (10 mg/kg) and proton therapy (30 CGE) q2w for 3 mo. Surgical evaluation at 3 mo. | 1 | 1 | 9 | Yes | 2017–2023 | Active, not recruiting | Immunogenicity Tumor size |
| Jiayi Huang | 03604978 | USA | Public | A. IV nivolumab; 28 d cycles for 1 y. Multi-fraction stereotactic radiosurgery (days 1,3, and 5) B. IV nivolumab q2w for 6 mo. followed by q4w for 6 mo. IV ipilimumab q6w for 6 mo. multi-fraction stereotactic radiosurgery (days 1,3, and 5) | 22 | 1/2 | 15 | Yes | 2018–2022 | Active, not recruiting | Toxicity RRR |
| Nancy Bush | 04659811 | USA | Private | IV pembrolizumab 200 mg q3w with SRS | 1 | 2 | 90 | Yes | 2020–2024 | Recruiting | PFS |
| Marta Penas-Prado | 03173950 | USA | Public | IV nivolumab 240 mg q2w for 2 cycles and then 480 mg q4w for a total 14 doses | 7 | 2 | 180 | No | 2017–2023 | Recruiting | PFS |
| NA | 05023018 | NA | Private | NEO100 self-administered qid on 28 d cycle for up to 12 cycles | NA | 2 | 30 | Yes | 2021–2026 | Not yet recruiting | Toxicity PFS |
| Priya Kumthekar | 02847559 | USA | Both | IV bevacizumab q2w for 4 cycles and then q2w or q3w + daily electric field therapy with Optune device | 9 | 2 | 27 | Yes | 2016–2024 | Recruiting | PFS |
| Agent | Dosage | Partial Radiographic Response (Range) | Stable Radiographic Response (Range) | 6-Month Progression-Free Survival (Range) | Common Grade III/IV/V Toxicities |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| Hydroxyurea | 15–30 mg/kg/d 1000–1500 mg/d | 0–10% | 0–88% | 85% | Hematological, dermatological |
| Temozolomide | 50–75 mg/m2/d | 0 | 81.25% | N/A | Hematological, constitutional, neurological |
| Irinotecan | 350–600 mg/m2/d | 0 | 81% | 6% | GI/hematological |
| Cyclophosphamide Doxorubicin Vincristine | 500 mg/m2/d 15 mg/m2/d 1.4 mg/m2/d | 21% | 79% | N/A | N/A |
| Imatinib | 500–800 mg/d | 0 | 47.3–100% | N/A | Hematological, fluid and electrolytes |
| Gefitinib Erlotinib | 500–1000 mg/d 150 mg/d | 0 | 32% | 28% | N/A |
| Vatalanib | 500–1000 mg/d | N/A | N/A | 37.5–64.3% | Hepatic, constitutional |
| Sunitinib | 50 mg/d | 5.55% | 69.4% | 42% | Hemorrhagic/thrombotic events |
| Hydroxyurea Imatinib | 1000 mg/d 400–600 mg/d | 0 | 57.1–66.6% | 61.9% | Hematological |
| Everolimus | 10 mg/d for 10 d preoperatively | N/A | N/A | N/A | None |
| Hormonal therapy | |||||
| Octreotide | 500 mg/d (regular) 30–60 mg monthly (LAR) | 0 | 33.33–75% | 32–44.4% | Hematological, metabolic, constitutional |
| 90Y-DOTATOC 177Lu-DOTATOC | 3 d every 6 or more wk | 0 | 65.6% | N/A | Hematological, renal |
| Mifepristone | 200 mg/d | 1.4–30.76% | 38.46–55% | N/A | N/A |
| Tamoxifen | 10–30 mg/d | 5–16.66% | 32–50% | 100% | N/A |
| Medroxyprogestrone acetate | 1000 mg weekly | 0 | N/A | 80% | N/A |
| Megestrol acetate | 160–320 mg/d | 0 | N/A | N/A | N/A |
| Biologic agents | |||||
| Interferon a | 1,500,000–5,000,000 IU/d 10,000,000 U/m2 every other day | 0 | 74.28% | 54% | N/A |
| Combined regimens | |||||
| Bevacizumab Everolumus, P.O. on days 1/15 of 28d cycles | 10 mg/kg 10 mg/d | 0 | 88% | 69% | Hematological, metabolic, renal, GI |
| Octreotide LAR Everolimus | 30 mg monthly 10 mg/d | 0 | N/A | 55% | Stomatitis |
| Types of Response | Definition |
|---|---|
| Complete Response (CR) | Complete disappearance of all enhancing tumors on consecutive CT or MRI scans at least 1 month apart, off steroids, and neurologically stable or improved. |
| Partial Response (PR) | ≥50% reduction in size of enhancing tumor on consecutive CT or MRI scans at least 1 month apart, steroids stable or reduced, and neurologically stable or improved |
| Progressive Disease (PD) | >25% increase in size of enhancing tumor or any new tumor on CT or MRI scans, or neurologically worse, and steroids stable or increased |
| Stable Disease (SD) | Imaging features do not qualify for CR, PR, or PD and neurologically stable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahbandi, A.; Shah, D.S.; Hadley, C.C.; Patel, A.J. The Role of Pharmacotherapy in Treatment of Meningioma: A Systematic Review. Cancers 2023, 15, 483. https://doi.org/10.3390/cancers15020483
Shahbandi A, Shah DS, Hadley CC, Patel AJ. The Role of Pharmacotherapy in Treatment of Meningioma: A Systematic Review. Cancers. 2023; 15(2):483. https://doi.org/10.3390/cancers15020483
Chicago/Turabian StyleShahbandi, Ataollah, Darsh S. Shah, Caroline C. Hadley, and Akash J. Patel. 2023. "The Role of Pharmacotherapy in Treatment of Meningioma: A Systematic Review" Cancers 15, no. 2: 483. https://doi.org/10.3390/cancers15020483
APA StyleShahbandi, A., Shah, D. S., Hadley, C. C., & Patel, A. J. (2023). The Role of Pharmacotherapy in Treatment of Meningioma: A Systematic Review. Cancers, 15(2), 483. https://doi.org/10.3390/cancers15020483







