Outcomes of Patients Treated for Hepatoblastoma with Low Alpha-Fetoprotein and/or Small Cell Undifferentiated Histology: A Report from the Children’s Hepatic Tumors International Collaboration (CHIC)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Rhabdoid Tumors
3.2. HBs with SCU Component
3.3. Low AFP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czauderna, P.; López-Terrada, D.; Hiyama, E.; Häberle, B.; Malogolowkin, M.H.; Meyers, R.L. Hepatoblastoma state of the art: Pathology, genetics, risk stratification, and chemotherapy. Curr. Opin. Pediatr. 2014, 26, 19–28. [Google Scholar] [CrossRef] [PubMed]
- López-Terrada, D.; Alaggio, R.; de Dávila, M.T.; Czauderna, P.; Hiyama, E.; Katzenstein, H.; Leuschner, I.; Malogolowkin, M.; Meyers, R.; Ranganathan, S.; et al. Towards an international pediatric liver tumor consensus classification: Proceedings of the Los Angeles COG liver tumors symposium. Mod. Pathol. 2014, 27, 472–491. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.E.; Muczynski, K.A.; Krailo, M.; Ablin, A.; Land, V.; Vietti, T.J.; Hammond, G.D. Histopathology and prognosis in childhood hepatoblastoma and hepatocarcinoma. Cancer 1989, 64, 1082–1095. [Google Scholar] [CrossRef]
- Haas, J.E.; Feusner, J.H.; Finegold, M.J. Small cell undifferentiated histology in hepatoblastoma may be unfavorable. Cancer 2001, 92, 3130–3134. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.L.; Maibach, R.; Hiyama, E.; Häberle, B.; Krailo, M.; Rangaswami, A.; Aronson, D.C.; Malogolowkin, M.H.; Perilongo, G.; von Schweinitz, D.; et al. Risk-stratified staging in paediatric hepatoblastoma: A unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017, 18, 122–131. [Google Scholar] [CrossRef] [PubMed]
- De Ioris, M.; Brugieres, L.; Zimmermann, A.; Keeling, J.; Brock, P.; Maibach, R.; Pritchard, J.; Shafford, L.; Zsiros, J.; Czauderna, P.; et al. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: The SIOPEL group experience. Eur. J. Cancer 2008, 44, 545–550. [Google Scholar] [CrossRef]
- Trobaugh-Lotrario, A.D.; Tomlinson, G.E.; Finegold, M.J.; Gore, L.; Feusner, J.H. Small cell undifferentiated variant of hepatoblastoma: Adverse clinical and molecular features similar to rhabdoid tumors. Pediatr. Blood Cancer 2009, 52, 328–334. [Google Scholar] [CrossRef]
- Vokuhl, C.; Oyen, F.; Häberle, B.; von Schweinitz, D.; Schneppenheim, R.; Leuschner, I. Small cell undifferentiated (SCUD) hepatoblastomas: All malignant rhabdoid tumors? Genes Chromosomes Cancer 2016, 55, 925–931. [Google Scholar] [CrossRef]
- Trobaugh-Lotrario, A.D.; Finegold, M.J.; Feusner, J.H. Rhabdoid tumors of the liver: Rare, aggressive, and poorly responsive to standard cytotoxic chemotherapy. Pediatr. Blood Cancer 2011, 57, 423–428. [Google Scholar] [CrossRef]
- Trobaugh-Lotrario, A.; Katzenstein, H.M.; Ranganathan, S.; López-Terrada, D.; Krailo, M.D.; Piao, J.; Chung, N.; Randazzo, J.; Malogolowkin, M.; Furman, W.L.; et al. Small Cell Undifferentiated Histology Does Not Adversely Affect Outcome in Hepatoblastoma: A Report from the Children’s Oncology Group (COG) AHEP0731 Study Committee. J. Clin. Oncol. 2021, 40, 459–467. [Google Scholar] [CrossRef]
- Zhou, S.; Gomulia, B.S.; Mascarenhas, L.; Wang, L. Is INI1-retained small cell undifferentiated histology in hepatoblastoma unfavorable? Hum. Pathol. 2015, 46, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, P.; Häberle, B.; Hiyama, E.; Rangaswami, A.; Krailo, M.; Maibach, R.; Rinaldi, E.; Feng, Y.; Aronson, D.; Malogolowkin, M.; et al. The Children’s Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur. J. Cancer 2016, 52, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Häberle, B.; Rangaswami, A.; Krailo, M.; Czauderna, P.; Hiyama, E.; Maibach, R.; López-Terrada, D.; Aronson, D.C.; Alaggio, R.; Ansari, M.; et al. The importance of age as prognostic factor for the outcome of patients with hepatoblastoma: Analysis from the Children’s Hepatic tumors International Collaboration (CHIC) database. Pediatr. Blood Cancer 2020, 67, 1–8. [Google Scholar]
- Tate, J.; Ward, G. Interferences in immunoassay. Clin. Biochem. Rev. 2004, 25, 105–120. [Google Scholar] [PubMed]
- Jassam, N.; Jones, C.M.; Briscoe, T.; Horner, J.H. The hook effect: A need for constant vigilance. Ann. Clin. Biochem. 2006, 43, 314–317. [Google Scholar] [CrossRef]
- Fernando, S.A.; Wilson, G.S. Studies of the ‘hook’ effect in the one-step sandwich immunoassay. J. Immunol. Methods 1992, 151, 47–66. [Google Scholar] [CrossRef]
- Ortega, J.A.; Douglass, E.C.; Feusner, J.H.; Reynolds, M.; Quinn, J.J.; Finegold, M.J.; Haas, J.E.; King, D.R.; Liu-Mares, W.; Sensel, M.G.; et al. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children’s Cancer Group and the Pediatric Oncology Group. J. Clin. Oncol. 2000, 18, 2665–2675. [Google Scholar] [CrossRef]
- Katzenstein, H.M.; Chang, K.W.; Krailo, M.; Chen, Z.; Finegold, M.J.; Rowland, J.; Reynolds, M.; Pappo, A.; London, W.B.; Malogolowkin, M. Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma: A report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children’s Oncology Group. Cancer 2009, 115, 5828–5835. [Google Scholar] [CrossRef]
- Zsíros, J.; Maibach, R.; Shafford, E.; Brugieres, L.; Brock, P.; Czauderna, P.; Roebuck, D.; Childs, M.; Zimmermann, A.; Laithier, V.; et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: Final results of the SIOPEL-3HR study. J. Clin. Oncol. 2010, 28, 2584–2590. [Google Scholar] [CrossRef]
- von Schweinitz, D.; Byrd, D.J.; Hecker, H.; Weinel, P.; Bode, U.; Bürger, D.; Erttmann, R.; Harms, D.; Mildenberger, H. Efficiency and toxicity of ifosfamide, cisplatin and doxorubicin in the treatment of childhood hepatoblastoma. Study Committee of the Cooperative Paediatric Liver Tumour Study HB89 of the German Society for Paediatric Oncology and Haematology. Eur. J. Cancer 1997, 33, 1243–1249. [Google Scholar] [CrossRef]
- Häberle, B.; Maxwell, R.; Schweinitz, D.V.; Schmid, I. High Dose Chemotherapy with Autologous Stem Cell Transplantation in Hepatoblastoma does not Improve Outcome. Results of the GPOH Study HB99. Klin. Padiatr. 2019, 231, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, F.; Matsunaga, T.; Iwafuchi, M.; Hayashi, Y.; Ohkawa, H.; Ohira, M.; Okamatsu, T.; Sugito, T.; Tsuchida, Y.; Toyosaka, A.; et al. Outcome of hepatoblastoma treated with the JPLT-1 (Japanese Study Group for Pediatric Liver Tumor) Protocol-1: A report from the Japanese Study Group for Pediatric Liver Tumor. J. Pediatr. Surg. 2002, 37, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Hishiki, T.; Matsunaga, T.; Sasaki, F.; Yano, M.; Ida, K.; Horie, H.; Kondo, S.; Watanabe, K.; Oue, T.; Tajiri, T.; et al. Outcome of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol-2: Report from the JPLT. Pediatr. Surg. Int. 2011, 27, 1–8. [Google Scholar] [CrossRef]
- Garcés-Iñigo, E.F.; Leung, R.; Sebire, N.J.; McHugh, K. Extrarenal rhabdoid tumours outside the central nervous system in infancy. Pediatr. Radiol. 2009, 39, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dagar, V.; Algar, E.; Muscat, A.; Bandopadhayay, P.; Ashley, D.; Wo Chow, C. Rhabdoid tumour: A malignancy of early childhood with variable primary site, histology and clinical behaviour. Pathology 2008, 40, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.V.; Cullinane, C.; Lewis, I.J.; Squire, B.R.; Stringer, M.D. Long-term survival after spontaneous rupture of a malignant rhabdoid tumor of the liver. J. Pediatr. Surg. 2002, 37, 1488–1490. [Google Scholar] [CrossRef]
- Scheimberg, I.; Cullinane, C.; Kelsey, A.; Malone, M. Primary hepatic malignant tumor with rhabdoid features. A histological, immunocytochemical, and electron microscopic study of four cases and a review of the literature. Am. J. Surg. Pathol. 1996, 20, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, A.; Finegold, M.J.; Parham, D.M.; Jasty, R. Successful management of rhabdoid tumor of the liver. J. Pediatr. Hematol. Oncol. 2007, 29, 406–408. [Google Scholar] [CrossRef]
- Blohm, M.E.; Vesterling-Hörner, D.; Calaminus, G.; Göbel, U. Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediat.r Hematol. Oncol. 1998, 15, 135–142. [Google Scholar] [CrossRef]
- Oda, Y.; Biegel, J.S.; Pfister, S.M. Soft Tissue and Bone Tumours, Extrarenal rhabdoid tumour. In WHO Classification of Tumours Editorial Board, 5th ed.; WHO Classification of Tumours Series; Paediatric Tumours; [Internet; beta version ahead of print]; International Agency for Research on Cancer: Lyon, France, 2022; Volume 7, Available online: https://tumourclassification.iarc.who.int/chapters/44 (accessed on 24 December 2022).

| Factor | Rhabdoid (n = 11) | SCU (n = 41) | Low AFP (n = 14) | ||||
|---|---|---|---|---|---|---|---|
| Sex | Male Female | 9 2 | 82% 18% | 24 17 | 59% 41% | 8 6 | 57% 43% |
| Age in years | Median Range | 0.71 0.32–1.35 | 1.45 0.22–4.80 | 1.80 0.18–10.34 | |||
| AFP in ng/mL | Median Range | 16 (n = 9) 1–756 | 63,540 (n = 5) 124–380,300 | 29 (n = 14) 0–63 | |||
| PRETEXT | 1 2 3 4 Missing | 0 3 7 1 | 0% 27% 64% 9% | 4 16 10 6 5 | 10% 39% 24% 15% 12% | 1 3 8 2 | 7% 21% 57% 14% |
| Evans stage | 1 2 3 4 | 0 2 3 6 | 0% 18% 27% 55% | 11 12 5 13 | 27% 29% 12% 32% | 2 2 8 2 | 14% 14% 57% 14% |
| Metastatic | 6 | 55% | 13 | 32% | 2 | 14% | |
| Treatment | Rhabdoid (n = 11) | SCU (n = 41) | Low AFP (n = 14) | |
|---|---|---|---|---|
| # neoadjuvant cycles | Median Range | 2 0–6 | 4 0–12 | 0 0–7 |
| # adjuvant cycles | Median Range | 0 0–5 | 4 0–12 | 0 0–6 |
| # patients with cisplatin | 9 (82%) | 41 (100%) | 7 (50%) | |
| # patients with doxorubicin | 9 (82%) | 4 (10%) | 5 (36%) | |
| # patients with carboplatin | 7 (64%) | 11 (27%) | 4 (29%) | |
| # patients with other chemo a | 3 (27%) | 28 (68%) | 1 (7%) | |
| Surgery | Upfront Delayed None/unknown | 3 (27%) 1 (9%) 7 (64%) | 13 (32%) 10 (24%) 18 (44%) | 3 (21%) 6 (43%) 5 (36%) |
| Result of surgery | Complete Micro residuals Macro residuals Unresectable None/unknown | 1 (9%) 2 (18%) 1 (9%) 4 (36%) 3 (27%) | 25 (61%) 0 0 3 (7%) 13 (32%) | 7 (50%) 1 (7%) 1 (7%) 0 5 (36%) |
| Metastectomy | 1 (9%) | 0 | 0 |
| Outcome | Rhabdoid (n = 11) | SCU (n = 41) | Low AFP (n = 14) | |
|---|---|---|---|---|
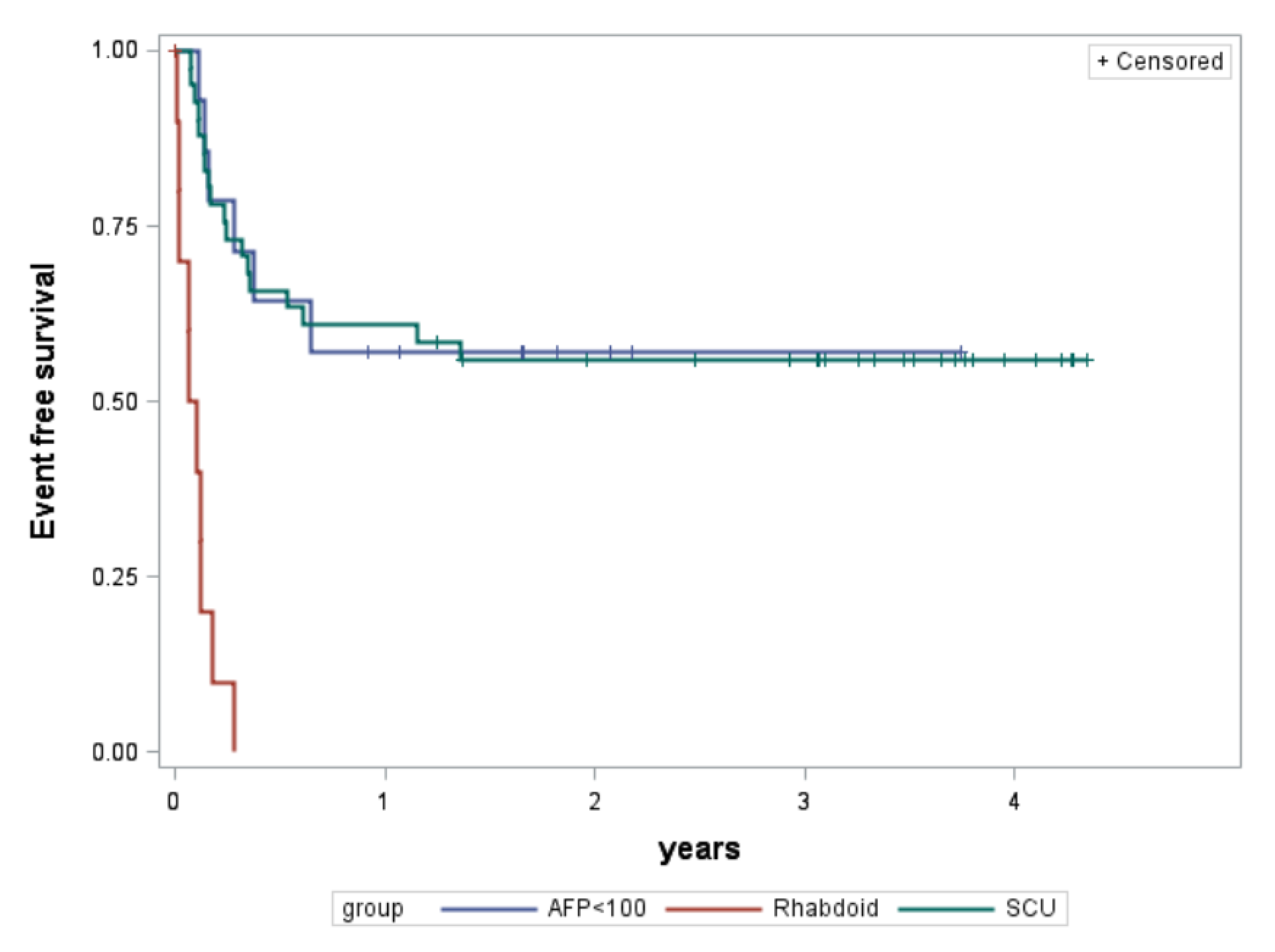
| 3-year event-free survival | Rate 95% conf.int. | 0% | 56% 40–70% | 57% 28–78% |
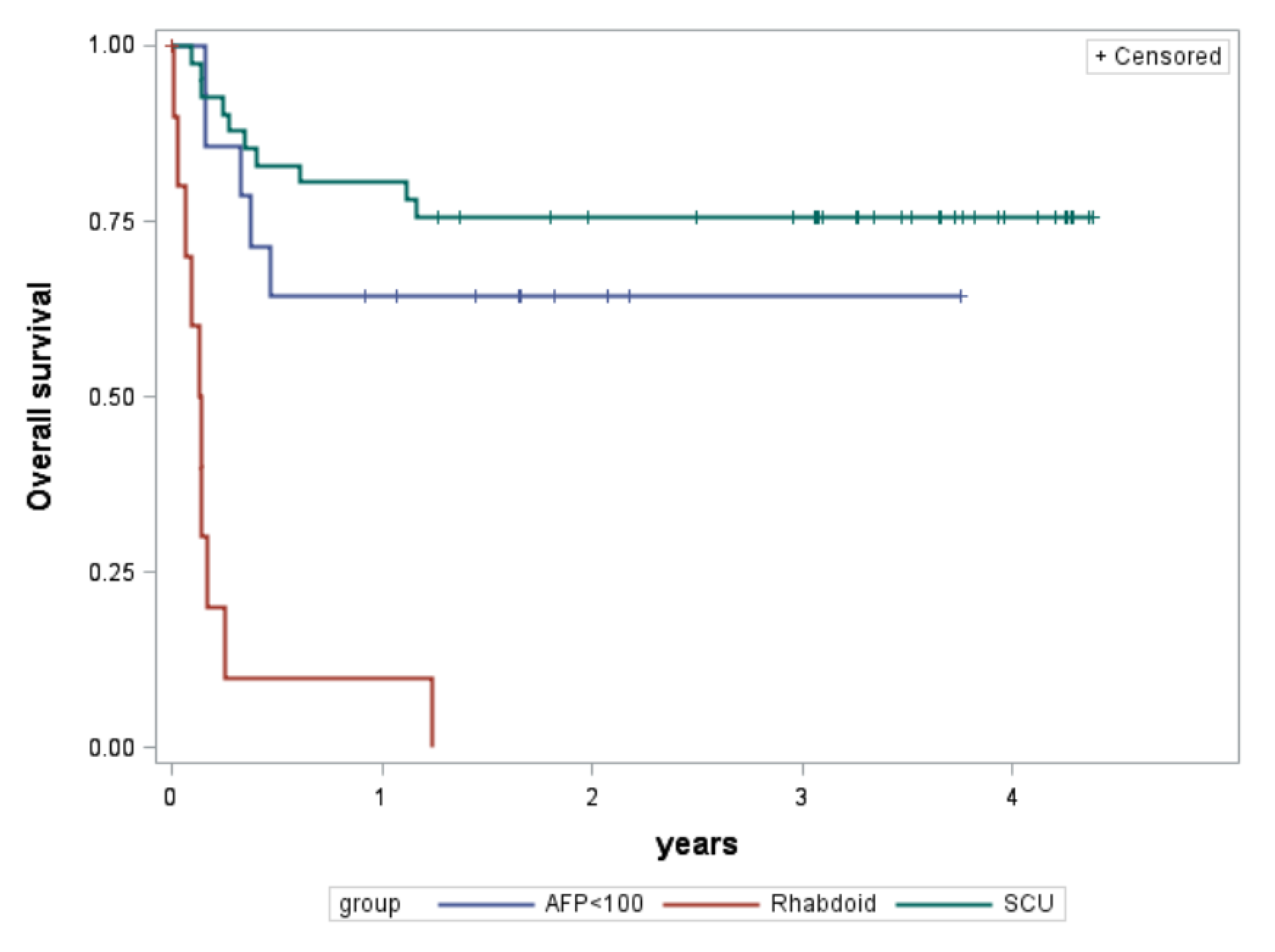
| 3-year overall survival | Rate 95% conf.int. | 0% | 76% 59–86% | 64% 34–83% |
| Backbone Group (CHIC Risk Stratification Development [2]) | EFS (CHIC) | HB with SCU Component (Current Study), 3-Year EFS |
|---|---|---|
| PRETEXT I/II, no mets, AFP > 100 | 86% | 73% (n = 15) |
| PRETEXT III, no mets, AFP > 100 | 82% | 57% (n = 7) |
| PRETEXT IV, no mets, AFP > 100 | 60% | 50% (n = 2) |
| Metastatic disease, AFP > 100 | 42% | 23% (n = 13) |
| (4 missing due to missing PRETEXT) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trobaugh-Lotrario, A.D.; Maibach, R.; Aronson, D.C.; Rangaswami, A.; Häberle, B.; O’Neill, A.F.; Schmid, I.; Ansari, M.; Hishiki, T.; Ranganathan, S.; et al. Outcomes of Patients Treated for Hepatoblastoma with Low Alpha-Fetoprotein and/or Small Cell Undifferentiated Histology: A Report from the Children’s Hepatic Tumors International Collaboration (CHIC). Cancers 2023, 15, 467. https://doi.org/10.3390/cancers15020467
Trobaugh-Lotrario AD, Maibach R, Aronson DC, Rangaswami A, Häberle B, O’Neill AF, Schmid I, Ansari M, Hishiki T, Ranganathan S, et al. Outcomes of Patients Treated for Hepatoblastoma with Low Alpha-Fetoprotein and/or Small Cell Undifferentiated Histology: A Report from the Children’s Hepatic Tumors International Collaboration (CHIC). Cancers. 2023; 15(2):467. https://doi.org/10.3390/cancers15020467
Chicago/Turabian StyleTrobaugh-Lotrario, Angela D., Rudolf Maibach, Daniel C. Aronson, Arun Rangaswami, Beate Häberle, Allison F. O’Neill, Irene Schmid, Marc Ansari, Tomoro Hishiki, Sarangarajan Ranganathan, and et al. 2023. "Outcomes of Patients Treated for Hepatoblastoma with Low Alpha-Fetoprotein and/or Small Cell Undifferentiated Histology: A Report from the Children’s Hepatic Tumors International Collaboration (CHIC)" Cancers 15, no. 2: 467. https://doi.org/10.3390/cancers15020467
APA StyleTrobaugh-Lotrario, A. D., Maibach, R., Aronson, D. C., Rangaswami, A., Häberle, B., O’Neill, A. F., Schmid, I., Ansari, M., Hishiki, T., Ranganathan, S., Alaggio, R., de Krijger, R. R., Tanaka, Y., Cho, S.-J., Vokuhl, C., Maxwell, R., Krailo, M., Hiyama, E., Czauderna, P., ... Lopez-Terrada, D. (2023). Outcomes of Patients Treated for Hepatoblastoma with Low Alpha-Fetoprotein and/or Small Cell Undifferentiated Histology: A Report from the Children’s Hepatic Tumors International Collaboration (CHIC). Cancers, 15(2), 467. https://doi.org/10.3390/cancers15020467







