VATS versus Open Lobectomy following Induction Therapy for Stage III NSCLC: A Propensity Score-Matched Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Unmatched Population
3.1.1. Patient Characteristics
3.1.2. Perioperative Outcomes
3.2. Oncologic Outcomes
3.3. Matched Population
3.3.1. Patient Characteristics and Perioperative Outcomes
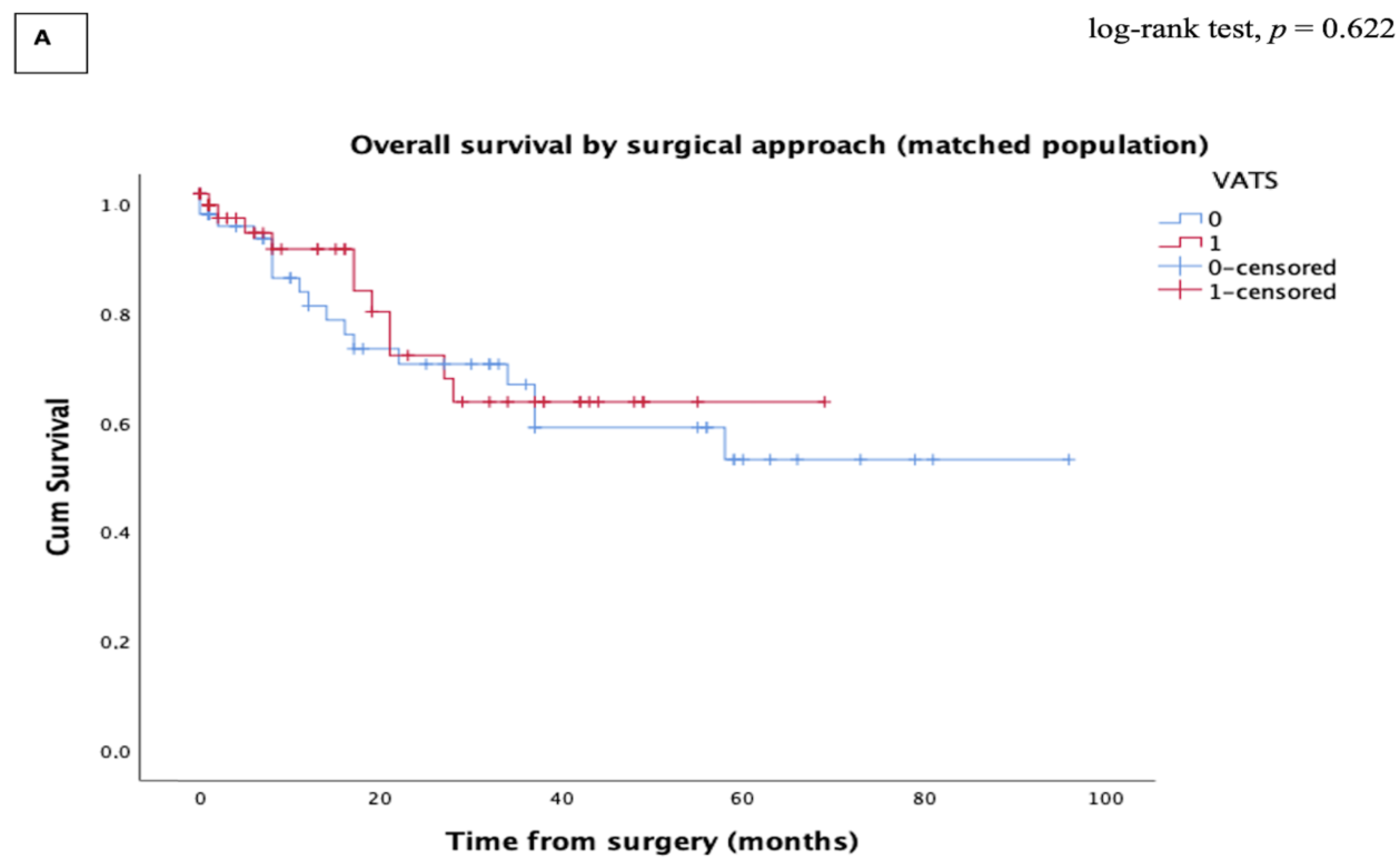
3.3.2. Oncologic Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SEER Stat Fact Sheets: Lung and Bronchus Cancer. Surveillance, Epidemiology End Results Program Cancer Stat 2021. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 22 December 2021).
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J. Natl. Compr. Canc. Netw. 2019, 17, 1464–1472. [Google Scholar] [CrossRef]
- Albain, K.S.; Rusch, V.W.; Crowley, J.J.; Rice, T.W.; Turrisi, A.T.; Weick, J.K.; Lonchyna, V.A.; Presant, C.A.; McKenna, R.J.; Gandara, D.R. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: Mature results of Southwest Oncology Group phase II study 8805. J. Clin. Oncol. 1995, 13, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.J.; Patel, A.P.; Crabtree, T.D.; Morgensztern, D.; Robinson, C.G.; Colditz, G.A.; Waqar, S.; Kreisel, D.; Krupnicka, A.S.; Patterson, G.A.; et al. Role for Surgical Resection in the Multidisciplinary Treatment of Stage IIIB Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2015, 99, 1921–1928. [Google Scholar] [CrossRef]
- David, E.A.; Canter, R.J.; Chen, Y.; Cooke, D.T.; Cress, R.D. Surgical Management of Advanced Non-Small Cell Lung Cancer Is Decreasing But Is Associated With Improved Survival. Ann. Thorac. Surg. 2016, 102, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, S.-G.; Xue, Q.; Guo, X.-T.; Wang, L.-X.; Yu, X.; Yang, Y.-K.; Mu, J.-W. Surgery of primary non-small cell lung cancer with oligometastasis: Analysis of 172 cases. J. Thorac. Dis. 2018, 10, 6540–6546. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet. Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Hernandez-Vaquero, D.; Vigil-Escalera, C.; Pérez-Méndez, I.; Gutiérrez, A.; Avanzas, P.; Wei, Y.; Diaz, R.; Silva, J.; Moris, C.; Pascual, I. Survival After Thoracoscopic Surgery or Open Lobectomy: Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2021, 111, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.P.; Pham, D.; Burfeind, W.R.; Hanish, S.I.; Toloza, E.M.; Harpole, D.H.; D’Amico, T.A. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann. Thorac. Surg. 2007, 83, 1245–1250. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, D.; Delgado, M.; Fieira, E.; Fernandez, R. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann. Cardiothorac. Surg. 2014, 3, E2. [Google Scholar] [CrossRef]
- Gao, H.-J.; Jiang, Z.-H.; Gong, L.; Ma, K.; Ren, P.; Yu, Z.-T.; Wei, Y.-C. Video-Assisted Vs Thoracotomy Sleeve Lobectomy for Lung Cancer: A Propensity Matched Analysis. Ann. Thorac. Surg. 2019, 108, 1072–1079. [Google Scholar] [CrossRef]
- Chen, K.; Wang, X.; Yang, F.; Li, J.; Jiang, G.; Liu, J.; Wang, J. Propensity-matched comparison of video-assisted thoracoscopic with thoracotomy lobectomy for locally advanced non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2017, 153, 967–976.e2. [Google Scholar] [CrossRef]
- Yang, C.-F.J.; Meyerhoff, R.R.; Mayne, N.R.; Singhapricha, T.; Toomey, C.B.; Speicher, P.J.; Hartwig, M.G.; Tong, B.C.; Onaitis, M.W.; Harpole, D.H.; et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2016, 49, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Hennon, M.; Sahai, R.K.; Yendamuri, S.; Tan, W.; Demmy, T.L.; Nwogu, C. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann. Surg. Oncol. 2011, 18, 3732–3736. [Google Scholar] [CrossRef] [PubMed]
- Hireche, K.; Moqaddam, M.; Lonjon, N.; Marty-Ané, C.; Solovei, L.; Ozdemir, B.A.; Canaud, L.; Alric, P. Combined video-assisted thoracoscopy surgery and posterior midline incision for en bloc resection of non-small-cell lung cancer invading the spine. Interact. Cardiovasc. Thorac. Surg. 2021, 34, 74–80. [Google Scholar] [CrossRef]
- Hanna, J.M.; Berry, M.F.; D’Amico, T.A. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J. Thorac. Dis. 2013, 5 (Suppl. S3), S182–S189. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, D.; Fieira, E.; Delgado, M.; Mendez, L.; Fernandez, R.; La Torre, M.d. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer. J. Thorac. Dis. 2014, 6, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Choi, Y.S.; Lee, K.J.; Lee, S.H.; Pyo, H.; Choi, J.Y. Outcomes of Pulmonary Resection and Mediastinal Node Dissection by Video-Assisted Thoracoscopic Surgery Following Neoadjuvant Chemoradiation Therapy for Stage IIIA N2 Non-Small Cell Lung Cancer. Korean. J. Thorac. Cardiovasc. Surg. 2018, 51, 29–34. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Chen, H.; Yin, W.; Shao, W.; Xiong, X.; He, J. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. S3), S267–S273. [Google Scholar] [CrossRef]
- Cao, C.; Manganas, C.; Ang, S.C.; Yan, T.D. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann. Cardiothorac. Surg. 2012, 1, 16–23. [Google Scholar] [CrossRef]
- Yang, C.-F.J.; Nwosu, A.; Mayne, N.R.; Wang, Y.-Y.; Raman, V.; Meyerhoff, R.R.; D’Amico, T.A.; Berry, M.F. A Minimally Invasive Approach to Lobectomy After Induction Therapy Does Not Compromise Survival. Ann. Thorac. Surg. 2020, 109, 1503–1511. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Choi, Y.S.; Cho, J.H.; Kim, H.K.; Kim, J.; Zo, J.I.; Shim, Y.M. Thoracoscopic Vs Open Surgery Following Neoadjuvant Chemoradiation for Clinical N2 Lung Cancer. Semin. Thorac. Cardiovasc. Surg. 2021, 34, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Agostini, P.; Lugg, S.T.; Adams, K.; Vartsaba, N.; Kalkat, M.S.; Rajesh, P.B.; Steyn, R.S.; Naidu, B.; Rushton, A.; Bishay, E. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: A propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomy. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J. Comparison of clinical outcomes for patients with clinical N0 and pathologic N2 non-small cell lung cancer after thoracoscopic lobectomy and open lobectomy: A retrospective analysis of 76 patients. J. Surg. Oncol. 2012, 106, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Yao, F.; Zhao, H. Clinical outcomes of thoracoscopic lobectomy for patients with clinical N0 and pathologic N2 non-small cell lung cancer. Ann. Thorac. Surg. 2013, 95, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, Y.S.; Kim, J.; Shim, Y.M.; Kim, K. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 1288–1293. [Google Scholar] [CrossRef]
- Watanabe, A.; Mishina, T.; Ohori, S.; Koyanagi, T.; Nakashima, S.; Mawatari, T.; Kurimoto, Y.; Higami, T. Is video-assisted thoracoscopic surgery a feasible approach for clinical N0 and postoperatively pathological N2 non-small cell lung cancer? Eur. J. Cardio-Thorac. Surg. 2008, 33, 812–818. [Google Scholar] [CrossRef]
- Wang, W.; Yin, W.; Shao, W.; Jiang, G.; Wang, Q.; Liu, L.; Liu, D.; Wang, Z.; Zhu, Z.; Chen, H.; et al. Comparative study of systematic thoracoscopic lymphadenectomy and conventional thoracotomy in resectable non-small cell lung cancer. J. Thorac. Dis. 2014, 6, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Medbery, R.L.; Gillespie, T.W.; Liu, Y.; Nickleach, D.C.; Lipscomb, J.; Sancheti, M.S.; Pickens, A.; Force, S.D.; Fernandez, F.G. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J. Thorac. Oncol. 2016, 11, 222–233. [Google Scholar] [CrossRef]
- Marty-Ané, C.-H.; Canaud, L.; Solovei, L.; Alric, P.; Berthet, J.-P. Video-assisted thoracoscopic lobectomy: An unavoidable trend? A retrospective single-institution series of 410 cases. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 36–43. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, T.A.; Niland, J.; Mamet, R.; Zornosa, C.; Dexter, E.U.; Onaitis, M.W. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann. Thorac. Surg. 2011, 92, 226–231. [Google Scholar] [CrossRef]
- Lee, P.C.; Kamel, M.; Nasar, A.; Ghaly, G.; Port, J.L.; Paul, S.; Stiles, B.M.; Andrews, W.G.; Altorki, N.K. Lobectomy for Non-Small Cell Lung Cancer by Video-Assisted Thoracic Surgery: Effects of Cumulative Institutional Experience on Adequacy of Lymphadenectomy. Ann. Thorac. Surg. 2016, 101, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Friedel, G.; Budach, W.; Dippon, J.; Spengler, W.; Eschmann, S.M.; Pfannenberg, C.; Al-Kamash, F.; Walles, T.; Aebert, H.; Kyriss, T.; et al. Phase II Trial of a Trimodality Regimen for Stage III Non-Small-Cell Lung Cancer Using Chemotherapy as Induction Treatment With Concurrent Hyperfractionated Chemoradiation With Carboplatin and Paclitaxel Followed by Subsequent Resection: A Single-Center Study. J. Clin. Oncol. 2010, 28, 942–948. [Google Scholar] [PubMed]
- Belani, C.P.; Choy, H.; Bonomi, P.; Scott, C.; Travis, P.; Haluschak, J.; Curran, W.J. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: A randomized phase II locally advanced multi-modality protocol. J. Clin. Oncol. 2005, 23, 5883–5891. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, T.; Shirakusa, T.; Hiratsuka, M.; Yamamoto, S.; Iwasaki, A. Video-assisted thoracoscopic surgery lobectomy for c-T1N0M0 primary lung cancer: Its impact on locoregional control. Ann. Thorac. Surg. 2006, 82, 1021–1026. [Google Scholar] [CrossRef]
- Flores, R.M.; Ihekweazu, U.N.; Rizk, N.; Dycoco, J.; Bains, M.S.; Downey, R.J.; Adusumilli, P.; Finley, D.J.; Huang, J.; Rusch, V.W.; et al. Patterns of recurrence and incidence of second primary tumors after lobectomy by means of video-assisted thoracoscopic surgery (VATS) versus thoracotomy for lung cancer. J. Thorac. Cardiovasc. Surg. 2011, 141, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, H.K.; Choi, Y.S.; Kim, K.; Kim, J.; Shim, Y.M. Pleural recurrence and long-term survival after thoracotomy and thoracoscopic lobectomy. Ann. Thorac. Surg. 2013, 96, 1769–1775. [Google Scholar] [CrossRef]


| Characteristics | All Included Patients | PSM Patients | ||||
|---|---|---|---|---|---|---|
| Open | VATS | p-Value | Open | VATS | p-Value | |
| Age, year ± SD | 57.6 ± 9.52 | 61.33 ± 8.54 | 0.031 | 63.09 ± 9.9 | 62.03 ± 8.03 | 0.293 |
| Sex, n (%) | 0.664 | 0.695 | ||||
| Male | 82 (64.1) | 47 (61) | 38 (59.3) | 36 (56.2) | ||
| FEV1%, mean ± SD | 90.50 ± 16.64 | 88.94 ± 16.98 | 0.008 | 87.16 ± 12.8 | 88.35 ± 17.2 | 0.579 |
| DLCO%, mean ± SD | 65.92 ± 13.76 | 67.34 ± 15.24 | 0.599 | 61.7 ± 12.56 | 68.09 ± 16.43 | 0.105 |
| Comorbidities, n (%) | ||||||
| Hypertension | 28 (21.9) | 19 (24.7) | 0.739 | 19 (29.6) | 17(26.5) | 0.653 |
| Diabetes | 17 (13.3) | 7 (9.1) | 0.041 | 7 (11) | 7 (11) | 1 |
| Cardiac disease | 21 (16.4) | 5 (6.5) | 0.039 | 5 (7.8) | 3 (4.6) | 0.358 |
| COPD | 25 (19.5) | 19 (24.7) | 0.385 | 10 (15.6) | 12 (18.7) | 0.633 |
| Smoking history, n (%) | 103 (80.5) | 65 (84.4) | 0.106 | 51 (79.7) | 54 (84.4) | 0.080 |
| cTm size, mm ± SD | 54.12 ± 35.77 | 31.16 ± 17.33 | 0.002 | 37.84 ± 20.8 | 35.01 ± 17.2 | 0.714 |
| cTNM, n (%) | 0.033 | 0.435 | ||||
| IIIA | 77(60.2) | 58 (75.3) | 43 (67.2) | 48 (75) | ||
| IIIB | 51 (39.8) | 19 (24.7) | 21(32.8) | 16 (25) | ||
| Induction therapy, n (%) | 128 (100) | 77 (100) | 1 | 64 (100) | 64 (100) | 1 |
| Time from preoperative therapy to surgery (days) | 95 (72–135) | 91(72–128) | 0.723 | 92 (72–130) | 89 (72–126) | 0.642 |
| Tumor location, n (%) | 0.880 | 0.983 | ||||
| Right upper | 50 (39.1) | 32 (41.6) | 27 (42.2) | 25 (39.1) | ||
| Right middle | 4 (3.1) | 4 (5.2) | 3 (4.7) | 4 (6.3) | ||
| Right lower | 21 (16.4) | 15 (19.5) | 11 (17.2) | 10 (15.6) | ||
| Left upper | 34 (26.6) | 17 (22.1) | 15 (23.4) | 17 (26.5) | ||
| Left lower | 16 (12.5) | 10 (13) | 8 (12.5) | 8 (12.5) | ||
| Surgical procedure | ||||||
| Lobectomy | 47 (36.7) | 59 (76.6) | 24 (37.5) | 48 (75) | ||
| Bilobectomy | 28 (21.9) | 10 (13) | 13 (20.3) | 8 (12.5) | ||
| Pneumonectomy | 32 (25) | 3 (3.9) | 16 (25) | 3 (4.7) | ||
| Sleeve lobectomy | 21 (16.4) | 5 (6.5) | 11 (17.2) | 5 (7.8) | ||
| Characteristics | All Included Patients | PSM Patients | ||||
|---|---|---|---|---|---|---|
| Open | VATS | p-Value | Open | VATS | p-Value | |
| Induction therapy, n (%) | ||||||
| Platinum doublet therapy only | 90 (70.3) | 48 (62.4) | 0.282 | 39 (60.9) | 38(59.4) | 1 |
| Platinum doublet therapy + Immunotherapy | 5 (3.9) | 3 (3.9) | 1 | 5 (7.8) | 3 (4.7) | 0.717 |
| Chemoradiotherapy | 33 (25.8) | 26 (33.7) | 0.265 | 20 (31.3) | 23 (35.9) | 0.708 |
| pTm size, mm ± SD | 42.3 ± 30 | 31.4 ± 18.9 | 0.002 | 33.2 ± 23.1 | 30.2 ± 16.3 | 0.696 |
| yp tumor stage, n (%) | <0.001 | 0.488 | ||||
| T0 | 14 (10.9) | 11 (14.3) | 8 (12.5) | 10 (15.6) | ||
| T1 | 23 (18) | 33 (42.8) | 18 (28.1) | 25 (39.1) | ||
| T2 | 34 (26.5) | 16 (20.8) | 16 (25) | 14 (21.9) | ||
| T3 | 37 (29) | 13 (16.9) | 15 (23.4) | 12 (18.7) | ||
| T4 | 20 (15.6) | 4 (5.2) | 7 (11) | 3 (4.7) | ||
| yp nodal stage, n (%) | 0.013 | 0.227 | ||||
| N0 | 76 (59.3) | 55 (71.4) | 30 (46.9) | 46 (71.8) | ||
| N1 | 27 (21.1) | 12 (15.6) | 16 (25) | 10 (15.6) | ||
| N2 | 25 (19.5) | 10 (12.9) | 18 (28.1) | 8 (12.5) | ||
| Histology | 0.910 | 0.983 | ||||
| Adenocarcinoma | 76 (59.4) | 48 (62.4) | 38 (59.4) | 37 (57.8) | ||
| Squamous cell | 47 (36.7) | 26 (33.8) | 24(37.5) | 25 (39.1) | ||
| Other | 5 (3.9) | 3 (3.8) | 2 (3.1) | 2 (3.1) | ||
| Lymph nodes, mean ± SD | ||||||
| Total stations | 5 ± 1.9 | 5.5 ± 1.6 | 0.078 | 4.8 ± 1.6 | 5.9 ± 1.7 | 0.011 |
| Total lymph nodes | 10.7 ± 6.7 | 12.2 ± 5.1 | 0.400 | 11.5 ± 6.3 | 12.5 ± 5.3 | 0.219 |
| Completeness of resection, n (%) | ||||||
| R0 | 105 (82) | 73 (94.8) | 0.012 | 58 (90.6) | 60 (93.7) | 0.443 |
| Perioperative Event | All Included Patients | PSM Patients | ||||
|---|---|---|---|---|---|---|
| Open | VATS | p-Value | Open | VATS | p-Value | |
| Conversion to open, n (%) | 12 (15.6) | 10 (15.6) | ||||
| Any complication, n (%) | 50 (39.1) | 23 (29.9) | 0.183 | 21 (32.8) | 13 (20.3) | 0.109 |
| Air leak > 5 days | 14 (11) | 10 (12.9) | 0.533 | 5 (7.8) | 5 (7.8) | 1 |
| Atrial arrhythmia | 6 (4.7) | 3 (3.9) | 0.879 | 3 (4.7) | 2 (3.1) | 1 |
| Pneumonia | 11 (8.6) | 5 (6.5) | 0.786 | 4 (6.2) | 3 (4.7) | 1 |
| ARDS, n (%) | 6 (4.7) | 2 (2.6) | 0.713 | 4 (6.2) | 2 (3.1) | 0.679 |
| BPF, n (%) | 6 (4.7) | 0 (0) | 0.148 | 3 (4.7) | 0 (0) | 0.244 |
| Pleural effusion, n (%) | 7 (5.4) | 3 (3.9) | 0.936 | 2 (3.1) | 1 (1.6) | 1 |
| Reoperation, n (%) | 12 (9.4) | 3 (3.9) | 0.129 | 3 (4.7) | 1 (1.6) | 0.619 |
| Surgery time, min, median (range) | 180 (60–520) | 175 (60–430) | 0.199 | 180 (60–348) | 180 (60–330) | 0.827 |
| Blood loss, mL, median (range) | 140 (50–2200) | 130 (50–1200) | 0.956 | 195 (50–1500) | 140 (50–1100) | 0.023 |
| Chest tube duration, days, median (range) | 3 (2–20) | 2 (1–22) | 0.311 | 3 (2–20) | 2 (1–15) | 0.160 |
| Length of stay, days, median (range) | 8 (3–40) | 4 (2–45) | <0.0001 | 7 (5–40) | 4 (2–35) | <0.0001 |
| 30 days in hospital death | 2 (1.5) | 1 (1.3) | 1 | 1 (1.6) | 0 (0) | 1 |
| Recurrence | All Included Patients | PSM Patients | ||||
|---|---|---|---|---|---|---|
| Open | VATS | Open | VATS | p-Value | ||
| Overall n (%) | 61 (47.6) | 31 (40.3) | 0.356 | 30 (46.9) | 25 (39.1) | 0.430 |
| Local (mediastinum) n (%) | 9 (7) | 3 (4) | 5 (7.8) | 3 (4.7) | ||
| Regional (lung) n (%) | 16 (12.5) | 5 (6.5) | 7 (10.9) | 3 (4.7) | ||
| Distant n (%) | 36 (28.1) | 23 (29.8) | 18 (28.2) | 19 (29.7) | ||
| Characteristics | All Included Patients | PSM Patients | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.023 | 0.988–1.059 | 0.195 | 1.041 | 0.993–1.091 | 0.095 |
| Sex (ref = female) | 0.686 | 0.356–1.321 | 0.260 | 0.349 | 0.134–0.911 | 0.031 |
| FEV1 | 0.998 | 0.979–1.017 | 0.815 | 0.980 | 0.953–1.008 | 0.158 |
| DLCO | 0.990 | 0.966–1.014 | 0.413 | 0.979 | 0.945–1.015 | 0.253 |
| Comorbidities | ||||||
| Hypertension | 1.024 | 0.485–2.164 | 0.950 | 0.543 | 0.218–1.351 | 0.189 |
| Diabetes | 1.348 | 0.451–2.024 | 0.593 | 0.716 | 0.117–1.365 | 0.717 |
| Cardiac disease | 1.240 | 0.495–2.104 | 0.646 | 1.347 | 0.716–1.639 | 0.125 |
| COPD | 0.654 | 0.325–1.314 | 0.233 | 2.502 | 0.949–3.598 | 0.064 |
| Smoking history | 0.999 | 0.984–1.014 | 0.903 | 1.002 | 0.982–1.023 | 0.860 |
| cTm size | 0.985 | 0.968–1.001 | 0.074 | 0.950 | 0.921–0.979 | 0.001 |
| Histology | ||||||
| Adenocarcinoma (ref) | 2.919 | 0.297–3.671 | 0.358 | 2.441 | 0.464–3.327 | 0.165 |
| Squamous cell | 1.381 | 0.494–2.608 | 0.167 | 2.126 | 0.385–3.554 | 0.199 |
| VATS (ref) | 1.589 | 0.812–2.109 | 0.176 | 2.359 | 0.946–2.883 | 0.066 |
| pTNM | 1.049 | 1.022–1.077 | <0.001 | 1.092 | 1.050–1.136 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hireche, K.; Lounes, Y.; Bacri, C.; Solovei, L.; Marty-Ané, C.; Canaud, L.; Alric, P. VATS versus Open Lobectomy following Induction Therapy for Stage III NSCLC: A Propensity Score-Matched Analysis. Cancers 2023, 15, 414. https://doi.org/10.3390/cancers15020414
Hireche K, Lounes Y, Bacri C, Solovei L, Marty-Ané C, Canaud L, Alric P. VATS versus Open Lobectomy following Induction Therapy for Stage III NSCLC: A Propensity Score-Matched Analysis. Cancers. 2023; 15(2):414. https://doi.org/10.3390/cancers15020414
Chicago/Turabian StyleHireche, Kheira, Youcef Lounes, Christophe Bacri, Laurence Solovei, Charles Marty-Ané, Ludovic Canaud, and Pierre Alric. 2023. "VATS versus Open Lobectomy following Induction Therapy for Stage III NSCLC: A Propensity Score-Matched Analysis" Cancers 15, no. 2: 414. https://doi.org/10.3390/cancers15020414
APA StyleHireche, K., Lounes, Y., Bacri, C., Solovei, L., Marty-Ané, C., Canaud, L., & Alric, P. (2023). VATS versus Open Lobectomy following Induction Therapy for Stage III NSCLC: A Propensity Score-Matched Analysis. Cancers, 15(2), 414. https://doi.org/10.3390/cancers15020414