Simple Summary
Therapeutic options for rare cancers are frequently limited and less effective than for common cancers. Therefore, novel therapeutic strategies to treat rare cancers are urgently required. Our clinical study on rare cancers showed that the biweekly WT1 Trio peptide vaccine comprising two WT1-cytotoxic T lymphocyte (CTL)-peptides and one WT1-helper T lymphocyte-peptide induced more robust immune responses targeting WT1 than the weekly WT1-CTL peptide (WT1-235) vaccine. In addition, the safety of WT1 Trio was confirmed without severe treatment-related adverse events, except grade 3 myasthenia gravis-like symptoms observed in a patient with thymic cancer (TC). Fifteen (33.3%) of the 45 patients with recurrent or advanced rare cancers, including malignant glioma, soft-tissue sarcoma, TC, and malignant pleural mesothelioma, achieved stable disease after 3 months of protocol treatment. Therefore, since WT1 is widely overexpressed in rare cancers, WT1-targeted immunotherapy may be a therapeutic strategy for rare cancers.
Abstract
No standard treatment has been established for most rare cancers. Here, we report a clinical trial of a biweekly WT1 tri-peptide-based vaccine for recurrent or advanced rare cancers. Due to the insufficient number of patients available for a traditional clinical trial, the trial was designed for rare cancers expressing shared target molecule WT1. The recruitment criteria included WT1-expressing tumors as well as HLA-A*24:02 or 02:01. The primary endpoints were immunoglobulin G (IgG) antibody (Ab) production against the WT1-235 cytotoxic T lymphocyte (CTL) epitope and delayed-type hypersensitivity (DTH) skin reactions to targeted WT1 CTL epitopes. The secondary endpoints were safety and clinical efficacy. Forty-five patients received WT1 Trio, and 25 (55.6%) completed the 3-month protocol treatment. WT1-235 IgG Ab was positive in 88.0% of patients treated with WT1 Trio at 3 months, significantly higher than 62.5% of the weekly WT1-235 CTL peptide vaccine. The DTH positivity rate in WT1 Trio was 62.9%, which was not significantly different from 60.7% in the WT1-235 CTL peptide vaccine. The WT1 Trio safety was confirmed without severe treatment-related adverse events, except grade 3 myasthenia gravis-like symptoms observed in a patient with thymic cancer. Fifteen (33.3%) patients achieved stable disease after 3 months of treatment. In conclusion, the biweekly WT1 Trio vaccine containing the WT1-332 helper T lymphocyte peptide induced more robust immune responses targeting WT1 than the weekly WT1-235 CTL peptide vaccine. Therefore, WT1-targeted immunotherapy may be a potential therapeutic strategy for rare cancers.
1. Introduction
Rare cancers comprise a diverse collection of less common cancers. According to the National Cancer Institute definition [1], all but 11 cancer types, including breast, lung, prostate, colon, and rectal cancers, are classified as rare in American adults [2]. Although the number of patients with cancer is small, 15–25% are diagnosed with rare cancers [3,4]. Compared with the more common malignancies, the 5-year survival rate is worse in patients with rare cancers. Several essential factors, including limited well-established therapies and delayed diagnosis, contribute to poor survival. Therefore, since treatment options for rare cancers are frequently more limited and less effective than those for more common cancers, novel therapeutic strategies to treat rare cancers are urgently needed.
The WT1 gene was initially isolated as a tumor suppressor gene responsible for Wilms’ tumor, which is a pediatric renal neoplasm [5]. However, WT1 has oncogenic functions [6,7,8,9,10] and is overexpressed in leukemia [11] and various types of solid tumors, including lung [12], colorectal [13], pancreatic [14], malignant gliomas [15], and bone and soft-tissue sarcomas [16]. WT1-targeted immunotherapy has been considered a promising therapeutic strategy for various cancers owing to its tumor-specific expression and high immunogenicity [17,18,19]. Consequently, we and others have demonstrated the clinical utility of WT1-targeted immunotherapies in multiple forms, including a WT1 peptide cancer vaccine [20,21,22,23], WT1 peptide-pulsed dendritic cell vaccine [24], or WT1 mRNA-electroporated dendritic cell vaccine [25], as well as WT1-specific T cell receptor-transduced T-cell therapy [26,27].
We conducted multiple clinical trials using the HLA-A*24:02 binding cytotoxic T lymphocyte (CTL) peptide WT1-235, which showed an association of favorable prognosis with a positive delayed-type hypersensitivity (DTH) skin test for the WT1-235 peptide [28,29,30] and production of WT1-235 peptide immunoglobulin G (IgG) antibody (Ab) [29,30]. As the class switch from IgM to IgG requires the help of helper T cells, the association between WT1 peptide IgG Ab production and favorable prognosis suggests the importance of cluster of differentiation 4 (CD4+) T cells in WT1 peptide vaccines. We identified WT1-332 as an HLA class II-binding helper T lymphocyte (HTL) helper peptide and demonstrated that it enhanced WT1 CTL responses in vitro [31]. Furthermore, we recently demonstrated that a combination of helper peptides enhances the intratumoral infiltration of lymphocytes in a tumor-bearing mouse model [32]. Therefore, based on these preclinical data, we conducted a clinical trial of the cocktail vaccine for recurrent glioblastoma multiforme, a weekly combinatorial vaccination of CTL peptide, and an HTL peptide vaccine at 2-week intervals. This clinical trial showed the clinical safety of a combination of WT1 CTL and HTL peptides [33]. The cocktail vaccine induced Th1-type, WT1-235-specific CD8+ CTLs, more efficiently than the WT1-235-CTL peptide vaccine at 4–7 weeks after the start of vaccination. In patients receiving the cocktail vaccine, the frequency of WT1 CD8+ CTLs correlated with that of WT1-332-specific CD4+ T cells [34]. These findings show that the HLA class II-binding HTL helper peptide WT1-332 can enhance the anti-tumor effects of the WT1-235 CTL vaccine in clinical settings.
Recent advances in understanding oncogenic molecular abnormalities and the ability to target them have provided a therapeutic spectrum across tumor types, including molecular targets shared by various tumor tissues [35]. Since all WT1-expressing tumors are targets of WT1-specific CTLs, WT1 may be a common target antigen for rare cancers, and WT1-targeting immunotherapy could be applied to rare cancers expressing WT1. Here, we report a clinical trial of a WT1 Trio peptide vaccine, which comprises two WT1 CTL peptides, WT1-126 and WT1-235, and an HTL peptide, WT1-332. In this clinical trial, we evaluated whether the WT1 Trio peptide vaccine enhanced the DTH response and IgG Ab production in patients with recurrent or advanced rare cancers where no standard treatment has been established as the primary endpoints. Additionally, we evaluated safety, clinical efficacy, and other immune responses as the secondary endpoints.
2. Materials and Methods
2.1. Peptides
The Good Manufacturing Practice grade WT1-235 (CYTWNQMNL) [20,21,22,23], WT1-126 (RMFPNAPYL) [17], and WT1-332 (KRYFKLSHLQMHSRKH) [33] for HLA-A*24:02, HLA-A*02:01, and HLA class II, respectively, were synthesized at the Peptide Institute (Ibaraki, Osaka, Japan). The biophysical properties of the peptides are presented in Table S1.
2.2. Treatment
Patients were recruited between May 2017 and December 2018. The eligibility criteria included the following: histologically confirmed rare cancers unamenable to potentially curative therapies. In addition, WT1 protein expression in tumor cells, HLA-A*24:02 or 02:01 positivity, the age range of 16–85 years, and good organ function were also assessed. The cancers included malignant glioma (grade III anaplastic astrocytoma and grade IV glioblastoma multiforme), soft tissue sarcoma, malignant pleural mesothelioma (MPM), thymic cancer (TC), thymoma, head and neck mucosal malignant melanoma, ophthalmic malignant melanoma, head and neck mucoepidermoid carcinoma, olfactory neuroblastoma, cancer of the external auditory canal, cancer of the small intestine, salivary gland cancer, testicular cancer, penile cancer, adrenal cancer, Merkel cell carcinoma, and cutaneous lymphoma. As previously described, WT1 protein expression was immunohistochemically assessed [8]. The WT1 Trio peptide vaccine comprised two WT1 CTL peptides, WT1-126 and WT1-235, and an HTL peptide, WT1-332, emulsified with Montanide ISA51 adjuvant (Sepic, Paris, France). We administered 2 mg of each of the three peptides emulsified with Montanide ISA51 adjuvant (Sepic, Paris, France) seven times at 2-week intervals (Figure 1A). The trials were registered as UMIN000023579 (registered on 10 September 2016) in the UMIN Clinical Trials Registry. The ethical review board of the Faculty of Medicine, Osaka University, approved this study (ID 15605, date of approval: 7 September 2016). Furthermore, written informed consent was obtained from all participants at Osaka University Hospital. These clinical trials were one-armed; therefore, they did not adhere to the CONSORT statement. We conducted our study following the Japanese Ethical Guidelines for Medical and Biological Research Involving Human Subjects. The procedure is audited externally.
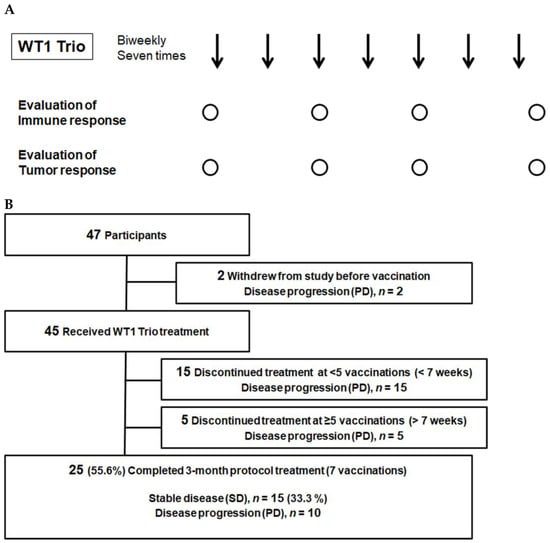
Figure 1.
Clinical study design and patient flow chart. (A) Clinical trial schema. Arrows, administration of WT1 Trio; circles, Collection of blood samples and image testing. (B) Flowchart of the enrolled patients. PD, progressive disease; SD, stable disease.
2.3. Evaluation of the Safety and Clinical Efficacy
Anti-tumor effects were assessed by determining the response of target lesions on computed tomography scans according to the Response Evaluation Criteria in Solid Tumors v1.1 [36]. Safety was assessed by monitoring and recording adverse events (AEs), vital signs, clinical chemistry, and hematology. AEs were graded according to the Common Terminology Criteria for Adverse Events v4.0 [37].
2.4. Collection of Samples for Immune Monitoring
A written informed consent for immune monitoring was obtained from the participants at Osaka University Hospital. The ethical review board of the Faculty of Medicine, Osaka University, approved this study (ID:13110 and 11293, date of approval: 17 October 2013 and 15 June 2012). Sera were obtained from patients with written informed consent at Osaka University Hospital at the indicated time points (Figure 1A). Serum samples were stored at −20 °C until use. Furthermore, blood samples were obtained from the patients to collect peripheral blood mononuclear cells (PBMC). PBMCs were isolated from heparinized whole blood using the standard Ficoll-Paque separation method and cryopreserved in liquid nitrogen until further use.
2.5. DTH Skin Test
WT1 DTH skin tests were performed monthly during the protocol treatment. Briefly, 10 μg of each WT1 peptide, WT1-126, WT1-235, and WT1-332, separately dissolved in saline, and saline alone (control) was intradermally injected into the forearm of the skin. DTH-positivity was defined as erythema ≥ 2 mm in diameter measured 48 h after injection.
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
WT1 epitope-specific IgG Abs in the serum were measured using ELISA, as described previously [29,38]. WT1 peptides or citrate (negative control) were immobilized on the bottom surface of each well using the MK100 Peptide Coating Kit (Takara, Shiga, Japan) according to the manufacturer’s instructions. After blocking, patient sera diluted 1:100 were added to each well and incubated at 4 °C overnight. All serum samples were measured in duplicates. After washing, horseradish peroxidase (HRP)-conjugated rabbit anti-human IgG Ab (Santa Cruz Biotechnology, Santa Cruz, TX, USA) diluted 1:2000 in TBST was added to each well and incubated. After washing, HRP-conjugated goat anti-rabbit IgG, diluted 1:5000 in TBST, was added and incubated. Furthermore, bound WT1 epitope-specific IgG Abs were colorimetrically detected using TMB substrate (KPL, Baltimore, MD, USA). Absorbance was measured at 450 nm using a microplate reader (MULTISKAN FC, Thermo Fisher Scientific, Waltham, MA, USA). The Ab titer for each serum sample was determined as the average absorbance value of duplicate wells after subtracting the absorbance value of the negative control well. The cutoff levels for the positivity of WT1-235 IgG, WT1-126, and WT1-332 IgG were set at 0.15 based on the absorbance values of the mean + 3SD from five independent assays in negative control serum samples.
2.7. Enzyme-Linked Immunospot (ELISPOT) Assay
As reported previously, the ELISPOT assay was performed [38,39]. Briefly, after hydrophilization, the bottom membrane of each well in a 96-well filtration plate (Merck) was incubated with capture Ab, anti-human interferon-gamma (IFN-γ) monoclonal Ab (anti-human IFN-γ mAb 1-D1K; MABTECH, Cincinnati, OH, USA, #3420-3-250, final concentration: 15 μg/mL in phosphate-buffered saline [PBS]), anti-human tumor necrosis factor-alpha (TNF-α) monoclonal Ab (anti-human TNF-α mAb TNF3/4; MABTECH, #3510-3-250, final concentration: 7.5 μg/mL of PBS), or anti-human interleukin 10 (IL-10) monoclonal Ab (anti-human IL-10 mAb; final concentration, 15 µg/mL in PBS; cat. no. 3430-3-250; Mabtech AB), at 4 °C overnight. After blocking the membranes, thawed PBMCs suspended in fetal bovine serum (FBS)-free RPMI1640 medium (5 × 104 cells per 100 μL) were seeded in each well in triplicate, stimulated with the antigen peptide at a final concentration of 10 μg/mL, and incubated at 37 °C for 48 h. After removing the cell suspension, each membrane was incubated with the corresponding detection Abs in PBS containing 1% bovine serum albumin and 0.05% tween-20, and a biotinylated anti-human IFN-γ monoclonal Ab (MABTECH, #3420-6-250, final concentration 1.3 μg/mL), a biotinylated-anti-human TNF-α monoclonal Ab (TNF5, MABTECH, #3510-6-250, final concentration of 1.5 μg/mL), or a biotinylated anti-human IL-10 monoclonal Ab (final concentration, 1.3 µg/mL; cat. no. 3430-6-250; Mabtech AB) was subsequently incubated with the sample at 4 °C overnight. After washing with PBS, each membrane was incubated with alkaline phosphatase-conjugated streptavidin (MABTECH, #3310-8, diluted 1:500 with 0.05% PBST) at room temperature for 1 h. After washing both sides of the membranes, the spots were colored using a BCIP/NBT solution (Nacalai Tesque, Kyoto, Japan), followed by washing with deionized water. Furthermore, after drying at 4 °C overnight, the membranes were punched out using an acrylic device, “ELI 8” (Create Ltd., Osaka, Japan). The membranes were scanned at a resolution of 1200 dpi. The generated digital images were analyzed for spot counting with the assistance of particle analysis using the NIH ImageJ software (version 1.50i; National Institutes of Health, Bethesda, MD, USA). WT1 antigen-specific IFN-γ production/secretion by PBMCs was described as the antigen-specific IR index as follows: (number of spot-forming cells in antigen-stimulated test conditions)/(number of spot-forming cells in antigen-free control conditions). The cutoff level for positive detection of antigen-specific cytokine production/secretion was 1.0 in the immune response index (IR index).
2.8. Statistical Analysis
Differences in WT1-235 IgG, WT1-126 IgG, and WT1-332 IgG Ab titers were analyzed using Welch’s t-test. Differences in the positivity rates of WT1-235 IgG, WT1-126 IgG, and WT1-332 IgG Abs and DTH skin reaction to WT1-126 or WT1-235 CTL epitopes between the WT1-235 CTL peptide vaccine and WT1 Trio at the end of the treatment protocol were analyzed using Fisher’s exact probability test. In addition, the association between immune factors before vaccination with the WT1 Trio and clinical outcomes in patients with malignant glioma was evaluated using Fisher’s exact probability test. The significant difference between Welch’s t-test and Fisher’s exact probability test was set at p < 0.05. The statistical analysis was performed using the Excel Statcel4 add-in software (OMS Publishing Ltd., Saitama, Japan).
3. Results
3.1. Patient Characteristics
This clinical trial included 80 participants. WT1 expression in tumor cells and HLA-A was examined after obtaining consent for testing. WT1 expression was confirmed by immunostaining in 73 (91.3%) of the 80 patients analyzed. HLA-A was adapted for 55 (68.8%) of the 80 participants. The enrolled patients included 30 males and 17 females, aged 18–81 (median 55 years). There were 23 malignant gliomas, nine soft tissue sarcomas, five TCs, six MPMs, one bronchial mucoepidermoid carcinoma, one salivary gland cancer, one thymoma, and one olfactory neuroblastoma (Table 1). Soft tissue sarcomas included two leiomyosarcomas, two rhabdomyosarcomas, two synovial sarcomas, one liposarcoma, one myxofibrosarcoma, and one solitary fibrous tumor.

Table 1.
Patient characteristics.
Number of patients who regularly received steroids and NSAIDs at the time of vaccination initiation is indicated.
The WT1 Trio vaccine was administered following a treatment-free period of at least 4 weeks. We administered one or more WT1 tripeptide vaccines to 45 out of 47 patients. However, 20 patients dropped out due to tumor progression; a total of 25 patients completed the 3-month protocol treatment. Of the 20 dropouts, 15 and 5 dropped out in the first (i.e., before the 7th week) and second (i.e., after the 7th week) half of the protocol treatment period, respectively (Figure 1B).
3.2. Immunological Responses
Here, the primary endpoints were the production of the WT1-235 IgG Ab and the DTH reaction to the WT1-235 peptide. This is because both endpoints have been shown to correlate with prognosis in previous studies [28,29,30].
The serum levels of IgG Abs against the three antigenic epitopes WT1-235, WT1-126, and WT1-332 were measured using ELISA before and at 1, 2, and 3 months (1 M, 2 M, and 3 M) after the start of vaccination. All three serum Ab levels were significantly higher at 2 M and 3 M than those before vaccination, confirming the induction of antigen epitope-specific IR (Figure 2A).
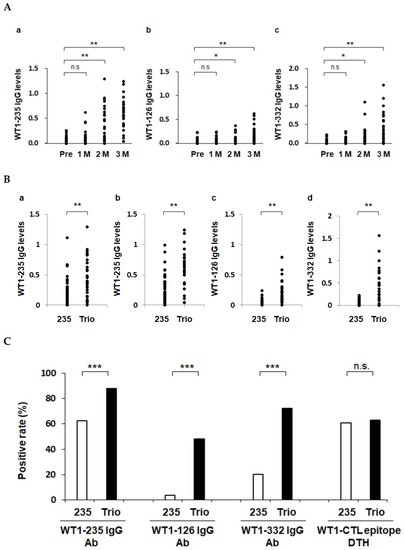
Figure 2.
Enhanced induction of WT1-specific immune responses by WT1 Trio. Serum IgG antibody levels against WT1-126, WT1-235, and WT1-332 epitopes were measured using ELISA. *, p < 0.05; **, p < 0.01, and ***, p < 0.001. n.s., not significant. (A) Induction of IgG responses during treatment with WT1 Trio. (B) Serum IgG levels in the WT1-235 CTL peptide vaccine (235) and the WT1 Trio peptide vaccine (Trio) were compared. Serum IgG levels at 2 months (a) and 3 months (b–d). (C) Positive rates of the primary endpoints: serum IgG antibody against the three WT1 epitopes and DTH against WT1-CTL epitopes (WT1-235 and WT1-126).
Next, we compared the biweekly WT1 Trio and weekly WT1-235 CTL peptide vaccines (Figure S1) regarding their induction of IgG Ab production against the target epitope WT1-235. Patients treated with the WT1-235 CTL peptide vaccine included 43 and 13 with malignant glioma [29] and non-small-cell lung cancer, respectively. Regarding Ab levels and positivity rates, WT1 Trio induced the production of WT1-235 IgG Abs more robustly than the WT1-235 peptide vaccine. We further compared the WT1 Trio and WT1-235 CTL peptide vaccines in terms of their induction of IgG Ab production against the WT1-126 and WT1-332 epitopes. Serum levels of WT1-126 and WT1-332 IgG Abs in patients treated with WT1 Trio were significantly higher than those in the WT1-235 CTL peptide vaccine at 3 M of vaccination. These results show that the WT1 Trio has more potential to induce the production of IgG Abs than the WT1-235 CTL peptide vaccine (Figure 2B,C).
We evaluated CTL epitope-specific DTH responses in 27 patients treated with WT1 Trio, for which data were available both before vaccination and 1 M after the vaccination began. In patients treated with WT1 Trio, 17 of the 27 (62.9%) were positive for the DTH skin test for HLA-matched CTL peptide. In patients treated with the WT1-235 vaccine in the control group, 34 of 56 (60.7%) patients were positive for the DTH skin test. The positivity rates were not significantly different between the WT1 Trio and WT1-235 CTL peptide vaccines (Figure 2C).
We further analyzed the cellular immune responses to the target antigen WT1-235 using the ELISPOT assay. The production and secretion of three cytokines from PBMCs collected before vaccination and 1 M, 2 M, and 3 M after the start of vaccination. WT1-235-specific cellular immune responses were induced in different patterns, depending on the disease group. Regarding WT1-235-specific IFN-γ production, the IR index increased in patients with GBM and AA after the initiation of the administration, although the timing after the start of vaccination differed. In patients with soft-tissue sarcoma (STS), the IR index increased slowly and became weakly positive at 3 months. In contrast, the IR index, which was positive before vaccination, decreased after the start of vaccination in patients with TC and MPM. For WT1-235-specific TNF-α production, the IR index was positive in patients with GBM, AA, or MPM before vaccination. However, the IR index declined after the start of administration in all three diseases and recovered at 3 M in GBM. Conversely, it continued to decline during the 3 months of protocol treatment in AA and MPM. The IR index of IL-10, which was a type 2 T helper cell (Th2)-type cytokine, was constant at approximately 1.0 in all diseases, and the antigen-specific Th2-type cellular immune responses were weak during the 3-month treatment period. In conclusion, we found that cellular immune responses changed dynamically after the start of the WT1 Trio vaccination, and that their mode differed depending on the specific disease (Figure 3).
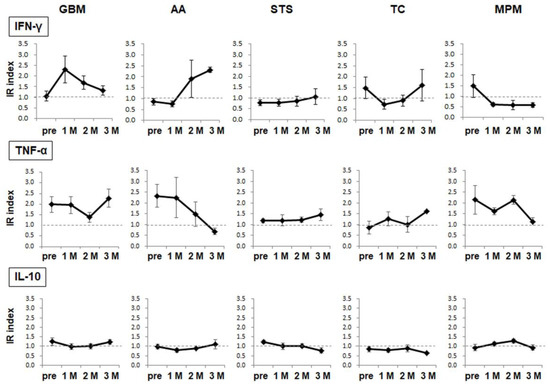
Figure 3.
WT1-specific cellular immune responses. The target epitope WT1-235-specific cytokine production/release from PBMCs was analyzed using the ELISPOT assay. The results are indicated in the IR index for each cytokine. The circles and error bars indicate the average and standard error, respectively.
3.3. Safety
Safety was analyzed in all 45 patients who received one or more doses of WT1 Trio. The AEs observed during the WT1 Trio treatment period are presented in Table 2. Skin reactions at the vaccination site (grade 1) were observed in 30 (66.7%) of the 45 patients. In addition, it was found that interstitial pneumonia (grade 1) was probably related to the WT1 Trio in a patient with MPM (Pt no. 17) and that myasthenia gravis-like symptoms (grade 3) were possibly associated with the WT1 Trio in a patient with TC (Pt no. 28). Two deaths were recorded in this study. One patient with synovial sarcoma died during the protocol treatment (Pt no. 13), and another with TC died within 30 days of the last vaccination with the WT1 Trio (Pt no. 45). Both deaths were attributed to disease progression (PD). Notably, no grade 4 or 5 AEs related to the WT1 Trio were observed (Table 2).

Table 2.
Adverse events.
3.4. Clinical efficacy
As outlined above, the WT1 Trio vaccine was administered following a treatment-free period of at least 4 weeks. The previous treatment regimens of the patients who were administered WT1 Trio are summarized in Table 3.

Table 3.
Previous treatment regimens.
3.4.1. Malignant Glioma
Here, 15 and 8 patients with glioblastoma multiforme and anaplastic astrocytoma, respectively, were enrolled. Before vaccination, one patient with glioblastoma multiforme dropped out because of PD. Seven (50.0%) of the 14 patients with glioblastoma multiforme completed the 3-month protocol treatment. Four of the seven patients who dropped out stopped the WT1 Trio until 7 weeks due to PD. Four patients achieved stable disease after 3 months of vaccination. Two (25.0%) of the eight patients with anaplastic astrocytoma completed the 3-month protocol treatment. Both patients achieved stable disease within 3 months of vaccination. Five of the six patients who dropped out stopped the WT1 Trio until 7 weeks due to PD.
3.4.2. STS
Before vaccination, one patient with STS dropped out because of PD. Five (62.5%) of the eight patients completed the 3-month protocol treatment. All three patients who dropped out stopped the WT1 Trio vaccine until 7 weeks due to PD. Two (25.0%) patients achieved stable disease after 3 months of vaccination.
3.4.3. MPM
Here, six patients were enrolled. Four (66.7%) of the six patients completed the 3-month protocol treatment. Two patients who dropped out stopped the WT1 Trio until 7 weeks due to PD. Three (50.0%) patients achieved stable disease after 3 months of vaccination.
3.4.4. TC
Here, five patients were enrolled. Three (60.0%) of the five patients completed the 3-month protocol treatment. Unfortunately, two patients dropped out due to PD during the protocol treatment period. One (20.0%) patient achieved stable disease after 3 months of vaccination.
3.4.5. Others
Here, four patients with four different tumors (olfactory neuroblastoma, broncho-mucoepidermal carcinoma, salivary adenocarcinoma, and thymoma) were enrolled. All patients completed the 3-month protocol treatment. Patients with olfactory neuroblastoma, broncho-mucoepidermal carcinoma, and salivary adenocarcinoma achieved SD. The tumor response in a patient with thymoma was PD.
3.5. Association between Immune-Related Factors before the Vaccination Start and Prognosis
Here, we analyzed the association between immune-related factors before vaccination and clinical outcomes in patients with malignant glioma who received WT1 Trio once or more than once. Immune-related factors included the neutrophil/lymphocyte ratio (N/L ratio), spontaneous IFN-γ, TNF-α, and IL-10 released from PBMCs, as well as WT1-332-specific IFN-γ, TNF-α, and IL-10 released from PBMCs in all patients with malignant glioma and WT1-235-specific IFN-γ and IL-10 released from PBMCs in patients with HLA-A*24:02. Clinical outcomes were assessed by the completion of the 3-month treatment protocol and tumor response during the study. Of these immune-related factors, the group with positive spontaneous IL-10 released from PBMCs had a significantly lower completion rate and a higher rate of tumor progression. In addition, the group with WT1-332-specific IL-10 released from PBMCs tended to have a lower completion rate and a higher rate of tumor progression. When the cutoff level of the WT1-332-specific IL-10 IR index was set as 1.15, the group with a higher WT1-332-specific IL-10 released from PBMCs had a significantly lower completion rate and a higher rate of tumor progression than the group with lower WT1-332-specific IL-10 released from PBMCs (Table 4).

Table 4.
Associations between immune factors before vaccination and clinical outcomes in patients with malignant glioma.
These results may imply that the immunosuppressive environment before vaccination is associated with poor clinical outcomes in patients treated with WT1 Trio.
4. Discussion
This clinical study revealed the following findings: (1) WT1, the target antigen of the WT1 Trio cancer vaccine, is highly expressed in the majority of rare cancers. (2) Biweekly administration of the WT1 Trio vaccine led to a more significant enhancement of IgG antibody production against the WT1-235 peptide compared to weekly administration of the WT1-235CTL peptide vaccine. (3) The WT1 Trio vaccine did not enhance CTL epitope-specific DTH, which is regarded as another primary endpoint. (4) The WT1 Trio vaccine is safe for patients without risk of autoimmune predisposition. (5) The WT1-332 HTL epitope-specific, Th2-type cellular immune response experienced prior to vaccination was associated with poor prognosis in WT1 Trio-treated recurrent malignant glioma.
When recruiting patients, we immunohistochemically examined the expression of WT1, the target antigen of the WT1 Trio cancer vaccine. WT1 protein expression in tumor cells was confirmed in most patients recruited for this clinical trial. These include GBM, AA, TC, soft tissue sarcoma, MPM, and olfactory neuroblastoma. Since no standard treatments have been established for most rare cancers, developing novel treatment strategies is urgently required [3,4]. All WT1-expressing tumor cells could be targets of WT1-specific CTLs. The high expression rate of the WT1 protein in tumor cells indicates that cancer immunotherapy targeting WT1 may be a therapeutic strategy for many rare cancers as an immunotherapy against a common target molecule.
We analyzed the production of IgG Abs against the WT1 peptide (the first primary endpoint of this clinical trial) by monitoring the immune responses against WT1, the target antigen of the WT1 Trio cancer vaccine. Abs are produced by activating antigen-specific B cells following cognate interactions with CD4+ T cells to help drive B-cell expansion, germinal center formation, and differentiation into Ab-secreting cells [40]. Therefore, the production of IgG Abs serves as an immune monitoring marker that indicates the activation of CD4+ T cells and antigen-specific B cell lineages, which play crucial roles in immune responses. Here, we analyzed the ability of WT1 Trio to induce WT1-specific immune responses as a primary endpoint. The enhanced WT1-235 IgG Ab production observed in this study showed that biweekly vaccination with WT1 Trio, which contains two WT1 CTL peptides, WT1-235 and WT1-126, as well as WT1 HTL peptide 332, induced immune responses mediated by helper T cells and WT1-235 epitope-specific B cell lineages more robustly than weekly vaccination with WT1-235 peptide vaccine.
WT1 Trio cancer vaccine-induced IgG antibody production against WT1 peptides indicates the activation of WT1-specific B-lymphocyte lineage cells. Recent evidence has shown that B cells play a crucial role in tumor cellular immune responses. Several studies have reported that some tumors generate adaptive immune responses in spatially well-organized structures called tertiary lymphoid structures (TLS). TLS has been observed in various types of tumors, including lung [41,42], breast [43,44], and sarcomas [45]. A positive association between the presence of TLS and B lymphocytes and therapeutic responses to immune checkpoint inhibitors has been reported in melanoma [46] and sarcoma [47]. One possible anti-tumor cellular immune mechanism relevant to B cells is antigen presentation. Compared with static B cells expressing scant co-stimulatory molecules, activated B cells have a strong antigen-presenting function that promotes the proliferation and IFN-γ production by CD8+ T cells [48]. Furthermore, as a cellular immune mechanism of B cells, it has been reported that B cells can produce and secrete cytokines and may function as helper cells [49]. Interestingly, we found that the WT1 Trio induced the production of IgG Abs against the three administered WT1 epitope peptides. These results indicate the existence of B cells with B cell receptors that recognize the CTL epitopes WT1-235 and WT1-126 and the HTL epitope WT1-332. These WT1-specific B cells are activated by the administered WT1 Trio and may affect clinical outcomes through antigen presentation or immunomodulation of the tumor immune microenvironment via cytokine production and release.
We also analyzed the DTH skin reaction to the WT1 peptide (the other primary endpoint of this clinical trial) by monitoring the immune response against WT1, the target antigen of the WT1 Trio cancer vaccine. We previously reported that DTH against the WT1 peptide correlated with favorable prognosis in WT1 peptide vaccines for patients with pancreatic cancer and glioblastoma multiforme [28,29]. However, the positive rates of DTH in patients receiving WT1 Trio did not differ significantly from those treated with the WT1-235 CTL peptide vaccine. These results show that WT1 Trio enhanced WT1-specific immune responses detected by WT1-235 IgG Ab production, rather than WT1-specific immune responses represented by DTH responses, as compared with the WT1-235 CTL peptide vaccine. Furthermore, these results indicate that the ability of the WT1 Trio to induce aWT1-specific cellular immune response detected by the DTH response is not superior to that of the WT1-235 CTL peptide vaccine. These findings show that the WT1 Trio peptide vaccine could be further improved to induce more robust WT1-specific cellular immune responses for better clinical outcomes.
As a secondary endpoint, we evaluated the safety of biweekly WT1 Trio administration. The WT1 Trio vaccine, which includes a helper peptide (WT1-332), was administered in combination in order to activate WT1-specific CD4+ T helper lymphocytes and enhance WT1-specific anti-tumor cellular immune responses. Since CD4+ T cells play a crucial role in regulating immune responses [50], their activation induced by the WT1 Trio might enhance immune responses toward the target and other antigens. WT1 protein is expressed in the pleura and renal podocytes in normal adult tissues. However, no treatment-related pleural or renal events were observed in this clinical study. As an AE in WT1-non-expressing tissues, acetylcholine receptor Ab-negative myasthenia gravis-like symptoms (G3) developed in a patient with TC (Pt no. 28) who received four doses of WT1 Trio. The symptoms improved to grade 1 after discontinuation of the WT1 Trio and administration of gamma globulin, implying that an immunological mechanism causes myasthenia gravis-like symptoms. Although myasthenia gravis is a well-known complication of TC as a paraneoplastic syndrome, activation of the general immune system by the WT1 Trio may enhance autoimmune responses in the patient. Therefore, careful considerations should be taken when recruiting patients at risk of autoimmune predisposition.
The observation period of this clinical study was 3 months. In a subsequent clinical trial, we will continue administering the WT1 Trio peptide vaccine to patients in whom the WT1 Trio is considered to slow tumor growth. In addition, future studies are required to examine the long-term induction and maintenance of WT1-specific immune responses, safety, and clinical efficacy in patients treated with WT1 Trio.
In addition to the primary endpoints investigated through immune monitoring, we examined the changes in WT1-specific cellular immune responses using an ELISPOT assay. ELISPOT assays revealed that WT1-specific cellular immune responses differed significantly in patients treated with WT1 Trio regarding robustness before vaccination, mode of immune response induction after vaccination, and maintenance of the cellular immune responses after their induction. These results indicate that each patient’s optimal dose and vaccination schedule may vary according to their immunological status. Therefore, individualized vaccination may be required based on biomarkers that can predict clinical efficacy before vaccination and monitor anti-tumor immune responses after the start of vaccination. In addition, the ELISPOT assay showed a decline in WT1-235 epitope-specific IFN-γ production from PBMCs one month after WT1 Trio initiation; this decline persisted throughout the 3-month treatment protocol in MPM cases. However, WT1-235Ab production increased in all four MPM cases that completed the treatment protocol. The DTH skin test demonstrated positive results in two of the four cases examined. Thus, it is evident that WT1 Trio induced WT1-specific immune responses in these cases. This discrepancy could be explained by the fact that most of the enrolled cases with MPM had large tumor burdens. This raises the possibility of migration of many activated WT1-235-specific immune cells from peripheral blood to the tumor site, which ultimately could have led to a decrease in WT1-235-specific cellular immune responses in PBMCs. On the other hand, WT1 Abs reflect the systemic immune responses of the B lymphocyte lineage, and the WT1 DTH skin test reflects the immune responses of the immune cells that infiltrated the skin tissue from peripheral blood. These differences in monitoring targets may have led to a discrepancy in the assessment of immune induction. Since it is impossible to monitor local immune dynamics in the tumor in real time, it is necessary to comprehensively evaluate the immune responses based on the characteristics of immune monitoring markers.
Predicting treatment response is essential for patient selection. In this study, we analyzed the association between WT1-specific cellular immune responses before vaccination and prognosis. This study showed that spontaneous IL-10 secretion and HTL epitope WT1-332-specific IL-10 secretion from PBMCs before vaccination were associated with a poor clinical prognosis in patients with recurrent malignant glioma treated with the WT1 Trio cancer vaccine. The inhibitory cytokine IL-10 acts on immune cells, including T cells and macrophages, directly suppressing cell activation and attenuating the antigen-presenting ability of macrophages, thereby lowering immune responses [51]. Therefore, it is reasonable to conclude that immunosuppressive immune status before vaccination is associated with a poor clinical prognosis. Another important point regarding epitope-specific IL-10 production is that HTL epitope-specific IL-10 production is significantly associated with a poor clinical prognosis. The WT1 Trio is a therapeutic vaccine that induces WT1-specific CTLs by combining the HTL epitope WT1-332 peptide with the CTL epitopes WT1-126 and WT235. However, if the WT1-332 peptide strongly induces immunosuppressive IL-10 production/secretion by HTLs, it may have a negative impact on the anti-tumor immune responses. Therefore, the two immune markers identified in this study may be helpful for prognostic prediction before the start of vaccination. However, further studies are required to examine whether they can be used as markers for patient selection.
5. Conclusions
In this study, we conducted a clinical trial evaluating a biweekly WT1 tri-peptide-based vaccine for recurrent or advanced rare cancers. Immunological analysis showed that the biweekly WT1 Trio vaccine containing the WT1-332 helper T lymphocyte peptide induced more robust immune responses targeting WT1 as compared with the weekly WT1-235 CTL peptide vaccine. The safety of the WT1 Trio vaccine was confirmed due to a lack of severe treatment-related adverse events, with the exception of grade 3 myasthenia gravis-like symptoms observed in a patient with thymic cancer. Fifteen (33.3%) patients achieved stable disease after 3 months of treatment. Therefore, we conclude that WT1-targeted immunotherapy may be a potential therapeutic strategy for rare cancers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15020393/s1, Table S1: Biophysical properties of WT1 peptides; Figure S1: WT1 peptide data.
Author Contributions
Conceptualization, Y.O. (Yusuke Oji); Design of the clinical study, Y.O. (Yusuke Oji), S.N., J.N., A.T. (Akihiro Tsuboi), N.H., Y.O. (Yoshihiro Oka), N.K., M.F., N.N. (Norio Nonomura), T.K., Y.S., Y.T., K.S., E.M., M.S., A.N., A.T. (Atsushi Tanemura), Y.U., T.M., and H.S. (Haruo Sugiyama); Investigation (Conducting the clinical trial), Y.O. (Yusuke Oji), N.K., H.A., N.N. (Norifumi Naka), K.-i.H., H.O., Y.S., Y.T., K.S., E.M., M.S., A.T. (Akihiro Tsuboi), S.N., J.N., N.H., Y.O. (Yoshihiro Oka), H.N., K.H. (Kana Hasegawa), S.M., F.F., H.I., S.O., H.K., and A.K.; Investigation (Immunological assays), Y.O. (Yusuke Oji), M.I. (Miki Iwai), S.H., R.I., S.I., M.K., M.I. (Masahiro Iwamoto), M.I. (Mayu Ikeda), K.Y., H.S. (Haruka Shimokado), M.O., and T.I.; Interpretation: Y.O. (Yusuke Oji), S.M., F.F., and S.N.; Writing and figure preparation, Y.O. (Yusuke Oji), and H.S. (Haruka Shimokado). Editing, Y.O. (Yoshihiro Oka), M.I. (Masahiro Iwamoto). All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by JSPS KAKENHI Grant Number 20K08732. No other external funding support.
Institutional Review Board Statement
The study was conducted under the approval of the ethical review board of the Faculty of Medicine, Osaka University (ID: 15605, 13110, and 11293, the date of approval: 7 September 2016, 17 October 2013, and 15 June 2012).
Informed Consent Statement
Written informed consent was obtained for participation in the study, as described in Section 2.
Data Availability Statement
The de-identified data supporting this manuscript are available upon reasonable request to the corresponding author.
Acknowledgments
We thank Masayoshi Inoue (Kyoto Prefectural Medical University, Thoracic Surgery) and Naoya Hashimoto (Kyoto Prefectural Medical University, Neurosurgery) for their contribution to the clinical trial through their participation on the Efficacy and Safety Evaluation Committee board. We would also like to thank Takashi Kijima (Hyogo College of Medicine, Respiratory Medicine and Hematology), Takahiro Yamauchi (University of Fukui, Department of Hematology and Oncology), Haruo Takigawa (Department of Neurosurgery, Matsue City Hospital), Mari Fukuda, Emi Harada, Akiko Ohta, Nobuko Toda, Shihoko Ito (Osaka University), and Ai Yamazaki for their support on the clinical trials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Greenlee, R.T.; Goodman, M.T.; Lynch, C.F.; Platz, C.E.; Havener, L.A.; Howe, H.L. The occurrence of rare cancers in U.S. adults, 1995–2004. Public Health Rep. 2010, 125, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.; Dancey, J.E.; Gilks, C.B.; Huntsman, D.G. Rare cancers: A sea of opportunity. Lancet Oncol. 2016, 17, e52–e61. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Trama, A. Rationale of the rare cancer list: A consensus paper from the Joint Action on Rare Cancers (JARC) of the European Union (EU). ESMO Open 2020, 5, e000666. [Google Scholar] [CrossRef]
- Matsuda, T.; Won, Y.J.; Chiang, R.C.; Lim, J.; Saika, K.; Fukui, K.; Lee, W.C.; Botta, L.; Bernasconi, A.; Trama, A. Rare cancers are not rare in Asia as well: The rare cancer burden in East Asia. Cancer Epidemiol. 2020, 67, 101702. [Google Scholar] [CrossRef]
- Call, K.M.; Glaser, T.M.; Ito, C.Y.; Buckler, A.J.; Pelletier, J.; Haber, D.A.; Rose, E.A.; Kral, A.; Yeger, H.; Lewis, W.H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 1990, 60, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Oji, Y.; Tatsumi, N.; Shimizu, S.; Kanai, Y.; Nakazawa, T.; Asada, M.; Jomgeow, T.; Aoyagi, S.; Nakano, Y.; et al. Antiapoptotic function of 17AA(+)WT1 (Wilms’ tumor gene) isoforms on the intrinsic apoptosis pathway. Oncogene 2006, 25, 4217–4229. [Google Scholar] [CrossRef] [PubMed]
- Jomgeow, T.; Oji, Y.; Tsuji, N.; Ikeda, Y.; Ito, K.; Tsuda, A.; Nakazawa, T.; Tatsumi, N.; Sakaguchi, N.; Takashima, S.; et al. Wilms’ tumor gene WT1 17AA(−)/KTS(−) isoform induces morphological changes and promotes cell migration and invasion in vitro. Cancer Sci. 2006, 97, 259–270. [Google Scholar] [CrossRef]
- Oji, Y.; Tatsumi, N.; Kobayashi, J.; Fukuda, M.; Ueda, T.; Nakano, E.; Saito, C.; Shibata, S.; Sumikawa, M.; Fukushima, H.; et al. Wilms’ tumor gene WT1 promotes homologous recombination-mediated DNA damage repair. Mol. Carcinog. 2015, 54, 1758–1771. [Google Scholar] [CrossRef]
- Han, Y.; Song, C.; Zhang, T.; Zhou, Q.; Zhang, X.; Wang, J.; Xu, B.; Zhang, X.; Liu, X.; Ying, X. Wilms’ tumor 1 (WT1) promotes ovarian cancer progression by regulating E-cadherin and ERK1/2 signaling. Cell Cycle 2020, 19, 2662–2675. [Google Scholar] [CrossRef]
- Dietachmayr, M.; Rathakrishnan, A.; Karpiuk, O.; von Zweydorf, F.; Engleitner, T.; Fernández-Sáiz, V.; Bassermann, F. Antagonistic activities of CDC14B and CDK1 on USP9X regulate WT1-dependent mitotic transcription and survival. Nat. Commun. 2020, 11, 1268. [Google Scholar] [CrossRef]
- Inoue, K.; Tamaki, H.; Ogawa, H.; Oka, Y.; Soma, T.; Tatekawa, T.; Oji, Y.; Tsuboi, A.; Kim, E.H.; Kawakami, M.; et al. Wilms’ tumor gene (WT1) competes with differentiation-inducing signal in hematopoietic progenitor cells. Blood 1998, 91, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Oji, Y.; Miyoshi, S.; Maeda, H.; Hayashi, S.; Tamaki, H.; Nakatsuka, S.; Yao, M.; Takahashi, E.; Nakano, Y.; Hirabayashi, H.; et al. Overexpression of the Wilms’ tumor gene WT1 in de novo lung cancers. Int. J. Cancer 2002, 100, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Oji, Y.; Yamamoto, H.; Nomura, M.; Nakano, Y.; Ikeba, A.; Nakatsuka, S.; Abeno, S.; Kiyotoh, E.; Jomgeow, T.; Sekimoto, M.; et al. Overexpression of the Wilms’ tumor gene WT1 in colorectal adenocarcinoma. Cancer Sci. 2003, 94, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Oji, Y.; Nakamori, S.; Fujikawa, M.; Nakatsuka, S.; Yokota, A.; Tatsumi, N.; Abeno, S.; Ikeba, A.; Takashima, S.; Tsujie, M.; et al. Overexpression of the Wilms’ tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci. 2004, 95, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Oji, Y.; Suzuki, T.; Nakano, Y.; Maruno, M.; Nakatsuka, S.; Jomgeow, T.; Abeno, S.; Tatsumi, N.; Yokota, A.; Aoyagi, S.; et al. Overexpression of the Wilms’ tumor gene WT1 in primary astrocytic tumors. Cancer Sci. 2004, 95, 822–827. [Google Scholar] [CrossRef]
- Ueda, T.; Oji, Y.; Naka, N.; Nakano, Y.; Takahashi, E.; Koga, S.; Asada, M.; Ikeba, A.; Nakatsuka, S.; Abeno, S.; et al. Overexpression of the Wilms’ tumor gene WT1 in human bone and soft-tissue sarcomas. Cancer Sci. 2003, 94, 271–276. [Google Scholar] [CrossRef]
- Oka, Y.; Elisseeva, O.A.; Tsuboi, A.; Ogawa, H.; Tamaki, H.; Li, H.; Oji, Y.; Kim, E.H.; Soma, T.; Asada, M.; et al. Human cytotoxic T lymphocyte responses specific for peptides of the wild-type Wilms’ tumor gene (WT1) product. Immunogenetics 2000, 51, 99–107. [Google Scholar] [CrossRef]
- Ohminami, H.; Yasukawa, M.; Fujita, S. HLA class I-restricted lysis of leukemia cells by a CD8+ cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood 2000, 95, 286–293. [Google Scholar] [CrossRef]
- Gao, L.; Bellantuono, I.; Elsässer, A.; Marley, S.B.; Gordon, M.Y.; Goldman, J.M.; Stauss, H.J. Selective elimination of leukemic CD34+ progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood 2000, 95, 2198–2203. [Google Scholar] [CrossRef]
- Oka, Y.; Tsuboi, A.; Taguchi, T.; Osaki, T.; Kyo, T.; Nakajima, H.; Elisseeva, O.A.; Oji, Y.; Kawakami, M.; Ikegame, K.; et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc. Natl. Acad. Sci. USA 2004, 101, 13885–13890. [Google Scholar] [CrossRef]
- Izumoto, S.; Tsuboi, A.; Oka, Y.; Suzuki, T.; Hashiba, T.; Kagawa, N.; Hashimoto, N.; Maruno, M.; Elisseeva, O.A.; Shirakata, T.; et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J. Neurosurg. 2008, 108, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Hashii, Y.; Sato-Miyashita, E.; Matsumura, R.; Kusuki, S.; Yoshida, H.; Ohta, H.; Hosen, N.; Tsuboi, A.; Oji, Y.; Oka, Y.; et al. WT1 peptide vaccination following allogeneic stem cell transplantation in pediatric leukemic patients with high risk for relapse: Successful maintenance of durable remission. Leukemia 2012, 26, 530–532. [Google Scholar] [CrossRef]
- Oji, Y.; Inoue, M.; Takeda, Y.; Hosen, N.; Shintani, Y.; Kawakami, M.; Harada, T.; Murakami, Y.; Iwai, M.; Fukuda, M.; et al. WT1 peptide-based immunotherapy for advanced thymic epithelial malignancies. Int. J. Cancer 2018, 142, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Tsuruta, T.; Haraguchi, K.; Okamoto, M.; Sugiyama, H.; Koido, S. Long-term survival of pancreatic cancer patients treated with multimodal therapy combined with WT1-targeted dendritic cell vaccines. Hum. Vaccines Immunother. 2019, 15, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Van de Velde, A.L.; Smits, E.L.; Van Tendeloo, V.F.; Juliusson, G.; Cools, N.; Nijs, G.; Stein, B.; Lion, E.; Van Driessche, A.; et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017, 130, 1713–1721. [Google Scholar] [CrossRef]
- Ochi, T.; Fujiwara, H.; Okamoto, S.; An, J.; Nagai, K.; Shirakata, T.; Mineno, J.; Kuzushima, K.; Shiku, H.; Yasukawa, M. Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood 2011, 118, 1495–1503. [Google Scholar] [CrossRef]
- Chapuis, A.G.; Egan, D.N.; Bar, M.; Schmitt, T.M.; McAfee, M.S.; Paulson, K.G.; Voillet, V.; Gottardo, R.; Ragnarsson, G.B.; Bleakley, M.; et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 2019, 25, 1064–1072. [Google Scholar] [CrossRef]
- Nishida, S.; Ishikawa, T.; Egawa, S.; Koido, S.; Yanagimoto, H.; Ishii, J.; Kanno, Y.; Kokura, S.; Yasuda, H.; Oba, M.S.; et al. Combination gemcitabine and WT1 peptide vaccination improves progression-free survival in advanced pancreatic ductal adenocarcinoma: A Phase II randomized study. Cancer Immunol. Res. 2018, 6, 320–331. [Google Scholar] [CrossRef]
- Oji, Y.; Hashimoto, N.; Tsuboi, A.; Murakami, Y.; Iwai, M.; Kagawa, N.; Chiba, Y.; Izumoto, S.; Elisseeva, O.; Ichinohasama, R.; et al. Association of WT1 IgG antibody against WT1 peptide with prolonged survival in glioblastoma multiforme patients vaccinated with WT1 peptide. Int. J. Cancer. 2016, 139, 1391–1401. [Google Scholar] [CrossRef]
- Nishida, S.; Morimoto, S.; Oji, Y.; Morita, S.; Shirakata, T.; Enomoto, T.; Tsuboi, A.; Ueda, Y.; Yoshino, K.; Shouq, A.; et al. Cellular and humoral immune responses induced by an HLA class I-restricted peptide cancer vaccine targeting WT1 are associated with favorable clinical outcomes in advanced ovarian cancer. J. Immunother. 2022, 45, 56–66. [Google Scholar] [CrossRef]
- Fujiki, F.; Oka, Y.; Tsuboi, A.; Kawakami, M.; Kawakatsu, M.; Nakajima, H.; Elisseeva, O.A.; Harada, Y.; Ito, K.; Li, Z.; et al. Identification and characterization of a WT1 (Wilms tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J. Immunother. 2007, 30, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Nakata, J.; Nakajima, H.; Hayashibara, H.; Imafuku, K.; Morimoto, S.; Fujiki, F.; Motooka, D.; Okuzaki, D.; Hasegawa, K.; Hosen, N.; et al. Extremely strong infiltration of WT1-specific CTLs into mouse tumor by the combination vaccine with WT1-specific CTL and helper peptides. Oncotarget 2018, 9, 36029–36038. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, A.; Hashimoto, N.; Fujiki, F.; Morimoto, S.; Kagawa, N.; Nakajima, H.; Hosen, N.; Nishida, S.; Nakata, J.; Morita, S.; et al. A phase I clinical study of a cocktail vaccine of Wilms’ tumor 1 (WT1) HLA class I and II peptides for recurrent malignant glioma. Cancer Immunol. Immunother. 2019, 68, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, F.; Tsuboi, A.; Morimoto, S.; Hashimoto, N.; Inatome, M.; Nakajima, H.; Nakata, J.; Nishida, S.; Hasegawa, K.; Hosen, N.; et al. Identification of two distinct populations of WT1-specific cytotoxic T lymphocytes in co-vaccination of WT1 killer and helper peptides. Cancer Immunol. Immunother. 2021, 70, 253–263. [Google Scholar] [CrossRef]
- Mittra, A.; Moscow, J.A. Future approaches to precision oncology-based clinical trials. Cancer J. 2019, 25, 300–304. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published: 2009 (v.4.03, 2010). Available online: https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf (accessed on 29 November 2022).
- Alzaaqi, S.; Naka, N.; Hamada, K.; Hosen, N.; Kanegae, M.; Outani, H.; Adachi, M.; Imanishi, R.; Morii, E.; Iwai, M.; et al. WT1 epitope-specific IgG and IgM antibodies for immune-monitoring in patients with advanced sarcoma treated with a WT1 peptide cancer vaccine. Oncol. Lett. 2022, 23, 65. [Google Scholar] [CrossRef]
- Hayashi, S.; Imanishi, R.; Adachi, M.; Ikejima, S.; Nakata, J.; Morimoto, S.; Fujiki, F.; Nishida, S.; Tsuboi, A.; Hosen, N.; et al. Reader-free ELISPOT assay for immuno-monitoring in peptide-based cancer vaccine immunotherapy. Biomed. Rep. 2020, 12, 244–250. [Google Scholar] [CrossRef]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- De Chaisemartin, L.; Goc, J.; Damotte, D.; Validire, P.; Magdeleinat, P.; Alifano, M.; Cremer, I.; Fridman, W.H.; Sautès-Fridman, C.; Dieu-Nosjean, M.C. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011, 71, 6391–6399. [Google Scholar] [CrossRef]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.Y.; Park, I.A.; Song, I.H.; Yu, J.H.; Ahn, J.H.; Gong, G. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am. J. Clin. Pathol. 2015, 144, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Oke, T.; Siegel, N.; Cojocaru, G.; Tam, A.J.; Blosser, R.L.; Swailes, J.; Ligon, J.A.; Lebid, A.; Morris, C.; et al. The immunosuppressive niche of soft-tissue sarcomas is sustained by tumor-associated macrophages and characterized by intratumoral tertiary lymphoid structures. Clin. Cancer Res. 2020, 26, 4018–4030. [Google Scholar] [CrossRef]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Zhang, L.; Bridle, B.W.; Chen, L.; Pol, J.; Spaner, D.; Boudreau, J.E.; Rosen, A.; Bassett, J.D.; Lichty, B.D.; Bramson, J.L.; et al. Delivery of viral-vectored vaccines by B cells represents a novel strategy to accelerate CD8+ T-cell recall responses. Blood 2013, 121, 2432–2439. [Google Scholar] [CrossRef]
- Becker, H.J.; Kondo, E.; Shimabukuro-Vornhagen, A.; Theurich, S.; von Bergwelt-Baildon, M.S. Processing and MHC class II presentation of exogenous soluble antigen involving a proteasome-dependent cytosolic pathway in CD40-activated B cells. Eur. J. Haematol. 2016, 97, 166–174. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015, 367, 103–107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).