Progresses, Challenges, and Prospects of CRISPR/Cas9 Gene-Editing in Glioma Studies
Abstract
Simple Summary
Abstract
1. Background
1.1. Current Glioma-Related Research
1.2. Overview of Studies Related to CRISPR/Cas9 Gene-Editing
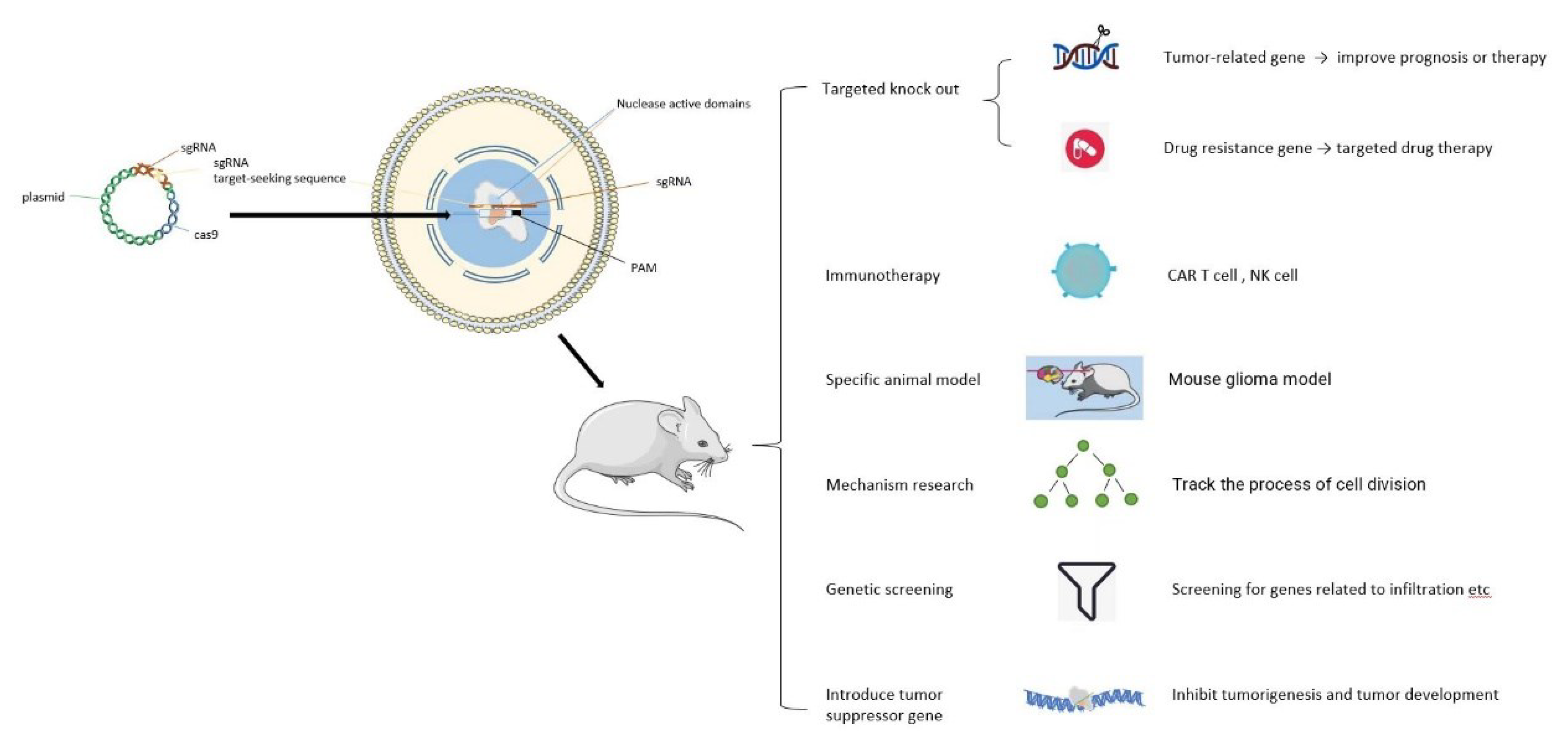
2. Roles of CRISPR/Cas9 in Glioma
2.1. Inhibition of Glioma Progression by Enhancing Expression of Tumor Suppressor Genes
2.2. Targeted Gene Knockout for Treatment of Glioma
2.3. Research on Targeted Drugs for Glioma
2.4. Application in Immunotherapy of Gliomas
2.5. Assistance on the Establishment of Specific Animal Models
2.6. Screening of Specific Functional Genes
2.7. Mechanism Research on Glioma
3. Challenges
- (1)
- CRISPR/Cas9 has off-target effects during operation.
- (2)
- CRISPR/Cas9 is not precise enough, and genome editing tool-induced DNA double-strand breaks (DSBS) can be repaired by a non-homologous terminal junction (NHEJ) and homologous recombination (HR), but the deflection of the former path in humans and other mammals often leads to imprecise repair [104]. Cas9 sometimes cuts DNA sequences that are similar to the ones it is looking for, but those sequences contain multiple different bases, which can lead to new mutations.
- (3)
- Efficiency and safety of the CRISPR/Cas9 system in the human body. If unedited tumor cells grow faster, the benefit of gene-editing therapy will be diminished.
- (4)
- The tumor is heterogeneous.
4. Conclusions and Outlook
Abbreviations
| CRISPR/Cas9 | clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 |
| DSB | double strand break |
| NHEJ | non-homologous end joining |
| HDR | homology directed repair |
| CHAF1A | chromatin assembly factor 1 subunit A |
| NK | Natural killer |
| GBM | glioblastoma |
| VMP1 | Vacuole membrane protein 1 |
| TRAC | T-cell receptor α constant |
| B7-H6 | Homologue 6 of B7 |
| GSC | GBM stem cells |
| TMZ | Temozolomide |
| AHR | aryl hydrocarbon receptor |
| iH2B-GFP | inducible histone2B-GFP |
Author Contributions
Funding
Conflicts of Interest
References
- Gittleman, H.; Boscia, A.; Ostrom, Q.T.; Truitt, G.; Fritz, Y.; Kruchko, C.; Barnholtz-Sloan, J. Survivorship in adults with malignant brain and other central nervous system tumor from 2000–2014. Neuro-Oncology 2018, 20, vii6–vii16. [Google Scholar] [CrossRef]
- Nicholson, J.; Fine, H. Diffuse Glioma Heterogeneity and Its Therapeutic Implications. Cancer Discov. 2021, 11, 575–590. [Google Scholar] [CrossRef]
- Ostrom, Q.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, iii1–iii105. [Google Scholar] [CrossRef]
- Northcott, P.A.; Robinson, G.w.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Prim. 2019, 5, 11. [Google Scholar] [CrossRef]
- Molinaro, A.; Taylor, J.; Wiencke, J.; Wrensch, M. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019, 15, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Moolten, F.; Cooperband, S. Selective destruction of target cells by diphtheria toxin conjugated to antibody directed against antigens on the cells. Science 1970, 169, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; Hassan, R.; FitzGerald, D.; Kreitman, R. Immunotoxin treatment of cancer. Annu. Rev. Med. 2007, 58, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Boldt, H.; Sørensen, M.; Blach, S.; Dahlrot, R.; Hansen, S.; Burton, M.; Thomassen, M.; Kruse, T.; Poulsen, F.; et al. Targeted next-generation sequencing of adult gliomas for retrospective prognostic evaluation and up-front diagnostics. Neuropathol. Appl. Neurobiol. 2021, 47, 108–126. [Google Scholar] [CrossRef]
- Cabral de Carvalho Corrêa, D.; Tesser-Gamba, F.; Oliveira, I.D.; da Silva, N.S.; Capellano, A.; de Seixas Alves, M.T.; Dastoli, P.; Cavalheiro, S.; de Toledo, S.C. Gliomas in children and adolescents: Investigation of molecular alterations with a potential prognostic and therapeutic impact. J. Cancer Res. Clin. Oncol. 2021, 148, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Miyoshi, H.; Yoshida, K.; Okano, H.; Toda, M. Recent progress in the research of suicide gene therapy for malignant glioma. Neurosurg. Rev. 2021, 44, 29–49. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Gan, H.; Wang, H.; Lee, J.; Fang, D.; Kitange, G.; He, L.; Hu, Z.; Parney, I.; et al. A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma. Nat. Commun. 2018, 9, 2949. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Nakazawa, T.; Natsume, A.; Nishimura, F.; Morimoto, T.; Matsuda, R.; Nakamura, M.; Yamada, S.; Nakagawa, I.; Motoyama, Y.; Park, Y.; et al. Effect of CRISPR/Cas9-Mediated PD-1-Disrupted Primary Human Third-Generation CAR-T Cells Targeting EGFRvIII on In Vitro Human Glioblastoma Cell Growth. Cells 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, R.; Zhang, H.; Liu, C.; Liu, C.; Lu, Y. Suppressing Dazl modulates tumorigenicity and stemness in human glioblastoma cells. BMC Cancer 2020, 20, 673. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, G.; di Valentin, E.; L’homme, L.; Lassence, C.; Dequiedt, F.; Fillet, M.; Coupienne, I.; Piette, J. RIP3 antagonizes a TSC2-mediated pro-survival pathway in glioblastoma cell death. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.; Vora, P.; Venugopal, C.; Sidhu, S.; Moffat, J.; Swanton, C.; Singh, S. Intratumoral heterogeneity: Pathways to treatment resistance and relapse in human glioblastoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1448–1456. [Google Scholar] [CrossRef]
- Lake, J.; Donson, A.; Prince, E.; Davies, K.; Nellan, A.; Green, A.; Levy, J.M.; Dorris, K.; Vibhakar, R.; Hankinson, T.; et al. Targeted fusion analysis can aid in the classification and treatment of pediatric glioma, ependymoma, and glioneuronal tumors. Pediatric Blood Cancer 2020, 67, e28028. [Google Scholar] [CrossRef] [PubMed]
- Zielke, S.; Meyer, N.; Mari, M.; Abou-El-Ardat, K.; Reggiori, F.; van Wijk, S.; Kögel, D.; Fulda, S. Loperamide, pimozide, and STF-62247 trigger autophagy-dependent cell death in glioblastoma cells. Cell Death Dis. 2018, 9, 994. [Google Scholar] [CrossRef]
- Hamis, S.; Nithiarasu, P.; Powathil, G. What does not kill a tumour may make it stronger: In silico insights into chemotherapeutic drug resistance. J. Theor. Biol. 2018, 454, 253–267. [Google Scholar] [CrossRef]
- Saydam, O.; Saydam, N. Deficiency of Ku Induces Host Cell Exploitation in Human Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 651818. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.; Dilliard, S.; Siegwart, D. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.; Schubert, M.; Friedmann-Morvinski, D.; Cohen, Z.; Behlke, M.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef]
- Al-Sammarraie, N.; Ray, S. Applications of CRISPR-Cas9 Technology to Genome Editing in Glioblastoma Multiforme. Cells 2021, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Zaman, N.; Mcgee, S.; Milanese, J.; Masoudi-Nejad, A.; O’Connor-McCourt, M. Predictive genomics: A cancer hallmark network framework for predicting tumor clinical phenotypes using genome sequencing data. Semin. Cancer Biol. 2015, 30, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Betge, J.; Ebert, M.; Boutros, M. CRISPR/Cas9 for cancer research and therapy. Semin. Cancer Biol. 2019, 55, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Ge, X.; Yang, F.; Wang, B.; Li, S.; Duan, J.; Lv, X.; Cheng, C.; Song, Z.; Liu, C.; et al. High-fidelity SaCas9 identified by directional screening in human cells. PLoS Biol. 2020, 18, e3000747. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.; Jinek, M. Mechanistic Insights into the cis- and trans-Acting DNase Activities of Cas12a. Mol. Cell 2019, 73, 589–600.e4. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Koo, T.; Park, S.; Kim, D.; Kim, K.; Cho, H.; Song, D.; Lee, K.; Jung, M.; Kim, S.; et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat. Commun. 2017, 8, 14500. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Embden, J.; Gaastra, W.; Schouls, L. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef]
- Ousterout, D.; Kabadi, A.; Thakore, P.; Majoros, W.; Reddy, T.; Gersbach, C. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat. Commun. 2015, 6, 6244. [Google Scholar] [CrossRef]
- O’Connell, M.R.; Oakes, B.L.; Sternberg, S.H.; East-Seletsky, A.; Kaplan, M.; Doudna, J.A. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature 2014, 516, 263–266. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.; Makarova, K.; Wolf, Y. Evolutionary Genomics of Defense Systems in Archaea and Bacteria. Annu. Rev. Microbiol. 2017, 71, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Hille, F.; Richter, H.; Wong, S.; Bratovič, M.; Ressel, S.; Charpentier, E. The Biology of CRISPR-Cas: Backward and Forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.; Dupuis, M.; Villion, M.; Romero, D.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef]
- Hwang, W.; Fu, Y.; Reyon, D.; Maeder, M.; Tsai, S.; Sander, J.; Peterson, R.; Yeh, J.; Joung, J. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Shen, B.; Cui, Y.; Chen, Y.; Wang, J.; Wang, L.; Kang, Y.; Zhao, X.; Si, W.; Li, W.; et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 2014, 156, 836–843. [Google Scholar] [CrossRef]
- Tamura, R.; Toda, M. Historic Overview of Genetic Engineering Technologies for Human Gene Therapy. Neurol. Med. Chir. 2020, 60, 483–491. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Li, M.; Xiang, H.; Yan, A. Harnessing the type I CRISPR-Cas systems for genome editing in prokaryotes. Environ. Microbiol. 2021, 23, 542–558. [Google Scholar] [CrossRef]
- Li, X.; Xiong, K.; Bi, D.; Zhao, C. A Novel CRISPR/Cas9 Screening Potential Index for Prognostic and Immunological Prediction in Low-Grade Glioma. Front. Genet. 2022, 25, 839884. [Google Scholar] [CrossRef]
- Bolotin, A.; Quinquis, B.; Sorokin, A.; Ehrlich, S. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 2005, 151, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Hale, C.; Zhao, P.; Olson, S.; Duff, M.; Graveley, B.; Wells, L.; Terns, R.; Terns, M. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell 2009, 139, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Mojica, F.; Díez-Villaseñor, C.; García-Martínez, J.; Almendros, C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 2009, 155, 733–740. [Google Scholar] [CrossRef]
- Mojica, F.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, C.; Salvignol, G.; Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 2005, 151, 653–663. [Google Scholar] [CrossRef]
- Waters, C.; Strande, N.; Pryor, J.; Strom, C.; Mieczkowski, P.; Burkhalter, M.; Oh, S.; Qaqish, B.; Moore, D.; Hendrickson, E.; et al. The fidelity of the ligation step determines how ends are resolved during nonhomologous end joining. Nat. Commun. 2014, 5, 4286. [Google Scholar] [CrossRef]
- Sander, J.; Joung, J. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Gohil, N.; García, R.R.; Braddick, D.; Fofié, C. Recent Advances in CRISPR-Cas9 Genome Editing Technology for Biological and Biomedical Investigations. J. Cell. Biochem. 2018, 119, 81–94. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, F.; Ding, Y. CRISPR/Cas9 Delivery System Engineering for Genome Editing in Therapeutic Applications. Pharmaceutics 2021, 13, 1649. [Google Scholar] [CrossRef] [PubMed]
- Jayavaradhan, R.; Pillis, D.M.; Goodman, M.; Zhang, F.; Zhang, Y.; Andreassen, P.R.; Malik, P. CRISPR-Cas9 fusion to dominant-negative 53BP1 enhances HDR and inhibits NHEJ specifically at Cas9 target sites. Nat. Commun. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Peng, H.; Du, B.; Jiang, H.; Gao, J. Over-expression of CHAF1A promotes cell proliferation and apoptosis resistance in glioblastoma cells via AKT/FOXO3a/Bim pathway. Biochem. Biophys. Res. Commun. 2016, 469, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhong, S.; Sun, B.; Sun, Q.; Hu, L.; Pan, S. Lnc-THOR silencing inhibits human glioma cell survival by activating MAGEA6-AMPK signaling. Cell Death Dis. 2019, 10, 866. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Nakazawa, T.; Matsuda, R.; Nishimura, F.; Nakamura, M.; Yamada, S.; Nakagawa, I.; Park, Y.; Tsujimura, T.; Nakase, H. CRISPR-Cas9-Mediated TIM3 Knockout in Human Natural Killer Cells Enhances Growth Inhibitory Effects on Human Glioma Cells. Int. J. Mol. Sci. 2021, 22, 3489. [Google Scholar] [CrossRef]
- Lin, W.; Sun, Y.; Qiu, X.; Huang, Q.; Kong, L.; Lu, J. VMP1, a novel prognostic biomarker, contributes to glioma development by regulating autophagy. J. Neuroinflamm. 2021, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Feng, X.; Mai, B.; Li, X.; Wang, F.; Liu, J.; Liu, X.; Zhang, K.; Wang, X. Bacterial-based cancer therapy: An emerging toolbox for targeted drug/gene delivery. Biomaterials 2021, 277, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Eyquem, J.; Mansilla-Soto, J.; Giavridis, T.; van der Stegen, S.J.C.; Hamieh, M.; Cunanan, K.M.; Odak, A.; Gönen, M.; Sadelain, M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–117. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Y.; Sun, J.; Dong, J.; Bao, Q.; Zhang, X.; Fu, F. Preferential Expression of B7-H6 in Glioma Stem-Like Cells Enhances Tumor Cell Proliferation via the c-Myc/RNMT Axis. J. Immunol. Res. 2020, 2020, 2328675. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Gutierrez, M.C.; Lozzi, B.; Huang-Hobbs, E.; Turner, W.; Tepe, B.; Zhang, Y.; Herman, A.; Rao, G.; Creighton, C.; et al. Identification of diverse tumor endothelial cell populations in malignant glioma. Neuro-Oncology 2021, 23, 932–944. [Google Scholar] [CrossRef]
- Prolo, L.; Li, A.; Owen, S.; Parker, J.; Foshay, K.; Nitta, R.; Morgens, D.; Bolin, S.; Wilson, C.; Vega, L.J.V.; et al. Targeted genomic CRISPR-Cas9 screen identifies MAP4K4 as essential for glioblastoma invasion. Sci. Rep. 2019, 9, 14020. [Google Scholar] [CrossRef]
- Rocha, C.; Rocha, A.R.; Silva, M.M.; Gomes, L.R.; Latancia, M.T.; Tomaz, M.A.; de Souza, I.; Monteiro, L.K.S.; Menck, C. Revealing Temozolomide Resistance Mechanisms via Genome-Wide CRISPR Libraries. Cells 2020, 9, 2573. [Google Scholar] [CrossRef]
- Ogawa, J.; Pao, G.; Shokhirev, M.; Verma, I. Glioblastoma Model Using Human Cerebral Organoids. Cell Rep. 2018, 23, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Oldrini, B.; Curiel-García, Á.; Marques, C.; Matia, V.; Uluçkan, Ö.; Graña-Castro, O.; Torres-Ruiz, R.; Rodriguez-Perales, S.; Huse, J.; Squatrito, M. Somatic genome editing with the RCAS-TVA-CRISPR-Cas9 system for precision tumor modeling. Nat. Commun. 2018, 9, 1466. [Google Scholar] [CrossRef] [PubMed]
- Tejero, R.; Huang, Y.; Katsyv, I.; Kluge, M.; Lin, J.; Tome-Garcia, J.; Daviaud, N.; Wang, Y.; Zhang, B.; Tsankova, N.; et al. Gene signatures of quiescent glioblastoma cells reveal mesenchymal shift and interactions with niche microenvironment. EBioMedicine 2019, 42, 252–269. [Google Scholar] [CrossRef]
- Mirgayazova, R.; Khadiullina, R.; Chasov, V.; Mingaleeva, R.; Miftakhova, R.; Rizvanov, A.; Bulatov, E. Therapeutic Editing of the Gene: Is CRISPR/Cas9 an Option? Genes 2020, 11, 704. [Google Scholar] [CrossRef]
- Zhou, L.; Li, P.; Cai, S.; Li, G.; Liu, F. Ninjurin2 overexpression promotes glioma cell growth. Aging 2019, 11, 11136–11147. [Google Scholar] [CrossRef] [PubMed]
- Mangraviti, A.; Gullotti, D.; Tyler, B.; Brem, H. Nanobiotechnology-based delivery strategies: New frontiers in brain tumor targeted therapies. J. Control. Release Off. J. Control. Release Soc. 2016, 240, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Szot, C.; Saha, S.; Zhang, X.; Zhu, Z.; Hilton, M.; Morris, K.; Seaman, S.; Dunleavey, J.; Hsu, K.; Yu, G.; et al. Tumor stroma-targeted antibody-drug conjugate triggers localized anticancer drug release. J. Clin. Investig. 2018, 128, 2927–2943. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef]
- Moreno, A.; Fu, X.; Zhu, J.; Katrekar, D.; Shih, Y.; Marlett, J.; Cabotaje, J.; Tat, J.; Naughton, J.; Lisowski, L.; et al. In Situ Gene Therapy via AAV-CRISPR-Cas9-Mediated Targeted Gene Regulation. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Lee, Y.; Hsieh, C.; Yeh, C.; Chao, T.; Chen, P.; Lin, I.; Hsieh, T.; Shih, J.; Cheng, C.; et al. Genome-wide CRISPR/Cas9 knockout screening uncovers a novel inflammatory pathway critical for resistance to arginine-deprivation therapy. Theranostics 2021, 11, 3624–3641. [Google Scholar] [CrossRef] [PubMed]
- Kurata, M.; Yamamoto, K.; Moriarity, B.; Kitagawa, M.; Largaespada, D. CRISPR/Cas9 library screening for drug target discovery. J. Hum. Genet. 2018, 63, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Meng, X.; Wu, P.; Li, Z.; Li, S.; Zhang, Y.; Zha, C.; Ye, Q.; Jiang, C.; Cai, J.; et al. ATRX/EZH2 complex epigenetically regulates FADD/PARP1 axis, contributing to TMZ resistance in glioma. Theranostics 2020, 10, 3351–3365. [Google Scholar] [CrossRef] [PubMed]
- Zindl, C.; Chaplin, D. Tumor immune evasion. Science 2010, 328, 697–698. [Google Scholar] [CrossRef]
- Chen, M.; Mao, A.; Xu, M.; Weng, Q.; Mao, J.; Ji, J. CRISPR-Cas9 for cancer therapy: Opportunities and challenges. Cancer Lett. 2019, 447, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Parkman, R. Tumor hybrid cells: An immunotherapeutic agent. J. Natl. Cancer Inst. 1974, 52, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Viitala, M.; Virtakoivu, R.; Tadayon, S.; Rannikko, J.; Jalkanen, S.; Hollmén, M. Immunotherapeutic Blockade of Macrophage Clever-1 Reactivates the CD8 T-cell Response against Immunosuppressive Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Janowicz, K.; Dyszkiewicz-Konwińska, M.; Hutchings, G.; Dompe, C.; Moncrieff, L.; Jankowski, M.; Machnik, M.; Oleksiewicz, U.; Kocherova, I.; et al. CRISPR/Cas9 in Cancer Immunotherapy: Animal Models and Human Clinical Trials. Genes 2020, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ouyang, W.; Kang, B.; Han, X.; Xiong, Y.; Ding, R.; Li, Y.; Wang, F.; Huang, L.; Chen, L.; et al. Selective targeting of the oncogenic G12S mutant allele by CRISPR/Cas9 induces efficient tumor regression. Theranostics 2020, 10, 5137–5153. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Kataoka, S.; Nakayama, M.; Ali, M.; Oshima, H.; Yamamoto, D.; Park, J.; Takegami, Y.; An, T.; Jenkins, N.; et al. CRISPR-Cas9-mediated gene knockout in intestinal tumor organoids provides functional validation for colorectal cancer driver genes. Proc. Natl. Acad. Sci. USA 2019, 116, 15635–15644. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Böll, B.; Schellongowski, P.; Valade, S.; Metaxa, V.; Azoulay, E.; von Bergwelt-Baildon, M. Critical care management of chimeric antigen receptor T-cell therapy recipients. CA Cancer J. Clin. 2021, 72, 78–93. [Google Scholar] [CrossRef]
- Georgiadis, C.; Qasim, W. Emerging applications of gene edited T cells for the treatment of leukemia. Expert Rev. Hematol. 2017, 10, 753–755. [Google Scholar] [CrossRef]
- Cloughesy, T.; Mochizuki, A.; Orpilla, J.; Hugo, W.; Lee, A.; Davidson, T.; Wang, A.; Ellingson, B.; Rytlewski, J.; Sanders, C.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Choi, B.; Yu, X.; Castano, A.; Darr, H.; Henderson, D.; Bouffard, A.; Larson, R.; Scarfò, I.; Bailey, S.; Gerhard, G.; et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J. Immunother. Cancer 2019, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Maddalo, D.; Manchado, E.; Concepcion, C.; Bonetti, C.; Vidigal, J.; Han, Y.; Ogrodowski, P.; Crippa, A.; Rekhtman, N.; de Stanchina, E.; et al. In vivo engineering of oncogenic chromosomal rearrangements with the CRISPR/Cas9 system. Nature 2014, 516, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; Martin, M.; Garcia, A.; Cigudosa, J.; Ramirez, J.; Rodriguez-Perales, S. Engineering human tumour-associated chromosomal translocations with the RNA-guided CRISPR-Cas9 system. Nat. Commun. 2014, 5, 3964. [Google Scholar] [CrossRef]
- Higuchi, F.; Nagashima, H.; Ning, J.; Koerner, M.; Wakimoto, H.; Cahill, D. Restoration of Temozolomide Sensitivity by PARP Inhibitors in Mismatch Repair Deficient Glioblastoma is Independent of Base Excision Repair. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1690–1699. [Google Scholar] [CrossRef]
- Platt, R.; Chen, S.; Zhou, Y.; Yim, M.; Swiech, L.; Kempton, H.; Dahlman, J.; Parnas, O.; Eisenhaure, T.; Jovanovic, M.; et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 2014, 159, 440–455. [Google Scholar] [CrossRef]
- Xue, W.; Chen, S.; Yin, H.; Tammela, T.; Papagiannakopoulos, T.; Joshi, N.; Cai, W.; Yang, G.; Bronson, R.; Crowley, D.; et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 2014, 514, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; van Jaarsveld, R.; Ponsioen, B.; Zimberlin, C.; van Boxtel, R.; Buijs, A.; Sachs, N.; Overmeer, R.; Offerhaus, G.; Begthel, H.; et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015, 521, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rivera, F.; Papagiannakopoulos, T.; Romero, R.; Tammela, T.; Bauer, M.; Bhutkar, A.; Joshi, N.; Subbaraj, L.; Bronson, R.; Xue, W.; et al. Rapid modelling of cooperating genetic events in cancer through somatic genome editing. Nature 2014, 516, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Carlson, J.; Huang, K.; Zheng, Y.; Oderup, C.; Gross, J.; Jang, A.; Burke, T.; Lewén, S.; Scholz, A.; et al. A Chimeric Antibody against ACKR3/CXCR7 in Combination with TMZ Activates Immune Responses and Extends Survival in Mouse GBM Models. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 1354–1365. [Google Scholar] [CrossRef]
- Miller, K.; Fidler-Benaoudia, M.; Keegan, T.; Hipp, H.; Jemal, A.; Siegel, R. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Miller, K.; Ostrom, Q.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.; Waite, K.; Jemal, A.; Siegel, R.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Miller, K.; Fuchs, H.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, B.; Priesterbach-Ackley, L.; Petersen, J.; Wesseling, P. Molecular pathology of tumors of the central nervous system. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1265–1278. [Google Scholar] [CrossRef]
- Reardon, D.; Wen, P. Glioma in 2014: Unravelling tumour heterogeneity-implications for therapy. Nat. Reviews. Clin. Oncol. 2015, 12, 69–70. [Google Scholar] [CrossRef]
- Westphal, M.; Lamszus, K. The neurobiology of gliomas: From cell biology to the development of therapeutic approaches. Nat. Rev. Neurosci. 2011, 12, 495–508. [Google Scholar] [CrossRef]
- Jin, U.; Karki, K.; Cheng, Y.; Michelhaugh, S.; Mittal, S.; Safe, S. The aryl hydrocarbon receptor is a tumor suppressor-like gene in glioblastoma. J. Biol. Chem. 2019, 294, 11342–11353. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Rosiene, J.; Che, A.; Becker, A.; LoTurco, J. Tracking and transforming neocortical progenitors by CRISPR/Cas9 gene targeting and piggyBac transposase lineage labeling. Development 2015, 142, 3601–3611. [Google Scholar] [CrossRef]
- Zuo, E.; Sun, Y.; Yuan, T.; He, B.; Zhou, C.; Ying, W.; Liu, J.; Wei, W.; Zeng, R.; Li, Y.; et al. A rationally engineered cytosine base editor retains high on-target activity while reducing both DNA and RNA off-target effects. Nat. Methods 2020, 17, 600–604. [Google Scholar] [CrossRef]
- Peretz, L.; Besser, E.; Hajbi, R.; Casden, N.; Ziv, D.; Kronenberg, N.; Gigi, L.; Sweetat, S.; Khawaled, S.; Aqeilan, R.; et al. Combined shRNA over CRISPR/cas9 as a methodology to detect off-target effects and a potential compensatory mechanism. Sci. Rep. 2018, 8, 93. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally engineered Cas9 nucleases with improved specificity. Science 2015, 351, 84–88. [Google Scholar] [CrossRef]
- Ray, U.; Raghavan, S.C. Modulation of DNA double-strand break repair as a strategy to improve precise genome editing. Oncogene 2020, 39, 6393–6405. [Google Scholar] [CrossRef] [PubMed]
- Badr, C.E.; Silver, D.J.; Siebzehnrubl, F.A.; Deleyrolle, L.P. Metabolic heterogeneity and adaptability in brain tumors. Cell. Mol. Life Sci. CMLS 2020, 77, 5101–5119. [Google Scholar] [CrossRef]
- Stoltz, K.; Sinyuk, M.; Hale, J.S.; Wu, Q.; Otvos, B.; Walker, K.; Vasanji, A.; Rich, J.N.; Hjelmeland, A.B.; Lathia, J.D. Development of a Sox2 reporter system modeling cellular heterogeneity in glioma. Neuro-Oncology 2014, 17, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.-F.; Li, C.; Xi, S.-Y.; Chen, F.-R.; Wang, J.; Zhang, Z.-Q.; Liu, Y.; Li, X.; Chen, Z.-P. Whole exome sequencing reveals the genetic heterogeneity and evolutionary history of primary gliomas and matched recurrences. Comput. Struct. Biotechnol. J. 2022, 20, 2235–2246. [Google Scholar] [CrossRef]
- MacLeod, G.; Bozek, D.; Rajakulendran, N.; Monteiro, V.; Ahmadi, M.; Steinhart, Z.; Kushida, M.; Yu, H.; Coutinho, F.; Cavalli, F.; et al. Genome-Wide CRISPR-Cas9 Screens Expose Genetic Vulnerabilities and Mechanisms of Temozolomide Sensitivity in Glioblastoma Stem Cells. Cell Rep. 2019, 27, 971–986.e9. [Google Scholar] [CrossRef]
- Lau, C.H.; Suh, Y. In vivo genome editing in animals using AAV-CRISPR system: Applications to translational research of human disease. F1000Research 2017, 6, 2153. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, J.; Luan, X.; Liu, X.; Li, X.; Yang, J.; Huang, T.; Sun, L.; Wang, Y.; Lin, Y.; et al. Near-infrared upconversion-activated CRISPR-Cas9 system: A remote-controlled gene editing platform. Sci. Adv. 2019, 5, eaav7199. [Google Scholar] [CrossRef] [PubMed]


| Year | Related Researches on CRISPR/CAS9 |
|---|---|
| In 1987 | Ishino et al. first found a clustered palindromic sequence with short spacers in Escherichia coli [32]. |
| In 2002 | Such a sequence family was officially named CRISPR by Jansen et al. |
| After 2011 | The mechanism of CRISPR-Cas in the bacteria-acquired immune system was basically elucidated, thus laying a solid foundation for further application. |
| In 2013 | Hwang et al. realized multigene knock-out in the embryo of zebrafish with efficiency at the highest of 59.4% [36]. |
| In 2014 | NIU et al. edited Ppar g and Rag1 genes, effectively realizing gene modification on specific loci [37]. |
| Since 2015 | CRISPR/CAS9 is beginning to be used in clinical studies [38]. |
| In 2017 | The smallest CRISPR/CAS9 was found in Campylobacter jejuni [28]. |
| In 2018 | CRISPR/CAS9 is beginning to be used for the study of autophagy [18]. |
| In 2020 | CRISPR-Cas9 using targeted lipid nanoparticles is being used for cancer therapy [22]. |
| In 2021 | Harnessing the type I CRISPR-Cas systems for genome editing in prokaryotes [39]. |
| In 2022 | Based on CRISPR/Cas9 to predict the clinical outcomes of patients with low-grade Glioma [40]. |
| Research Direction | Locus of Action by CRISPR/Cas9 | Effect | References |
|---|---|---|---|
| Inhibition of tumor progression by enhancing the expression of cancer suppressor genes | RNA inhibition in combination with CRISPR/Cas9 | Could be used for identifying possible off-targets and taking a potential compensating action | [50] |
| Targeting gene knockout | Knock out CHAF1A | Result in G1 phase arrest and apoptosis of glioma cells (U251 and U87) as well as blocking the AKT/FOXO3a /Bim signaling pathway | [51] |
| Knock out Ninjurin2 shRNA | Inhibit the cell survival, growth, proliferation, migration, and invasion while inducing apoptosis | [52] | |
| Knock out IGF2BP1 | Activate the MAGEA6-AMPK signaling, resulting in the apoptosis of glioma cells (A172) | [53] | |
| Knock out TIM3 gene | Enhance the cytotoxicity to GBM cells medicated by human NK cells | [54] | |
| Knock out VMP1 | Block the autophagic flux so as to make GBM cells sensitive to radiotherapy and chemotherapy | [55] | |
| Research on drugs targeting neuroglioma | Knock out ATRX gene | Explore its influence on TMZ resistance | [53] |
| Immunotherapy | CD19-specific CAR targeting TRAC loci | Not only result in uniform CAR expression in human peripheral blood T lymphocytes but also enhance the activities of T cells | [56] |
| Down-regulate PD-1 signaling | Enhance the efficacy of CAR- T immunotherapy | [13] | |
| Knock out B7-H6 | Study the immunotherapy targeting of glioma stem cells | [57] | |
| Establishment of specific animal models | Specific disease genes | Establish a natural mouse glioma model with immune activities | [58] |
| Screening of specific functional genes | Screen genes that could promote tumor cells to invade into normal tissues | Explore a therapy to block tumor invasion | [53] |
| Explore functions of encoding genomes | Determine pathways related to the growth of a tumorigenic population | [59] | |
| Screen a few drug-resistance-related genes of which candidate genes can be targeted by inhibitors or small molecules | Weaken resistance of glioblastoma to TMZ | [60] | |
| Research on tumor mechanism | Target an HRas-IRES-tdTomato construct by homologous recombination into the TP53 gene locus | Observe the tumor progression in human organoids | [61] |
| Mediate the directed mutation of neuroglioma cells | Study the potential pathogenesis of neuroglioma | [62] | |
| Knock in an inducible histone 2B-GFP (iH2B-GFP) | Track cell division history and study pathogenesis | [63] | |
| Knock out DAZL gene | Find out that DAZL contributes to the tumorigenicity of glioblastoma via reducing cell stemness | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, X.; Wang, Y.; Liu, P.; Huang, B.; Zhou, B.; Lu, S.; Geng, W.; Tang, H. Progresses, Challenges, and Prospects of CRISPR/Cas9 Gene-Editing in Glioma Studies. Cancers 2023, 15, 396. https://doi.org/10.3390/cancers15020396
Kang X, Wang Y, Liu P, Huang B, Zhou B, Lu S, Geng W, Tang H. Progresses, Challenges, and Prospects of CRISPR/Cas9 Gene-Editing in Glioma Studies. Cancers. 2023; 15(2):396. https://doi.org/10.3390/cancers15020396
Chicago/Turabian StyleKang, Xianhui, Yijian Wang, Pan Liu, Baojun Huang, Baofeng Zhou, Shufang Lu, Wujun Geng, and Hongli Tang. 2023. "Progresses, Challenges, and Prospects of CRISPR/Cas9 Gene-Editing in Glioma Studies" Cancers 15, no. 2: 396. https://doi.org/10.3390/cancers15020396
APA StyleKang, X., Wang, Y., Liu, P., Huang, B., Zhou, B., Lu, S., Geng, W., & Tang, H. (2023). Progresses, Challenges, and Prospects of CRISPR/Cas9 Gene-Editing in Glioma Studies. Cancers, 15(2), 396. https://doi.org/10.3390/cancers15020396





