Dysfunctional Lipid Metabolism—The Basis for How Genetic Abnormalities Express the Phenotype of Aggressive Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. General Lipid Metabolism
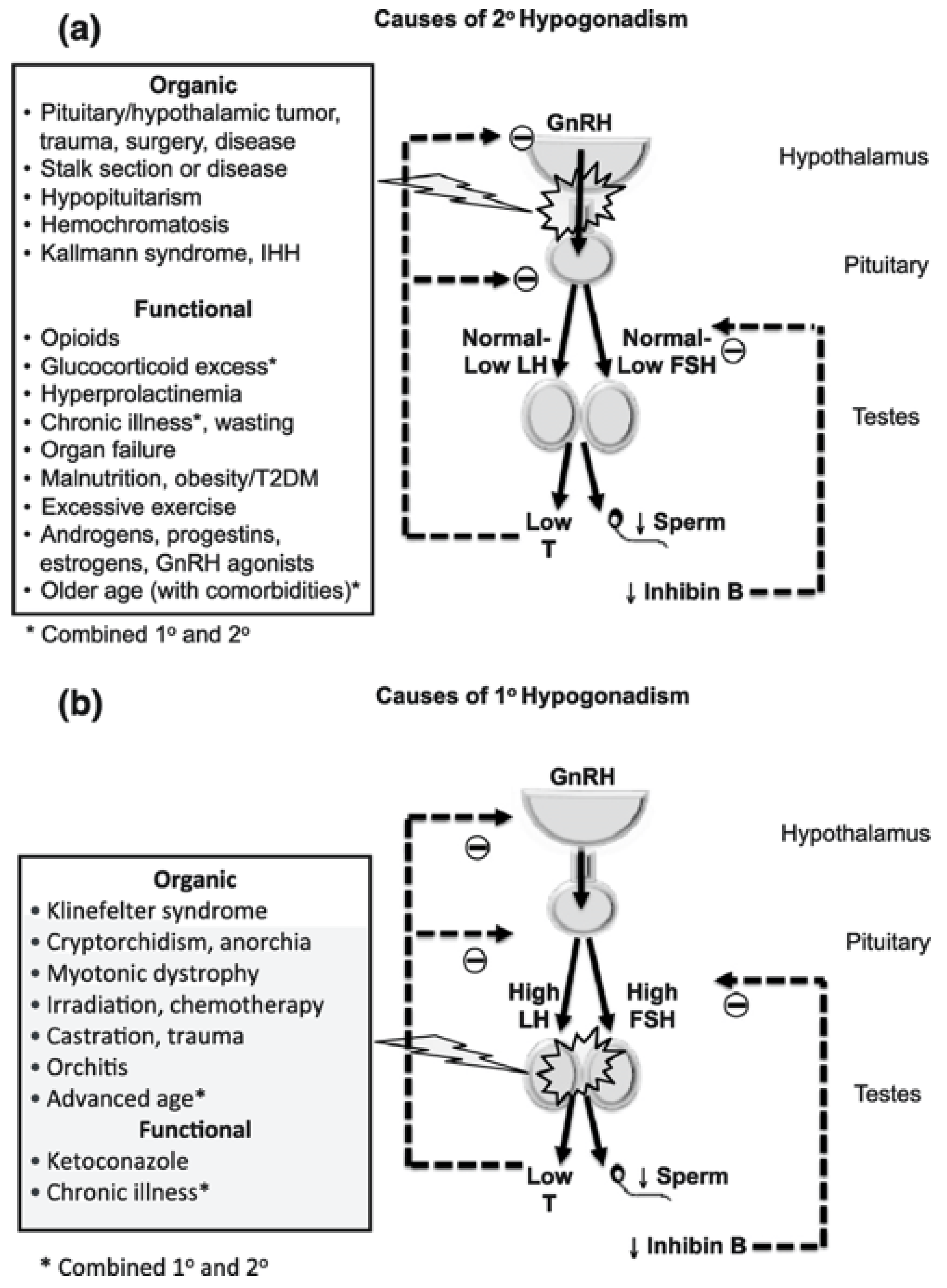
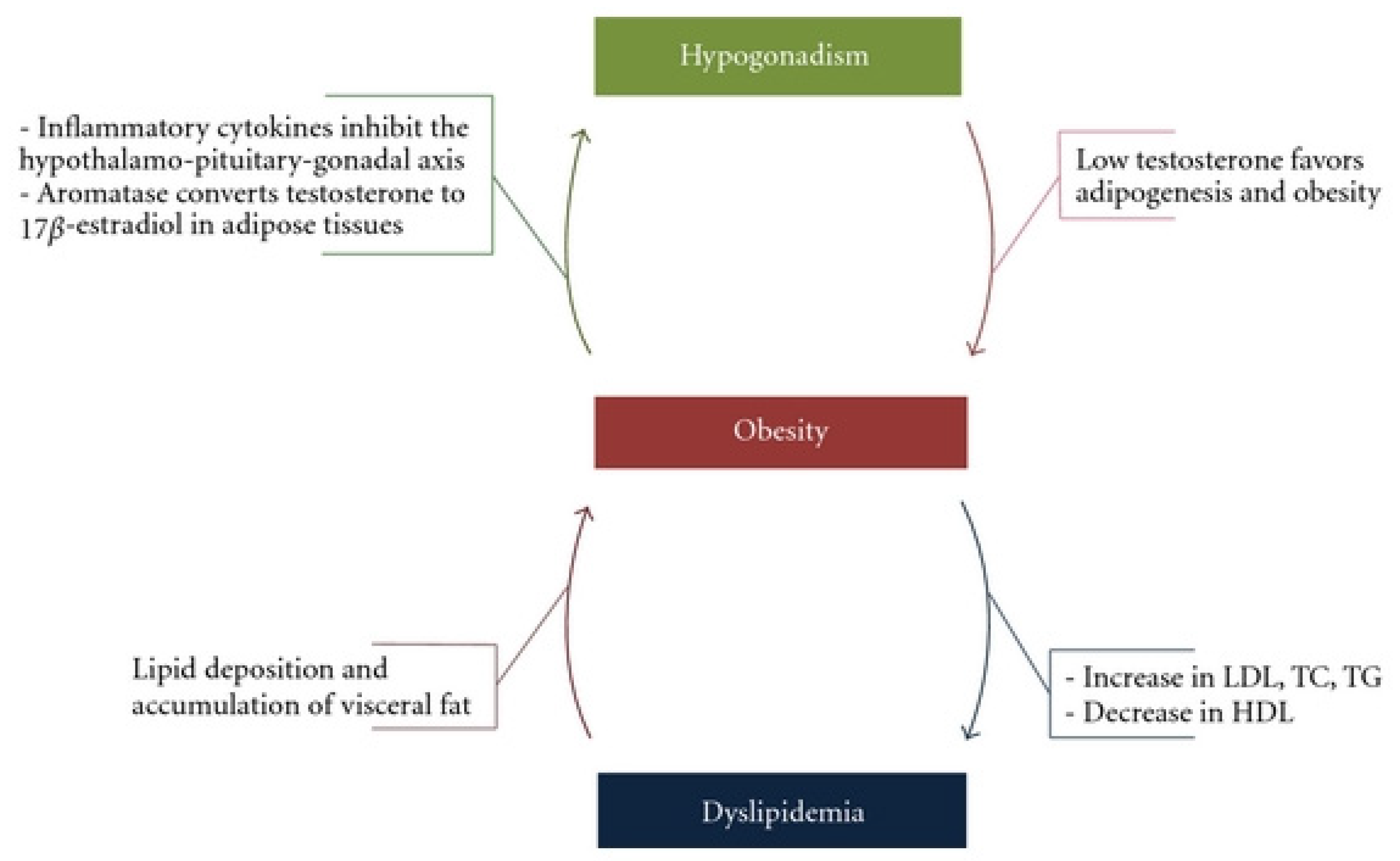
3. Lipid Metabolism, Hypogonadism and Testosterone Disorders—Chicken or the Egg?
4. Prostate Cancer and Dysregulated Lipid Metabolism
5. Lipid Metabolism and Androgens in Prostate Cancer

6. Androgen Deprivation Therapy
7. Cellular Mechanisms of Prostate Cancer and Dysfunctional Lipid Metabolism
8. Novel Pharmacology Treatments
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate cancer incidence and mortality: Global status and temporal trends in 89 countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, D.; Ofori Awuah, E.; Owusu, S. Chapter 2–lipid metabolism: An overview. In The Molecular Nutrition of Fats; Patel, V.B., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 17–32. [Google Scholar]
- Butler, L.; Centenera, M.; Swinnen, J.V. Androgen control of lipid metabolism in prostate cancer: Novel insights and future applications. Endocr. Relat. Cancer 2016, 23, R219–R227. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.; Shen, W.-J.; Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Mounier, C.; Bouraoui, L.; Rassart, E. Lipogenesis in cancer progression (Review). Int. J. Oncol. 2014, 45, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.; Vreken, P.; den Boer, M.E.; Wijburg, F.A.; van Gennip, A.H.; IJlst, L. Disorders of mitochondrial fatty acyl-CoA beta-oxidation. J. Inherit. Metab. Dis. 1999, 22, 442–487. [Google Scholar] [CrossRef]
- Haffner, S.M.; Mykkänen, L.; A Valdez, R.; Katz, M.S. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J. Clin. Endocrinol. Metab. 1993, 77, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Malkin, C.J.; Pugh, P.J.; Jones, R.D.; Kapoor, D.; Channer, K.S.; Jones, T.H. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metab. 2004, 89, 3313–3318. [Google Scholar] [CrossRef]
- Pivonello, R.; Menafra, D.; Riccio, E.; Garifalos, F.; Mazzella, M.; de Angelis, C.; Colao, A. Metabolic disorders and male hypogonadotropic hypogonadism. Front. Endocrinol. 2019, 10, 345. [Google Scholar] [CrossRef]
- Basaria, S. Male hypogonadism. Lancet 2014, 383, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, C.W.; Lee, J.S.; Seo, J.T. Hypogonadism makes dyslipidemia in klinefelter’s syndrome. J. Korean Med. Sci. 2017, 32, 1848–1851. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M. Androgen deficiency in older men. Aust. J. Gen. Pract. 2019, 48, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Matsumoto, A.M. A Perspective on Middle-aged and older men with functional hypogonadism: Focus on holistic management. J. Clin. Endocrinol. Metab. 2017, 102, 1067–1075. [Google Scholar] [CrossRef]
- Sartorius, G.; Spasevska, S.; Idan, A.; Turner, L.; Forbes, E.; Zamojska, A.; Allan, C.A.; Ly, L.P.; Conway, A.J.; McLachlan, R.I.; et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: The healthy man study. Clin. Endocrinol. 2012, 77, 755–763. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Lin, J.; Johnson-Levonas, A.O.; Shah, A.K.; Meehan, A.G. Increased occurrence of marked elevations of lipoprotein(a) in ageing, hypercholesterolaemic men with low testosterone. Aging Male 2010, 13, 40–43. [Google Scholar] [CrossRef]
- Kapoor, D.; Aldred, H.; Clark, S.; Channer, K.S.; Jones, T.H. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: Correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007, 30, 911–917. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Charchar, F.; Maric, C.; Kuzniewicz, R.; Gola, M.; Grzeszczak, W.; Samani, N.J.; Zukowska-Szczechowska, E. Association between lipid profile and circulating concentrations of estrogens in young men. Atherosclerosis 2009, 203, 257–262. [Google Scholar] [CrossRef][Green Version]
- Choong, K.; Basaria, S. Emerging cardiometabolic complications of androgen deprivation therapy. Aging Male 2010, 13, 1–9. [Google Scholar] [CrossRef]
- Fahed, A.C.; Gholmieh, J.M.; Azar, S.T. Connecting the lines between hypogonadism and atherosclerosis. Int. J. Endocrinol. 2012, 2012, 793953. [Google Scholar] [CrossRef] [PubMed]
- Medes, G.; Thomas, A.; Weinhouse, S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953, 13, 27–29. [Google Scholar]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Kuemmerle, N.B.; Rysman, E.; Lombardo, P.S.; Flanagan, A.J.; Lipe, B.C.; Wells, W.A.; Pettus, J.R.; Froehlich, H.M.; Memoli, V.A.; Morganelli, P.M.; et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol. Cancer Ther. 2011, 10, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zuckier, L.S.; Ghesani, N.V. Dominant uptake of fatty acid over glucose by prostate cells: A potential new diagnostic and therapeutic approach. Anticancer Res. 2010, 30, 369–374. [Google Scholar]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and prostate cancer: Weighing the evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef]
- Migita, T.; Ruiz, S.; Fornari, A.; Fiorentino, M.; Priolo, C.; Zadra, G.; Inazuka, F.; Grisanzio, C.; Palescandolo, E.; Shin, E.; et al. Fatty acid synthase: A metabolic enzyme and candidate oncogene in prostate cancer. Gynecol. Oncol. 2009, 101, 519–532. [Google Scholar] [CrossRef]
- Sadowski, M.C.; Pouwer, R.H.; Gunter, J.H.; Lubik, A.A.; Quinn, R.J.; Nelson, C.C. The fatty acid synthase inhibitor triclosan: Repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget 2014, 5, 9362–9381. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.-Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by pten loss and pi3k/akt activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Van Veldhoven, P.P.; Esquenet, M.; Heyns, W.; Verhoeven, G. Androgens markedly stimulate the accumulation of neutral lipids in the human prostatic adenocarcinoma cell line LNCaP. Endocrinology 1996, 137, 4468–4474. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Ulrix, W.; Heyns, W.; Verhoeven, G. Coordinate regulation of lipogenic gene expression by androgens: Evidence for a cascade mechanism involving sterol regulatory element binding proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 12975–12980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hou, X.; Shao, C.; Li, J.; Cheng, J.-X.; Kuang, S.; Ahmad, N.; Ratliff, T.; Liu, X. Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer. Cancer Res 2014, 74, 6635–6647. [Google Scholar] [CrossRef]
- Wu, X.; Daniels, G.; Lee, P.; Monaco, M.E. Lipid metabolism in prostate cancer. Am. J. Clin. Exp. Urol. 2014, 2, 111–120. [Google Scholar] [PubMed]
- Sharifi, N. Androgen Deprivation Therapy for Prostate Cancer. JAMA 2005, 294, 238. [Google Scholar] [CrossRef] [PubMed]
- Stoykova, G.E.; Schlaepfer, I.R. Lipid metabolism and endocrine resistance in prostate cancer, and new opportunities for therapy. Int. J. Mol. Sci. 2019, 20, 2626. [Google Scholar] [CrossRef]
- Wang, L.; Paller, C.J.; Hong, H.; De Felice, A.; Alexander, G.C.; Brawley, O. Comparison of systemic treatments for metastatic castration-sensitive prostate cancer. JAMA Oncol. 2021, 7, 412–420. [Google Scholar] [CrossRef]
- Weiner, A.B.; Cohen, J.E.; DeLancey, J.O.; Schaeffer, E.M.; Auffenberg, G.B. Surgical versus medical castration for metastatic prostate cancer: Use and overall survival in a national cohort. J. Urol. 2020, 203, 933–939. [Google Scholar] [CrossRef]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef]
- Haseen, F.; Murray, L.J.; Cardwell, C.; O’Sullivan, J.M.; Cantwell, M.M. The effect of androgen deprivation therapy on body composition in men with prostate cancer: Systematic review and meta-analysis. J. Cancer Surviv. 2010, 4, 128–139. [Google Scholar] [CrossRef]
- Edmunds, K.; Tuffaha, H.; A Galvão, D.; Scuffham, P.; Newton, R.U. Incidence of the adverse effects of androgen deprivation therapy for prostate cancer: A systematic literature review. Support. Care Cancer 2020, 28, 2079–2093. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Kam, S.C. Metabolic effects of androgen deprivation therapy. Korean J. Urol. 2015, 56, 12–18. [Google Scholar] [CrossRef]
- Mitsuzuka, K.; Arai, Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int. J. Urol. 2018, 25, 45–53. [Google Scholar] [CrossRef]
- Shore, N.D. Experience with degarelix in the treatment of prostate cancer. Ther. Adv. Urol. 2013, 5, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guccini, I.; Di Mitri, D.; Brina, D.; Revandkar, A.; Sarti, M.; Pasquini, E.; Alajati, A.; Pinton, S.; Losa, M.; et al. Compartmentalized activities of the pyruvate dehydrogenase complex sustain lipogenesis in prostate cancer. Nat. Genet. 2018, 50, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.-S.; Lee, Y.-R.; Fung, J.; Katon, J.M.; et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Cutz, J.-C.; Nuin, P.A.; Joshua, A.M.; Bayani, J.; Evans, A.J.; Zielenska, M.; Squire, J.A. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet. Cytogenet. 2006, 169, 128–137. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.-M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- Nunes-Xavier, C.E.; Mingo, J.; Emaldi, M.; Flem-Karlsen, K.; Mælandsmo, G.M.; Fodstad, Ø.; Llarena, R.; López, J.I.; Pulido, R. Heterogeneous expression and subcellular localization of pyruvate dehydrogenase complex in prostate cancer. Front. Oncol. 2022, 12, 873516. [Google Scholar] [CrossRef]
- Sun, J.-X.; Liu, C.-Q.; Zhong, X.-Y.; Xu, J.-Z.; An, Y.; Xu, M.-Y.; Hu, J.; Zhang, Z.-B.; Xia, Q.-D.; Wang, S.-G. Statin use and the risk of prostate cancer biochemical recurrence following definitive therapy: A systematic review and meta-analysis of cohort studies. Front. Oncol. 2022, 12, 887854. [Google Scholar] [CrossRef] [PubMed]
- Parrales, A.; Iwakuma, T. p53 as a regulator of lipid metabolism in cancer. Int. J. Mol. Sci. 2016, 17, 2074. [Google Scholar] [CrossRef] [PubMed]
- Mandigo, A.C.; Yuan, W.; Xu, K.; Gallagher, P.; Pang, A.; Guan, Y.F.; Shafi, A.A.; Thangavel, C.; Sheehan, B.; Bogdan, D.; et al. RB/E2F1 as a master regulator of cancer cell metabolism in advanced disease. Cancer Discov. 2021, 11, 2334–2353. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Hahm, E.-R.; Kim, S.-H.; Wendell, S.G.; Singh, S.V. A novel metabolic function of Myc in regulation of fatty acid synthesis in prostate cancer. Oncogene 2021, 40, 592–602. [Google Scholar] [CrossRef]
- Montironi, R.; Cimadamore, A.; Lopez-Beltran, A.; Scarpelli, M.; Aurilio, G.; Santoni, M.; Massari, F.; Cheng, L. Morphologic, molecular and clinical features of aggressive variant prostate cancer. Cells 2020, 9, 1073. [Google Scholar] [CrossRef]
- Aparicio, A.M.; Shen, L.; Tapia, E.L.N.; Lu, J.-F.; Chen, H.-C.; Zhang, J.; Wu, G.; Wang, X.; Troncoso, P.; Corn, P.; et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin. Cancer Res. 2016, 22, 1520–1530. [Google Scholar] [CrossRef]
- Aparicio, A.M.; Harzstark, A.L.; Corn, P.G.; Wen, S.; Araujo, J.C.; Tu, S.-M.; Pagliaro, L.C.; Kim, J.; Millikan, R.E.; Ryan, C.; et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 3621–3630. [Google Scholar] [CrossRef]
- Karimaa, M.; Riikonen, R.; Kettunen, H.; Taavitsainen, P.; Ramela, M.; Chrusciel, M.; Karlsson, S.; Rummakko, P.; Simola, O.; Wohlfahrt, G.; et al. First-in-class small molecule to inhibit cyp11a1 and steroid hormone biosynthesis. Mol. Cancer Ther. 2022, 21, 1765–1776. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberto, M.; Yim, A.; Lawrentschuk, N.; Bolton, D. Dysfunctional Lipid Metabolism—The Basis for How Genetic Abnormalities Express the Phenotype of Aggressive Prostate Cancer. Cancers 2023, 15, 341. https://doi.org/10.3390/cancers15020341
Alberto M, Yim A, Lawrentschuk N, Bolton D. Dysfunctional Lipid Metabolism—The Basis for How Genetic Abnormalities Express the Phenotype of Aggressive Prostate Cancer. Cancers. 2023; 15(2):341. https://doi.org/10.3390/cancers15020341
Chicago/Turabian StyleAlberto, Matthew, Arthur Yim, Nathan Lawrentschuk, and Damien Bolton. 2023. "Dysfunctional Lipid Metabolism—The Basis for How Genetic Abnormalities Express the Phenotype of Aggressive Prostate Cancer" Cancers 15, no. 2: 341. https://doi.org/10.3390/cancers15020341
APA StyleAlberto, M., Yim, A., Lawrentschuk, N., & Bolton, D. (2023). Dysfunctional Lipid Metabolism—The Basis for How Genetic Abnormalities Express the Phenotype of Aggressive Prostate Cancer. Cancers, 15(2), 341. https://doi.org/10.3390/cancers15020341





