Current and Emerging Strategies to Treat Urothelial Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Overview
1.2. Environmental and Hereditary Risk Factors for Urothelial Cancers
1.3. Novel Approaches for Urothelial Cancers
2. Divergent Mechanisms Underlying Urothelial Tumorigenesis and Treatment Resistance
2.1. Invasive and Non-Invasive UCC
2.2. Cell Cycle Alterations
2.3. Influence of Angiogenesis on Invasion and Metastasis
2.4. Effect of Hypoxia on UCC Invasion and Metastasis
2.5. Immune Dysregulation in UCC
3. Screening, Diagnostic Approach and Staging of Urothelial Carcinoma
4. Current Treatment of UCC
4.1. Initial Treatment
4.2. Bladder Preservation in UCC
4.3. Surgical
4.4. Single Agent and Combination Chemotherapy
5. Emerging Strategies to Treat UCC
5.1. Targeting FGFR
5.2. Immune Checkpoint Inhibitors
5.3. Antibody-Drug Conjugates
5.4. Cellular Vaccines and Oncolytic Viruses
5.5. CAR-T Therapy
5.6. Antiangiogenics
5.7. Herbal Medicines
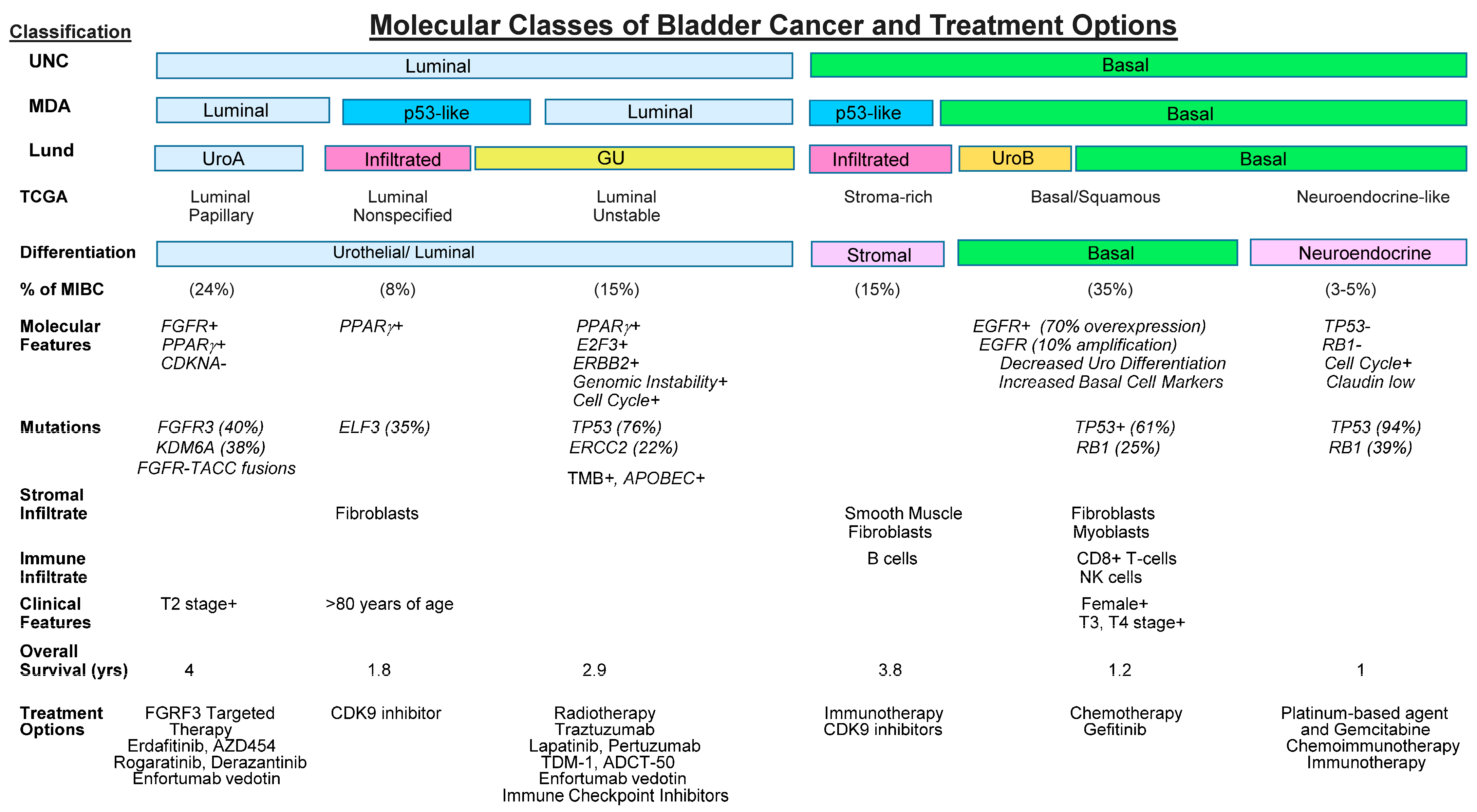
6. Molecular Classification as a Prognostic to Guide Treatment Decisions
| Chemotherapy | No. of Patients | Patient Positivity, % | Stage of Disease | Comments |
|---|---|---|---|---|
| DNA Damage Repair Pathways Nucleotide Excision Repair ERCC1 Expression, ERCC2 Mutations | 100 Patients | 47 | Locally advanced and mUCC | mRNA obtained from patient tumor biopsies and analyzed by reverse transcriptase polymerase chain reaction (RT-PCR) was used to measure mRNA levels of several DNA repair genes. Deleterious mutations in an NER pathway helicase, ERRC2, are predictive of cisplatin sensitivity in bladder cancer patients. DNA repair deficiency phenotype predicts benefit from platinum-based chemotherapy. |
| Microsatellite Instability (MSI) | 44 patients | 40.6 | NMIBC and MIBC | DNA from patient tumor biopsies was used to assess microsatellite sequence length using Polymerase chain reaction (PCR). MSI-H status in UC predicts deep and durable responses to CPI and is associated with inferior chemotherapy responses. CPI should be considered for first-line treatment in this subset of patients [169] |
| APOBEC mutational signature | 307 patients | Up to 70 | mUCC | Using patient tumor biopsies, genomic DNA was isolated and assayed using whole-exome sequencing (WES) and targeted next-generation sequencing (NGS) techniques to assess for APOBEC mutational signature. APOBEC-high are more likely to have mutations in DNA damage response genes (TP53, ATR, BRCA2) and chromatin regulatory genes (ARID1A, MLL, MLL3), potentially leading to a hypermutation phenotype and subsequent enhanced immune response against the tumor. IMvigor-130 trial- mUC patients with APOBEC mutational signature had significantly higher TMB and improved OS with atezolizumab containing regimens in the first-line cisplatin-ineligible scenario [170] |
| Immunotherapy Markers | ||||
| PD-L1—IHC | 40 patients | 20–72 | Locally advanced and mUCC | Patient tumor biopsies were assayed for PDL1 expression using immunohistochemistry (IHC) and expressed as a combined positive score (CPS, positive tumor cells and immune cells divided by the total number of cells). PD-L1 expression and high (TMB) may predict better responses to ICIs, but patients without these biomarkers may still respond to immunotherapy. Additional caveats include a lack of standardization, tumor heterogeneity and other factors influencing the TME. |
| PD-L1 IHC PD-L1 (CD274)—Amplification TMB MSI | Genitourinary tumors (0.4%, 10/2420) | Protein expression- using a CPS 1 cutoff for UC, the positive prevalence was 83.6% (989/1183) Prevalence of 0.7% from 1183 patients TMB- Urothelial carcinoma (36.0%, 426/1183). MSI-H Prevalence 1.2% of 1183 patients | Locally advanced and mUCC | Retrospective pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases. analysis of all cases in which both PD-L1 IHC (using the DAKO 22C3 IHC assay with either tumor proportion score (TPS) or combined positive score (CPS); or the VENTANA SP142 assay with infiltrating immune cell score (IC)) and comprehensive genomic profiling (CGP) were tested at Foundation Medicine between January 2016 and November 2019. PD-L1 positivity was defined per the CDx indication and tumor proportion score (TPS ≥ 1) for indications without a CDx claim; and TMB positivity is defined as ≥10 mutations/Mb. A total of 48,782 cases were tested for PD-L1 IHC and CGP. Patient tumor biopsies were assayed for PD-L1 amplification using PD-L1 RNA in situ hybridization (RNAish). PD-L1 amplification was detected in only 0.7% of solid tumors. Amplification had a low correlation with PD-L1 IHC and did not correlate with TMB. |
| Tumor Mutational Burden (TMB) | 401 patients | 30.4 | Locally advanced and mUCC | Genomic DNA was isolated from patient tumor biopsies and characterized for mutational burden using NGS. TMB ≥ 10 mutations per megabase was detected in 122 of 401 (30.4%) patients. Total of 191 linked to response to ICI. High TMB correlated with response in certain solid tumor types: melanoma and NSCLC. May correlate mUCC. TMB assessment is multi-factorial. |
| Inflammatory Gene Signatures | Checkmate-275 trial indicated a better response to Nivolumab therapy. | |||
| ARID1A mutation + CXCL13 expression levels | 275 patients + 348 patients | 50.5% 62% | Locally advanced and mUCC | DNA and RNA were isolated from patient tumor biopsies and characterized using NGS. Interrogated CXCL13 expression and ARID1A mutation as a combination biomarker in predicting response to ICT in CheckMate275 and IMvigor210. The combination of the two biomarkers in baseline tumor tissues suggested improved OS compared to either single biomarker. Cumulatively, this study revealed that the combination of CXCL13 plus ARID1A may improve prediction capability for patients receiving ICT. |
| TRAF2 loss | 116 patients | 73% | Patient tumor biopsies were analyzed using whole-exome sequencing, RNA-seq, proteomic, and phosphoproteomic analysis to describe TRAF2 status. Proteomic analysis identified three groups reflecting distinct clinical prognoses and molecular signatures. Immune subtypes of UC tumors revealed a complex immune landscape and suggested that TRAF2 amplification is related to the increased expression of PD-L1. Increased GARS was validated to promote the pentose phosphate pathway by inhibiting activities of PGK1 and PKM2. | |
| CCND1 amplification | 152 patients | Primary homogeneous 15% Primary- heterogeneous 6% Metastasis- homogeneous 22% Metastasis- heterogeneous 2% | Lymph node (LN)-positive UCC pts | CCND1 and expression of CyclinD1 were evaluated by fluorescence in situ hybridization and immunohistochemistry on patient tumor biopsies obtained from node-positive urothelial bladder cancers. |
| Targeted Therapies | ||||
| FGFR alterations | 87 patients | 10–30% | Locally advanced and mUCC | DNA was isolated from patient tumor biopsies and characterized using NGS for FGFR single nucleotide variants, gene fusions or copy number abnormalities. Aberrantly activated through single-nucleotide variants, gene fusions and CNA in 5–10% of all human cancers, frequency increases to 10–30% in UC. Numerous FGFR inhibitors are currently being assessed in preclinical, Phase 1, Phase 2 and Phase 3 clinical trials. Erdafitinib and pemigatinib are currently the only approved inhibitors for use in the treatment of patients with FGFR-altered UC and Cholangiocarcinoma. UC patients with an increased frequency of FGFR3 point mutations tend to respond better to TKI therapy FGFR fusions- Clinical-grade NGS diagnostics to detect FGFR fusions and SNVs using tissue and ctDNA. Rapid identification of patients for targeted therapies and the real-time detection of acquired mutations that signal impending treatment resistance and cancer progression. |
| NECTIN4 | 169 patients | 59.7% | All NMIBC and MIBC were included | Patient tumor biopsies were analyzed using IHC for Nectin-4 expression. High expression of Nectin-4 in squamous cell carcinoma and adenocarcinoma may guide treatment with novel Nectin-4-directed ADCs and provide a high-risk patient collective with a new promising therapeutic option. Nectin-4-directed therapy enfortumab vedotin, an ADC comprised of a fully human monoclonal antibody specific for nectin-4 conjugated through a cleavable linker to the microtubule inhibitor MMAE. Nectin-4 was not prognostic in histological subtypes of BC [171] |
| TROP2 | >1400 patients | Up to 82% | Refractory mUCC | Patient tumor biopsies were analyzed using IHC for TROP2 expression. Sacituzumab Govitecan (SG) is an ADC targeting TROP2, approved for treatment-refractory mUC. Using gene expression data from four clinical cohorts with >1400 patient samples of muscle-invasive BC and a BC tissue microarray, we found that TROP2 mRNA and protein are highly expressed across basal, luminal, and stroma-rich subtypes, but depleted in the neuroendocrine subtype. High levels of TROP2 in most subtypes were detected except in the neuroendocrine subtype. TROP2 expression is higher than NECTIN4 expression, and cells resistant to enfortumab vedotin, remain sensitive to SG. |
| Erb2 (Her2, EGFR) | 128 patients | 10.5% | LN-positive disease and mUCC | Patient tumor biopsies were analyzed using IHC for ERBB2 expression. ERBB2 overexpression and amplification were linked with high-grade and high-stage upper-tract urothelial CA (UTUCs) tumors and with tumor progression. Results suggest that ERBB2 is a biomarker for progression in UTUCs. |
| PI3K/AKT/mTOR/MAPK | 45 patients | ~42% in PI3K/AKT/mTOR 17% activating point mutations in PIK3CA 10% overexpression of AKT3 9% with mutations or deletions of TSC1/2 | Refractory UC | DNA was isolated from patient tumor biopsies and mutations in mTOR genes were assayed by NGS. Limited clinical benefit with targeting this pathway in advanced UC. Phase II, single-arm, non-randomized study with everolimus in refractory UC showed minimal response with median PFS 2.6 months, median OS 8.3 months, and 2 responses seen in 45 patients. |
| DNA Damage Repair (DDR) Gene Abnormalities | 19 patients | Alterations in DDR in up to 25% | mUCC | DNA was isolated from patient tumor biopsies and alterations in DNA damage repair genes were assayed by NGS. Single-agent olaparib showed limited antitumor activity in patients with mUC and DDR alterations. May relate to poorly characterized functional implications of particular DDR alterations and/or cross-resistance with platinum-based chemotherapy [159] |
| VEGF | 40 patients | 82% | LN-positive disease and mUCC | Patient tumor biopsies were analyzed using IHC for VEGF expression. The proportion of VEGF+ cells were defined to calculate a proportion score. The relative intensity was quantitated into an intensity score. Finally, a total immunostaining score was determined as the product of a proportion score and intensity score. Elevated VEGF correlates with worse outcome. VEGF pathway inhibition attenuates tumor proliferation and invasion. Ramucirumab, a fully humanized monoclonal antibody that binds VEGF receptor 2, has shown benefit both in randomized phase II and III trials. Results with sunitinib, pazopanib, vandetinib, or cabozantinib were not convincing. |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krakhmal, N.V.; Zavyalova, M.V.; Denisov, E.V.; Vtorushin, S.V.; Perelmuter, V.M. Cancer Invasion: Patterns and Mechanisms. Acta Nat. 2015, 7, 17–28. [Google Scholar] [CrossRef]
- Katims, A.B.; Reisz, P.A.; Nogueira, L.; Truong, H.; Lenis, A.T.; Pietzak, E.J.; Kim, K.; Coleman, J.A. Targeted Therapies in Advanced and Metastatic Urothelial Carcinoma. Cancers 2022, 14, 5431. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Compérat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current best practice for bladder cancer: A narrative review of diagnostics and treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef]
- Akhtar, M.; Al-Bozom, I.; Ben Gashir, M.; Taha, N.M.; Rashid, S.; Al-Nabet, A.D.M.H. Urothelial Carcinoma In Situ (CIS): New Insights. Adv. Anat. Pathol. 2019, 26, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Deuker, M.; Nocera, L.; Collà Ruvolo, C.; Tian, Z.; Shariat, S.F.; Saad, F.; Briganti, A.; Becker, A.; Kluth, L.A.; et al. Comparison Between Urothelial and Non-Urothelial Urethral Cancer. Front. Oncol. 2021, 10, 629692. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Fujita, K.; Hayashi, Y.; Hatano, K.; Kawashima, A.; McConkey, D.J.; Nonomura, N. Mutational Landscape and Environmental Effects in Bladder Cancer. Int. J. Mol. Sci. 2020, 21, 6072. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between Smoking and Risk of Bladder Cancer among Men and Women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.; Rota, M.; Catto, J.W.F.; La Vecchia, C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-Analysis of Incidence and Mortality Risks. Eur. Urol. 2016, 70, 458–466. [Google Scholar] [CrossRef]
- Letašiová, S.; Medve’ová, A.; Šovčíková, A.; Dušinská, M.; Volkovová, K.; Mosoiu, C.; Bartonová, A. Bladder cancer, a review of the environmental risk factors. Environ. Health 2012, 11 (Suppl. S1), S11. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Rosenberg, R.E.; Motzer, R.J. Bladder and Renal Cell Carcinomas. In Harrison’s Principles of Internal Medicine; McGraw Hill: New York, NY, USA, 2015; p. 17e. [Google Scholar]
- Hubosky, S.G.; Boman, B.M.; Charles, S.; Bibbo, M.; Bagley, D.H. Ureteroscopic management of upper tract urothelial carcinoma (UTUC) in patients with Lynch Syndrome (hereditary nonpolyposis colorectal cancer syndrome). BJU Int. 2013, 112, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Shafique, M.A.; Haseeb, A.; Siddiq, M.A.; Mussarat, A.; Rangwala, H.S.; Mustafa, M.S. Current and Emerging Treatments for Urothelial Carcinoma: A Focus on Enfortumab Vedotin. Cancer Manag. Res. 2023, 15, 699–706. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Daro-Faye, M.; Kassouf, W.; Souhami, L.; Marcq, G.; Cury, F.; Niazi, T.; Sargos, P. Combined radiotherapy and immunotherapy in urothelial bladder cancer: Harnessing the full potential of the anti-tumor immune response. World J. Urol. 2021, 39, 1331–1343. [Google Scholar] [CrossRef]
- Shah, S.; Zhang, C.A.; Hancock, S.; Fan, A.; Skinner, E.; Srinivas, S. Consolidative Radiotherapy in Metastatic Urothelial Cancer. Clin. Genitourin. Cancer 2017, 15, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J. Control. Release 2022, 352, 793–812. [Google Scholar]
- Liu, Y.; Zhou, Z.; Hou, J.; Xiong, W.; Kim, H.; Chen, J.; Zheng, C.; Jiang, X.; Yoon, J.; Shen, J. Tumor Selective Metabolic Reprogramming as a Prospective PD-L1 Depression Strategy to Reactivate Immunotherapy. Adv. Mater. 2022, 34, e2206121. [Google Scholar] [PubMed]
- Adamczyk, P.; Pobłocki, P.; Kadlubowski, M.; Ostrowski, A.; Wróbel, A.; Mikołajczak, W.; Adamowicz, J.; Drewa, T.; Juszczak, K. A Comprehensive Approach to Clinical Staging of Bladder Cancer. J. Clin. Med. 2022, 11, 761. [Google Scholar] [CrossRef]
- McConkey, D.J.; Lee, S.; Choi, W.; Tran, M.; Majewski, T.; Lee, S.; Siefker-Radtke, A.; Dinney, C.; Czerniak, B. Molecular genetics of bladder cancer: Emerging mechanisms of tumor initiation and progression. Urol. Oncol. 2010, 28, 429–440. [Google Scholar] [CrossRef]
- Mitra, A.P. Molecular substratification of bladder cancer: Moving towards individualized patient management. Ther. Adv. Urol. 2016, 8, 215–233. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556. [Google Scholar]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-Invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Akbani, R.; Broom, B.M.; Wang, W.; Verhaak, R.G.W.; McConkey, D.; Lerner, S.; Morgan, M.; Creighton, C.J.; Smith, C.; et al. Comprehensive Molecular Characterization of Urothelial Bladder Carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Ren, X.; Guo, S.; Guan, X.; Kang, Y.; Liu, J.; Yang, X. Immunological Classification of Tumor Types and Advances in Precision Combination Immunotherapy. Front. Immunol. 2022, 13, 790113. [Google Scholar] [CrossRef] [PubMed]
- Nordentoft, I.; Lamy, P.; Birkenkamp-Demtröder, K.; Shumansky, K.; Vang, S.; Hornshøj, H.; Juul, M.; Villesen, P.; Hedegaard, J.; Roth, A.; et al. Mutational Context and Diverse Clonal Development in Early and Late Bladder Cancer. Cell Rep. 2014, 7, 1649–1663. [Google Scholar] [CrossRef]
- Mitra, A.P.; Jordà, M.; Cote, R.J. Pathological Possibilities and Pitfalls in Detecting Aggressive Bladder Cancer. Curr. Opin. Urol. 2012, 22, 397–404. [Google Scholar]
- Droller, M.J. FGFR3 and P53 Characterize Alternative Genetic Pathways in the Pathogenesis of Urothelial Cell Carcinoma. J. Urol. 2004, 172, 1911–1914. [Google Scholar] [CrossRef]
- Homami, A.; Ataei Kachoei, Z.; Asgarie, M.; Ghazi, F. Analysis of FGFR3 and HRAS genes in patients with bladder cancer. Med. J. Islamic Repub. Iran 2020, 34, 108. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.; Choi, W.; Pal, S.; Trabulsi, E.; Kelly, W.K.; Hahn, N.M.; McConkey, D.; Comperat, E.; Matoso, A.; Cussenot, O.; et al. Urothelial Cancers with Small Cell Variant Histology Have Confirmed High Tumor Mutational Burden, Frequent TP53 and RB Mutations, and a Unique Gene Expression Profile. Eur. Urol. Oncol. 2021, 4, 297–300. [Google Scholar] [CrossRef]
- Kerzeli, I.K.; Kostakis, A.; Türker, P.; Malmström, P.U.; Hemdan, T.; Mezheyeuski, A.; Ward, D.G.; Bryan, R.T.; Segersten, U.; Lord, M.; et al. Elevated levels of MMP12 sourced from macrophages are associated with poor prognosis in urothelial bladder cancer. BMC Cancer 2023, 23, 605. [Google Scholar] [CrossRef]
- Mitra, A.P.; Hansel, D.E.; Cote, R.J. Prognostic Value of Cell-Cycle Regulation Biomarkers in Bladder Cancer. Semin. Oncol. 2012, 39, 524–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hammam, O.; Magdy, M.; Badawy, M.; Osili, K.A.; Kholy, A.E.; LeitHy, T.E. Expression of MDM2 mRNA, MDM2, P53 and P16 Proteins in Urothelial Lesions in the View of the WHO 4th Edition Guidelines as a Molecular Insight towards Personalized Medicine. Maced. J. Med. Sci. 2017, 5, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Sathe, A.; Black, P.C.; Goebell, P.J.; Kamat, A.M.; Schmitz-Draeger, B.; Nawroth, R. CDK4/6 Inhibitors in Cancer Therapy: A Novel Treatement Strategy for Bladder Cancer. Bladder Cancer 2017, 3, 79–88. [Google Scholar] [CrossRef]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef]
- Ong, F.; Moonen, L.M.; Gallee, M.P.; ten Bosch, C.; Zerp, S.F.; Hart, A.A.; Bartelink, H.; Verheij, M. Prognostic factors in transitional cell cancer of the bladder: An emerging role for Bcl-2 and p53. Radiother. Oncol. 2001, 61, 169–175. [Google Scholar] [CrossRef]
- Thomas, J.; Sonpavde, G. Molecularly Targeted Therapy towards Genetic Alterations in Advanced Bladder Cancer. Cancers 2022, 14, 1795. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Al-Rohil, R.N.; Nazeer, T.; Sheehan, C.E.; Otto, G.A.; He, J.; Palmer, G.; Yelensky, R.; Lipson, D.; et al. Advanced urothelial carcinoma: Next-generation sequencing reveals diverse genomic alterations and targets of therapy. Mod. Pathol. 2014, 27, 271–280. [Google Scholar] [CrossRef]
- Rose, T.L.; Chism, D.D.; Alva, A.S.; Deal, A.M.; Maygarden, S.J.; Whang, Y.E.; Kardos, J.; Drier, A.; Basch, E.; Godley, P.A.; et al. Phase II trial of palbociclib in patients with metastatic urothelial cancer after failure of first-line chemotherapy. Br. J. Cancer 2018, 119, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.G.; Granier, C.; Zhao, Y.; Pan, Q.; Tong, Z.; Gschwend, J.E.; Holm, P.S.; Nawroth, R. Combination of Talazoparib and Palbociclib as a Potent Treatment Strategy in Bladder Cancer. J. Pers. Med. 2021, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Dangle, P.P.; Zaharieva, B.; Jia, H.; Pohar, K.S. Ras-MAPK pathway as a therapeutic target in cancer—Emphasis on bladder cancer. Recent Pat. Anticancer Drug Discov. 2009, 4, 125–136. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Van Rhijn, B.W.G.; Zuiverloon, T.C.M.; Vis, A.N.; Radvanyi, F.; Van Leenders, G.J.L.H.; Ooms, B.C.M.; Kirkels, W.J.; Lockwood, G.A.; Boevé, E.R.; Jöbsis, A.C.; et al. Molecular Grade (FGFR3/MIB-1) and EORTC Risk Scores Are Predictive in Primary Non-Muscle-Invasive Bladder Cancer. Eur. Urol. 2010, 58, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, J.R.; Brems-Eskildsen, A.S.; Nordentoft, I.; Fristrup, N.; Schepeler, T.; Ulhøi, B.P.; Agerbæk, M.; Hartmann, A.; Bertz, S.; Wittlinger, M.; et al. Expression of TIP60 (Tat-Interactive Protein) and MRE11 (Meiotic Recombination 11 Homolog) Predict Treatment-Specific Outcome of Localised Invasive Bladder Cancer. BJU Int. 2012, 110, E1228–E1236. [Google Scholar] [CrossRef]
- Ide, H.; Inoue, S.; Miyamoto, H. Histopathological and Prognostic Significance of the Expression of Sex Hormone Receptors in Bladder Cancer: A Meta-Analysis of Immunohistochemical Studies. PLoS ONE 2017, 12, e0174746. [Google Scholar] [CrossRef]
- Tuygun, C.; Kankaya, D.; Imamoglu, A.; Sertcelik, A.; Zengin, K.; Oktay, M.; Sertcelik, N. Sex-Specific Hormone Receptors in Urothelial Carcinomas of the Human Urinary Bladder: A Comparative Analysis of Clinicopathological Features and Survival Outcomes According to Receptor Expression. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 43–51. [Google Scholar] [CrossRef]
- Boorjian, S.; Ugras, S.; Mongan, N.P.; Gudas, L.J.; You, X.; Tickoo, S.K.; Scherr, D.S. Androgen Receptor Expression Is Inversely Correlated with Pathologic Tumor Stage in Bladder Cancer. Urology 2004, 64, 383–388. [Google Scholar] [CrossRef]
- Mitra, A.P.; Pagliarulo, V.; Yang, D.; Waldman, F.M.; Datar, R.H.; Skinner, D.G.; Groshen, S.; Cote, R.J. Generation of a Concise Gene Panel for Outcome Prediction in Urinary Bladder Cancer. J. Clin. Oncol. 2009, 27, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Miyamoto, H. The Role of Estrogen Receptors in Urothelial Cancer. Front. Endocrinol. 2021, 12, 643870. [Google Scholar] [CrossRef]
- Golus, M.; Bugajski, P.; Chorbińska, J.; Krajewski, W.; Lemiński, A.; Saczko, J.; Kulbacka, J.; Szydełko, T.; Małkiewicz, B. STAT3 and Its Pathways’ Dysregulation-Underestimated Role in Urological Tumors. Cells 2022, 11, 3024. [Google Scholar] [CrossRef] [PubMed]
- Crew, J.P.; O’Brien, T.; Bradburn, M.; Fuggle, S.; Bicknell, R.; Cranston, D.; Harris, A.L. Vascular Endothelial Growth Factor Is a Predictor of Relapse and Stage Progression in Superficial Bladder Cancer. Cancer Res. 1997, 57, 5281–5285. [Google Scholar] [CrossRef][Green Version]
- Jaeger, T.M.; Weidner, N.; Chew, K.; Moore, D.H.; Kerschmann, R.L.; Waldman, F.M.; Carroll, P.R. Tumor Angiogenesis Correlates with Lymph Node Metastases in Invasive Bladder Cancer. J. Urol. 1995, 154, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Youssef, R.F.; Gupta, A.; Chade, D.C.; Karakiewicz, P.I.; Isbarn, H.; Jeldres, C.; Sagalowsky, A.I.; Ashfaq, R.; Lotan, Y. Association of Angiogenesis Related Markers With Bladder Cancer Outcomes and Other Molecular Markers. J. Urol. 2010, 183, 1744–1750. [Google Scholar] [CrossRef]
- Bryan, R.T. Cell adhesion and urothelial bladder cancer: The role of cadherin switching and related phenomena. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140042. [Google Scholar] [CrossRef]
- Rodriguez Faba, O.; Palou-Redorta, J.; Fernández-Gómez, J.M.; Algaba, F.; Eiró, N.; Villavicencio, H.; Vizoso, F.J. Matrix Metalloproteinases and Bladder Cancer: What is New? ISRN Urol. 2012, 2012, 581539. [Google Scholar] [PubMed]
- Bazargani, S.T.; Clifford, T.; Djaladat, H.; Schuckman, A.; Sadeghi, S.; Dorff, T.; Quinn, D.; Daneshmand, S. Association between Epithelial Tumor Markers’ Trends during the Course of Treatment and Oncological Outcomes in Urothelial Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 609–611. [Google Scholar] [CrossRef]
- Theodoropoulos, V.E.; Lazaris, A.C.; Sofras, F.; Gerzelis, I.; Tsoukala, V.; Ghikonti, I.; Manikas, K.; Kastriotis, I. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur. Urol. 2004, 46, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Palit, V.; Phillips, R.M.; Puri, R.; Shah, T.; Bibby, M.C. Expression of HIF-1alpha and Glut-1 in human bladder cancer. Oncol. Rep. 2005, 14, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Nanzhang, U.; Xiao, J.; Wu, H.; Ding, K. Hypoxia-Induced Autophagy Enhances Cisplatin Resistance in Human Bladder Cancer Cells by Targeting Hypoxia-Inducible Factor-1α J. Immunol. Res. 2021, 2021, 8887437. [Google Scholar]
- Hong, S.; Zhang, Y.; Cao, M.; Lin, A.; Yang, Q.; Zhang, J.; Luo, P.; Guo, L. Hypoxic Characteristic Genes Predict Response to Immunotherapy for Urothelial Carcinoma. Front. Cell Dev. Biol. 2021, 9, 762478. [Google Scholar] [CrossRef]
- Krzywinska, E.; Stockmann, C. Hypoxia, Metabolism and Immune Cell Function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef]
- Ohta, A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front. Immunol. 2016, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Yuen, V.W.-H.; Wong, C.C.-L. Hypoxia-inducible Factors and Innate Immunity in Liver Cancer. J. Clin. Investig. 2020, 130, 5052–5062. [Google Scholar] [CrossRef] [PubMed]
- Matulay, J.T.; Kamat, A.M. Advances in risk stratification of bladder cancer to guide personalized medicine. F1000Research 2018, 7, 1137. [Google Scholar] [CrossRef]
- de Jong, J.J.; Liu, Y.; Boorjian, S.A.; Bivalacqua, T.J.; Porten, S.P.; Wheeler, T.; Davicioni, E.; Svatek, R.S.; Boormans, J.L.; Black, P.C.; et al. A Genomic Classifier for Predicting Clinically Aggressive Luminal Bladder Tumors with Higher Rates of Pathological Up Staging. J. Urol. 2020, 204, 239–246. [Google Scholar] [PubMed]
- Donin, N.M.; Lenis, A.T.; Holden, S.; Drakaki, A.; Pantuck, A.; Belldegrun, A.; Chamie, K. Immunotherapy for the Treatment of Urothelial Carcinoma. J. Urol. 2017, 197, 14–22. [Google Scholar]
- Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Ahmadi Najafabadi, M.; Yousefi, F.; Mirarefin, S.M.J.; Rahbarizadeh, F. Recent Advances in Solid Tumor CAR-T Cell Therapy: Driving Tumor Cells From Hero to Zero? Front. Immunol. 2022, 13, 795164. [Google Scholar]
- Nakanishi, J.; Wada, Y.; Matsumoto, K.; Azuma, M.; Kikuchi, K.; Ueda, S. Overexpression of B7-H1 (PD-L1) Significantly Associates with Tumor Grade and Postoperative Prognosis in Human Urothelial Cancers. Cancer Immunol. Immunother. 2007, 56, 1173–1182. [Google Scholar] [CrossRef]
- Ding, X.; Chen, Q.; Yang, Z.; Li, J.; Zhan, H.; Lu, N.; Chen, M.; Yang, Y.; Wang, J.; Yang, D. Clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: A meta-analysis. Cancer Manag. Res. 2019, 11, 4171–4184. [Google Scholar] [CrossRef]
- Lyu, X.; Wang, P.; Qiao, Q.; Jiang, Y. Genomic stratification based on microenvironment immune types and PD-L1 for tailoring therapeutic strategies in bladder cancer. BMC Cancer 2021, 21, 646. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; He, D.; Cheng, Y.; Zhu, Y.; Xiao, M.; Lan, H.; Wang, Z.; Cao, K. Analysis of Tumor Microenvironment Characteristics in Bladder Cancer: Implications for Immune Checkpoint Inhibitor Therapy. Front. Immunol. 2021, 12, 672158. [Google Scholar] [CrossRef]
- Kołodziej, A.; Krajewski, W.; Matuszewski, M.; Tupikowski, K. Review of current optical diagnostic techniques for non-muscle-invasive bladder cancer. Central Eur. J. Urol. 2016, 69, 150. [Google Scholar]
- Kim, L.H.C.; Patel, M.I. Transurethral resection of bladder tumor (TURBT). Trans. Androl. Urol. 2020, 9, 3056–3072. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Riches, J.; Schöder, H.; Akin, O.; Trout, A.; Milowsky, M.I.; Bajorin, D.F. Clinical Value of Fluorine-18 2-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography/Computed Tomography in Bladder Cancer. J. Clin. Oncol. 2010, 28, 3973–3978. [Google Scholar] [CrossRef]
- Soubra, A.; Hayward, D.; Dahm, P.; Goldfarb, R.; Froehlich, J.; Jha, G.; Konety, B.R. The Diagnostic Accuracy of 18F-Fluorodeoxyglucose Positron Emission Tomography and Computed Tomography in Staging Bladder Cancer: A Single-Institution Study and a Systematic Review with Meta-Analysis. World J. Urol. 2016, 34, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Koutsilieris, M. Diagnostic Value of Circulating Tumor Cell Detection in Bladder and Urothelial Cancer: Systematic Review and Meta-Analysis. BMC Cancer 2011, 11, 336. [Google Scholar] [CrossRef]
- Shelley, M.; Court, J.B.; Kynaston, H.; Wilt, T.J.; Fish, R.; Mason, M. Intravesical Bacillus Calmette-Guérin in Ta and T1 Bladder Cancer. Cochrane Database Syst. Rev. 2000, 2000, CD001986. [Google Scholar] [CrossRef]
- Bevers, R.F.M.; Kurth, K.H.; Schamhart, D.H.J. Role of Urothelial Cells in BCG Immunotherapy for Superficial Bladder Cancer. Br. J. Cancer 2004, 91, 607–612. [Google Scholar] [PubMed]
- SEER Cancer Stat Facts: Bladder Cancer. In Surveillance, Epidemiology and End Results Program; National Cancer Institute: Rockville Pike Bethesda, MD, USA, 2018.
- Scher, H.I.; Yagoda, A.; Herr, H.W.; Sternberg, C.N.; Morse, M.J.; Sogani, P.C.; Watson, R.C.; Reuter, V.; Whitmore, W.F.; Fair, W.R. Neoadjuvant M-VAC (Methotrexate, Vinblastine, Doxorubicin and Cisplatin) for Extravesical Urinary Tract Tumors. J. Urol. 1988, 139, 474–477. [Google Scholar] [CrossRef]
- Park, J.C.; Citrin, D.E.; Agarwal, P.K.; Apolo, A.B. Multimodal Management of Muscle-Invasive Bladder Cancer. Curr. Probl. Cancer 2014, 38, 80–108. [Google Scholar] [CrossRef]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.N.; Bajorin, D.F.; et al. Treatment of Patients with Metastatic Urothelial Cancer “Unfit” for Cisplatin-Based Chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438. [Google Scholar] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar]
- Chen, R.C.; Shipley, W.U.; Efstathiou, J.A.; Zietman, A.L. Trimodality Bladder Preservation Therapy for Muscle-Invasive Bladder Cancer. JNCCN J. Natl. Compr. Cancer Netw. 2013, 11, 952–960. [Google Scholar] [CrossRef]
- Donat, S.M.; Shabsigh, A.; Savage, C.; Cronin, A.M.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; Milowsky, M.I. Potential Impact of Postoperative Early Complications on the Timing of Adjuvant Chemotherapy in Patients Undergoing Radical Cystectomy: A High-Volume Tertiary Cancer Center Experience. Eur. Urol. 2009, 55, 177–185. [Google Scholar] [CrossRef]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. Eur. Urol. 2009, 55, 164–174. [Google Scholar] [CrossRef]
- James, N.D.; Hussain, S.A.; Hall, E.; Jenkins, P.; Tremlett, J.; Rawlings, C.; Crundwell, M.; Sizer, B.; Sreenivasan, T.; Hendron, C.; et al. Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. N. Engl. J. Med. 2012, 366, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, G.; Arcangeli, S.; Strigari, L. A Systematic Review and Meta-Analysis of Clinical Trials of Bladder-Sparing Trimodality Treatment for Muscle-Invasive Bladder Cancer (MIBC). Crit. Rev. Oncol. Hematol. 2015, 94, 105–115. [Google Scholar] [PubMed]
- Mak, R.H.; Hunt, D.; Shipley, W.U.; Efstathiou, J.A.; Tester, W.J.; Hagan, M.P.; Kaufman, D.S.; Heney, N.M.; Zietman, A.L. Long-Term Outcomes in Patients with Muscle-Invasive Bladder Cancer after Selective Bladder-Preserving Combined-Modality Therapy: A Pooled Analysis of Radiation Therapy Oncology Group Protocols 8802, 8903, 9506, 9706, 9906, and 0233. J. Clin. Oncol. 2014, 32, 3801–3809. [Google Scholar] [CrossRef]
- Griffiths, G. International Phase III Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: Long-Term Results of the BA06 30894 Trial. J. Clin. Oncol. 2011, 29, 2171–2177. [Google Scholar] [CrossRef]
- Kapoor, A.; Niazi, T.; Noonan, K.; Rendon, R.A.; Alimohamed, N.; Kassouf, W.; Berlin, A.; Chu, W.; Kollmannsberger, C.; So, A.I. 2022 American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium: Meeting Highlights. Yosetsu Gakkai Shi/J. Jpn. Weld. Soc. 2022, 16, 125–131. [Google Scholar] [CrossRef]
- Plimack, E.R.; Dunbrack, R.L.; Brennan, T.A.; Andrake, M.D.; Zhou, Y.; Serebriiskii, I.G.; Slifker, M.; Alpaugh, K.; Dulaimi, E.; Palma, N.; et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-Based Chemotherapy in Muscle-Invasive Bladder Cancer. Eur. Urol. 2015, 68, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Geynisman, D.M.; Abbosh, P.; Ross, E.A.; Zibelman, M.R.; Ghatalia, P.; Anari, F.; Ansel, K.; Mark, J.R.; Stamatakis, L.; Hoffman-Censits, J.H.; et al. A Phase II Trial of Risk-Enabled Therapy after Initiating Neoadjuvant Chemotherapy for Bladder Cancer (RETAIN). J. Clin. Oncol. 2023, 41, 438. [Google Scholar] [CrossRef]
- Geynisman, D.M.; Abbosh, P.; Ross, E.A.; Zibelman, M.R.; Ghatalia, P.; Anari, F.; Ansel, K.; Mark, J.R.; Stamatakis, L.; Hoffman-Censits, J.H.; et al. A Phase II Trial of Risk Enabled Therapy after Initiating Neoadjuvant Chemotherapy for Bladder Cancer (RETAIN BLADDER): Interim Analysis. J. Clin. Oncol. 2021, 39, 397. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar]
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Lotan, Y.; Meeks, J.J.; Michalski, J.M.; Morgan, T.M.; et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J. Urol. 2017, 198, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Friedlander, D.F.; Patel, S.; Hu, J.C. The Current Status of Robotic Oncologic Surgery. CA Cancer J. Clin. 2013, 63, 45–56. [Google Scholar] [CrossRef]
- Falagario, U.; Veccia, A.; Weprin, S.; Albuquerque, E.V.; Nahas, W.; Carrieri, G.; Pansadoro, V.; Hampton, L.; Porpiglia, F.; Autorino, R. Robotic-Assisted Surgery for the Treatment of Urologic Cancers: Recent Advances. Expert Rev. Med. Devices 2020, 17, 579–590. [Google Scholar]
- Jocham, D.; von Wietersheim, J.; Pflüger, H.; Steiner, H.; Doehn, C.; Büttner, H.; Böhle, A.; Kausch, I. BCG versus Photodynamic Therapy (PDT) for Nonmuscle Invasive Bladder Cancer—A Multicentre Clinical Phase III Study. Aktuelle Urol. 2009, 40, 91–99. [Google Scholar]
- Von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and Cisplatin versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized Phase II/III Trial Assessing Gemcitabine/Carboplatin and Methotrexate/Carboplatin/Vinblastine in Patients with Advanced Urothelial Cancer Who Are Unfit for Cisplatin-Based Chemotherapy: EORTC Study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef]
- Gitlitz, B.J.; Baker, C.; Chapman, Y.; Allen, H.J.; Bosserman, L.D.; Patel, R.; Sanchez, J.D.; Shapiro, R.M.; Figlin, R.A. A Phase II Study of Gemcitabine and Docetaxel Therapy in Patients with Advanced Urothelial Carcinoma. Cancer 2003, 98, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Witte, R.S.; Elson, P.; Bono, B.; Knop, R.; Richardson, R.R.; Dreicer, R.; Loehrer, P.J. Eastern Cooperative Oncology Group Phase II Trial of Ifosfamide in the Treatment of Previously Treated Advanced Urothelial Carcinoma. J. Clin. Oncol. 1997, 15, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; He, G.; Goh, S.; Low, A.W.X.; Tay, K.J.; Lim, T.K.H.; Yeong, J.; Khor, L.Y.; Lim, T.S. Biomarkers for Precision Urothelial Carcinoma Diagnosis: Current Approaches and the Application of Single-Cell Technologies. Cancers 2021, 13, 260. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Bedke, J.; Maas, M. Re: Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. Eur. Urol. 2021, 80, 6017. [Google Scholar]
- Zibelman, M.; Ramamurthy, C.; Plimack, E.R. Emerging Role of Immunotherapy in Urothelial Carcinoma—Advanced Disease. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 538–547. [Google Scholar]
- Stenehjem, D.D.; Tran, D.; Nkrumah, M.A.; Gupta, S. PD1/PDL1 Inhibitors for the Treatment of Advanced Urothelial Bladder Cancer. Oncol. Targets Ther. 2018, 11, 5973–5989. [Google Scholar]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without Chemotherapy in Metastatic Urothelial Cancer (IMvigor130): A Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Faltas, B.M. Treatment resistance in urothelial carcinoma: An evolutionary perspective. Nat. Rev. Clin. Oncol. 2018, 15, 495–509. [Google Scholar] [PubMed]
- Massari, F.; Santoni, M.; Ciccarese, C.; Brunelli, M.; Conti, A.; Santini, D.; Montironi, R.; Cascinu, S.; Tortora, G. Emerging concepts on drug resistance in bladder cancer: Implications for future strategies. Crit. Rev. Oncol. Hematol. 2015, 96, 81–90. [Google Scholar] [PubMed]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell. 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Padua, T.C.; Moschini, M.; Martini, A.; Pederzoli, F.; Nocera, L.; Marandino, L.; Raggi, D.; Briganti, A.; Montorsi, F.; Necchi, A. Efficacy and toxicity of antibody-drug conjugates in the treatment of metastatic urothelial cancer: A scoping review. Urol. Oncol. 2022, 40, 413–423. [Google Scholar]
- Hoimes, C.J.; Flaig, T.W.; Milowsky, M.I.; Friedlander, T.W.; Bilen, M.A.; Gupta, S.; Srinivas, S.; Merchan, J.R.; McKay, R.R.; Petrylak, D.P.; et al. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J. Clin. Oncol. 2023, 41, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Chou, J.; Trepka, K.; Sjöström, M.; Egusa, E.A.; Chu, C.E.; Zhu, J.; Chan, E.; Gibb, E.A.; Badura, M.L.; Contreras-Sanz, A.; et al. TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-resistant Cells. Eur. Urol. Oncol. 2022, 5, 714–718. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Petrulio, C.A.; Kaufman, H.L. Development of the PANVACTM-VF Vaccine for Pancreatic Cancer. Expert Rev. Vaccines 2006, 5, 9–19. [Google Scholar]
- Grandi, P.; Darilek, A.; Moscu, A.; Pradhan, A.; Li, R. Intravesical Infusion of Oncolytic Virus CG0070 in the Treatment of Bladder Cancer. Methods Mol. Biol. 2023, 2684, 303–317. [Google Scholar] [PubMed]
- Kloss, C.C.; Condomines, M.; Cartellieri, M.; Bachmann, M.; Sadelain, M. Combinatorial Antigen Recognition with Balanced Signaling Promotes Selective Tumor Eradication by Engineered T Cells. Nat. Biotechnol. 2013, 31, 71–75. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Yun, H.; Liu, W.; Chai, K.; Tong, J.; Zeng, T.; Gao, Z.; Xie, Y. CAR-T Cells in the Treatment of Urologic Neoplasms: Present and Future. Front. Oncol. 2022, 12, 915171. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Powles, T.; Li, S.; Hennicken, D.; Sonpavde, G. A phase 3, open-label, randomized study of nivolumab plus ipilimumab or standard of care (SOC) versus SOC alone in patients (pts) with previously untreated unresectable or metastatic urothelial carcinoma (mUC.; CheckMate 901). J. Clin. Oncol. 2018, 36, TPS539. [Google Scholar]
- Powles, T.; Gschwend, J.E.; Loriot, Y.; Bellmunt, J.; Geczi, L.; Vulsteke, C.; Abdelsalam, M.; Gafanov, R.; Bae, W.K.; Revesz, J.; et al. Phase 3 KEYNOTE-361 trial: Pembrolizumab (pembro) with or without chemotherapy versus chemotherapy alone in advanced urothelial cancer. J. Clin. Oncol. 2017, 35, TPS4590. [Google Scholar]
- Jackson-Spence, F.E.; Powles, T.; Loriot, Y.; Toms, C.; Jovaisaite, A.; Choy, J.; Szabados, B.E.; Nudds, H.; Ackerman, C.; Mousa, K. P126 Trial In Progress: DISCUS: A randomized phase II study comparing 3 vs. 6 cycles of platinum-based chemotherapy prior to maintenance avelumab in advanced urothelial cancer. Eur. Urol. Open Sci. 2022, 42, S219. [Google Scholar] [CrossRef]
- Gupta, S.; Ballman, K.V.; Galsky, M.D.; Morris, M.J.; Chen, R.C.; Chan, T.A.; Dercle, L.; Wen, Y.; Sridhar, S.S.; Yen, A.R.; et al. MAIN-CAV: Phase III randomized trial of maintenance cabozantinib and avelumab versus avelumab after first-line platinum-based chemotherapy in patients with metastatic urothelial cancer (mUC) (Alliance A032001). J. Clin. Oncol. 2022, 40, TPS4607. [Google Scholar]
- Petrylak, D.; de Wit, R.; Chi, K.N.; Drakaki, A.; Sternberg, C.N.; Nishiyama, H.; Castellano, D.; Hussain, S.; Fléchon, A.; Bamias, A.; et al. Ramucirumab plus Docetaxel versus Placebo plus Docetaxel in Patients with Locally Advanced or Metastatic Urothelial Carcinoma after Platinum-Based Therapy (RANGE): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2017, 390, 2266–2277. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Ballman, K.A.; Halabi, S.; Atherton, P.J.; Mortazavi, A.; Sweeney, C.; Stadler, W.M.; Teply, B.A.; Picus, J.; Tagawa, S.T.; et al. Randomized Phase III Trial of Gemcitabine and Cisplatin with Bevacizumab or Placebo in Patients With Advanced Urothelial Carcinoma: Results of CALGB 90601 (Alliance). J. Clin. Oncol. 2021, 39, 2486–2496. [Google Scholar] [CrossRef]
- Apolo, A.B.; Nadal, R.; Tomita, Y.; Davarpanah, N.N.; Cordes, L.M.; Steinberg, S.M.; Cao, L.; Parnes, H.L.; Costello, R.; Merino, M.J.; et al. Cabozantinib in Patients with Platinum-Refractory Metastatic Urothelial Carcinoma: An Open-Label, Single-Centre, Phase 2 Trial. Lancet Oncol. 2020, 21, 1099–1109. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular Mechanisms of Sanguinarine in Cancer Prevention and Treatment. Anticancer Agents Med. Chem. 2023, 23, 765–778. [Google Scholar] [PubMed]
- Desai, N.B.; Zabor, E.C.; Cha, E.K.; Hreiki, J.; Sfakianos, J.P.; Ramirez, R.; Bagrodia, A.; Rosenberg, J.E.; Bajorin, D.F.; Berger, M.F.; et al. Genomic characterization of response to chemoradiation in urothelial bladder cancer. Cancer 2016, 122, 3715–3723. [Google Scholar] [PubMed]
- Hedegaard, J.; Lamy, P.; Nordentoft, I.; Algaba, F.; Høyer, S.; Ulhøi, B.P.; Vang, S.; Reinert, T.; Hermann, G.G.; Mogensen, K.; et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 2016, 30, 27–42. [Google Scholar] [PubMed]
- Fong, M.H.Y.; Feng, M.; McConkey, D.J.; Choi, W. Update on bladder cancer molecular subtypes. Transl. Androl. Urol. 2020, 9, 2881–2889. [Google Scholar] [CrossRef]
- Williams, S.B.; Black, P.C.; Dyrskjøt, L.; Seiler, R.; Schmitz-Dräger, B.; Nawroth, R.; Todenhöfer, T.; Kamat, A.M.; Goebell, P.J. Re: Aurélie Kamoun, Aurélien de Reyniès, Yves Allory, et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. A Statement from the International Bladder Cancer Network. Eur. Urol. 2020, 77, e105–e106. [Google Scholar] [CrossRef]
- Alifrangis, C.; McGovern, U.; Freeman, A.; Powles, T.; Linch, M. Molecular and histopathology directed therapy for advanced bladder cancer. Nat. Rev. Urol. 2019, 16, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Faltas, B.M.; Prandi, D.; Tagawa, S.T.; Molina, A.M.; Nanus, D.M.; Sternberg, C.; Rosenberg, J.; Mosquera, J.M.; Robinson, B.; Elemento, O.; et al. Clonal evolution of chemotherapy-resistant urothelial carcinoma. Nat. Genet. 2016, 48, 1490–1499. [Google Scholar] [PubMed]
- Mohanty, S.K.; Lobo, A.; Mishra, S.K.; Cheng, L. Precision Medicine in Bladder Cancer: Present Challenges and Future Directions. J. Pers. Med. 2023, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Marzouka, N.A.; Eriksson, P.; Bernardo, C.; Hurst, C.D.; Knowles, M.A.; Sjödahl, G.; Liedberg, F.; Höglund, M. The Lund Molecular Taxonomy Applied to Non-Muscle-Invasive Urothelial Carcinoma. J. Mol. Diagn. 2022, 24, 992–1008. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, C.; Eriksson, P.; Marzouka, N.A.; Liedberg, F.; Sjödahl, G.; Höglund, M. Molecular pathology of the luminal class of urothelial tumors. J. Pathol. 2019, 249, 308–318. [Google Scholar] [CrossRef]
- Van Allen, E.M.; Mouw, K.W.; Kim, P.; Iyer, G.; Wagle, N.; Al-Ahmadie, H.; Zhu, C.; Ostrovnaya, I.; Kryukov, G.V.; O’Connor, K.W.; et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014, 4, 1140–1153. [Google Scholar]
- Sarfaty, M.; Teo, M.Y.; Al-Ahmadie, H.; Funt, S.A.; Lee, C.-H.; Aggen, D.H.; Solit, D.B.; Ratna, N.; Regazzi, A.M.; Hechtman, J.F.; et al. Microsatellite instability (MSI-H) in metastatic urothelial carcinoma (mUC): A biomarker of divergent responses to systemic therapy. J. Clin. Oncol. 2020, 38 (Suppl. 6), 566. [Google Scholar]
- Sarfaty, M.; Golkaram, M.; Funt, S.A.; Al-Ahmadie, H.; Kaplan, S.; Song, F.; Regazzi, A.; Makarov, V.; Kuo, F.; Ostrovnaya, I.; et al. Novel Genetic Subtypes of Urothelial Carcinoma With Differential Outcomes on Immune Checkpoint Blockade. J. Clin. Oncol. 2023, 41, 3225–3235. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.M.; de Souza, Z.S.; Gongora, A.B.L.; de Galiza Barbosa, F.; Buchpiguel, C.A.; de Castro, M.G.; de Macedo, M.P.; Coelho, R.F.; Sokol, E.S.; Camargo, A.A.; et al. Emerging biomarkers in metastatic urothelial carcinoma: Tumour mutational burden, PD-L1 expression and APOBEC polypeptide-like signature in a patient with complete response to anti-programmed cell death protein-1 inhibitor. Ecancermedicalscience 2021, 15, 1306. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Chen, Y.; Anandhan, S.; Szabo, P.M.; Basu, S.; Blando, J.M.; Liu, W.; Zhang, J.; Natarajan, S.M.; Xiong, L.; et al. ARID1A mutation plus CXCL13 expression act as combinatorial biomarkers to predict responses to immune checkpoint therapy in mUCC. Sci. Transl. Med. 2020, 12, eabc4220. [Google Scholar] [PubMed]
- Xu, N.; Yao, Z.; Shang, G.; Ye, D.; Wang, H.; Zhang, H.; Qu, Y.; Xu, F.; Wang, Y.; Qin, Z.; et al. Integrated proteogenomic characterization of urothelial carcinoma of the bladder. J. Hematol. Oncol. 2022, 15, 76. [Google Scholar] [CrossRef]
- Seiler, R.; Thalmann, G.N.; Rotzer, D.; Perren, A.; Fleischmann, A. CCND1/CyclinD1 status in metastasizing bladder cancer: A prognosticator and predictor of chemotherapeutic response. Mod. Pathol. 2014, 27, 87–95. [Google Scholar] [CrossRef]
- Krook, M.A.; Reeser, J.W.; Ernst, G.; Barker, H.; Wilberding, M.; Li, G.; Chen, H.-Z.; Roychowdhury, S. Fibroblast growth factor receptors in cancer: Genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br. J. Cancer 2021, 124, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Rodler, S.; Eismann, L.; Schlenker, B.; Casuscelli, J.; Brinkmann, I.; Sendelhofert, A.; Waidelich, R.; Buchner, A.; Stief, C.; Schulz, G.B.; et al. Expression of Nectin-4 in Variant Histologies of Bladder Cancer and Its Prognostic Value-Need for Biomarker Testing in High-Risk Patients? Cancers 2022, 14, 4411. [Google Scholar] [CrossRef]
- Wong, J.L.; Rosenberg, J.E. Targeting nectin-4 by antibody-drug conjugates for the treatment of urothelial carcinoma. Expert Opin. Biol. Ther. 2021, 21, 863–873. [Google Scholar] [CrossRef]
- Zimpfer, A.; Kdimati, S.; Mosig, M.; Rudolf, H.; Zettl, H.; Erbersdobler, A.; Hakenberg, O.W.; Maruschke, M.; Schneider, B. ERBB2 Amplification as a Predictive and Prognostic Biomarker in Upper Tract Urothelial Carcinoma. Cancers 2023, 15, 2414. [Google Scholar] [CrossRef]
- Mendiratta, P.; Grivas, P. Emerging biomarkers and targeted therapies in urothelial carcinoma. Ann. Transl. Med. 2018, 6, 250. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Grivas, P. The utility of next generation sequencing in advanced urothelial carcinoma. Eur. Urol. Focus 2020, 6, 41–44. [Google Scholar] [CrossRef]
- Peng, J.; Sridhar, S.; Siefker-Radtke, A.O.; Selvarajah, S.; Jiang, D.M. Targeting the FGFR Pathway in Urothelial Carcinoma: The Future Is Now. Curr. Treat Opt. Oncol. 2022, 23, 1269–1287. [Google Scholar] [CrossRef]
- Doroshow, D.B.; O’Donnell, P.H.; Hoffman-Censits, J.H.; Gupta, S.V.; Vaishampayan, U.; Heath, E.I.; Garcia, P.; Zhao, Q.; Yu, M.; Milowsky, M.I.; et al. Phase II Trial of Olaparib in Patients with Metastatic Urothelial Cancer Harboring DNA Damage Response Gene Alterations. JCO Precis. Oncol. 2023, 7, e2300095. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Lerner, S.P. Poly (ADP-ribose) Polymerase Inhibition in Advanced Urothelial Carcinoma. JCO Precis. Oncol. 2023, 7, e2300293. [Google Scholar] [CrossRef]
- Torres-Jiménez, J.; Albarrán-Fernández, V.; Pozas, J.; Román-Gil, M.S.; Esteban-Villarrubia, J.; Carrato, A.; Rosero, A.; Grande, E.; Alonso-Gordoa, T.; Molina-Cerrillo, J. Novel Tyrosine Kinase Targets in Urothelial Carcinoma. Int. J. Mol. Sci. 2021, 22, 747. [Google Scholar] [CrossRef]
- Schmidt, A.L.; Siefker-Radtke, A.; McConkey, D.; McGregor, B. Renal Cell and Urothelial Carcinoma: Biomarkers for New Treatments. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e197–e206. [Google Scholar] [CrossRef]
- Narayanan, S.; Srinivas, S. Incorporating VEGF-targeted therapy in advanced urothelial cancer. Ther. Adv. Med. Oncol. 2017, 9, 33–45. [Google Scholar] [CrossRef]
- Gallagher, D.J.; Milowsky, M.I.; Gerst, S.R.; Ishill, N.; Riches, J.; Regazzi, A.; Boyle, M.G.; Trout, A.; Flaherty, A.M.; Bajorin, D.F. Phase II study of sunitinib in patients with metastatic urothelial cancer. J. Clin. Oncol. 2010, 28, 1373–1379. [Google Scholar]
- Geldart, T.; Chester, J.; Casbard, A.; Crabb, S.; Elliott, T.; Protheroe, A.; Huddart, R.A.; Mead, G.; Barber, J.; Jones, R.J.; et al. SUCCINCT: An open-label, single-arm, non-randomised, phase 2 trial of gemcitabine and cisplatin chemotherapy in combination with sunitinib as first-line treatment for patients with advanced urothelial carcinoma. Eur. Urol. 2015, 67, 599–602. [Google Scholar]
- Bellmunt, J.; Powles, T.B.; van der Heijden, M.S.; Galsky, M.D.; He, P.; Wang, X.; Xiao, F.; Jones, F.; Scott, M.; Walker, J.; et al. 708P PD-L1 as a predictor of survival in patients with metastatic urothelial carcinoma (mUC) from the phase III DANUBE trial of durvalumab (D) or durvalumab plus tremelimumab (D + T) versus standard of care chemotherapy (SoC). Ann. Oncol. 2021, 32, S716–S717. [Google Scholar]
- Goodman, A.M.; Piccioni, D.; Kato, S.; Boichard, A.; Wang, H.Y.; Frampton, G.; Lippman, S.M.; Connelly, C.; Fabrizio, D.; Miller, V.; et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol. 2018, 4, 1237–1244. [Google Scholar]
- Graf, R.P.; Fisher, V.; Huang, R.S.P.; Hamdani, O.; Gjoerup, O.V.; Stanke, J.; Creeden, J.; Levy, M.A.; Oxnard, G.R.; Gupta, S. Tumor Mutational Burden as a Predictor of First-Line Immune Checkpoint Inhibitor Versus Carboplatin Benefit in Cisplatin-Unfit Patients with Urothelial Carcinoma. JCO Precis. Oncol. 2022, 6, e2200121. [Google Scholar] [CrossRef]
- Van der Fels, C.A.M.; Leliveld, A.; Buikema, H.; van den Heuvel, M.C.; de Jong, I.J. VEGF, EGFR and PSMA as possible imaging targets of lymph node metastases of urothelial carcinoma of the bladder. BMC Urol. 2022, 22, 213. [Google Scholar]
- Huang, R.S.P.; Haberberger, J.; Severson, E.; Duncan, D.L.; Hemmerich, A.; Edgerly, C.; Ferguson, N.L.; Williams, E.; Elvin, J.; Vergilio, J.-A.; et al. A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases. Mod. Pathol. 2021, 34, 252–263. [Google Scholar]
- Koutros, S.; Rao, N.; Moore, L.E.; Nickerson, M.L.; Lee, D.; Zhu, B.; Pardo, L.A.; Baris, D.; Schwenn, M.; Johnson, A.; et al. Targeted deep sequencing of bladder tumors reveals novel associations between cancer gene mutations and mutational signatures with major risk factors. Clin. Cancer Res. 2021, 27, 3725–3733. [Google Scholar] [PubMed]
- Hoffman-Censits, J.H.; Lombardo, K.A.; Parimi, V.; Kamanda, S.; Choi, W.; Hahn, N.M.; McConkey, D.J.; McGuire, B.M.; Bivalacqua, T.J.; Kates, M.; et al. Expression of nectin-4 in bladder urothelial carcinoma, in morphologic variants, and nonurothelial histotypes. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 619–625. [Google Scholar]
- Sarafidis, M.; Lambrou, G.I.; Zoumpourlis, V.; Koutsouris, D. An Integrated Bioinformatics Analysis towards the Identification of Diagnostic, Prognostic, and Predictive Key Biomarkers for Urinary Bladder Cancer. Cancers 2022, 14, 3358. [Google Scholar] [CrossRef]
- Eckstein, M.; Cimadamore, A.; Hartmann, A.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Gevaert, T. PD-L1 assessment in urothelial carcinoma: A practical approach. Ann. Transl. Med. 2019, 7, 690. [Google Scholar] [CrossRef]
- Wang, L.; Sfakianos, J.P.; Beaumont, K.G.; Akturk, G.; Horowitz, A.; Sebra, R.P.; Farkas, A.M.; Gnjatic, S.; Hake, A.; Izadmehr, S.; et al. Myeloid Cell-associated Resistance to PD-1/PD-L1 Blockade in Urothelial Cancer Revealed Through Bulk and Single-cell RNA Sequencing. Clin. Cancer Res. 2021, 27, 4287–4300. [Google Scholar] [CrossRef] [PubMed]
- Pfannstiel, C.; Strissel, P.L.; Chiappinelli, K.B.; Sikic, D.; Wach, S.; Wirtz, R.M.; Wullweber, A.; Taubert, H.; Breyer, J.; Otto, W.; et al. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes. Cancer Immunol. Res. 2019, 7, 923–938. [Google Scholar]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef]
- Lourenço, C.; Constâncio, V.; Henrique, R.; Carvalho, Â.; Jerónimo, C. Urinary Extracellular Vesicles as Potential Biomarkers for Urologic Cancers: An Overview of Current Methods and Advances. Cancers 2021, 13, 1529. [Google Scholar] [CrossRef]
- Hölzel, M.; Bovier, A.; Tüting, T. Plasticity of tumour and immune cells: A source of heterogeneity and a cause for therapy resistance? Nat. Rev. Cancer 2013, 13, 365–376. [Google Scholar]
- Gupta, S.; Bellmunt, J.; Plimack, E.R.; Sonpavde, G.P.; Grivas, P.; Apolo, A.B.; Pal, S.K.; Siefker-Radtke, A.O.; Flaig, T.W.; Galsky, M.D.; et al. Defining “platinum-ineligible” patients with metastatic urothelial cancer (mUC). J. Clin. Oncol. 2022, 40 (Suppl. S16), 4577. [Google Scholar]
- Zhu, A.; Garcia, J.A.; Faltas, B.; Grivas, P.; Barata, P.; Shoag, J.E. Immune Checkpoint Inhibitors and Long-term Survival of Patients With Metastatic Urothelial Cancer. JAMA Netw. Open 2023, 6, e237444. [Google Scholar]
- Sun, J.Y.; Zhang, D.; Wu, S.; Xu, M.; Zhou, X.; Lu, X.; Ji, J. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: Mechanisms, predictive factors, and future perspectives. Biomark. Res. 2020, 8, 35. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Y.; Bi, Y.; Zhang, N.; Wang, H.; Xing, T.; Bai, S.; Shen, Z.; Naz, F.; Zhang, Z.; et al. Immune escape mechanisms and immunotherapy of urothelial bladder cancer. J. Clin. Transl. Res. 2021, 7, 485–500. [Google Scholar] [PubMed]
- Özdemir, B.C.; Siefker-Radtke, A.O.; Campbell, M.T.; Subudhi, S.K. Current and Future Applications of Novel Immunotherapies in Urological Oncology: A Critical Review of the Literature. Eur. Urol. Focus 2018, 4, 442–454. [Google Scholar] [CrossRef]
- Guercio, B.J.; Iyer, G.; Rosenberg, J.E. Developing Precision Medicine for Bladder Cancer. Hematol. Oncol. Clin. N. Am. 2021, 35, 633–653. [Google Scholar] [CrossRef]
| Gene | Chromosomal Location | Genetic Alteration | Frequency Observed in MIBC Tumors |
|---|---|---|---|
| Chromosome | |||
| 9p | Deletion | 21–30% | |
| 9q | Deletion | 17% | |
| Oncogenes | |||
| HRAS | 11p15 | Activating mutation | 10–15% |
| FGFR3 | 4p16 | Activating mutation | ~50% Overexpression |
| 15% Mutation | |||
| PIK3CA | 3q26 | Activating mutation | 25% |
| MDM2 | 12q13 | Overexpression | 4% Overexpression |
| Tumor suppressor genes | |||
| TP53 | 17p13 | Deletion or mutation | 70% |
| RB1 | 13q14 | Deletion or mutation | 37% |
| PTEN | 10q23 | Homozygous deletion or mutation | LOH 30–35% |
| Mutation 17% | |||
| CDKN2A | 9p21 | Homozygous deletion or methylation or mutation | HD 20–30% |
| LOH ~60% | |||
| PTCH | 9q22 | Deletion or mutation | LOH ~60% |
| Mutation rare | |||
| DBC1 | 9q32–33 | Deletion or methylation | LOH ~60% |
| TSC1 | 9q34 | Deletion or mutation | LOH ~60% |
| Mutation ~15% |
| Trial | Patient Characteristics | Regimen | Primary & Secondary End Points | Common AEs | Results |
|---|---|---|---|---|---|
| BLC2001 Phase 2 study in mUCC patients | 99 patients with FGFR alteration, who have progressed on chemotherapy or immunotherapy | Erdafitinib 8 mg in either an intermittent or continuous regimen | Primary end point was ORR and secondary end points were PFS, OS and duration of response. | Hyperphosphatemia, Stomatitis and diarrhea. | ORR was 40%. |
| KEYNOTE-045 Phase 3 trial in mUCC | 542 patients who recurred or progressed after platinum-based chemotherapy | Pembrolizumab at a dose of 200 mg every 3 weeks or the investigator’s choice of chemotherapy with paclitaxel, docetaxel, or vinflunine | Co-primary endpoints were OS and PFS, among all patients and among patients who had PD-L1 CPS of 10% or more. | Pruritus, fatigue, and nausea | OS was 8 vs. 5.2 mos. PFS did not demonstrate a significant difference. |
| JAVELIN Bladder-100—Phase 3 trial in unresectable locally advanced & mUCC | 700 patients who completed 1st line chemotherapy without progression. | Maintenance avelumab 10 mg/kg IV q2 weekly vs. best supportive care | Primary end point was OS and secondary end points included PFS and safety. | Fatigue, pruritus and urinary tract infections. | OS at 1 year was 71.3 compared to 58.4%. Median PFS was 3.7 vs. 2.0 mos. |
| CheckMate-274 Phase 3 trial with MIBC. | 709 patients with MIBC who had undergone radical cystectomy. Neoadjuvant cisplatin-based chemotherapy before trial entry was allowed. | Adjuvant Nivolumab 240 mg IV or placebo q2 weeks for up to 1 year vs. Placebo. | Primary end point was DFS. Secondary end point was survival free from recurrence outside the urothelial tract. | Pruritus, fatigue, and diarrhea | DFS was 20.8 mos with nivolumab and 10.8 mos with placebo. Patients who were free from recurrence outside the urothelial tract at 6 mos was 77 vs. 63%. |
| EV-301 Phase 3 trial in locally advanced or mUCC | 608 patients who had previously received platinum-containing CHT and had disease progression during or after treatment with a PD-1 or PD-L1 inhibitor | Enfortumab vedotin 1.25 mg/kg on days 1, 8, 15 of a 28-day cycle or investigator-chosen CHT on day 1 of a 21-day cycle. | The primary end point was overall survival. | Alopecia, Peripheral sensory neuropathy, Pruritus. | Median OS was 12.8 vs. 8.9 mos. |
| TROPHY-U- Phase II in mUCC | 113 patients who previously received platinum-containing CHT and had disease progression during or after treatment with a PD-1 or PD-L1 inhibitor | Sacituzumab govitecan 10 mg/kg on days 1 and 8 of 21-day cycles | objective response rate (ORR) secondary end points were PFS, OS, duration of response, and safety | neutropenia (3%) leukopenia (18%), anemia | ORR of 27% |
| DANUBE- Phase 3 trial | 1032 patients that had received Durvalumab (346), Durvalumab+ Tremelimumab(342), or chemotherapy(344) in patients with untreated, unresectable or locally advance mUCC | Durvalumab (1500 mg) IV q4 weeks; Durvalumab (1500 mg)+ Tremelimumab (75 mg) IV q4 weeks for up to 4 doses, followed by durvalumab maintenance (1500 mg) q4 weeks; or SOC chemotherapy (gemcitabine + cisplatin/carboplatin) IV for up to 6 cycles. | Co-primary endpoints were OS compared b/w durvalumab and CT in pts whose tumor cells and/or tumor-infiltrating immune cells express high levels of PD-L1 (≥25%) and between durvalumab + tremelimumab and CT regardless of PD-L1 expression | Increased lipase in the Durvalumab Group and neutropenia in the chemotherapy group. | Did not meet either of the co-primary endpoints. |
| Checkmate 901 | 608 patients were randomized to either Nivolumab + Ipilumimab with chemotherapy or chemotherapy alone. | Nivolumab 360 mg combined with CHT every 3 weeks or CHT alone. | Dual endpoints were PFS and OS | Pruritis, fatigue, diarrhea, pneumonitis | Met its dual primary end points |
| KEYNOTE-361 Phase 3 trial | 1010 patients were randomly assigned to receive Pembrolizumab with CHT or Pembrolizumab alone or CHT alone | Pembrolizumab 200 mg q3 weeks for a maximum of 35 cycles + IV CHT on D 1 and 8 vs. CHT on day 1 of every 3-week cycle for a maximum of 6 cycles | Dual primary endpoints of OS and PFS. Secondary endpoints included duration of response, disease control rate, overall response rate and safety | Fatigue, musculoskeletal pain, decreased appetite, constipation, rash, and diarrhea | Did not meet either of the endpoints. |
| DISCUS (ONGOING) | 224 eligible and evaluable patients (112 in each arm) to receive 3 vs. 6 cycles of platinum-based CHT + Avelumab in 1st line of mUCC | Gemcitabine on D1 and D8 with Carboplatin/Cisplatin on D1 and Avelumab every 2weekly. | QoL as measured by the change from baseline in EORTC QLQ-C30 questionnaire GHS/QoL scale scores from baseline to the completion of 6 cycles of treatment | ||
| Main-CAV Alliance A032001 (ONGOING) | Maintenance Cabozantinib+Avelumab vs. Avelumab in 1st line mUCC with clinical benefit after platinum-based CHT | Avelumab 800 mg IV q2 wk or combination of Avelumab and CABO 40 mg orally daily for up to 2 yrs | OS |
| Type of Treatment | Examples | Mechanism of Action | Indications | Adverse Events |
|---|---|---|---|---|
| Chemotherapy | Gemcitabine, Cisplatin, Methotrexate | Kills rapidly dividing cancer cells | Advanced/metastatic stages. Contraindications include ECOG, hearing disorder, heart failure, peripheral neuropathy and Creatinine Cl <60. | Nausea and vomiting. Loss of appetite, Hair loss, Mouth sores, Diarrhea, Constipation |
| Immunotherapy (PD-1/PD-L1 inhibitors) | Pembrolizumab, Atezolizumab | Blocks PD-1/PD-L1 interaction, boosting immune response | Advanced/metastatic stages. Unresponsive to other treatments. Indicated as frontline for platinum-ineligible patients. | Fatigue, Nausea, Loss of appetite, Fever, Urinary tract infections (UTIs) |
| FGFR3 Inhibitors | Erdafitinib | Inhibits FGFR3, a gene mutation common in UCC | Advanced/metastatic with FGFR3 mutation. Progressed on prior treatment | Hyperphosphatemia, Stomatitis and diarrhea. |
| Antibody-Drug Conjugates | Enfortumab vedotin | Targeted delivery of toxic agents to tumor cells that express Nectin-4 | Advanced/metastatic stages. Progressed on prior cisplatin-based therapy or immunotherapy. | Alopecia, Peripheral sensory neuropathy, pruritus. |
| CAR-T Cell Therapy | Currently not approved for mUCC | Autologous patient T-cells engineered to express a chimeric antigen receptor (CAR) directed against a cancer cell target. Potential targets include EGFR, MUC1, PD-1, HER2 and PSMA. | Under clinical investigation | Immune effector cell-associated neurotoxicity syndrome (ICANS), Cytokine Release Syndrome (CRS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, B.; Ignatz-Hoover, J.J.; Rana, P.S.; Driscoll, J.J. Current and Emerging Strategies to Treat Urothelial Carcinoma. Cancers 2023, 15, 4886. https://doi.org/10.3390/cancers15194886
Rani B, Ignatz-Hoover JJ, Rana PS, Driscoll JJ. Current and Emerging Strategies to Treat Urothelial Carcinoma. Cancers. 2023; 15(19):4886. https://doi.org/10.3390/cancers15194886
Chicago/Turabian StyleRani, Berkha, James J. Ignatz-Hoover, Priyanka S. Rana, and James J. Driscoll. 2023. "Current and Emerging Strategies to Treat Urothelial Carcinoma" Cancers 15, no. 19: 4886. https://doi.org/10.3390/cancers15194886
APA StyleRani, B., Ignatz-Hoover, J. J., Rana, P. S., & Driscoll, J. J. (2023). Current and Emerging Strategies to Treat Urothelial Carcinoma. Cancers, 15(19), 4886. https://doi.org/10.3390/cancers15194886






