Fatty Pancreas Is a Risk Factor for Pancreatic Cancer: A Systematic Review and Meta-Analysis of 2956 Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection
2.6. Study Risk of Bias Assessment
2.7. Statistical Analysis
2.8. Quality of Evidence
3. Results
3.1. Study Selection of the Included Studies
3.2. Study Characteristics
3.3. No Relationship between Patients with FP and the Existence of PC
3.4. Fatty Pancreas Is Six Times More Common among Individuals Diagnosed with PC
3.5. Fatty Pancreas Is Found in 62% of the PC Patients
3.6. Risk of Bias Assessment
3.7. Publication Bias and Heterogeneity
3.8. Certainty of Evidence
4. Discussion
4.1. Strengths and Limitations
4.2. Implication for Practice
4.3. Implication for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrov, M.S. Fatty change of the pancreas: The Pandora’s box of pancreatology. Lancet Gastroenterol. Hepatol. 2023, 8, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Goral, V. Pancreatic Cancer: Pathogenesis and Diagnosis. Asian Pac. J. Cancer Prev. 2015, 16, 5619–5624. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Korc, M.; Jeon, C.Y.; Edderkaoui, M.; Pandol, S.J.; Petrov, M.S. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Hur, C.; Tramontano, A.C.; Dowling, E.C.; Brooks, G.A.; Jeon, A.; Brugge, W.R.; Gazelle, G.S.; Kong, C.Y.; Pandharipande, P.V. Early Pancreatic Ductal Adenocarcinoma Survival Is Dependent on Size. Pancreas 2016, 45, 1062–1066. [Google Scholar] [CrossRef]
- Klatte, D.C.F.; Boekestijn, B.; Onnekink, A.M.; Dekker, F.W.; Van Der Geest, L.G.; Wasser, M.N.J.M.; Feshtali, S.; Mieog, J.S.D.; Luelmo, S.A.C.; Morreau, H.; et al. Surveillance for Pancreatic Cancer in High-Risk Individuals Leads to Improved Outcomes: A Propensity Score-Matched Analysis. Gastroenterology 2023, 164, 1223–1231. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Gou, Y.-W.; Jin, W.-W.; Xiao, M.; Fang, H.-Y. Association between alcohol intake and the risk of pancreatic cancer: A dose–response meta-analysis of cohort studies. BMC Cancer 2016, 16, 212. [Google Scholar] [CrossRef]
- Parkin, D.M.; Boyd, L.; Walker, L.C. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer 2011, 105, S77–S81. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Bilici, A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J. Gastroenterol. 2014, 20, 10802. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, J.L.; Li, M.L.; Zhou, J.; Sun, X.L. The association between pancreas steatosis and metabolic syndrome: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2019, 35, e3142. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, U.L.; DeSouza, S.V.; Park, B.; Petrov, M.S. A Systematic Review of Intra-pancreatic Fat Deposition and Pancreatic Carcinogenesis. J. Gastrointest. Surg. 2020, 24, 2560–2569. [Google Scholar] [CrossRef]
- Takahashi, M.; Hori, M.; Ishigamori, R.; Mutoh, M.; Imai, T.; Nakagama, H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018, 109, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.S. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol. Microbiol. Scand. A 1978, 86a, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Zhou, J.; Chen, X.; Sun, Y.; Mao, Z.; Chai, K. Prevalence and factors associated with nonalcoholic fatty pancreas disease and its severity in China. Medicine 2018, 97, e11293. [Google Scholar] [CrossRef]
- Lesmana, C.R.; Pakasi, L.S.; Inggriani, S.; Aidawati, M.L.; Lesmana, L.A. Prevalence of Non-Alcoholic Fatty Pancreas Disease (NAFPD) and its risk factors among adult medical check-up patients in a private hospital: A large cross sectional study. BMC Gastroenterol. 2015, 15, 174. [Google Scholar] [CrossRef]
- Eibl, G.; Cruz-Monserrate, Z.; Korc, M.; Petrov, M.S.; Goodarzi, M.O.; Fisher, W.E.; Habtezion, A.; Lugea, A.; Pandol, S.J.; Hart, P.A.; et al. Diabetes Mellitus and Obesity as Risk Factors for Pancreatic Cancer. J. Acad. Nutr. Diet. 2018, 118, 555–567. [Google Scholar] [CrossRef]
- Hori, M.; Kitahashi, T.; Imai, T.; Ishigamori, R.; Takasu, S.; Mutoh, M.; Sugimura, T.; Wakabayashi, K.; Takahashi, M. Enhancement of carcinogenesis and fatty infiltration in the pancreas in N-nitrosobis(2-oxopropyl)amine-treated hamsters by high-fat diet. Pancreas 2011, 40, 1234–1240. [Google Scholar] [CrossRef]
- Mahyoub, M.A.; Elhoumed, M.; Maqul, A.H.; Almezgagi, M.; Abbas, M.; Jiao, Y.; Wang, J.; Alnaggar, M.; Zhao, P.; He, S. Fatty infiltration of the pancreas: A systematic concept analysis. Front. Med. 2023, 10, 1227188. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S.; Cochrane, C. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Collaboration: Oxford, UK, 2022. [Google Scholar]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. citationchaser: An R Package for Forward and Backward Citations Chasing in Academic Searching, Version 0.0.3; R Foundation: Ames, IW, USA, 2021. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Joanna Briggs, I. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar]
- Hayden, J.A.; Cote, P.; Bombardier, C. Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 2006, 144, 427–437. [Google Scholar] [CrossRef]
- Higgins JPT, T.S. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Peters, J.L. Comparison of Two Methods to Detect Publication Bias in Meta-analysis. JAMA 2006, 295, 676. [Google Scholar] [CrossRef]
- Harbord, R.M.; Harris, R.J.; Sterne, J.A.C. Updated Tests for Small-study Effects in Meta-analyses. Stata J. 2009, 9, 197–210. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D. Doing Meta-Analysis with R: A Hands-On Guide, 1st ed.; Chapman & Hall/CRC Press: London, UK; Boca Raton, FL, USA, 2021. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation: Ames, IW, USA, 2023. [Google Scholar]
- Schwarzer, G. Meta: General Package for Meta-Analysis; R Foundation: Ames, IW, USA, 2023. [Google Scholar]
- Cuijpers, P.F.T.; Ebert, D.D. Dmetar: Companion R Package for the Guide Doing Meta-Analysis in R; R Foundation: Ames, IW, USA, 2023. [Google Scholar]
- Schünemann, H.B.J.; Oxman, A.G.G. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; The GRADE Working Group. Available online: Guidelinedevelopment.org/handbook (accessed on 24 August 2023).
- GRADEpro, G. D. T. GRADEpro Guideline Development Tool; McMaster University: Hamilton, ON, Canada, 2021. [Google Scholar]
- Sepe, P.S.; Ohri, A.; Sanaka, S.; Berzin, T.M.; Sekhon, S.; Bennett, G.; Mehta, G.; Chuttani, R.; Kane, R.; Pleskow, D.; et al. A prospective evaluation of fatty pancreas by using EUS. Gastrointest. Endosc. 2011, 73, 987–993. [Google Scholar] [CrossRef]
- Dei, H.; Natsume, S.; Okuno, M.; Kawakatsu, S.; Hosoda, W.; Matsuo, K.; Hara, K.; Ito, S.; Komori, K.; Abe, T.; et al. Impact of pancreatic fat infiltration on postoperative pancreatic fistula occurrence in patients undergoing invagination pancreaticojejunostomy. HPB 2022, 24, 2119–2124. [Google Scholar] [CrossRef]
- Sbeit, W.; Greener, T.; Kadah, A.; Mari, A.; Goldin, E.; Khoury, T.; Mahamid, M. Pancreatobiliary manifestations of nonalcoholic fatty liver disease: A retrospective case-control multicenter study. Eur. J. Gastroenterol. Hepatol. 2021, 33, 722–726. [Google Scholar] [CrossRef]
- Khoury, T.; Sbeit, W. Fatty Pancreas and Pancreatic Cancer: An Overlooked Association? J. Clin. Med. 2022, 11, 763. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, S.A.; Bolan, C.W.; Chuprin, A.; Raimondo, M.T.; van Hooft, J.E.; Wallace, M.B.; Raimondo, M. Pancreatic steatosis on computed tomography is an early imaging feature of pre-diagnostic pancreatic cancer: A preliminary study in overweight patients. Pancreatology 2021, 21, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Takahashi, M.; Hiraoka, N.; Yamaji, T.; Mutoh, M.; Ishigamori, R.; Furuta, K.; Okusaka, T.; Shimada, K.; Kosuge, T.; et al. Association of pancreatic Fatty infiltration with pancreatic ductal adenocarcinoma. Clin. Transl. Gastroenterol. 2014, 5, e53. [Google Scholar] [CrossRef]
- Tomita, Y.; Azuma, K.; Nonaka, Y.; Kamada, Y.; Tomoeda, M.; Kishida, M.; Tanemura, M.; Miyoshi, E. Pancreatic fatty degeneration and fibrosis as predisposing factors for the development of pancreatic ductal adenocarcinoma. Pancreas 2014, 43, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Mandai, K.; Uno, K.; Nakase, K.; Kawamura, T.; Yasuda, K. Association between hyperechogenic pancreas and pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasms. J. Med. Ultrason. 2019, 46, 435–439. [Google Scholar] [CrossRef]
- Noda, Y.; Goshima, S.; Suzui, N.; Miyazaki, T.; Kajita, K.; Kawada, H.; Kawai, N.; Tanahashi, Y.; Matsuo, M. Pancreatic MRI associated with pancreatic fibrosis and postoperative fistula: Comparison between pancreatic cancer and non-pancreatic cancer tissue. Clin. Radiol. 2019, 74, 490.e491–490.e496. [Google Scholar] [CrossRef]
- Lesmana, C.R.A.; Gani, R.A.; Lesmana, L.A. Non-alcoholic fatty pancreas disease as a risk factor for pancreatic cancer based on endoscopic ultrasound examination among pancreatic cancer patients: A single-center experience. JGH Open 2018, 2, 4–7. [Google Scholar] [CrossRef]
- Miura, S.; Kume, K.; Kikuta, K.; Hamada, S.; Takikawa, T.; Yoshida, N.; Hongo, S.; Tanaka, Y.; Matsumoto, R.; Sano, T.; et al. Focal Parenchymal Atrophy and Fat Replacement Are Clues for Early Diagnosis of Pancreatic Cancer with Abnormalities of the Main Pancreatic Duct. Tohoku J. Exp. Med. 2020, 252, 63–71. [Google Scholar] [CrossRef]
- Kawamura, A.; Takakura, K.; Torisu, Y.; Kinoshita, Y.; Tomita, Y.; Nakano, M.; Yamauchi, T.; Suka, M.; Sumiyama, K.; Koido, S.; et al. Impact of qualitative endoscopic ultrasonography on fatty pancreas at a referral medical center. JGH Open 2022, 6, 44–49. [Google Scholar] [CrossRef]
- Kobashi, Y.; Uchiyama, M.; Matsui, J. The “K-Sign”-A Novel CT Finding Suggestive before the Appearance of Pancreatic Cancer. Cancers 2021, 13, 4222. [Google Scholar] [CrossRef]
- Gaujoux, S.; Torres, J.; Olson, S.; Winston, C.; Gonen, M.; Brennan, M.F.; Klimstra, D.S.; D’Angelica, M.; DeMatteo, R.; Fong, Y.; et al. Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann. Surg. Oncol. 2012, 19, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Izumi, Y.; Hanada, K.; Okazaki, A.; Minami, T.; Hirano, N.; Ikemoto, J.; Kanemitsu, K.; Nakadoi, K.; Shishido, T.; Katamura, Y.; et al. Endoscopic ultrasound findings and pathological features of pancreatic carcinoma in situ. Endosc. Int. Open 2019, 7, E585–E593. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Hernandez, J.; Shaheen, F.; Shroff, M.; Dahal, S.; Morton, C.; Farrior, T.; Kedar, R.; Rosemurgy, A. Preoperative computed tomography measurements of pancreatic steatosis and visceral fat: Prognostic markers for dissemination and lethality of pancreatic adenocarcinoma. HPB 2011, 13, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; van Geenen, E.J. The clinical significance of pancreatic steatosis. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 169–177. [Google Scholar] [CrossRef]
- Tariq, H.; Nayudu, S.; Akella, S.; Glandt, M.; Chilimuri, S. Non-Alcoholic Fatty Pancreatic Disease: A Review of Literature. Gastroenterol. Res. 2016, 9, 87–91. [Google Scholar] [CrossRef]
- Truong, E.; Pandol, S.; Jeon, C. Uniting epidemiology and experimental models: Pancreatic steatosis and pancreatic cancer. eBioMedicine 2022, 79, 103996. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.; Mouralidarane, A.; Soeda, J.; Ray, S.; Pombo, J.; Saraswati, R.; Novelli, M.; Fusai, G.; Rappa, F.; Saracino, C.; et al. Non-Alcoholic Fatty Pancreas Disease Pathogenesis: A Role for Developmental Programming and Altered Circadian Rhythms. PLoS ONE 2014, 9, e89505. [Google Scholar] [CrossRef] [PubMed]
- Soeda, J.; Mouralidarane, A.; Cordero, P.; Li, J.; Nguyen, V.; Carter, R.; Kapur, S.R.; Pombo, J.; Poston, L.; Taylor, P.D.; et al. Maternal obesity alters endoplasmic reticulum homeostasis in offspring pancreas. J. Physiol. Biochem. 2016, 72, 281–291. [Google Scholar] [CrossRef]
- Mathur, A.; Marine, M.; Lu, D.; Swartz-Basile, D.A.; Saxena, R.; Zyromski, N.J.; Pitt, H.A. Nonalcoholic fatty pancreas disease. HPB 2007, 9, 312–318. [Google Scholar] [CrossRef]
- Liu, L.; Mei, M.; Yang, S.; Li, Q. Roles of chronic low-grade inflammation in the development of ectopic fat deposition. Mediat. Inflamm. 2014, 2014, 418185. [Google Scholar] [CrossRef] [PubMed]
- Stamm, B.H. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: A systematic study of 112 autopsies in patients without known pancreatic disease. Hum. Pathol 1984, 15, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Garcia, T.S.; Rech, T.H.; Leitao, C.B. Pancreatic size and fat content in diabetes: A systematic review and meta-analysis of imaging studies. PLoS ONE 2017, 12, e0180911. [Google Scholar] [CrossRef] [PubMed]
- Schepis, T.; Tringali, A.; Spada, C.; Costamagna, G.; Boškoski, I. Intrapancreatic Fat Deposition: Cause or Consequence of First Acute Pancreatitis Attack? AJG 2023, 118, 910–911. [Google Scholar] [CrossRef]
- Kim, G.A.; Lee, H.C.; Choe, J.; Kim, M.J.; Lee, M.J.; Chang, H.S.; Bae, I.Y.; Kim, H.K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2017, 68, 140–146. [Google Scholar] [CrossRef]
- Pinnick, K.E.; Collins, S.C.; Londos, C.; Gauguier, D.; Clark, A.; Fielding, B.A. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity 2008, 16, 522–530. [Google Scholar] [CrossRef]
- Wu, W.C.; Wang, C.Y. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: Case-control retrospective study. Cardiovasc. Diabetol. 2013, 12, 77. [Google Scholar] [CrossRef]
- van Geenen, E.J.; Smits, M.M.; Schreuder, T.C.; van der Peet, D.L.; Bloemena, E.; Mulder, C.J. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas 2010, 39, 1185–1190. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694. [Google Scholar] [CrossRef]
- Khoury, T.; Mari, A.; Sbeit, W. A Novel Clinical Score Predicting the Presence of Fatty Pancreas. J. Clin. Med. 2021, 10, 5843. [Google Scholar] [CrossRef]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, P.; Petersen, O.H.; Holgate, S.; Erőss, B.; Garami, A.; Szakács, Z.; Dobszai, D.; Balaskó, M.; Kemény, L.; Peng, S.; et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. J. Clin. Med. 2020, 9, 1532. [Google Scholar] [CrossRef]
- Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J.; Rucker, G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods 2019, 10, 476–483. [Google Scholar] [CrossRef]
- Stijnen, T.; Hamza, T.H.; Ozdemir, P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010, 29, 3046–3067. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical Aspects of the Analysis of Data From Retrospective Studies of Disease. JNCI J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [CrossRef]
- Robins, J.; Greenland, S.; Breslow, N.E. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am. J. Epidemiol. 1986, 124, 719–723. [Google Scholar] [CrossRef]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. (Eds.) The Handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Russell Sage Foundation: New York, NY, USA, 2009. [Google Scholar]
- J. Sweeting, M.; J. Sutton, A.; C. Lambert, P. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 2004, 23, 1351–1375. [Google Scholar] [CrossRef]
- Knapp, G.; Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003, 22, 2693–2710. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Jackson, D.; Law, M.; Rucker, G.; Schwarzer, G. The Hartung-Knapp modification for random-effects meta-analysis: A useful refinement but are there any residual concerns? Stat. Med. 2017, 36, 3923–3934. [Google Scholar] [CrossRef]
- Paule, R.C.; Mandel, J. Consensus Values and Weighting Factors. J. Res. Natl. Bur. Stand. (1977) 1982, 87, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A.; Coull, B.A. Approximate Is Better than “Exact” for Interval Estimation of Binomial Proportions. Am. Stat. 1998, 52, 119–126. [Google Scholar] [CrossRef]
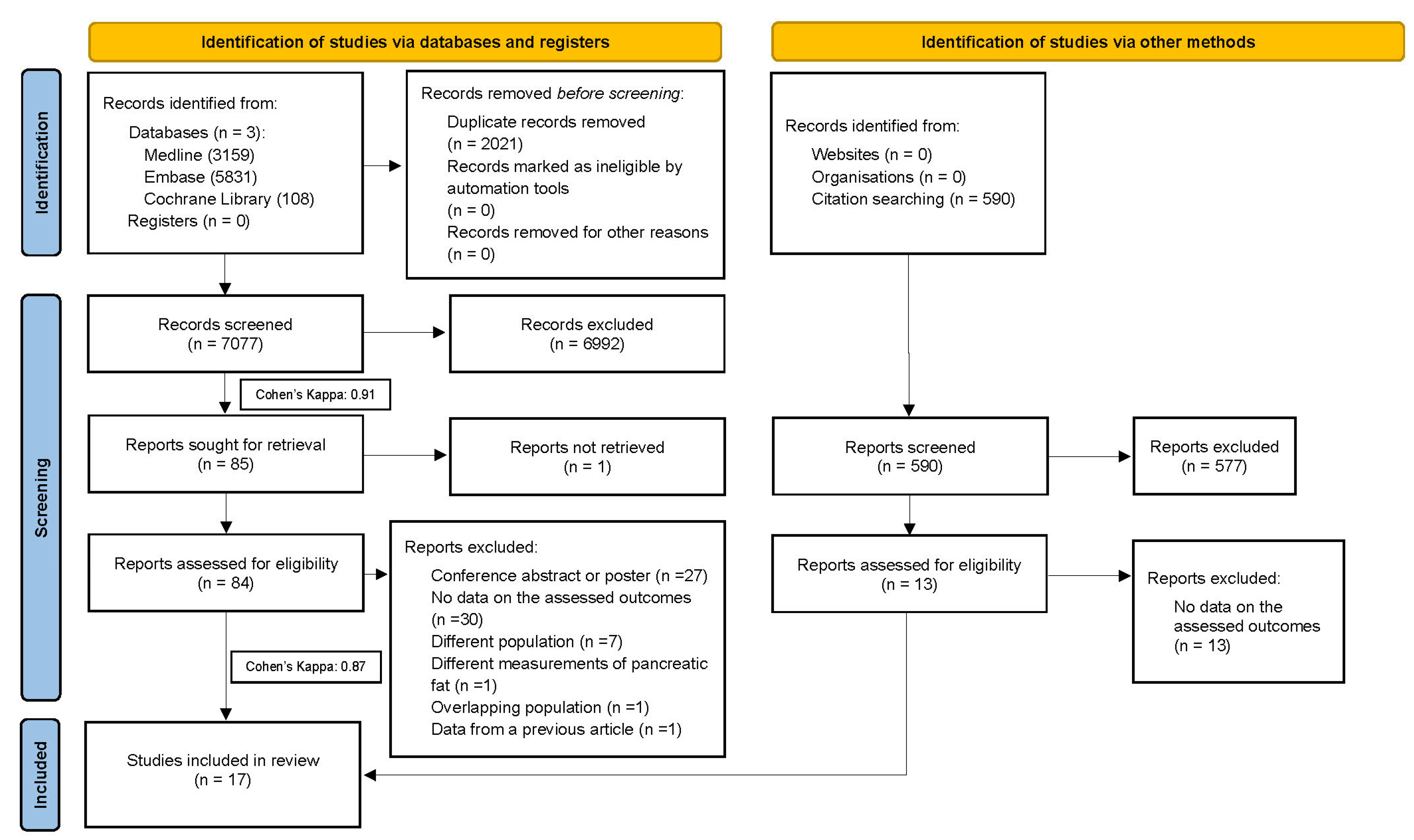

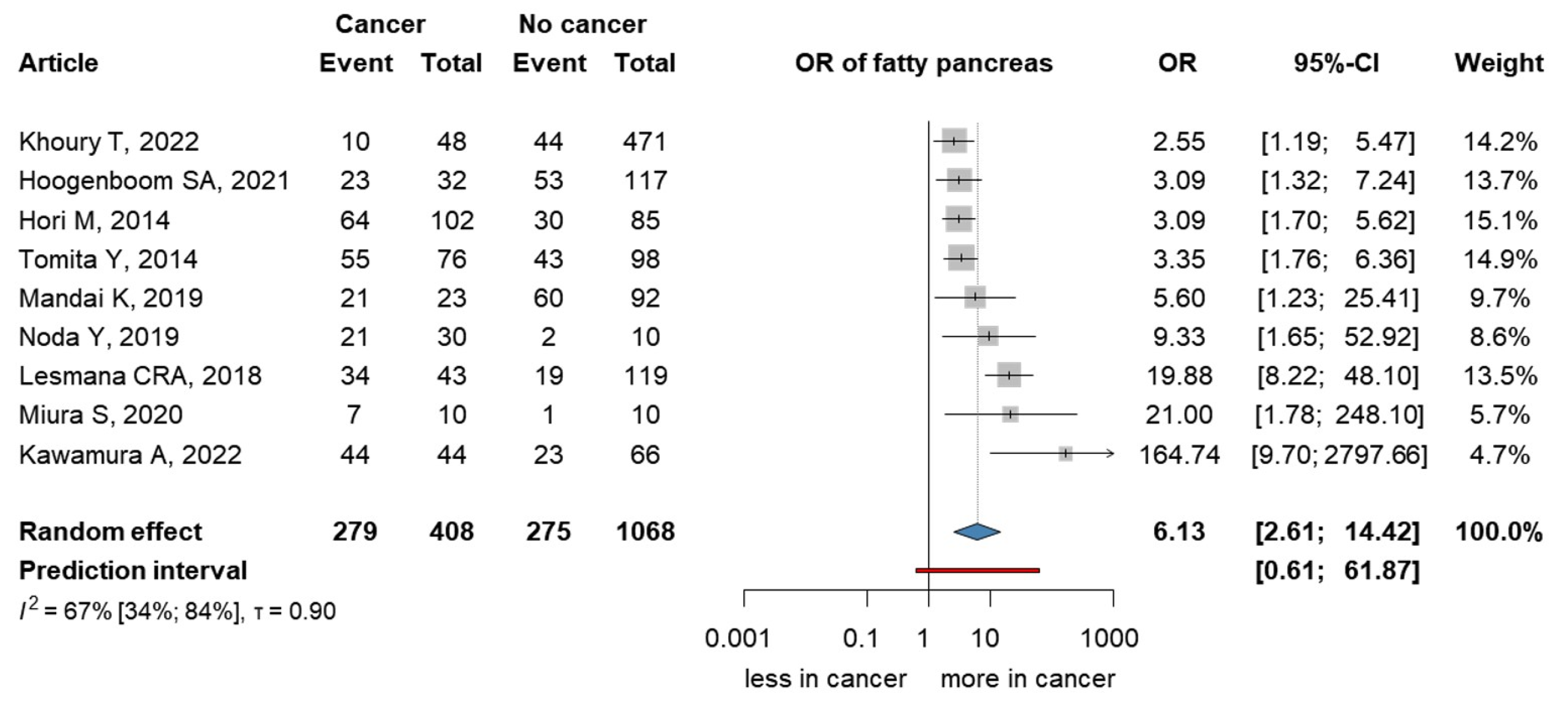
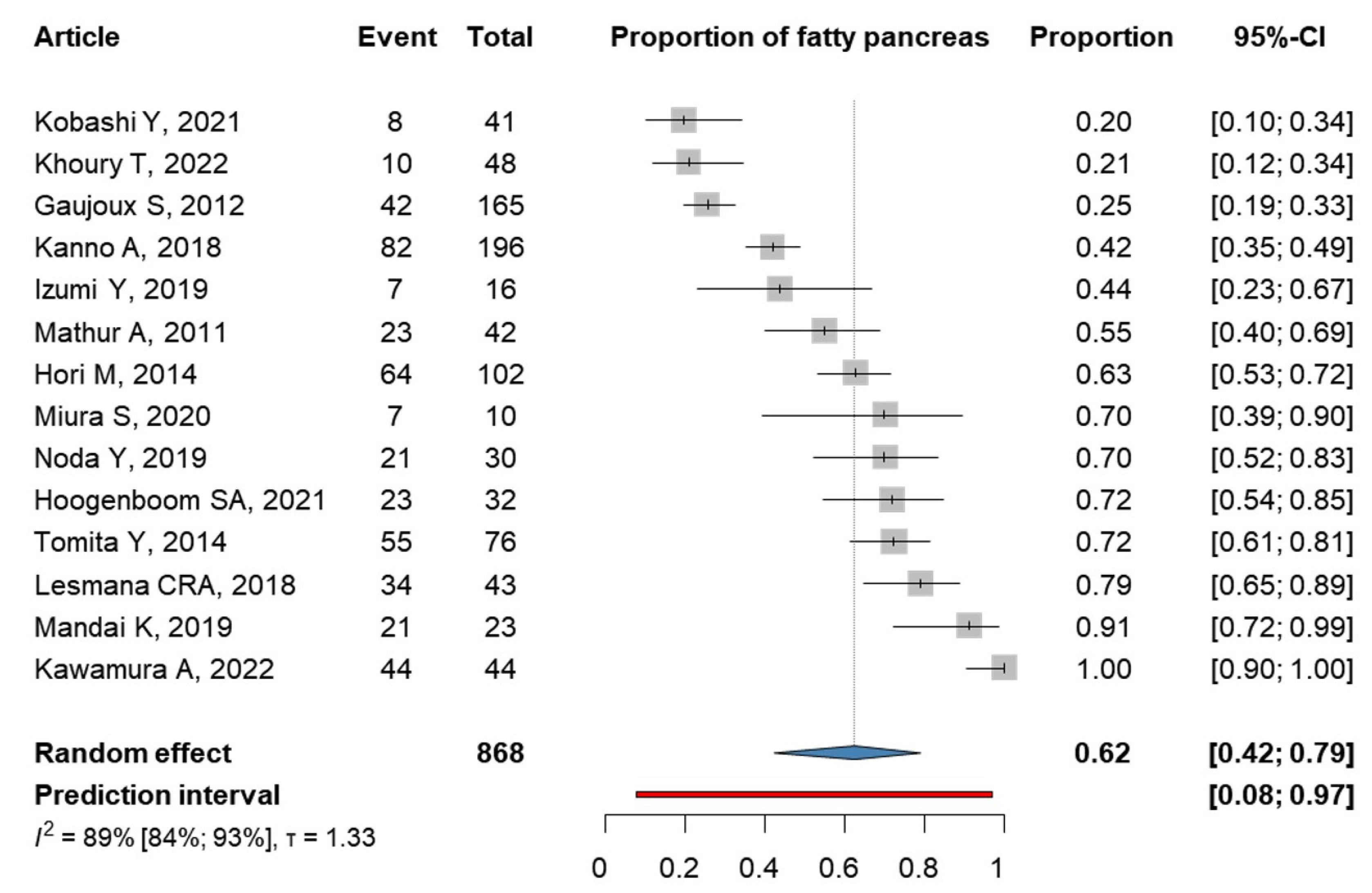
| Study | Design | Demography | Imaging Modality Used to Assess FP | Total Number of PC Individuals | Total Number of FP in PC Individuals | |||
|---|---|---|---|---|---|---|---|---|
| Population | ||||||||
| Country | FP Patients | Control c | Mean Age | |||||
| Sepe P. S. et al. (2011) [36] | Cross-sectional | USA | 64 | 198 | 62.9 a (13.9) b | EUS | 32 | 7 |
| Sbeit W. et al. (2021) [38] | Cross-sectional | Israel | 78 | 716 | 62.8 d (14.1) e | EUS | 50 | 10 |
| Dei H. et al. (2022) [37] | Cross-sectional | Japan | 92 | 172 | 144 f | CT, Histology | 49 | 25 |
| Scheme | Design | Demography | Imaging Modality Used to Assess FP | Total Number of PC Individuals with FP | Total Number of FP Individuals in Non-PC Group | |||
|---|---|---|---|---|---|---|---|---|
| Population | ||||||||
| Country | PC Patients | Control a | Mean Age | |||||
| Mathur A. et al. (2011) [52] | Cross-sectional | USA | 42 | N/A | 46 b (11) c | CT | 23 | N/A |
| Gaujoux S. et al. (2012) [49] | Cross-sectional | USA | 165 | N/A | 71 d (63–77) e | Histology | 42 | N/A |
| Hori M. et al. (2014) [41] | Cross-sectional | Japan | 102 | 85 | 63.5 g (56–69) i, 68.0 h (63–79) j | Histology | 64 | 30 |
| Tomita Y. et al. (2014) [42] | Case–control | Japan | 76 | 98 | 64.04 k (11.84) l | Histology | 55 | 43 |
| Kanno A. et al. (2018) [50] | Cross-sectional | Japan | 196 | N/A | N/A | CT | 82 | N/A |
| Lesmana C. et al. (2018) [45] | Cross-sectional | Indonesia | 43 | 119 | 57 b (15.9)c | EUS | 34 | 19 |
| Izumi Y. et al. (2019) [51] | Cross-sectional | Japan | 16 | N/A | 68.4 b (52–84 f; 9.1 c) | EUS, Histology | 7 | N/A |
| Mandai K. et al. (2019) [43] | Case–control | Japan | 23 | 92 | 73 g, 73 h (69–79) e | EUS | 21 | 60 |
| Noda Y. et al. (2019) [44] | Cross-sectional | Japan | 30 | 10 | 69.9 b (52–82 f; 7.9 c) | MRI, Histology | 21 | 2 |
| Miura S. et al. (2020) [46] | Cross-sectional | Japan | 10 | 10 | 68.15 k (10.23) l | CT | 7 | 1 |
| Hoogenboom S. et al. (2021) [40] | Case–control | USA | 32 | 117 | 68.49 k (10.55) l | CT | 23 | 53 |
| Kobashi Y. et al. (2021) [48] | Case–control | Japan | 41 | N/A | 74.8 b (10.5) c | CT | 8 | N/A |
| Kawamura A. et al. (2022) [47] | Cross-sectional | Japan | 44 | 66 | 64.79 k (13.52) l | EUS | 44 | 23 |
| Khoury T. et al. (2022) [39] | Cross-sectional | Israel | 48 | 471 | 63.07 k (14.01) l | EUS | 10 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipp, M.; Tarján, D.; Lee, J.; Zolcsák, Á.; Szalai, E.; Teutsch, B.; Faluhelyi, N.; Erőss, B.; Hegyi, P.; Mikó, A. Fatty Pancreas Is a Risk Factor for Pancreatic Cancer: A Systematic Review and Meta-Analysis of 2956 Patients. Cancers 2023, 15, 4876. https://doi.org/10.3390/cancers15194876
Lipp M, Tarján D, Lee J, Zolcsák Á, Szalai E, Teutsch B, Faluhelyi N, Erőss B, Hegyi P, Mikó A. Fatty Pancreas Is a Risk Factor for Pancreatic Cancer: A Systematic Review and Meta-Analysis of 2956 Patients. Cancers. 2023; 15(19):4876. https://doi.org/10.3390/cancers15194876
Chicago/Turabian StyleLipp, Mónika, Dorottya Tarján, Jimin Lee, Ádám Zolcsák, Eszter Szalai, Brigitta Teutsch, Nándor Faluhelyi, Bálint Erőss, Péter Hegyi, and Alexandra Mikó. 2023. "Fatty Pancreas Is a Risk Factor for Pancreatic Cancer: A Systematic Review and Meta-Analysis of 2956 Patients" Cancers 15, no. 19: 4876. https://doi.org/10.3390/cancers15194876
APA StyleLipp, M., Tarján, D., Lee, J., Zolcsák, Á., Szalai, E., Teutsch, B., Faluhelyi, N., Erőss, B., Hegyi, P., & Mikó, A. (2023). Fatty Pancreas Is a Risk Factor for Pancreatic Cancer: A Systematic Review and Meta-Analysis of 2956 Patients. Cancers, 15(19), 4876. https://doi.org/10.3390/cancers15194876





