Long-Term Survival Associated with Direct Oral Feeding Following Minimally Invasive Esophagectomy: Results from a Randomized Controlled Trial (NUTRIENT II)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Surgical Procedure
2.3. Nutritional Intervention
2.4. Follow-Up
2.5. Outcome and Definitions
2.6. Data Collection and Statistical Analysis
3. Results
3.1. Study Population
3.2. Baseline Characteristics and Postoperative Outcomes
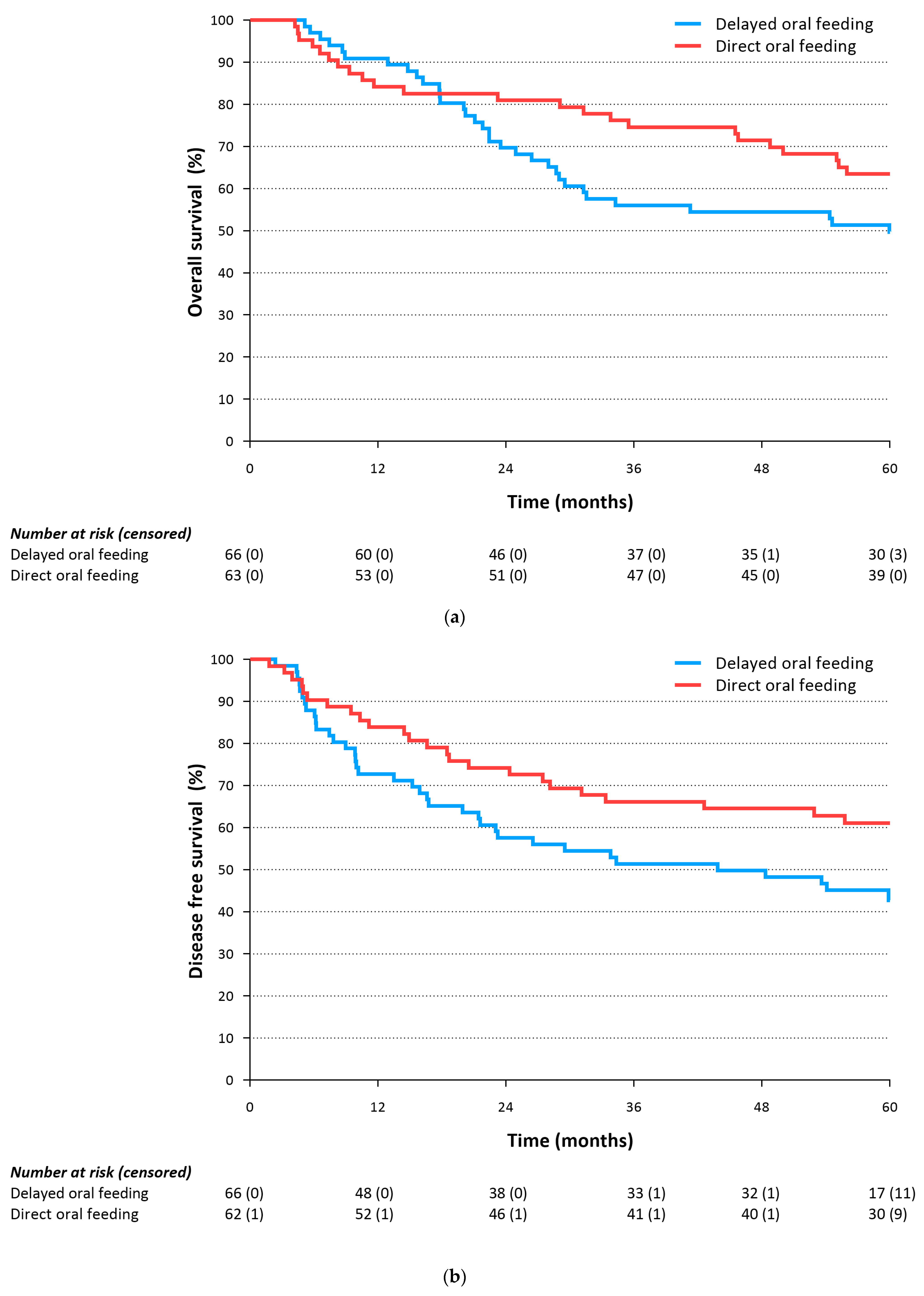
3.3. Primary Outcomes
3.4. Secondary Outcomes
3.5. Impact of Complications on Overall Survival
3.6. Impact of Caloric Intake on Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faiz, Z.; Lemmens, V.E.; Siersema, P.D.; Nieuwenhuijzen, G.A.; Wouters, M.W.; Rozema, T.; Coebergh, J.W.; Wijnhoven, B.P. Increased resection rates and survival among patients aged 75 years and older with esophageal cancer: A Dutch nationwide population-based study. World J. Surg. 2012, 36, 2872–2878. [Google Scholar] [PubMed]
- Kumagai, K.; Rouvelas, I.; Tsai, J.; Mariosa, D.; Lind, P.; Lindblad, M.; Ye, W.; Lundell, L.; Schuhmacher, C.; Mauer, M.; et al. Survival benefit and additional value of preoperative chemoradiotherapy in resectable gastric and gastro-oesophageal junction cancer: A direct and adjusted indirect comparison meta-analysis. Eur. J. Surg. Oncol. 2015, 41, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [PubMed]
- Voeten, D.M.; Busweiler, L.A.D.; van der Werf, L.R.; Wijnhoven, B.P.L.; Verhoeven, R.H.A.; van Sandick, J.W.; van Hillegersberg, R.; Henegouwen, M.I.v.B. Outcomes of Esophagogastric Cancer Surgery during Eight Years of Surgical Auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann. Surg. 2021, 274, 866–873. [Google Scholar] [CrossRef]
- Fransen, L.F.C.; Luyer, M.D.P. Effects of improving outcomes after esophagectomy on the short- and long-term: A review of literature. J. Thorac. Dis. 2019, 11, S845–S850. [Google Scholar] [CrossRef]
- Straatman, J.; Van Der Wielen, N.; Cuesta, M.A.; Daams, F.; Garcia, J.R.; Bonavina, L.; Rosman, C.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Van Der Peet, D.L. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: The TIME Trial. Ann. Surg. 2017, 266, 232–236. [Google Scholar] [CrossRef]
- Findlay, J.M.; Gillies, R.S.; Millo, J.; Sgromo, B.; Marshall, R.E.; Maynard, N.D. Enhanced recovery for esophagectomy: A systematic review and evidence-based guidelines. Ann. Surg. 2014, 259, 413–431. [Google Scholar]
- Berkelmans, G.H.; Fransen, L.F.; Dolmans-Zwartjes, A.C.; Kouwenhoven, E.A.; van Det, M.J.; Nilsson, M.; Nieuwenhuijzen, G.A.; Luyer, M.D. Direct Oral Feeding Following Minimally Invasive Esophagectomy (NUTRIENT II trial): An International, Multicenter, Open-label Randomized Controlled Trial. Ann. Surg. 2020, 271, 41–47. [Google Scholar]
- Fransen, L.F.; Janssen, T.H.; Aarnoudse, M.; Nieuwenhuijzen, G.A.; Luyer, M.D. Direct Oral Feeding after a Minimally Invasive Esophagectomy: A Single-Center Prospective Cohort Study. Ann. Surg. 2022, 275, 919–923. [Google Scholar] [CrossRef]
- Fransen, L.F.; Verhoeven, R.H.; Janssen, T.H.; van Det, M.J.; Gisbertz, S.S.; van Hillegersberg, R.; Klarenbeek, B.; Kouwenhoven, E.A.; Nieuwenhuijzen, G.A.; Rosman, C.; et al. The association between postoperative complications and long-term survival after esophagectomy: A multicenter cohort study. Dis. Esophagus. 2022, 36, doac086. [Google Scholar]
- Fransen, L.F.; Berkelmans, G.H.; Asti, E.; van Berge Henegouwen, M.I.; Berlth, F.; Bonavina, L.; Brown, A.; Bruns, C.; van Daele, E.; Gisbertz, S.S.; et al. The Effect of Postoperative Complications after Minimally Invasive Esophagectomy on Long-term Survival: An International Multicenter Cohort Study. Ann. Surg. 2021, 274, e1129–e1137. [Google Scholar] [CrossRef] [PubMed]
- Booka, E.; Takeuchi, H.; Suda, K.; Fukuda, K.; Nakamura, R.; Wada, N.; Kawakubo, H.; Kitagawa, Y. Meta-analysis of the impact of postoperative complications on survival after oesophagectomy for cancer. BJS Open 2018, 2, 276–284. [Google Scholar] [CrossRef]
- Markar, S.; Gronnier, C.; Duhamel, A.; Mabrut, J.Y.; Bail, J.P.; Carrere, N.; Lefevre, J.H.; Brigand, C.; Vaillant, J.C.; Adham, M.; et al. The Impact of Severe Anastomotic Leak on Long-term Survival and Cancer Recurrence after Surgical Resection for Esophageal Malignancy. Ann. Surg. 2015, 262, 972–980. [Google Scholar] [CrossRef]
- Rutegård, M.; Lagergren, P.; Rouvelas, I.; Mason, R.; Lagergren, J. Surgical complications and long-term survival after esophagectomy for cancer in a nationwide Swedish cohort study. Eur. J. Surg. Oncol. 2012, 38, 555–561. [Google Scholar] [CrossRef]
- Berkelmans, G.H.K.; Wilts, B.J.W.; Kouwenhoven, E.A.; Kumagai, K.; Nilsson, M.; Weijs, T.J.; Nieuwenhuijzen, G.A.P.; van Det, M.J.; Luyer, M.D.P. Nutritional route in oesophageal resection trial II (NUTRIENT II): Study protocol for a multicentre open-label randomised controlled trial. BMJ Open 2016, 6, e011979. [Google Scholar] [CrossRef] [PubMed]
- Soop, M.; Carlson, G.L.; Hopkinson, J.; Clarke, S.; Thorell, A.A.; Nygren, J.; Ljungqvist, O.O. Randomized clinical trial of the effects of immediate enteral nutrition on metabolic responses to major colorectal surgery in an enhanced recovery protocol. Br. J. Surg. 2004, 91, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Brodner, G.; Van Aken, H.; Hertle, L.; Fobker, M.; Von Eckardstein, A.; Goeters, C.; Buerkle, H.; Harks, A.; Kehlet, H. Multimodal perioperative management--combining thoracic epidural analgesia, forced mobilization, and oral nutrition—Reduces hormonal and metabolic stress and improves convalescence after major urologic surgery. Anesth. Analg. 2001, 92, 1594–1600. [Google Scholar] [CrossRef]
- Visioni, A.; Shah, R.; Gabriel, E.; Attwood, K.; Kukar, M.; Nurkin, S. Enhanced Recovery after Surgery for Noncolorectal Surgery?: A Systematic Review and Meta-analysis of Major Abdominal Surgery. Ann. Surg. 2018, 267, 57–65. [Google Scholar] [CrossRef]
- Balayla, J.; Bujold, E.; Lapensée, L.; Mayrand, M.-H.; Sansregret, A. Early Versus Delayed Postoperative Feeding after Major Gynaecological Surgery and its Effects on Clinical Outcomes, Patient Satisfaction, and Length of Stay: A Randomized Controlled Trial. J. Obstet. Gynaecol. Can. 2015, 37, 1079–1085. [Google Scholar] [CrossRef]
- Dag, A.; Colak, T.; Turkmenoglu, O.; Gundogdu, R.; Aydin, S. A randomized controlled trial evaluating early versus traditional oral feeding after colorectal surgery. Clinics 2011, 66, 2001–2005. [Google Scholar] [CrossRef]
- Lewis, S.J.; Egger, M.; Sylvester, P.A.; Thomas, S. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: Systematic review and meta-analysis of controlled trials. BMJ 2001, 323, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, H.; Shoar, S.; Sirati, F.; Khorgami, Z. Early initiation of oral feeding following upper gastrointestinal tumor surgery: A randomized controlled trial. Surg. Today 2015, 45, 203–208. [Google Scholar] [CrossRef]
- Sun, H.B.; Li, Y.; Liu, X.B.; Zhang, R.X.; Wang, Z.F.; Lerut, T.; Liu, C.C.; Fiorelli, A.; Chao, Y.K.; Molena, D.; et al. Early Oral Feeding Following McKeown Minimally Invasive Esophagectomy: An Open-label, Randomized, Controlled, Noninferiority Trial. Ann. Surg. 2018, 267, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Willcutts, K.F.; Chung, M.C.; Erenberg, C.L.; Finn, K.L.; Schirmer, B.D.; Byham-Gray, L.D. Early Oral Feeding as Compared With Traditional Timing of Oral Feeding after Upper Gastrointestinal Surgery: A Systematic Review and Meta-analysis. Ann. Surg. 2016, 264, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Pattamatta, M.; Fransen, L.F.C.; Dolmans-Zwartjes, A.C.P.; Nieuwenhuijzen, G.A.P.; Evers, S.M.A.A.; Kouwenhoven, E.A.; van Det, M.J.; Hiligsmann, M.; Luyer, M.D.P. Effect of direct oral feeding following minimally invasive esophagectomy on costs and quality of life. J. Med. Econ. 2021, 24, 54–60. [Google Scholar] [CrossRef]
- Berkelmans, G.H.K.; Fransen, L.; Weijs, T.J.; Lubbers, M.; Nieuwenhuijzen, G.A.P.; Ruurda, J.P.; Kouwenhoven, E.A.; van Det, M.J.; Rosman, C.; van Hillegersberg, R.; et al. The long-term effects of early oral feeding following minimal invasive esophagectomy. Dis. Esophagus 2018, 31, dox114. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.H.; Yanni, F.; Dorrington, M.S.; Bowman, C.R.; Vohra, R.S.; Parsons, S.L. Impact of postoperative complications on disease recurrence and long-term survival following oesophagogastric cancer resection. Br. J. Surg. 2020, 107, 103–112. [Google Scholar] [CrossRef]
- Tam, V.; Luketich, J.D.; Winger, D.G.; Sarkaria, I.S.; Levy, R.M.; Christie, N.A.; Awais, O.; Shende, M.R.; Nason, K.S. Cancer Recurrence after Esophagectomy: Impact of Postoperative Infection in Propensity-Matched Cohorts. Ann. Thorac. Surg. 2016, 102, 1638–1646. [Google Scholar] [CrossRef]
- Bundred, J.R.; Hollis, A.C.; Evans, R.; Hodson, J.; Whiting, J.L.; Griffiths, E.A. Impact of postoperative complications on survival after oesophagectomy for oesophageal cancer. BJS Open 2020, 4, 405–415. [Google Scholar] [CrossRef]
- Hagens, E.R.C.; Feenstra, M.L.; Eshuis, W.J.; Hulshof, M.C.C.M.; van Laarhoven, H.W.M.; Henegouwen, M.I.v.B.; Gisbertz, S.S. Conditional survival after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br. J. Surg. 2020, 107, 1053–1061. [Google Scholar] [CrossRef]
- Milito, P.; Chmelo, J.; Dunn, L.; Kamarajah, S.K.; Madhavan, A.; Wahed, S.; Immanuel, A.; Griffin, S.M.; Phillips, A.W. Chyle Leak Following Radical En Bloc Esophagectomy with Two-Field Nodal Dissection: Predisposing Factors, Management, and Outcomes. Ann. Surg. Oncol. 2021, 28, 3963–3972. [Google Scholar] [CrossRef] [PubMed]
- Pomatto-Watson, L.C.D.; Bodogai, M.; Bosompra, O.; Kato, J.; Wong, S.; Carpenter, M.; Duregon, E.; Chowdhury, D.; Krishna, P.; Ng, S.; et al. Daily caloric restriction limits tumor growth more effectively than caloric cycling regardless of dietary composition. Nat. Commun. 2021, 12, 6201. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.M.; Al-Foheidi, M.H.; Al-Mansour, M.M. Energy and caloric restriction, and fasting and cancer: A narrative review. Support Care Cancer 2021, 29, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.E.; Tang, Z.; Kerbois, C.; Delville, C.; Konstantopedos, P.; Bruel, A.; Derous, D.; Green, C.; Aspden, R.M.; Goodyear, S.R.; et al. The effects of graded levels of calorie restriction: I. impact of short term calorie and protein restriction on body composition in the C57BL/6 mouse. Oncotarget 2015, 6, 15902–15930. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, C.; Ferraresi, A.; Esposito, A.; Maheshwari, C.; Dhanasekaran, D.N.; Mollace, V.; Isidoro, C. Calorie Restriction for Cancer Prevention and Therapy: Mechanisms, Expectations, and Efficacy. J. Cancer Prev. 2021, 26, 224–236. [Google Scholar] [CrossRef]
- Dan, T.D.; Wright, C.M.; Simone, N.L. What benefits could caloric restriction bring to cancer patients? Future Oncol. 2014, 10, 2543–2546. [Google Scholar] [CrossRef]
- Wright, J.L.; Plymate, S.; D’Oria-Cameron, A.; Bain, C.; Haugk, K.; Xiao, L.; Lin, D.W.; Stanford, J.L.; McTiernan, A. A study of caloric restriction versus standard diet in overweight men with newly diagnosed prostate cancer: A randomized controlled trial. Prostate 2013, 73, 1345–1351. [Google Scholar] [CrossRef]
- Lope, V.; Martín, M.; Castelló, A.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Antolín, S.; Ramos-Vázquez, M.; García-Sáenz, J.Á.; Muñoz, M.; et al. Overeating, caloric restriction and breast cancer risk by pathologic subtype: The EPIGEICAM study. Sci. Rep. 2019, 9, 3904. [Google Scholar] [CrossRef]
- Steenhagen, E. Preoperative nutritional optimization of esophageal cancer patients. J. Thorac. Dis. 2019, 11, S645–S653. [Google Scholar] [CrossRef]
- Cao, Y.; Han, D.; Zhou, X.; Han, Y.; Zhang, Y.; Li, H. Effects of preoperative nutrition on postoperative outcomes in esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2022, 35, doab028. [Google Scholar] [CrossRef]
- Okada, G.; Momoki, C.; Habu, D.; Kambara, C.; Fujii, T.; Matsuda, Y.; Lee, S.; Osugi, H. Effect of Postoperative Oral Intake on Prognosis for Esophageal Cancer. Nutrients 2019, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Berger, M.M.; Berghe, G.V.D.; Biolo, G.; Calder, P.; Forbes, A.; Griffiths, R.; Kreyman, G.; Leverve, X.; Pichard, C. ESPEN Guidelines on Parenteral Nutrition: Intensive care. Clin. Nutr. 2009, 28, 387–400. [Google Scholar] [CrossRef] [PubMed]


| Delayed Oral Feeding n = 66 | Direct Oral Feeding n = 63 | p-Value * | |
|---|---|---|---|
| 57 (86.4) | 54 (85.7) | 0.915 | |
| Age at randomization, years | 65 [61–70] | 66 [59–70] | 0.853 |
| BMI at diagnosis, kg/m2 | 26 [24–29] | 26 [23–29] | 0.441 |
| ASA score | 0.749 | ||
| I | 9 (13.6) | 6 (9.5) | |
| II | 42 (63.5) | 43 (68.3) | |
| III-IV | 15 (22.7) | 14 (22.2) | |
| Comorbidities | |||
| Overall | 48 (72.7) | 40 (63.5) | 0.260 |
| Vascular | 21 (31.8) | 20 (31.7) | 0.993 |
| Cardiac | 17 (25.8) | 8 (12.7) | 0.061 |
| Pulmonary | 7 (10.6) | 8 (12.7) | 0.711 |
| Diabetes | 6 (9.1) | 8 (12.7) | 0.510 |
| Tumor location | 0.565 | ||
| Mid | 3 (4.5) | 1 (1.6) | |
| Distal | 46 (69.7) | 43 (68.3) | |
| GEJ | 17 (25.8) | 19 (30.2) | |
| Tumor histology | 0.549 | ||
| Adenocarcinoma | 54 (81.8) | 54 (85.7) | |
| Squamous-cell carcinoma | 12 (18.2) | 9 (14.3) | |
| Neoadjuvant treatment | 63 (95.5) | 56 (88.9) | 0.163 |
| Clinical tumor stage | 0.787 | ||
| I | 12 (18.5) | 11 (17.5) | |
| II | 20 (30.8) | 23 (36.5) | |
| III | 33 (50.8) | 29 (46.0) | |
| Lymph nodes harvested, number | 22 [17–27] | 23 [18–30] | 0.252 |
| Total positive lymph nodes, number | 0 [0–1] | 0 [0–1] | 0.775 |
| Radicality of resection | |||
| R0: microscopic radical | 65 (98.5) | 63 (100) | 0.327 |
| R1: microscopic irradical | 1 (1.5) | 0 (-) | |
| Pathological T stage | 0.202 | ||
| T0 | 11 (19.7) | 20 (31.7) | |
| T1 | 18 (27.3) | 15 (23.8) | |
| T2 | 10 (15.2) | 10 (15.9) | |
| T3 | 27 (40.9) | 18 (28.6) | |
| Pathological N stage | 0.102 | ||
| N0 | 47 (71.2) | 41 (65.1) | |
| N1 | 7 (10.6) | 15 (23.8) | |
| N2-3 | 12 (18.2) | 7 (11.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geraedts, T.C.M.; Weijs, T.J.; Berkelmans, G.H.K.; Fransen, L.F.C.; Kouwenhoven, E.A.; van Det, M.J.; Nilsson, M.; Lagarde, S.M.; van Hillegersberg, R.; Markar, S.R.; et al. Long-Term Survival Associated with Direct Oral Feeding Following Minimally Invasive Esophagectomy: Results from a Randomized Controlled Trial (NUTRIENT II). Cancers 2023, 15, 4856. https://doi.org/10.3390/cancers15194856
Geraedts TCM, Weijs TJ, Berkelmans GHK, Fransen LFC, Kouwenhoven EA, van Det MJ, Nilsson M, Lagarde SM, van Hillegersberg R, Markar SR, et al. Long-Term Survival Associated with Direct Oral Feeding Following Minimally Invasive Esophagectomy: Results from a Randomized Controlled Trial (NUTRIENT II). Cancers. 2023; 15(19):4856. https://doi.org/10.3390/cancers15194856
Chicago/Turabian StyleGeraedts, Tessa C. M., Teus J. Weijs, Gijs H. K. Berkelmans, Laura F. C. Fransen, Ewout A. Kouwenhoven, Marc J. van Det, Magnus Nilsson, Sjoerd M. Lagarde, Richard van Hillegersberg, Sheraz R. Markar, and et al. 2023. "Long-Term Survival Associated with Direct Oral Feeding Following Minimally Invasive Esophagectomy: Results from a Randomized Controlled Trial (NUTRIENT II)" Cancers 15, no. 19: 4856. https://doi.org/10.3390/cancers15194856
APA StyleGeraedts, T. C. M., Weijs, T. J., Berkelmans, G. H. K., Fransen, L. F. C., Kouwenhoven, E. A., van Det, M. J., Nilsson, M., Lagarde, S. M., van Hillegersberg, R., Markar, S. R., Nieuwenhuijzen, G. A. P., & Luyer, M. D. P. (2023). Long-Term Survival Associated with Direct Oral Feeding Following Minimally Invasive Esophagectomy: Results from a Randomized Controlled Trial (NUTRIENT II). Cancers, 15(19), 4856. https://doi.org/10.3390/cancers15194856







