WNT4 Gene and Protein Expression in Endometrial Cancer and Its Significance
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Material Collection
2.3. RNA Isolation and cDNA Synthesis
2.4. Real-Time PCR
2.5. Immunohistochemistry
2.6. The Staining Heterogeneity Evaluation
2.7. Database Analysis
2.8. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Patients with EEC and Non-EEC
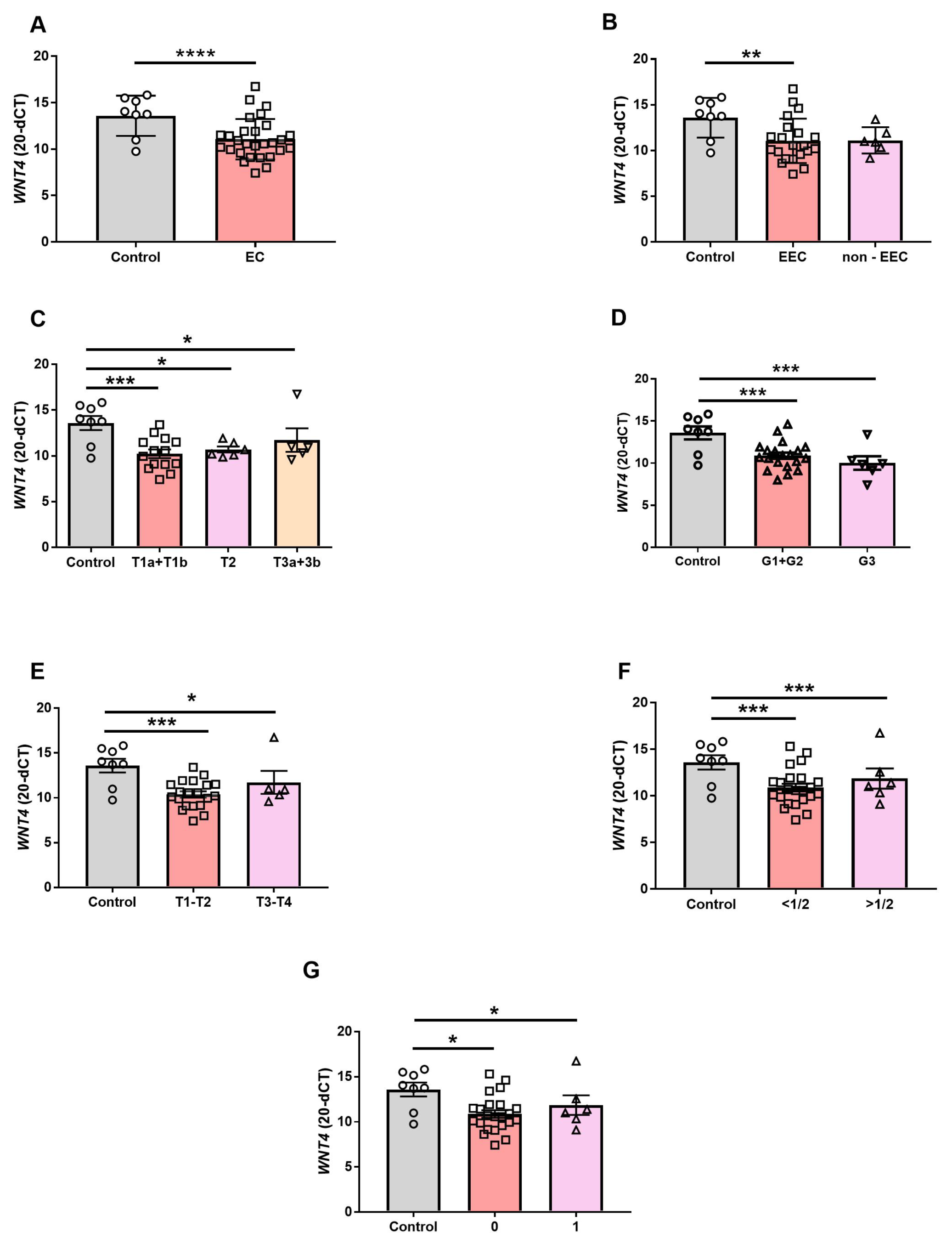
3.2. WNT4 Gene Expression in EC
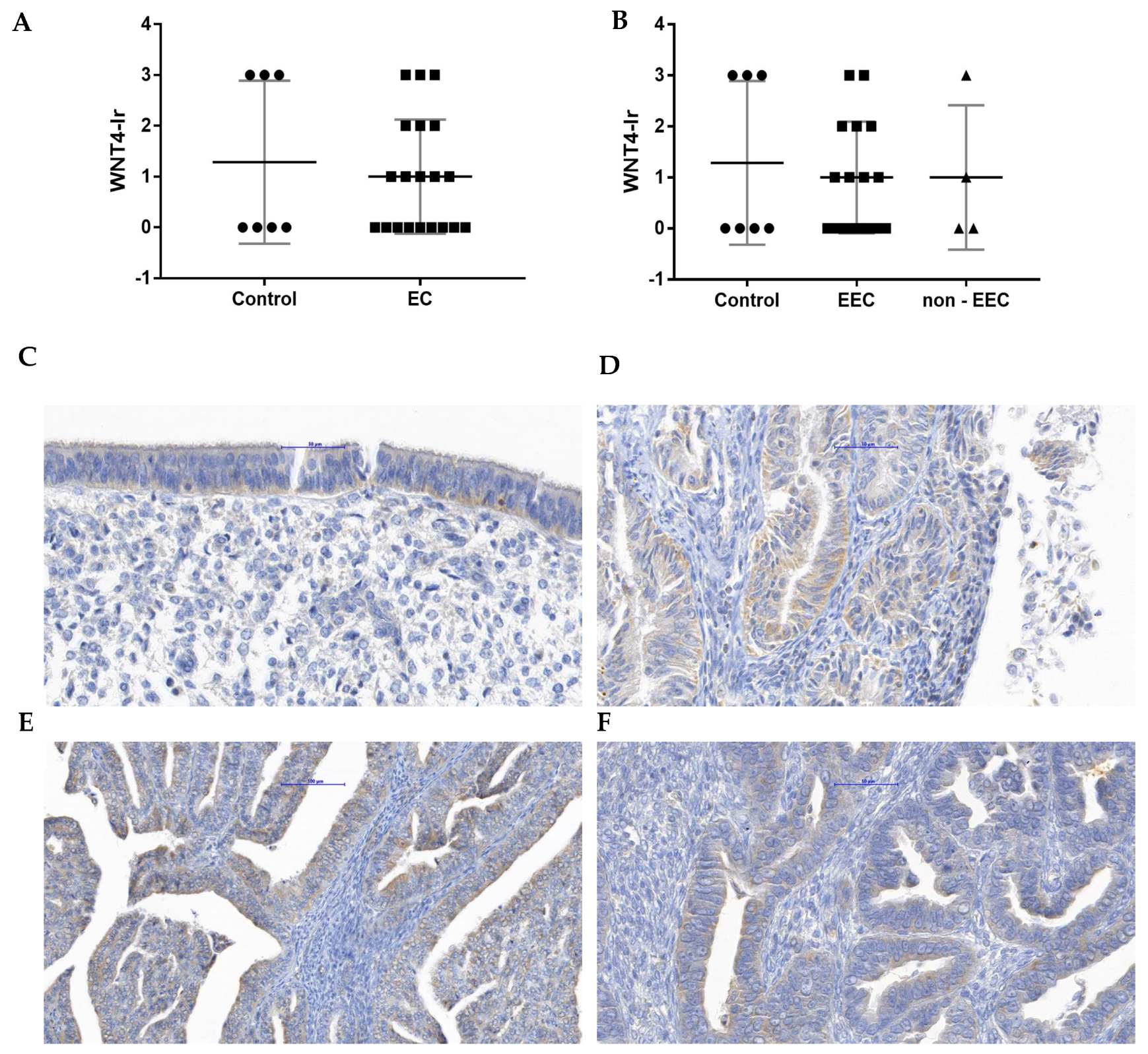
3.3. The WNT4-Ir in EC
3.4. Blood Biochemical Parameters Correlation with WNT4 Gene Expression and WNT4-Ir in EC
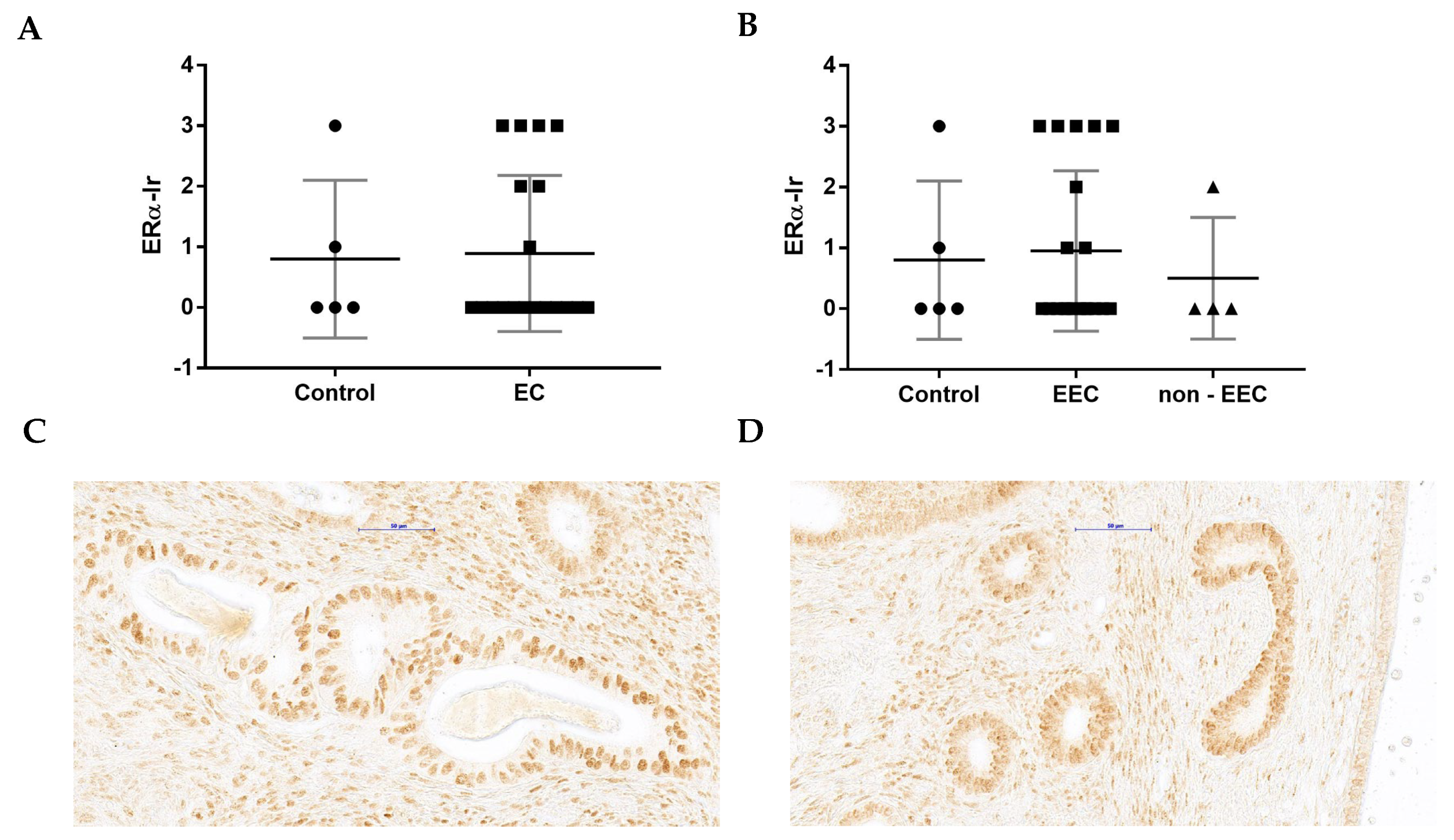
3.5. The Comparison of WNT4-Ir and ERα-Ir in Control and EC Samples
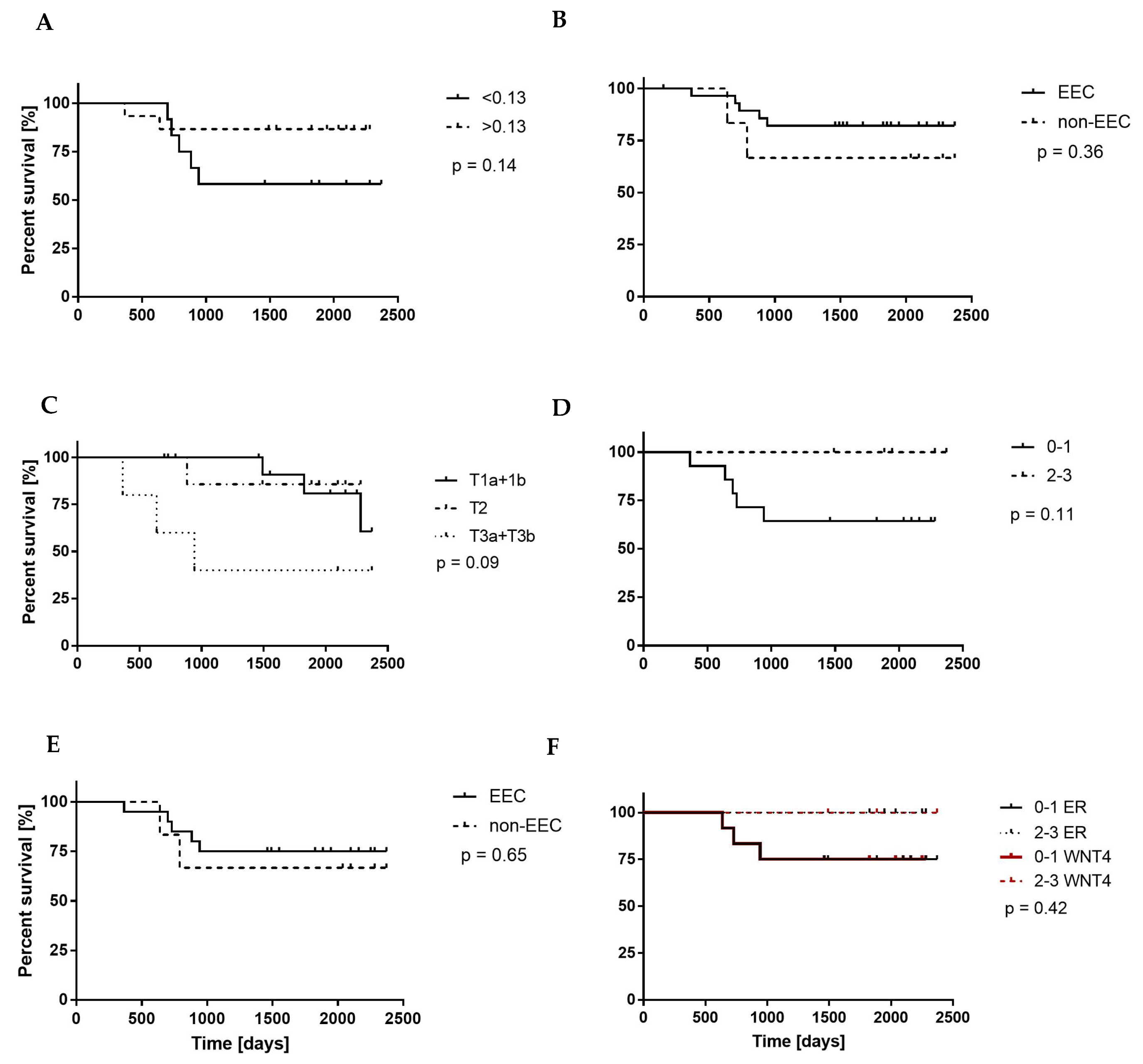
3.6. WNT4 mRNA Level and WNT4-Ir Correlation with OS of EC Patients
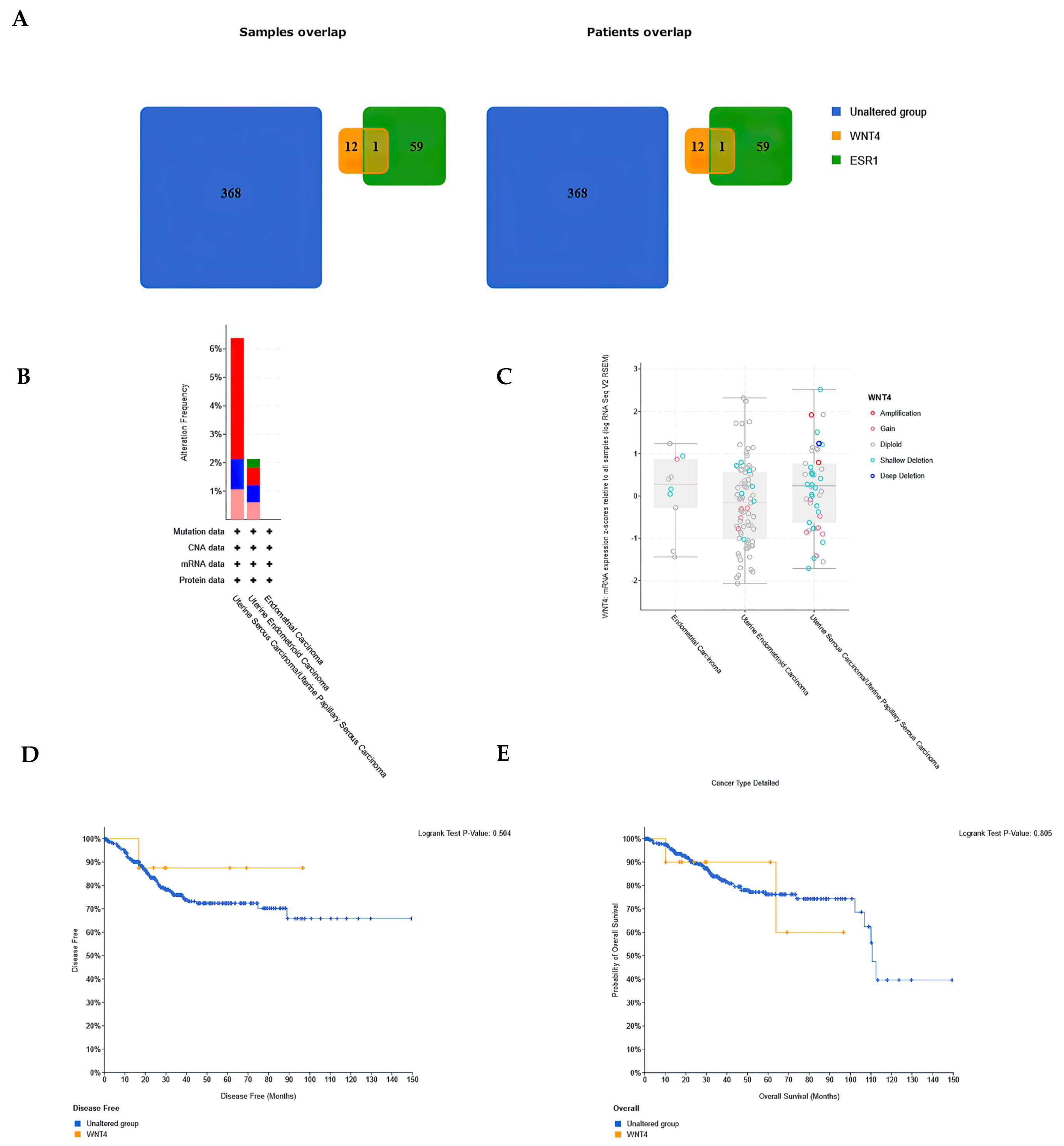
3.7. WNT4 Expression Diversity in a Large Patient Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, Endogenous Hormones, and Endometrial Cancer Risk: A Synthetic Review. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1531–1543. [Google Scholar]
- Renehan, A.G.; Roberts, D.L.; Dive, C. Obesity and Cancer: Pathophysiological and Biological Mechanisms. Arch. Physiol. Biochem. 2008, 114, 71–83. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Brewer, M.A. Endometrial Cancer: Is This a New Disease? Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Cancer Facts & Figures 2010|American Cancer Society. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html (accessed on 15 August 2023).
- Pathology Outlines—Endometrial Carcinoma-General. Available online: https://www.pathologyoutlines.com/topic/uterusendometrialcarc.html (accessed on 13 September 2023).
- Masood, M.; Singh, N. Endometrial Carcinoma: Changes to Classification (WHO 2020). Diagn. Histopathol. 2021, 27, 493–499. [Google Scholar] [CrossRef]
- Endometrial Cancer|Williams Gynecology, 3e|AccessMedicine|McGraw Hill Medical. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=1758§ionid=118172898 (accessed on 9 May 2023).
- Samarnthai, N.; Hall, K.; Yeh, I.-T. Molecular Profiling of Endometrial Malignancies. Obstet. Gynecol. Int. 2010, 2010, 162363. [Google Scholar] [CrossRef]
- Onstad, M.A.; Pakish, J.B.; Lu, K.H. Chapter 32: Tumors of the Uterine Corpus. In The MD Anderson Manual of Medical Oncology; McGraw-Hill: New York, NY, USA, 2016; pp. 1–39. [Google Scholar]
- Hou, X.; Tan, Y.; Li, M.; Dey, S.K.; Das, S.K. Canonical Wnt Signaling Is Critical to Estrogen-Mediated Uterine Growth. Mol. Endocrinol. 2004, 18, 3035–3049. [Google Scholar] [CrossRef]
- Stark, K.; Vainio, S.; Vassileva, G.; McMahon, A.P. Epithelial Transformation of Metanephric Mesenchyme in the Developing Kidney Regulated by Wnt-4. Nature 1994, 372, 679–683. [Google Scholar] [CrossRef]
- Boyer, A.; Lapointe, É.; Zheng, X.; Cowan, R.G.; Li, H.; Quirk, S.M.; Demayo, F.J.; Richards, J.S.; Boerboom, D. WNT4 Is Required for Normal Ovarian Follicle Development and Female Fertility. FASEB J. 2010, 24, 3010–3025. [Google Scholar] [CrossRef]
- Vainio, S.; Heikkilä, M.; Kispert, A.; Chin, N.; McMahon, A.P. Female Development in Mammals Is Regulated by Wnt-4 Signalling. Nature 1999, 397, 405–409. [Google Scholar] [CrossRef]
- Tulac, S.; Nayak, N.R.; Kao, L.C.; Van Waes, M.; Huang, J.; Lobo, S.; Germeyer, A.; Lessey, B.A.; Taylor, R.N.; Suchanek, E.; et al. Identification, Characterization, and Regulation of the Canonical Wnt Signaling Pathway in Human Endometrium. J. Clin. Endocrinol. Metab. 2003, 88, 3860–3866. [Google Scholar] [CrossRef]
- Bui, T.D.; Zhang, L.; Rees, M.C.P.; Bicknell, R.; Harris, A.L. Expression and Hormone Regulation of Wnt2, 3, 4, 5a, 7a, 7b and 10b in Normal Human Endometrium and Endometrial Carcinoma. Br. J. Cancer 1997, 75, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Pitzer, L.M.; Moroney, M.R.; Nokoff, N.J.; Sikora, M.J. Mini-Review WNT4 Balances Development vs Disease in Gynecologic Tissues and Women’s Health. Endocrinology 2021, 162, bqab093. [Google Scholar] [CrossRef] [PubMed]
- Takama, F.; Kanuma, T.; Wang, D.; Kagami, I.; Mizunuma, H. Oestrogen Receptor Beta Expression and Depth of Myometrial Invasion in Human Endometrial Cancer. Br. J. Cancer 2001, 84, 545–549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saegusa, M.; Okayasu, I. Changes in Expression of Estrogen Receptors Alpha and Beta in Relation to Progesterone Receptor and PS2 Status in Normal and Malignant Endometrium. Jpn. J. Cancer Res. 2000, 91, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Jazaeri, A.A.; Nunes, K.J.; Dalton, M.S.; Xu, M.; Shupnik, M.A.; Rice, L.W. Well-Differentiated Endometrial Adenocarcinomas and Poorly Differentiated Mixed Mullerian Tumors Have Altered ER and PR Isoform Expression. Oncogene 2001, 20, 6965–6969. [Google Scholar] [CrossRef]
- Skrzypczak, M.; Bieche, I.; Szymczak, S.; Tozlu, S.; Lewandowski, S.; Girault, I.; Radwanska, K.; Szczylik, C.; Jakowicki, J.A.; Lidereau, R.; et al. Evaluation of MRNA Expression of Estrogen Receptor β and Its Isoforms in Human Normal and Neoplastic Endometrium. Int. J. Cancer 2004, 110, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Sikora, M.J.; Cooper, K.L.; Bahreini, A.; Luthra, S.; Wang, G.; Chandran, U.R.; Davidson, N.E.; Dabbs, D.J.; Welm, A.L.; Oesterreich, S. Invasive Lobular Carcinoma Cell Lines Are Characterized by Unique Estrogen-Mediated Gene Expression Patterns and Altered Tamoxifen Response. Cancer Res. 2014, 74, 1463–1474. [Google Scholar] [CrossRef]
- Sikora, M.J.; Jacobsen, B.M.; Levine, K.; Chen, J.; Davidson, N.E.; Lee, A.V.; Alexander, C.M.; Oesterreich, S. WNT4 Mediates Estrogen Receptor Signaling and Endocrine Resistance in Invasive Lobular Carcinoma Cell Lines. Breast Cancer Res. 2016, 18, 92. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, J.; Zhou, X.; Liu, J.; Wang, Q.; Zhang, B. Roles of Estrogen Receptor α and β in the Regulation of Proliferation in Endometrial Carcinoma. Pathol. Res. Pract. 2020, 216, 153149. [Google Scholar] [CrossRef] [PubMed]
- Wasniewski, T.; Kiezun, J.; Krazinski, B.E.; Kowalczyk, A.E.; Szostak, B.; Wierzbicki, P.M.; Kiewisz, J. WNT5A Gene and Protein Expression in Endometrial Cancer. Folia Histochem. Cytobiol. 2019, 57, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Romani, C.; Calza, S.; Todeschini, P.; Tassi, R.A.; Zanotti, L.; Bandiera, E.; Sartori, E.; Pecorelli, S.; Ravaggi, A.; Santin, A.D.; et al. Identification of Optimal Reference Genes for Gene Expression Normalization in a Wide Cohort of Endometrioid Endometrial Carcinoma Tissues. PLoS ONE 2014, 9, e113781. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Charafe-Jauffret, E.; Tarpin, C.; Bardou, V.J.; Bertucci, F.; Ginestier, C.; Braud, A.C.; Puig, B.; Geneix, J.; Hassoun, J.; Bimbaum, D.; et al. Immunophenotypic Analysis of Inflammatory Breast Cancers: Identification of an “Inflammatory Signature”. J. Pathol. 2004, 202, 265–273. [Google Scholar] [CrossRef]
- Crowe, A.R.; Yue, W. Semi-Quantitative Determination of Protein Expression Using Immunohistochemistry Staining and Analysis: An integrated protocol. Bio-protocol 2023, 9, e3465. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Coopes, A.; Henry, C.E.; Llamosas, E.; Ford, C.E. An Update of Wnt Signalling in Endometrial Cancer and Its Potential as a Therapeutic Target. Endocr. Relat. Cancer 2018, 25, R647–R662. [Google Scholar] [CrossRef]
- Zmarzły, N.; Hermyt, E.; Kruszniewska-Rajs, C.; Gola, J.; Witek, A.; Mazurek, U.; Ostenda, A.; Boroń, D. Expression Profile of EMT-Related Genes and MiRNAs Involved in Signal Transduction via the Wnt Pathway and Cadherins in Endometrial Cancer. Curr. Pharm. Biotechnol. 2021, 22, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Jiang, X.; Du, X.; Hu, W.; Bai, S.; Wang, X.; Xu, B.; Zhao, W. Integrated Transcriptome and Multiple Activated Pathways in Endometrial Cancer. Front. Genet. 2021, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Vouyovitch, C.M.; Perry, J.K.; Liu, D.X.; Bezin, L.; Vilain, E.; Diaz, J.J.; Lobie, P.E.; Mertani, H.C. WNT4 Mediates the Autocrine Effects of Growth Hormone in Mammary Carcinoma Cells. Endocr. Relat. Cancer 2016, 23, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Liu, Y.; Wang, Y.; Wang, H.; Lu, C.; Zhang, P. Decreased Wnt4 Expression Inhibits Thymoma Development through Downregulation of FoxN1. J. Thorac. Dis. 2017, 9, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Feng, Y. Exosomes Derived from Hypoxic Colorectal Cancer Cells Promote Angiogenesis through Wnt4-Induced β-Catenin Signaling in Endothelial Cells. Oncol. Res. 2017, 25, 651–661. [Google Scholar] [CrossRef]
- Nadine Markowski, D.; Bartnitzke, S.; Drieschner, N.; Maria Helmke, B.; Bullerdiek, J. MED12 Mutations in Uterine Fibroids-Their Relationship to Cytogenetic Subgroups. Int. J. Cancer 2012, 131, 1528–1536. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Zhang, C.; Liu, Z.; Piao, Y.; Yan, J.; Xiang, R.; Yao, Y.; Shi, Y. E6-Induced Selective Translation of WNT4 and JIP2 Promotes the Progression of Cervical Cancer via a Noncanonical WNT Signaling Pathway. Signal Transduct. Target. Ther. 2019, 4, 32. [Google Scholar] [CrossRef]
- Shackleford, M.T.; Rao, D.M.; Bordeaux, E.K.; Hicks, H.M.; Towers, C.G.; Sottnik, J.L.; Oesterreich, S.; Sikora, M.J. Estrogen Regulation of MTOR Signaling and Mitochondrial Function in Invasive Lobular Carcinoma Cell Lines Requires WNT4. Cancers 2020, 12, 2931. [Google Scholar] [CrossRef]
- Jordan, K.R.; Sikora, M.J.; Slansky, J.E.; Minic, A.; Richer, J.K.; Moroney, M.R.; Hu, J.; Wolsky, R.J.; Watson, Z.L.; Yamamoto, T.M.; et al. The Capacity of the Ovarian Cancer Tumor Microenvironment to Integrate Inflammation Signaling Conveys a Shorter Disease-Free Interval. Clin. Cancer Res. 2020, 26, 6362–6373. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, Y.; Ji, J.; Xu, Y.; Zhang, Q.; Qin, L. Roles and Action Mechanisms of WNT4 in Cell Differentiation and Human Diseases: A Review. Cell Death Discov. 2021, 7, 287. [Google Scholar] [CrossRef]
- Schindler, A.E. Progestogen Deficiency and Endometrial Cancer Risk. Maturitas 2009, 62, 334–337. [Google Scholar] [CrossRef]
- Zhang, G.; Feenstra, B.; Bacelis, J.; Liu, X.; Muglia, L.M.; Juodakis, J.; Miller, D.E.; Litterman, N.; Jiang, P.-P.; Russell, L.; et al. Genetic Associations with Gestational Duration and Spontaneous Preterm Birth. N. Engl. J. Med. 2017, 377, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Liang, J.; Yang, X.; Wang, Y.; Zhao, Y.; Wu, H.; Sun, L.; Zhang, Y.; Chen, Y.; Li, R.; et al. Integration of Estrogen and Wnt Signaling Circuits by the Polycomb Group Protein EZH2 in Breast Cancer Cells. Mol. Cell. Biol. 2007, 27, 5105–5119. [Google Scholar] [CrossRef] [PubMed]
- Kiewisz, J.; Kaczmarek, M.M.; Andronowska, A.; Blitek, A.; Ziecik, A.J. Gene Expression of WNTs, β-Catenin and E-Cadherin during the Periimplantation Period of Pregnancy in Pigs—Involvement of Steroid Hormones. Theriogenology 2011, 76, 687–699. [Google Scholar] [CrossRef]
- Kiewisz, J.; Kaczmarek, M.M.; Morawska, E.; Blitek, A.; Kapelanski, W.; Ziecik, A.J. Estrus Synchronization Affects WNT Signaling in the Porcine Reproductive Tract and Embryos. Theriogenology 2011, 76, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cong, R.; Kong, F.; Ma, J.; Wu, Q.; Ma, X. Fibrinogen is a coagulation marker associated with the prognosis of endometrial cancer. Onco-Targets Ther. 2019, 12, 9947–9956. [Google Scholar] [CrossRef]
- Marieb, E.; Hoehn, K. Human Anatomy & Physiology, 8th ed.; Benjamin Cummings: San Francisco, CA, USA, 2010. [Google Scholar]
- Lin, R.J.; Afshar-Kharghan, V.; Schafer, A.I. Paraneoplastic Thrombocytosis: The Secrets of Tumor Self-Promotion. Blood 2014, 124, 184–187. [Google Scholar] [CrossRef]
| Characteristics | Units | EC | ||
|---|---|---|---|---|
| EEC | non-EEC | |||
| Total number of patients | - | 22 | 6 | |
| Demographical status | Age | years | 65.04 ± 10.25 | 69.5 ± 9.73 |
| BMI | kg/m | 32.35 ± 5.19 | 27.88 ± 5.43 | |
| Blood biochemical characteristic | RBC | 106/μL | 4.8 ± 0.43 | 4.68 ± 0.52 |
| Hb | g/dL | 13.9 ± 1.43 | 13.7 ± 1.13 | |
| Ht | % | 42.1 ± 3.25 | 41.08 ± 3.01 | |
| WBC | 103/μL | 7.57 ± 2.19 | 6.62 ± 1.94 | |
| Granulocytes | % | 59.95 ± 8.64 | 61.68 ± 9.76 | |
| Lymphocytes | % | 30.81 ± 7.74 | 29.15 ± 8.72 | |
| Monocytes | % | 6.68 ± 2.05 | 7.00 ± 1.67 | |
| Platelets | 103/μL | 233.5 ± 61.59 | 216.5 ± 58.31 | |
| MPV | fl | 11.06 ± 0.75 | 10.45 ± 0.75 | |
| APTT | s | 26.77 ± 2.15 | 30.15 ± 6.35 | |
| General characteristics | Histological subtypes | G1 | 2 | 0 |
| G2 | 16 | 3 | ||
| G3 | 4 | 3 | ||
| FIGO stage | T1 | 13 | 3 | |
| T2 | 7 | 0 | ||
| T3 | 2 | 3 | ||
| T4 | 0 | 0 | ||
| Vital status | Alive | 17 | 4 | |
| Dead | 5 | 2 | ||
| WNT4 mRNA | WNT4-Ir | |||
|---|---|---|---|---|
| EEC | non-EEC | EEC | non-EEC | |
| RBC | −0.14 | −0.24 | 0.06 | −0.40 |
| Hb | −0.37 | −0.16 | 0.41 | −0.20 |
| Ht | −0.43 | −0.12 | 0.49 | −0.20 |
| WBC | −0.48 | 0.24 | −0.38 | 0.40 |
| Granulocytes | −0.03 | 0.31 | 0.23 | 0.80 |
| Lymphocytes | 0.37 | −0.32 | −0.28 | −0.80 |
| Monocytes | 0.83 | 0.06 | −0.13 | −1 |
| Platelets | −0.6 | 0.17 | −0.25 | −0.20 |
| MPV | −0.14 | 0.39 | 0.23 | 0.40 |
| APTT | 0.37 | −0.06 | −0.51 * | −0.80 |
| No. | WNT4-Ir | ERα-Ir | SURVIVAL (0/1) |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 1 |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 |
| 6 | 1 | 0 | 1 |
| 7 | 1 | 0 | 1 |
| 8 | 1 | 0 | 0 |
| 9 | 2 | 0 | 0 |
| 10 | 3 | 0 | 0 |
| 11 | 3 | 0 | 0 |
| 12 | 3 | 0 | 0 |
| 13 | 0 | 2 | 0 |
| 14 | 0 | 3 | 0 |
| 15 | 2 | 2 | 0 |
| 16 | 1 | 3 | 0 |
| 17 | 1 | 3 | 0 |
| 18 | 2 | 3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiewisz, J.; Waśniewski, T.; Kieżun, J.; Skowrońska, A.; Kaczmarek, M.M.; Szóstak, B.; Kowalczyk, A.E.; Kmieć, Z. WNT4 Gene and Protein Expression in Endometrial Cancer and Its Significance. Cancers 2023, 15, 4780. https://doi.org/10.3390/cancers15194780
Kiewisz J, Waśniewski T, Kieżun J, Skowrońska A, Kaczmarek MM, Szóstak B, Kowalczyk AE, Kmieć Z. WNT4 Gene and Protein Expression in Endometrial Cancer and Its Significance. Cancers. 2023; 15(19):4780. https://doi.org/10.3390/cancers15194780
Chicago/Turabian StyleKiewisz, Jolanta, Tomasz Waśniewski, Jacek Kieżun, Agnieszka Skowrońska, Monika M. Kaczmarek, Błażej Szóstak, Anna E. Kowalczyk, and Zbigniew Kmieć. 2023. "WNT4 Gene and Protein Expression in Endometrial Cancer and Its Significance" Cancers 15, no. 19: 4780. https://doi.org/10.3390/cancers15194780
APA StyleKiewisz, J., Waśniewski, T., Kieżun, J., Skowrońska, A., Kaczmarek, M. M., Szóstak, B., Kowalczyk, A. E., & Kmieć, Z. (2023). WNT4 Gene and Protein Expression in Endometrial Cancer and Its Significance. Cancers, 15(19), 4780. https://doi.org/10.3390/cancers15194780








