Endoscopic Management of Dysplastic Barrett’s Oesophagus and Early Oesophageal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
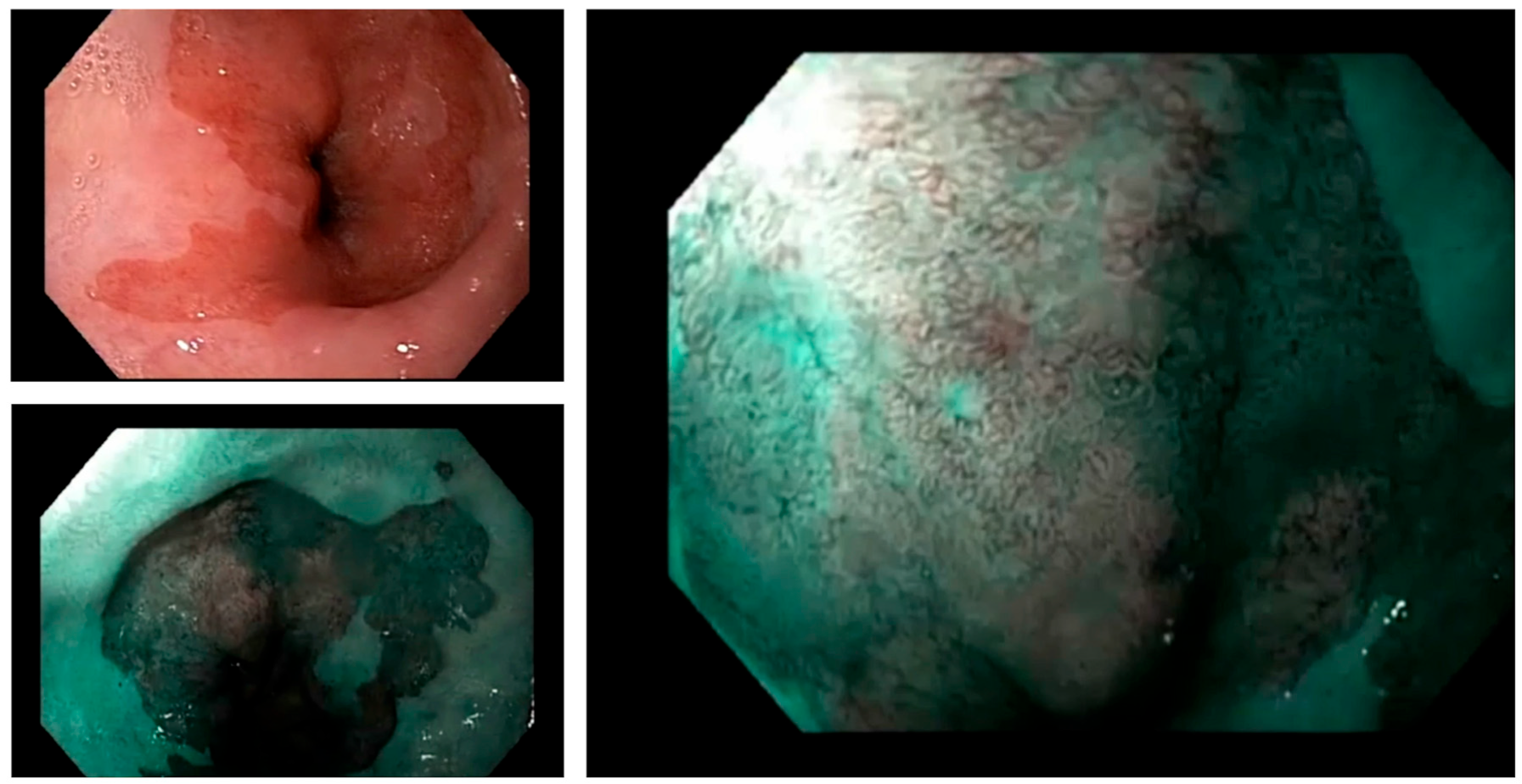
2. Diagnosis of Barrett’s Oesophagus
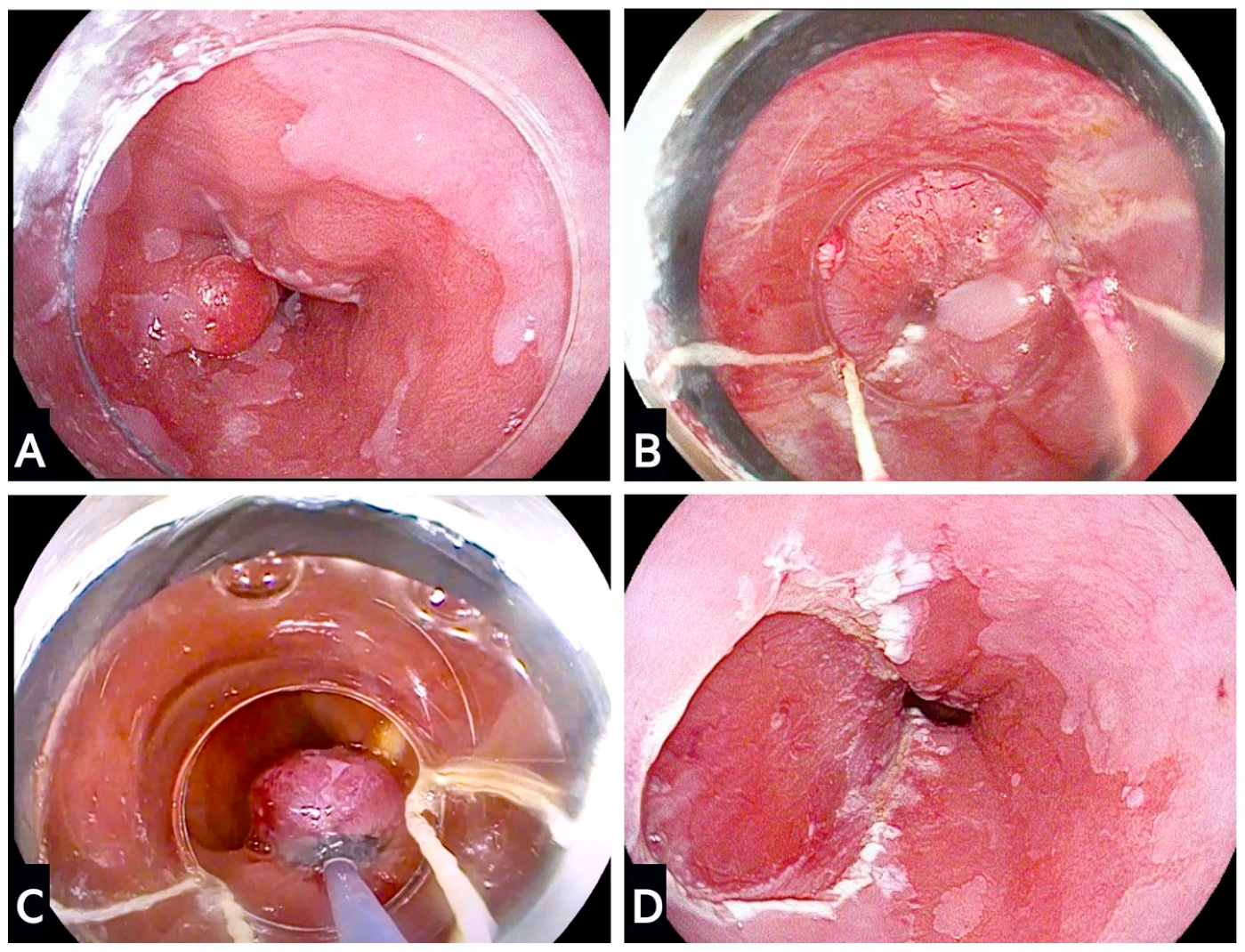
3. Endoscopic Resection Techniques for Dysplastic Barrett’s and Early Oesophageal Adenocarcinoma
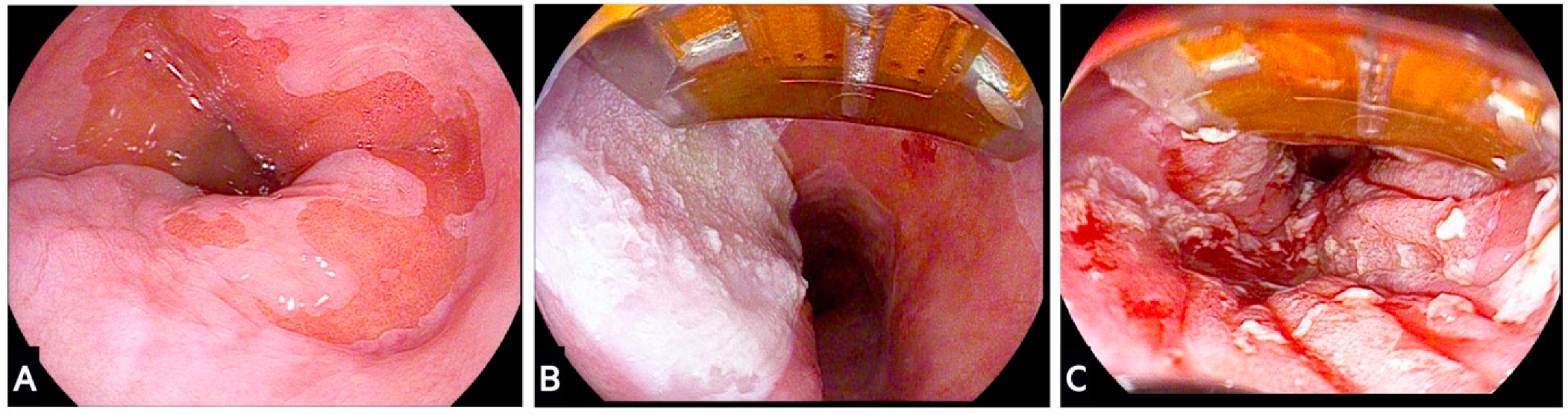
4. Ablation Treatments for Barrett’s Oesophagus
5. Failure of Therapy
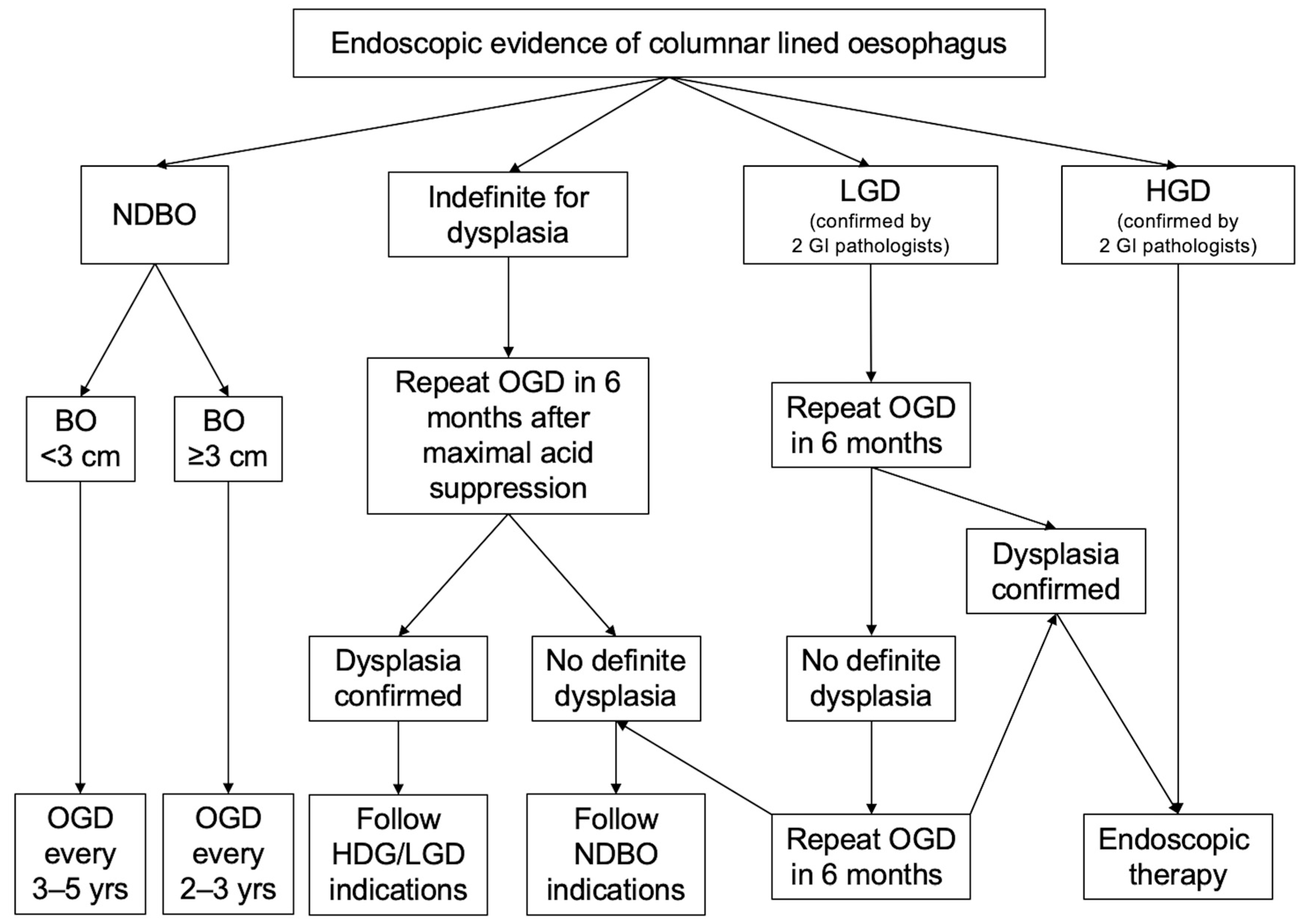
6. Follow-up after Ablation
7. Medical Management after the Endoscopic Treatment
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Gerson, L.B. Acg Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am. J. Gastroenterol. 2016, 111, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Burke, Z.D.; Tosh, D. Barrett’s Metaplasia as a Paradigm for Understanding the Development of Cancer. Curr. Opin. Genet. Dev. 2012, 22, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Winters, C., Jr.; Spurling, T.J.; Chobanian, S.J.; Curtis, D.J.; Esposito, R.L.; Hacker, J.F., 3rd; Johnson, D.A.; Cruess, D.F.; Cotelingam, J.D.; Gurney, M.S.; et al. Barrett’s Esophagus. A Prevalent, Occult Complication of Gastroesophageal Reflux Disease. Gastroenterology 1987, 92, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, L.H.; Telese, A.; Cirota, G.G.; Haidry, R.; Zagari, R.M.; Bazzoli, F.; Ford, A.C. Effect of Gastro-Esophageal Reflux Symptoms on the Risk of Barrett’s Esophagus: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2022, 37, 1507–1516. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the Epidemiology of Gastro-Oesophageal Reflux Disease: A Systematic Review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Ratnakumaran, R.; Yuan, Y.; Solaymani-Dodaran, M.; Bazzoli, F.; Ford, A.C. Global Prevalence of, and Risk Factors for, Gastro-Oesophageal Reflux Symptoms: A Meta-Analysis. Gut 2018, 67, 430–440. [Google Scholar] [CrossRef]
- Qumseya, B.J.; Bukannan, A.; Gendy, S.; Ahemd, Y.; Sultan, S.; Bain, P.; Gross, S.A.; Iyer, P.; Wani, S. Systematic Review and Meta-Analysis of Prevalence and risk factors for Barrett’s Esophagus. Gastrointest. Endosc. 2019, 90, 707–717.E1. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Cirota, G.G.; Zagari, R.M.; Ford, A.C. Global Prevalence of Barrett’s Oesophagus and Oesophageal Cancer in Individuals with Gastro-Oesophageal Reflux: A Systematic Review and Meta-Analysis. Gut 2021, 70, 456–463. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Hasanpour, A.H.; Eusebi, L.H.; Ford, A.C. Systematic Review with Meta-Analysis: Association of Helicobacter Pylori Infection with Gastro-Oesophageal Reflux and Its Complications. Aliment. Pharmacol. Ther. 2021, 54, 988–998. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Telese, A.; Cirota, G.G.; Haidry, R.; Zagari, R.M.; Bazzoli, F.; Ford, A.C. Systematic Review with Meta-Analysis: Risk Factors for Barrett’s Oesophagus in Individuals with Gastro-Oesophageal Reflux Symptoms. Aliment. Pharmacol. Ther. 2021, 53, 968–976. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; Di Pietro, M.; Ragunath, K.; Ang, Y.; Kang, J.Y.; Watson, P.; Trudgill, N.; Patel, P.; Kaye, P.V.; Sanders, S.; et al. British Society of Gastroenterology Guidelines on the Diagnosis and Management of Barrett’s Oesophagus. Gut 2014, 63, 7–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Manickam, P.; Amin, A.V.; Samala, N.; Schouten, L.J.; Iyer, P.G.; Desai, T.K. Incidence of Esophageal Adenocarcinoma in Barrett’s Esophagus with Low-Grade Dysplasia: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2014, 79, 897–909.E4. [Google Scholar] [CrossRef]
- Rastogi, A.; Puli, S.; El-Serag, H.B.; Bansal, A.; Wani, S.; Sharma, P. Incidence of Esophageal Adenocarcinoma in Patients with Barrett’s Esophagus and High-Grade Dysplasia: A Meta-Analysis. Gastrointest. Endosc. 2008, 67, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Anand, O.; Wani, S.; Sharma, P. When and How to Grade Barrett’s Columnar Metaplasia: The Prague System. Best. Pract. Res. Clin. Gastroenterol. 2008, 22, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J. Barrett Esophagus and Risk of Esophageal Cancer: A Clinical Review. JAMA 2013, 310, 627–636. [Google Scholar] [CrossRef]
- Sampliner, R.E. Updated Guidelines for the Diagnosis, Surveillance, and Therapy of Barrett’s Esophagus. Am. J. Gastroenterol. 2002, 97, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Maione, F.; Chini, A.; Maione, R.; Manigrasso, M.; Marello, A.; Cassese, G.; Gennarelli, N.; Milone, M.; De Palma, G.D. Endoscopic Diagnosis and Management of Barrett’s Esophagus with Low-Grade Dysplasia. Diagnostics 2022, 12, 1295. [Google Scholar] [CrossRef]
- Mcclave, S.A.; Boyce, H.W., Jr.; Gottfried, M.R. Early Diagnosis of Columnar-Lined Esophagus: A New Endoscopic Diagnostic Criterion. Gastrointest. Endosc. 1987, 33, 413–416. [Google Scholar] [CrossRef]
- Sharma, P.; Morales, T.G.; Sampliner, R.E. Short Segment Barrett’s Esophagus—The Need for Standardization of the Definition and of Endoscopic Criteria. Am. J. Gastroenterol. 1998, 93, 1033–1036. [Google Scholar] [CrossRef]
- Cotton, C.C.; Eluri, S.; Shaheen, N.J. Management of Dysplastic Barrett’s Esophagus and Early Esophageal Adenocarcinoma. Gastroenterol. Clin. N. Am. 2022, 51, 485–500. [Google Scholar] [CrossRef]
- Zagari, R.M.; Eusebi, L.H.; Galloro, G.; Rabitti, S.; Neri, M.; Pasquale, L.; Bazzoli, F. Attending Training Courses on Barrett’s Esophagus Improves Adherence to Guidelines: A Survey from the Italian Society of Digestive Endoscopy. Dig. Dis. Sci. 2021, 66, 2888–2896. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dent, J.; Armstrong, D.; Bergman, J.J.; Gossner, L.; Hoshihara, Y.; Jankowski, J.A.; Junghard, O.; Lundell, L.; Tytgat, G.N.; et al. The Development and Validation of an Endoscopic Grading System for Barrett’s Esophagus: The Prague C & M Criteria. Gastroenterology 2006, 131, 1392–1399. [Google Scholar] [CrossRef]
- Kim, J.B.; Shin, S.R.; Shin, W.G.; Choi, M.H.; Jang, H.J.; Kim, K.O.; Park, C.H.; Baek, I.H.; Baik, G.H.; Kim, K.H.; et al. Prevalence of Minimal Change Lesions in Patients with Non-Erosive Reflux Disease: A Case-Control Study. Digestion 2012, 85, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Dickman, R.; Levi, Z.; Vilkin, A.; Zvidi, I.; Niv, Y. Predictors of Specialized Intestinal Metaplasia in Patients with an Incidental Irregular Z Line. Eur. J. Gastroenterol. Hepatol. 2010, 22, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J.; Sharma, P.; Souza, R.F.; Inadomi, J.M.; Shaheen, N.J. American Gastroenterological Association Technical Review on the Management of Barrett’s Esophagus. Gastroenterology 2011, 140, E18–E52. [Google Scholar] [CrossRef]
- Canto, M.I.; Setrakian, S.; Willis, J.E.; Chak, A.; Petras, R.E.; Sivak, M.V. Methylene Blue Staining of Dysplastic and Nondysplastic Barrett’s Esophagus: An in Vivo and Ex Vivo Study. Endoscopy 2001, 33, 391–400. [Google Scholar] [CrossRef]
- Ngamruengphong, S.; Sharma, V.K.; Das, A. Diagnostic Yield of Methylene Blue Chromoendoscopy for Detecting Specialized Intestinal Metaplasia and Dysplasia in Barrett’s Esophagus: A Meta-Analysis. Gastrointest. Endosc. 2009, 69, 1021–1028. [Google Scholar] [CrossRef]
- Wong Kee Song, L.M.; Adler, D.G.; Chand, B.; Conway, J.D.; Croffie, J.M.; Disario, J.A.; Mishkin, D.S.; Shah, R.J.; Somogyi, L.; Tierney, W.M.; et al. Chromoendoscopy. Gastrointest. Endosc. 2007, 66, 639–649. [Google Scholar] [CrossRef]
- Guelrud, M.; Herrera, I.; Essenfeld, H.; Castro, J. Enhanced Magnification Endoscopy: A New Technique to Identify Specialized Intestinal Metaplasia in Barrett’s Esophagus. Gastrointest. Endosc. 2001, 53, 559–565. [Google Scholar] [CrossRef]
- Canto, M.I. Vital Staining and Barrett’s Esophagus. Gastrointest. Endosc. 1999, 49, S12–S16. [Google Scholar] [CrossRef]
- Horwhat, J.D.; Maydonovitch, C.L.; Ramos, F.; Colina, R.; Gaertner, E.; Lee, H.; Wong, R.K. A Randomized Comparison of Methylene Blue-Directed Biopsy Versus Conventional Four-Quadrant Biopsy for the Detection of Intestinal Metaplasia and Dysplasia in Patients with Long-Segment Barrett’s Esophagus. Am. J. Gastroenterol. 2008, 103, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Olliver, J.R.; Wild, C.P.; Sahay, P.; Dexter, S.; Hardie, L.J. Chromoendoscopy with Methylene Blue and Associated DNA Damage in Barrett’s Oesophagus. Lancet 2003, 362, 373–374. [Google Scholar] [CrossRef]
- Sharma, P.; Topalovski, M.; Mayo, M.S.; Weston, A.P. Methylene Blue Chromoendoscopy for Detection of Short-Segment Barrett’s Esophagus. Gastrointest. Endosc. 2001, 54, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Wolfsen, H.C.; Crook, J.E.; Krishna, M.; Achem, S.R.; Devault, K.R.; Bouras, E.P.; Loeb, D.S.; Stark, M.E.; Woodward, T.A.; Hemminger, L.L.; et al. Prospective, Controlled Tandem Endoscopy Study of Narrow Band Imaging for Dysplasia Detection in Barrett’s Esophagus. Gastroenterology 2008, 135, 24–31. [Google Scholar] [CrossRef]
- Hajelssedig, O.E.; Zorron Cheng Tao Pu, L.; Thompson, J.Y.; Lord, A.; El Sayed, I.; Meyer, C.; Shaukat Ali, F.; Abdulazeem, H.M.; Kheir, A.O.; Siepmann, T.; et al. Diagnostic Accuracy of Narrow-Band Imaging Endoscopy with Targeted Biopsies Compared with Standard Endoscopy with Random Biopsies in Patients with Barrett’s Esophagus: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2021, 36, 2659–2671. [Google Scholar] [CrossRef]
- Curvers, W.L.; Bohmer, C.J.; Mallant-Hent, R.C.; Naber, A.H.; Ponsioen, C.I.; Ragunath, K.; Singh, R.; Wallace, M.B.; Wolfsen, H.C.; Song, L.M.; et al. Mucosal Morphology in Barrett’s Esophagus: Interobserver Agreement and Role of Narrow Band Imaging. Endoscopy 2008, 40, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.R.; Wani, S.; Gyawali, C.P.; Komanduri, S.; Participants, C.B.S.E.C.C. Aga Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett’s Esophagus: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 2696–2706.E1. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Meining, A.R.; Coron, E.; Lightdale, C.J.; Wolfsen, H.C.; Bansal, A.; Bajbouj, M.; Galmiche, J.P.; Abrams, J.A.; Rastogi, A.; et al. Real-Time Increased Detection of Neoplastic Tissue in Barrett’s Esophagus with Probe-Based Confocal Laser Endomicroscopy: Final Results of an International Multicenter, Prospective, Randomized, Controlled Trial. Gastrointest. Endosc. 2011, 74, 465–472. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Ma, S.J.; Zhou, J.H.; Zhong, X.S.; Chen, Q. A Meta-Analysis of Confocal Laser Endomicroscopy for the Detection of Neoplasia in Patients with Barrett’s Esophagus. J. Gastroenterol. Hepatol. 2016, 31, 1102–1110. [Google Scholar] [CrossRef]
- Mcardle, J.E.; Lewin, K.J.; Randall, G.; Weinstein, W. Distribution of Dysplasias and Early Invasive Carcinoma in Barrett’s Esophagus. Hum. Pathol. 1992, 23, 479–482. [Google Scholar] [CrossRef]
- Abrams, J.A.; Kapel, R.C.; Lindberg, G.M.; Saboorian, M.H.; Genta, R.M.; Neugut, A.I.; Lightdale, C.J. Adherence to Biopsy Guidelines for Barrett’s Esophagus Surveillance in the Community Setting in the United States. Clin. Gastroenterol. Hepatol. 2009, 7, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Fléjou, J.F.; Geboes, K.; et al. The Vienna Classification of Gastrointestinal Epithelial Neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F. Gastrointestinal Epithelial Neoplasia: Vienna Revisited. Gut 2002, 51, 130–131. [Google Scholar] [CrossRef]
- Paull, A.; Trier, J.S.; Dalton, M.D.; Camp, R.C.; Loeb, P.; Goyal, R.K. The Histologic Spectrum of Barrett’s Esophagus. N. Engl. J. Med. 1976, 295, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J.; Souza, R.F. Barrett’s Esophagus. N. Engl. J. Med. 2014, 371, 836–845. [Google Scholar] [CrossRef]
- Levine, D.S.; Blount, P.L.; Rudolph, R.E.; Reid, B.J. Safety of a Systematic Endoscopic Biopsy Protocol in Patients with Barrett’s Esophagus. Am. J. Gastroenterol. 2000, 95, 1152–1157. [Google Scholar] [CrossRef]
- Di Pietro, M.; Fitzgerald, R.C. Revised British Society of Gastroenterology Recommendation on the Diagnosis and Management of Barrett’s Oesophagus with Low-Grade Dysplasia. Gut 2018, 67, 392–393. [Google Scholar] [CrossRef]
- Weusten, B.; Bisschops, R.; Coron, E.; Dinis-Ribeiro, M.; Dumonceau, J.M.; Esteban, J.M.; Hassan, C.; Pech, O.; Repici, A.; Bergman, J.; et al. Endoscopic Management of Barrett’s Esophagus: European Society of Gastrointestinal Endoscopy (Esge) Position Statement. Endoscopy 2017, 49, 191–198. [Google Scholar] [CrossRef]
- Sharma, P.; Shaheen, N.J.; Katzka, D.; Bergman, J. Aga Clinical Practice Update on Endoscopic Treatment of Barrett’s Esophagus with Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020, 158, 760–769. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Souza, R.F.; Yadlapati, R.H.; Sauer, B.G.; Wani, S. Diagnosis and Management of Barrett’s Esophagus: An Updated Acg Guideline. Am. J. Gastroenterol. 2022, 117, 559–587. [Google Scholar] [CrossRef]
- Fock, K.M.; Talley, N.; Goh, K.L.; Sugano, K.; Katelaris, P.; Holtmann, G.; Pandolfino, J.E.; Sharma, P.; Ang, T.L.; Hongo, M.; et al. Asia-Pacific Consensus on the Management of Gastro-Oesophageal Reflux Disease: An Update Focusing on Refractory Reflux Disease and Barrett’s Oesophagus. Gut 2016, 65, 1402–1415. [Google Scholar] [CrossRef]
- De Matos, M.V.; Da Ponte-Neto, A.M.; De Moura, D.T.H.; Maahs, E.D.; Chaves, D.M.; Baba, E.R.; Ide, E.; Sallum, R.; Bernardo, W.M.; De Moura, E.G.H. Treatment of High-Grade Dysplasia and Intramucosal Carcinoma Using Radiofrequency Ablation or Endoscopic Mucosal Resection + Radiofrequency Ablation: Meta-Analysis and Systematic Review. World J. Gastrointest. Endosc. 2019, 11, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Libânio, D.; Bastiaansen, B.A.J.; Bhandari, P.; Bisschops, R.; Bourke, M.J.; Esposito, G.; Lemmers, A.; Maselli, R.; Messmann, H.; et al. Endoscopic Submucosal Dissection for Superficial Gastrointestinal Lesions: European Society of Gastrointestinal Endoscopy (Esge) Guideline—Update 2022. Endoscopy 2022, 54, 591–622. [Google Scholar] [CrossRef] [PubMed]
- Vantanasiri, K.; Iyer, P.G. State-of-the-Art Management of Dysplastic Barrett’s Esophagus. Gastroenterol. Rep. 2022, 10, Goac068. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, K.B.; Spechler, S.J. The Risk of Lymph-Node Metastases in Patients with High-Grade Dysplasia or Intramucosal Carcinoma in Barrett’s Esophagus: A Systematic Review. Am. J. Gastroenterol. 2012, 107, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Konda, V.J.; Gonzalez Haba Ruiz, M.; Koons, A.; Hart, J.; Xiao, S.Y.; Siddiqui, U.D.; Ferguson, M.K.; Posner, M.; Patti, M.G.; Waxman, I. Complete Endoscopic Mucosal Resection Is Effective and Durable Treatment for Barrett’s-Associated Neoplasia. Clin. Gastroenterol. Hepatol. 2014, 12, 2002–2010.E2. [Google Scholar] [CrossRef]
- May, A.; Gossner, L.; Behrens, A.; Kohnen, R.; Vieth, M.; Stolte, M.; Ell, C. A Prospective Randomized Trial of Two Different Endoscopic Resection Techniques for Early Stage Cancer of the Esophagus. Gastrointest. Endosc. 2003, 58, 167–175. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Y.M.; Wang, T.T.; Gao, D.J.; Hu, B. Endotherapy versus Surgery for Early Neoplasia in Barrett’s Esophagus: A Meta-Analysis. Gastrointest. Endosc. 2014, 79, 233–241.E2. [Google Scholar] [CrossRef]
- Van Munster, S.N.; Verheij, E.P.D.; Nieuwenhuis, E.A.; Offerhaus, J.; Meijer, S.L.; Brosens, L.A.A.; Weusten, B.; Alkhalaf, A.; Schenk, E.B.E.; Schoon, E.J.; et al. Extending Treatment Criteria for Barrett’s Neoplasia: Results of a Nationwide Cohort of 138 Endoscopic Submucosal Dissection Procedures. Endoscopy 2022, 54, 531–541. [Google Scholar] [CrossRef]
- Terheggen, G.; Horn, E.M.; Vieth, M.; Gabbert, H.; Enderle, M.; Neugebauer, A.; Schumacher, B.; Neuhaus, H. A Randomised Trial of Endoscopic Submucosal Dissection versus Endoscopic Mucosal Resection for Early Barrett’s Neoplasia. Gut 2017, 66, 783–793. [Google Scholar] [CrossRef]
- Codipilly, D.C.; Dhaliwal, L.; Oberoi, M.; Gandhi, P.; Johnson, M.L.; Lansing, R.M.; Harmsen, W.S.; Wang, K.K.; Iyer, P.G. Comparative Outcomes of Cap Assisted Endoscopic Resection and Endoscopic Submucosal Dissection in Dysplastic Barrett’s Esophagus. Clin. Gastroenterol. Hepatol. 2022, 20, 65–73.E1. [Google Scholar] [CrossRef] [PubMed]
- Zeki, S.S.; Bergman, J.J.; Dunn, J.M. Endoscopic Management of Dysplasia and Early Oesophageal Cancer. Best. Pract. Res. Clin. Gastroenterol. 2018, 36–37, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Phoa, K.N.; Van Vilsteren, F.G.; Weusten, B.L.; Bisschops, R.; Schoon, E.J.; Ragunath, K.; Fullarton, G.; Di Pietro, M.; Ravi, N.; Visser, M.; et al. Radiofrequency Ablation vs. Endoscopic Surveillance for Patients with Barrett Esophagus and Low-Grade Dysplasia: A Randomized Clinical Trial. JAMA 2014, 311, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Pouw, R.E.; Klaver, E.; Phoa, K.N.; Van Vilsteren, F.G.; Weusten, B.L.; Bisschops, R.; Schoon, E.J.; Pech, O.; Manner, H.; Ragunath, K.; et al. Radiofrequency Ablation for Low-Grade Dysplasia in Barrett’s Esophagus: Long-Term Outcome of A Randomized Trial. Gastrointest. Endosc. 2020, 92, 569–574. [Google Scholar] [CrossRef]
- Brandt, L.J.; Kauvar, D.R. Laser-Induced Transient Regression of Barrett’s Epithelium. Gastrointest. Endosc. 1992, 38, 619–622. [Google Scholar] [CrossRef]
- Sampliner, R.E.; Hixson, L.J.; Fennerty, M.B.; Garewal, H.S. Regression of Barrett’s Esophagus by Laser Ablation in an Anacid Environment. Dig. Dis. Sci. 1993, 38, 365–368. [Google Scholar] [CrossRef]
- Berenson, M.M.; Johnson, T.D.; Markowitz, N.R.; Buchi, K.N.; Samowitz, W.S. Restoration of Squamous Mucosa after Ablation of Barrett’s Esophageal Epithelium. Gastroenterology 1993, 104, 1686–1691. [Google Scholar] [CrossRef]
- Overholt, B.; Panjehpour, M.; Tefftellar, E.; Rose, M. Photodynamic Therapy for Treatment of Early Adenocarcinoma in Barrett’s Esophagus. Gastrointest. Endosc. 1993, 39, 73–76. [Google Scholar] [CrossRef]
- Overholt, B.F.; Panjehpour, M. Photodynamic Therapy for Barrett’s Esophagus: Clinical Update. Am. J. Gastroenterol. 1996, 91, 1719–1723. [Google Scholar]
- Dumoulin, F.L.; Terjung, B.; Neubrand, M.; Scheurlen, C.; Fischer, H.P.; Sauerbruch, T. Treatment of Barrett’s Esophagus By Endoscopic Argon Plasma Coagulation. Endoscopy 1997, 29, 751–753. [Google Scholar] [CrossRef]
- Sampliner, R.E.; Fennerty, B.; Garewal, H.S. Reversal of Barrett’s Esophagus with Acid Suppression and Multipolar Electrocoagulation: Preliminary Results. Gastrointest. Endosc. 1996, 44, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mcquaid, K.; Dent, J.; Fennerty, M.B.; Sampliner, R.; Spechler, S.; Cameron, A.; Corley, D.; Falk, G.; Goldblum, J.; et al. A Critical Review of the Diagnosis and Management of Barrett’s Esophagus: The Aga Chicago Workshop. Gastroenterology 2004, 127, 310–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Sampliner, R.E.; Gastroenterology, P.P.C.O.T.A.C.O. Updated Guidelines 2008 for the Diagnosis, Surveillance and Therapy of Barrett’s Esophagus. Am. J. Gastroenterol. 2008, 103, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J.; Sharma, P.; Souza, R.F.; Inadomi, J.M.; Shaheen, N.J.; Association, A.G. American Gastroenterological Association Medical Position Statement on the Management of Barrett’s Esophagus. Gastroenterology 2011, 140, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Luigiano, C.; Iabichino, G.; Eusebi, L.H.; Arena, M.; Consolo, P.; Morace, C.; Opocher, E.; Mangiavillano, B. Outcomes of Radiofrequency Ablation for Dysplastic Barrett’s Esophagus: A Comprehensive Review. Gastroenterol. Res. Pract. 2016, 2016, 4249510. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Sharma, P.; Overholt, B.F.; Wolfsen, H.C.; Sampliner, R.E.; Wang, K.K.; Galanko, J.A.; Bronner, M.P.; Goldblum, J.R.; Bennett, A.E.; et al. Radiofrequency Ablation in Barrett’s Esophagus with Dysplasia. N. Engl. J. Med. 2009, 360, 2277–2288. [Google Scholar] [CrossRef]
- Haidry, R.J.; Dunn, J.M.; Butt, M.A.; Burnell, M.G.; Gupta, A.; Green, S.; Miah, H.; Smart, H.L.; Bhandari, P.; Smith, L.A.; et al. Radiofrequency Ablation and Endoscopic Mucosal Resection for Dysplastic Barrett’s Esophagus and Early Esophageal Adenocarcinoma: Outcomes of the Uk National Halo Rfa Registry. Gastroenterology 2013, 145, 87–95. [Google Scholar] [CrossRef]
- Haidry, R.J.; Butt, M.A.; Dunn, J.M.; Gupta, A.; Lipman, G.; Smart, H.L.; Bhandari, P.; Smith, L.; Willert, R.; Fullarton, G.; et al. Improvement over Time in Outcomes for Patients Undergoing Endoscopic Therapy for Barrett’s Oesophagus-Related Neoplasia: 6-Year Experience from the First 500 Patients Treated in the Uk Patient Registry. Gut 2015, 64, 1192–1199. [Google Scholar] [CrossRef]
- Barret, M.; Pioche, M.; Terris, B.; Ponchon, T.; Cholet, F.; Zerbib, F.; Chabrun, E.; Le Rhun, M.; Coron, E.; Giovannini, M.; et al. Endoscopic Radiofrequency Ablation or Surveillance in Patients with Barrett’s Oesophagus with Confirmed Low-Grade Dysplasia: A Multicentre Randomised Trial. Gut 2021, 70, 1014–1022. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, B.; Yang, S.; Li, W.; Li, P. Efficacy and Safety of Radiofrequency Ablation vs. Endoscopic Surveillance for Barrett’s Esophagus with Low-Grade Dysplasia: Meta-Analysis of Randomized Controlled Trials. Front. Oncol. 2022, 12, 801940. [Google Scholar] [CrossRef]
- Benjamin, S.B.; Maher, K.A.; Cattau, E.L., Jr.; Collen, M.J.; Fleischer, D.E.; Lewis, J.H.; Ciarleglio, C.A.; Earll, J.M.; Schaffer, S.; Mirkin, K.; et al. Double-Blind Controlled Trial of the Garren-Edwards Gastric Bubble: An Adjunctive Treatment for Exogenous Obesity. Gastroenterology 1988, 95, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Dubrouskaya, K.; Hagenstein, L.; Ramai, D.; Adler, D.G. Clinical Adverse Events and Device Failures for the Barrx™ Radiofrequency Ablation Catheter System: A Maude Database Analysis. Ann. Gastroenterol. 2022, 35, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Manner, H.; May, A.; Miehlke, S.; Dertinger, S.; Wigginghaus, B.; Schimming, W.; Krämer, W.; Niemann, G.; Stolte, M.; Ell, C. Ablation of Nonneoplastic Barrett’s Mucosa Using Argon Plasma Coagulation with Concomitant Esomeprazole Therapy (Apbanex): A Prospective Multicenter Evaluation. Am. J. Gastroenterol. 2006, 101, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Peerally, M.F.; Bhandari, P.; Ragunath, K.; Barr, H.; Stokes, C.; Haidry, R.; Lovat, L.; Smart, H.; Harrison, R.; Smith, K.; et al. Radiofrequency Ablation Compared with Argon Plasma Coagulation after Endoscopic Resection of High-Grade Dysplasia or Stage T1 Adenocarcinoma in Barrett’s Esophagus: A Randomized Pilot Study (Bride). Gastrointest. Endosc. 2019, 89, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Manner, H.; Neugebauer, A.; Scharpf, M.; Braun, K.; May, A.; Ell, C.; Fend, F.; Enderle, M.D. The Tissue Effect of Argon-Plasma Coagulation with Prior Submucosal Injection (Hybrid-Apc) Versus Standard Apc: A Randomized Ex-Vivo Study. United Eur. Gastroenterol. J. 2014, 2, 383–390. [Google Scholar] [CrossRef]
- Manner, H.; May, A.; Kouti, I.; Pech, O.; Vieth, M.; Ell, C. Efficacy and Safety of Hybrid-Apc for the Ablation of Barrett’s Esophagus. Surg. Endosc. 2016, 30, 1364–1370. [Google Scholar] [CrossRef]
- Knabe, M.; Beyna, T.; Rösch, T.; Bergman, J.; Manner, H.; May, A.; Schachschal, G.; Neuhaus, H.; Kandler, J.; Weusten, B.; et al. Hybrid Apc in Combination with Resection for the Endoscopic Treatment of Neoplastic Barrett’s Esophagus: A Prospective, Multicenter Study. Am. J. Gastroenterol. 2022, 117, 110–119. [Google Scholar] [CrossRef]
- Shah, S.N.; Chehade, N.E.H.; Tavangar, A.; Choi, A.; Monachese, M.; Chang, K.J.; Samarasena, J.B. Hybrid Argon Plasma Coagulation in Barrett’s Esophagus: A Systematic Review and Meta-Analysis. Clin. Endosc. 2023, 56, 38–49. [Google Scholar] [CrossRef]
- Trindade, A.J.; Wee, D.; Wander, P.; Stewart, M.; Lee, C.; Benias, P.C.; Mckinley, M.J. Successful Treatment of Refractory Barrett’s Neoplasia with Hybrid Argon Plasma Coagulation: A Case Series. Endoscopy 2020, 52, 812–813. [Google Scholar] [CrossRef]
- Künzli, H.T.; Schölvinck, D.W.; Meijer, S.L.; Seldenrijk, K.A.; Bergman, J.G.H.M.; Weusten, B.L.A.M. Efficacy of the Cryoballoon Focal Ablation System for the Eradication of Dysplastic Barrett’s Esophagus Islands. Endoscopy 2017, 49, 169–175. [Google Scholar] [CrossRef]
- Canto, M.I.; Trindade, A.J.; Abrams, J.; Rosenblum, M.; Dumot, J.; Chak, A.; Iyer, P.; Diehl, D.; Khara, H.S.; Corbett, F.S.; et al. Multifocal Cryoballoon Ablation for Eradication of Barrett’s Esophagus-Related Neoplasia: A Prospective Multicenter Clinical Trial. Am. J. Gastroenterol. 2020, 115, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Westerveld, D.R.; Nguyen, K.; Banerjee, D.; Jacobs, C.; Kadle, N.; Draganov, P.V.; Yang, D. Safety and Effectiveness of Balloon Cryoablation for Treatment of Barrett’s Associated Neoplasia: Systematic Review and Meta-Analysis. Endosc. Int. Open 2020, 8, E172–E178. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Alshelleh, M.; Scott, J.; Dhaliwal, L.; Codipilly, D.C.; Dierkhising, R.; Leggett, C.L.; Wang, K.K.; Otaki, F.A.; Trindade, A.J.; et al. Comparative Outcomes of Radiofrequency Ablation and Cryoballoon Ablation in Dysplastic Barrett’s Esophagus: A Propensity Score-Matched Cohort Study. Gastrointest. Endosc. 2022, 95, 422–431.E2. [Google Scholar] [CrossRef] [PubMed]
- Frederiks, C.N.; Overwater, A.; Alvarez Herrero, L.; Alkhalaf, A.; Schenk, E.; Repici, A.; Bergman, J.J.G.H.; Pouw, R.E.; Bisschops, R.; Haidry, R.J.; et al. Comparison of Focal Cryoballoon Ablation with 10- and 8-Second Doses for Treatment of Barrett’s Esophagus-Related Neoplasia: Results from A Prospective European Multicenter Study (with Video). Gastrointest. Endosc. 2022, 96, 743–751.E4. [Google Scholar] [CrossRef] [PubMed]
- Overwater, A.; Van Munster, S.N.; Nagengast, W.B.; Pouw, R.E.; Bergman, J.J.G.H.; Schoon, E.J.; Weusten, B.L.A.M. Novel Cryoballoon 180° Ablation System for Treatment of Barrett’s Esophagus-Related Neoplasia: A First-in-Human Study. Endoscopy 2022, 54, 64–70. [Google Scholar] [CrossRef]
- Van Munster, S.N.; Overwater, A.; Raicu, M.G.M.; Seldenrijk, K.C.A.; Nagengast, W.B.; Schoon, E.J.; Bergman, J.J.G.H.; Weusten, B.L.A.M. A Novel Cryoballoon Ablation System for Eradication of Dysplastic Barrett’s Esophagus: A First-In-Human Feasibility Study. Endoscopy 2020, 52, 193–201. [Google Scholar] [CrossRef]
- Mittal, C.; Muthusamy, V.R.; Simon, V.C.; Brauer, B.C.; Mullady, D.K.; Hollander, T.; Sloan, I.; Kushnir, V.; Early, D.; Rastogi, A.; et al. Threshold Evaluation for Optimal Number of Endoscopic Treatment Sessions to Achieve Complete Eradication of Barrett’s Metaplasia. Endoscopy 2022, 54, 927–933. [Google Scholar] [CrossRef]
- Van Munster, S.N.; Frederiks, C.N.; Nieuwenhuis, E.A.; Alvarez Herrero, L.; Bogte, A.; Alkhalaf, A.; Schenk, B.E.; Schoon, E.J.; Curvers, W.L.; Koch, A.D.; et al. Incidence and Outcomes of Poor Healing and Poor Squamous Regeneration after Radiofrequency Ablation Therapy for Early Barrett’s Neoplasia. Endoscopy 2022, 54, 229–240. [Google Scholar] [CrossRef]
- Van Munster, S.N.; Nieuwenhuis, E.; Bisschops, R.; Willekens, H.; Weusten, B.L.A.M.; Herrero, L.A.; Bogte, A.; Alkhalaf, A.; Schenk, E.B.E.; Schoon, E.J.; et al. Development and External Validation of A Model to Predict Complex Treatment after Radiofrequency Ablation for Barrett’s Esophagus with Early Neoplasia. Clin. Gastroenterol. Hepatol. 2022, 20, 2495–2504.E5. [Google Scholar] [CrossRef]
- Wolfson, P.; Ho, K.M.A.; Wilson, A.; Mcbain, H.; Hogan, A.; Lipman, G.; Dunn, J.; Haidry, R.; Novelli, M.; Olivo, A.; et al. Endoscopic Eradication Therapy for Barrett’s Esophagus-Related Neoplasia: A Final 10-Year Report from the Uk National Halo Radiofrequency Ablation Registry. Gastrointest. Endosc. 2022, 96, 223–233. [Google Scholar] [CrossRef]
- Menon, S.; Norman, R.; Mannath, J.; Iyer, P.G.; Ragunath, K. Comparative Cost-Effectiveness of Three Post-Radiofrequency Ablation Surveillance Intervals for Barrett’s Esophagus. Endosc. Int. Open 2022, 10, E1053–E1064. [Google Scholar] [CrossRef] [PubMed]
- Van Munster, S.N.; Nieuwenhuis, E.; Bisschops, R.; Willekens, H.; Weusten, B.L.A.M.; Herrero, L.A.; Bogte, A.; Alkhalaf, A.; Schenk, E.B.E.; Schoon, E.J.; et al. Dysplastic Recurrence after Successful Treatment for Early Barrett’s Neoplasia: Development and Validation of a Prediction Model. Gastroenterology 2022, 163, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Verheij, E.P.D.; Van Munster, S.N.; Nieuwenhuis, E.A.; Cotton, C.C.; Weusten, B.L.A.M.; Alvarez Herrero, L.; Alkhalaf, A.; Schenk, B.E.; Schoon, E.J.; Curvers, W.; et al. All-Cause Mortality after Successful Endoscopic Eradication Therapy for Barrett’s Related Neoplasia in a Nationwide Cohort of 1154 Patients. Endoscopy 2022, 54, S93. [Google Scholar]
- Phoa, K.N.; Pouw, R.E.; Bisschops, R.; Pech, O.; Ragunath, K.; Weusten, B.L.; Schumacher, B.; Rembacken, B.; Meining, A.; Messmann, H.; et al. Multimodality Endoscopic Eradication for Neoplastic Barrett Oesophagus: Results of an European Multicentre Study (Euro-Ii). Gut 2016, 65, 555–562. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Li, Y.; Lu, G.; Lu, X.; Guo, D.; Wang, W.; Liu, C.; Xiao, Y.; Han, N.; et al. Efficacy and Safety of Steroid in the Prevention of Esophageal Stricture after Endoscopic Submucosal Dissection: A Network Meta-Analysis. J. Gastroenterol. Hepatol. 2019, 34, 985–995. [Google Scholar] [CrossRef]
- Bartel, M.J.; Mousa, O.Y.; Brahmbhatt, B.; Coffman, D.L.; Patel, K.; Repici, A.; Tokar, J.L.; Wolfsen, H.C.; Wallace, M.B. Impact of Topical Budesonide on Prevention of Esophageal Stricture after Mucosal Resection. Gastrointest. Endosc. 2021, 93, 1276–1282. [Google Scholar] [CrossRef]
| Scientific Society | Authors, Year [Ref] | ND-BO | LGD-BO | HGD-BO | |
|---|---|---|---|---|---|
| European | BSG | Fitzgerald et al., 2014 [11] Di Pietro et al., 2018 [47] |
|
|
|
| ESGE | Weusten et al., 2017 [48] |
|
|
| |
| American | AGA | Sharma et al., 2020 [49] |
|
|
|
| ACG | Shaheen et al., 2022 [50] |
|
|
| |
| Asian Pacific | Asia-Pacific consensus | Fock et al., 2016 [51] |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eusebi, L.H.; Telese, A.; Castellana, C.; Engin, R.M.; Norton, B.; Papaefthymiou, A.; Zagari, R.M.; Haidry, R. Endoscopic Management of Dysplastic Barrett’s Oesophagus and Early Oesophageal Adenocarcinoma. Cancers 2023, 15, 4776. https://doi.org/10.3390/cancers15194776
Eusebi LH, Telese A, Castellana C, Engin RM, Norton B, Papaefthymiou A, Zagari RM, Haidry R. Endoscopic Management of Dysplastic Barrett’s Oesophagus and Early Oesophageal Adenocarcinoma. Cancers. 2023; 15(19):4776. https://doi.org/10.3390/cancers15194776
Chicago/Turabian StyleEusebi, Leonardo Henry, Andrea Telese, Chiara Castellana, Rengin Melis Engin, Benjamin Norton, Apostolis Papaefthymiou, Rocco Maurizio Zagari, and Rehan Haidry. 2023. "Endoscopic Management of Dysplastic Barrett’s Oesophagus and Early Oesophageal Adenocarcinoma" Cancers 15, no. 19: 4776. https://doi.org/10.3390/cancers15194776
APA StyleEusebi, L. H., Telese, A., Castellana, C., Engin, R. M., Norton, B., Papaefthymiou, A., Zagari, R. M., & Haidry, R. (2023). Endoscopic Management of Dysplastic Barrett’s Oesophagus and Early Oesophageal Adenocarcinoma. Cancers, 15(19), 4776. https://doi.org/10.3390/cancers15194776






