Simple Summary
In this research study, the authors investigated the impact of specific genetic mutations on the survival of lung cancer patients with brain metastases who underwent surgical resection. These mutations, known as anaplastic lymphoma kinase (ALK)-rearranged and epidermal growth factor receptor (EGFR)-amplified mutations, have shown potential for targeted treatments. The study analyzed data from patients who received surgical treatment at Emory University Hospital between 2012 and 2022. Results showed that the overall survival and progression-free survival rates in this group were better than those seen in earlier studies. The study suggests that as more targeted therapies become available, the survival rates for lung cancer patients with brain metastases may continue to improve. The findings emphasize the importance of individualized treatments based on genetic mutations.
Abstract
In the context of the post-genomic era, where targeted oncological therapies like monoclonal antibodies (mAbs) and tyrosine-kinase inhibitors (TKIs) are gaining prominence, this study investigates whether these therapies can enhance survival for lung carcinoma patients with specific genetic mutations—EGFR-amplified and ALK-rearranged mutations. Prior to this study, no research series had explored how these mutations influence patient survival in cases of surgical lung brain metastases (BMs). Through a multi-site retrospective analysis, the study examined patients who underwent surgical resection for BM arising from primary lung cancer at Emory University Hospital from January 2012 to May 2022. The mutational statuses were determined from brain tissue biopsies, and survival analyses were conducted. Results from 95 patients (average age: 65.8 ± 10.6) showed that while 6.3% had anaplastic lymphoma kinase (ALK)-rearranged mutations and 20.0% had epidermal growth factor receptor (EGFR)-amplified mutations—with 9.5% receiving second-line therapies—these mutations did not significantly correlate with overall survival. Although the sample size of patients receiving targeted therapies was limited, the study highlighted improved overall survival and progression-free survival rates compared to earlier trials, suggesting advancements in systemic lung metastasis treatment. The study suggests that as more targeted therapies emerge, the prospects for increased overall survival and progression-free survival in lung brain metastasis patients will likely improve.
1. Introduction
Multimodal treatment of metastatic cancer has changed in the genomic era. Targeted therapies have improved systemic disease control in subsets of metastatic disease [1,2], most notably for breast cancer, lung cancer, and melanoma [3,4,5]. As systemic disease is better controlled and overall survival (OS) of patients with metastatic disease improves, the incidence of brain metastases (BMs) has also risen [5,6,7].
Meanwhile, significant progress has been made in function-sparing microsurgical techniques for the resection of solitary, dominant, and multiple symptomatic BMs [8,9]. Combining microsurgery with radiation therapy in the form of whole brain radiotherapy (WBRT), focused radiation stereotactic radiosurgery (SRS), and fractionated SRS has resulted in local control rates of 80%, 82%, and 84%, respectively [10,11,12,13,14]. New systemic immune-modulating therapies or tyrosine kinase inhibitors (TKIs) have demonstrated clinical evidence of at least partial blood–brain barrier (BBB) penetration and efficacy, unlike prior chemotherapeutic agents [15]. New guidelines from the National Comprehensive Cancer Network (NCCN) now mention the emerging role of systemic therapy in brain disease [16]. Multiple ongoing clinical trials in lung, breast, and melanoma are underway to better elucidate the role of systemic therapy in the multimodal algorithm for brain metastases treatment [17,18,19,20,21,22,23,24,25,26,27,28,29].
The incidence of anaplastic lymphoma kinase (ALK)-rearranged mutations and epidermal growth factor receptor (EGFR)-rearranged mutations in lung cancer patients is around 5% and 15%, respectively. Approximately 20 to 40% of patients with metastatic lung cancer will develop BMs [30,31,32,33]. Among these, 25% of lung cancer patients develop ALK-rearranged mutations [34] and 33% of patients develop activating mutations [35]. Both ALK and EGFR mutations strongly correlate with favorable systemic therapeutic responses to TKIs and increased OS [36,37]. The incidence of BMs in EGFR and ALK patients is also significantly higher than other mutational types [17,38], with approximately 30% of ALK and 40% of EGFR patients having BMs [39,40]. Notably, retrospective, single-institution analysis has suggested that systemic mutational status in patients with melanoma BM correlates to improved local control (LC) and OS in patients undergoing craniotomy for resection [41]. This is significant given that melanoma was one of the first systemic malignancies to demonstrate the cerebral penetrance of systemic therapy and role of molecular drivers (BRAF) in prognostication. The role of mutational status has not been well described in patients with lung BMs.
Therefore, this study investigates the role of mutational status in lung cancer BM patients undergoing surgical resection with respect to LC and OS. With our study, we aim to (1) review the relationship between the extent of resection (EOR) and OS with lung cancer BM after undergoing surgical resection; (2) evaluate the relationship among ALK and EGFR mutations with the rate of gross total resection (GTR), local control, and OS; and (3) discuss the targeted therapies administered for mutational lung cancer management and their potential role in local control and OS.
2. Materials and Methods
2.1. Study Design
This surgical series was a multi-site, retrospective study of all cerebral lung cancer patients undergoing resection at Emory University Healthcare hospital between January 2012 and May 2022, including two tertiary, academic referral hospitals, and two mid-sized, community hospitals. We collected data from the CNS Tumor Outcomes Registry at Emory (CTORE), a prospectively managed patient outcomes database for central nervous system (CNS) tumors treated at participating sites. Our study was approved by the institutional review board at Emory University and had obtained an informed consent waiver.
2.2. Patient Selection and Data Collection
Patients undergoing surgical resection for BM with a diagnosis of primary lung cancer within the study period were included. Eligible patients were identified from the CTORE database and data were collected from primary review of the electronic medical record, including clinical, pathology/genomic, and imaging data. These were reviewed for demographic data, Karnofsky Performance Scale (KPS), surgical procedure details and frequency including EOR, complications (i.e., motor weakness or speech trouble after surgery, wound infection, CSF leak, and/or seizures), local recurrence, pathology (i.e., primary tumor and mutational statuses), extent of systemic disease (i.e., disease in bone, adrenal glands, liver or other organs) and control of disease, prior therapy (i.e., chemotherapy, radiation, and/or immunotherapy), adjuvant/postoperative therapy, and discharge/readmission information. Imaging data were used for BM characterization, including location, number of metastatic lesions, and volume of dominant lesion. Data regarding preoperative as well as postoperative targeted therapy and radiation were also collected. The type of radiation administered was characterized as either standard-fractionated, WBRT, or SRS. Genomic data were obtained from pathology reports and Caris molecular profiling (Caris Life Sciences, Dallas, TX, USA). Patient driver mutational statuses were categorized as either EGFR-amplified, ALK-rearranged mutations present in the biopsied brain tissue, or neither.
2.3. Statistical Analysis
Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Descriptive statistics were performed for all demographic data. The primary end points of the study were OS and local recurrence free survival (LRFS). OS was defined as the time from the date of surgery to the date of death or the date of last follow-up. LRFS was defined as the time from the date of surgery to the date of local recurrence or the date of last follow-up. Univariate and multivariable analyses were performed by Cox proportional hazard models to assess factors associated with OS and LRFS. Multivariable models were selected using the backward variable selection approach with an alpha of removal of 0.2. To account for the small sample size and low event rates, Firth’s penalized likelihood bias-reduction approach was used. The Kaplan–Meier method was used to generate OS and LRFS curves and survival curves were compared between different groups using the log-rank test. Statistical significance was set at an alpha value of 0.05.
3. Results
3.1. Patient Population
During the study period between January 2012 and May 2022, 95 patients (Table 1) underwent surgical resection of cerebral lung cancer with accessible molecular testing of the biopsied brain tissue and were available to review in our electronic medical record. This patient cohort had a mean age of 63.2 ± 10.4 years with 54 (56.8%) female patients and 41 (43.2%) male patients. Median preoperative KPS for the patient cohort was 80 (Table 1). A total of 43 (45.3%) patients had local recurrence of the resection site post-surgery. A total of 7 (7.4%) patients had ALK-rearranged mutations and 19 (20.0%) patients had EGFR-amplified mutations. The mean number of metastatic brain lesions for patients was 2.64 ± 2.97 prior to surgery and the median number of metastatic lesions that developed post-surgery was 1 (0–3). The median time from lung cancer diagnosis to BM was 244 (12–932) days. A total of 72 (79.1%) patients had GTR of the BM during their first craniotomy. Six patients required reoperation for BM resection and, of those, 83.3% of patients had GTR. A total of 54 (58.7%) patients were readmitted after discharge with presentations that included hypotension, tachycardia, seizures, altered mental status, speech and motor impairments, and disease worsening (Table 2).

Table 1.
Clinical characteristics of cerebral lung cancer patients with pertinent demographic and surgical variables.

Table 2.
Complications of cerebral lung cancer patients with pertinent outcome variables.
In our study, three (42.9%) patients with ALK mutations (Table 1) received targeted therapy in the form of TKIs and had a mean OS of 0.97 years. Of these patients, three (42.9%) had adjuvant targeted therapies in the form of Crizotinib (TKI), Alectinib (TKI), and Lorlatinib (TKI). Two (28.6%) ALK patients had targeted TKI therapy prior to surgical resection in the form of Crizotinib with a mean OS of 2.93 years prior to requiring surgical intervention.
Furthermore, eight (42.1%) patients with EGFR mutations (Table 1) received targeted therapy in the form of TKIs and mAbs and had a mean OS of 3.06 years after surgical resection of BMs. Of these patients, eight (88.9%) had adjuvant targeted therapies in the form of Atezolizumab (mAb), Osimertinib (TKI), Pembrolizumab (mAb), and Erlotinib (TKI). Six (31.6%) patients had targeted therapy prior to surgical resection in the form of Gefitinib (TKI), Erlotinib, and Osimertinib with a mean OS of 4.39 years prior to requiring surgical intervention.
In addition, 30 (31.6%) patients without records of ALK or EGFR mutations in their biopsied brain tissue received immunotherapy (Table 1) and had a mean OS of 2.50 years. Of these patients, 21 (70.0%) had adjuvant targeted therapies in the form of Atezolizumab, Ipilimumab (mAb), Pembrolizumab, Nivolumab (mAb), Ramucirumab (mAb), and Erlotinib. Ten (33.3%) patients had immunotherapy prior to surgical resection in the form of Durvalumab, Nivolumab, Atezolizumab, Pembrolizumab, Crizotinib, and Denosumab (mAb) with a mean OS of 1.57 years prior to requiring surgical intervention.
The median survival from the start of treatment administration was 3.55 (95% CI: 1.70-NA) years (Figure 1). When stratified, the median survival was 6.01 (95% CI: 2.75-NA) years for patients who had immunotherapy for their NSCLC and an ALK or EGFR mutation that required targeted therapy in the form of TKIs and mAbs. On the other hand, the median survival from the start of treatment administration was 4.08 (95% CI: 1.59-NA) years for patients who had immunotherapy for their NSCLC but not ALK or EGFR mutation-related targeted therapy, and 1.70 (95% CI: 1.30-NA) years for patients who did not have immunotherapy or ALK or EGFR mutation-related targeted therapy (Figure 2). These levels were not statistically significant from each other, however.
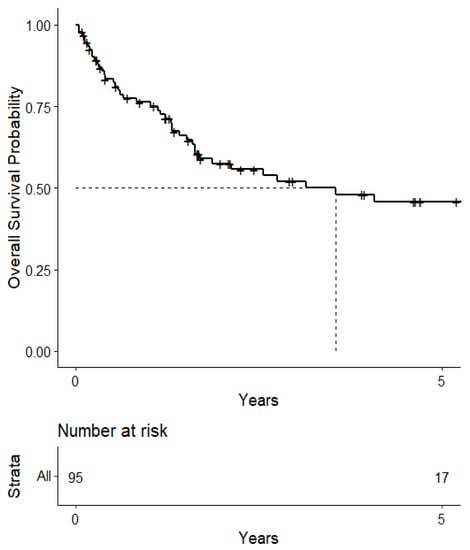
Figure 1.
Kaplan–Meier curve of survival from 1st treatment administration.
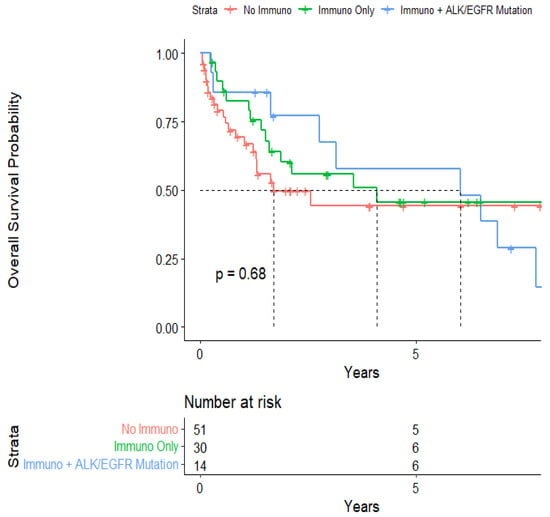
Figure 2.
Kaplan–Meier curve of survival from 1st treatment administration stratified by immunotherapy and mutational status.
3.2. Univariate Association with Overall Survival and Local Recurrence-Free Survival
Univariate analysis of our surgical cohort found adjuvant radiation therapy (HR: 0.26; 95% CI: 0.12–0.52; p-value = <0.001), adjuvant chemotherapy (HR: 0.54; 95% CI: 0.30–0.98; p-value = 0.040), new brain metastases within a year of surgery (HR: 3.37; 95% CI: 1.66–6.85; p-value = <0.001), systemic progressive disease (HR: 3.82; 95% CI: 1.84–7.92; p-value = <0.001), and type of radiation therapy (SRS vs. standard fractionated) (HR: 0.44; 95% CI: 0.20–0.94; p-value = 0.030) as prognostic factors for overall survival (Table 3). ALK-rearranged (HR: 2.26; 95% CI: 0.87–5.84; p-value = 0.085) and EGFR-amplified (HR: 1.63; 95% CI: 0.76–3.49; p-value = 0.692) mutations were not significantly associated with overall survival. Furthermore, there was no statistical difference in OS between patients with GTR versus near-total resection.

Table 3.
Univariate association with predictors of overall survival.
In addition, univariate analysis of our surgical cohort found number of new brain metastases within a year of surgery (HR: 1.07; 95% CI: 1.01–1.14; p-value = 0.030) and volume of tumor resected in the first craniotomy (HR: 1.02; 95% CI: 1.00–1.03; p-value = 0.024) as prognostic factors for LRFS (Table 4). EGFR/ALK mutations and EOR were not significant prognostic factors for LRFS.

Table 4.
Univariate association with predictors of local recurrence-free survival.
The overall median survival was 2.12 years (Figure 3). The median survival for patients with ALK-rearranged mutations was 1.29 years (Figure 4), and the median survival for patients with EGFR-amplified mutations was 5.19 years (Figure 5). Additionally, the median survival for patients with GTR was 2.12 years (Figure 6). Furthermore, the overall LRFS was 1.89 years (Figure 7). Median LRFS could not be calculated for ALK patients due to limited sample size (Figure 8). The median LRFS for EGFR patients was 4.35 years (Figure 9). The median LRFS for patients with GTR was 1.76 years (Figure 10).
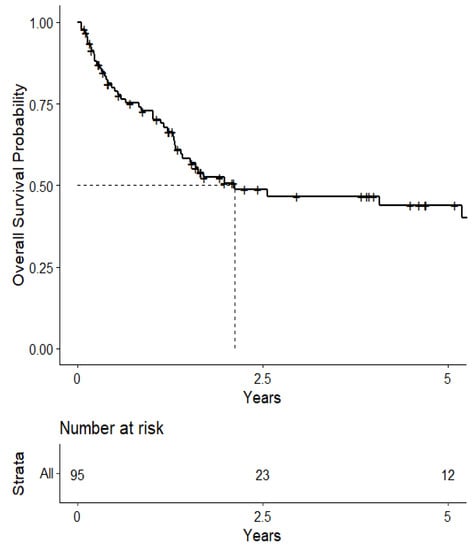
Figure 3.
Kaplan–Meier curve of overall survival.
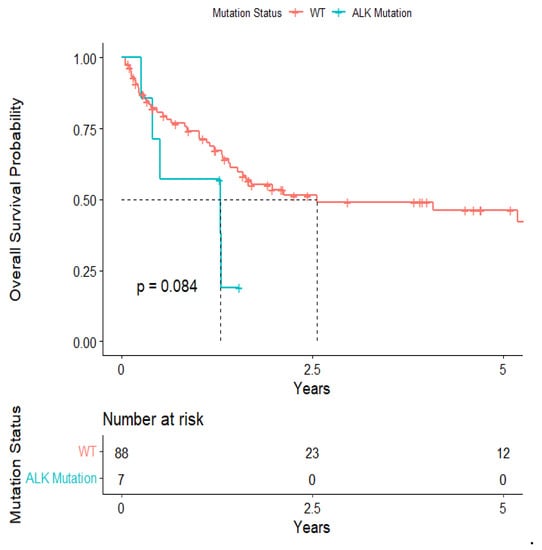
Figure 4.
Kaplan–Meier survival curve of ALK-rearranged mutational status predicting overall survival.
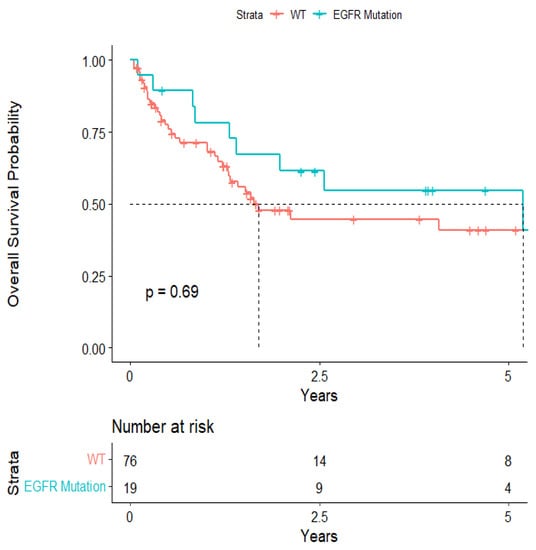
Figure 5.
Kaplan–Meier survival curve of EGFR-amplified mutational status predicting overall survival.
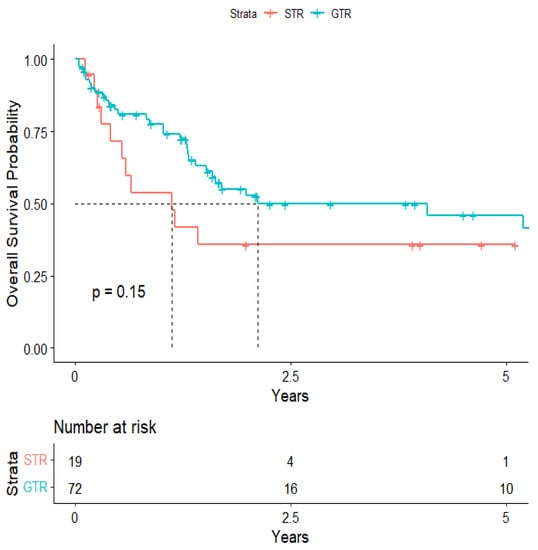
Figure 6.
Kaplan–Meier curve of extent of resection from 1st craniotomy predicting overall survival.
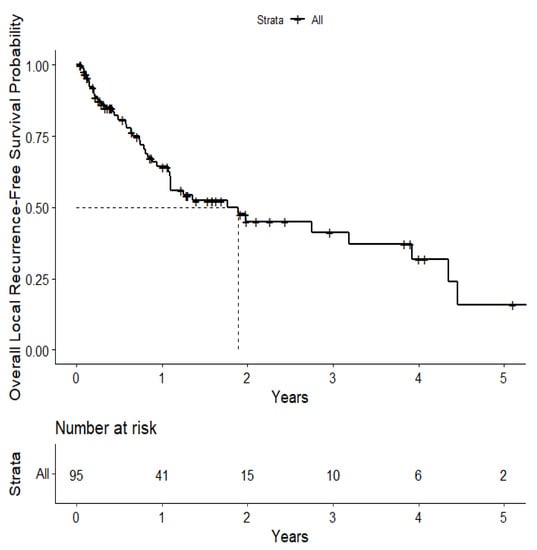
Figure 7.
Kaplan–Meier curve of local recurrence-free survival.
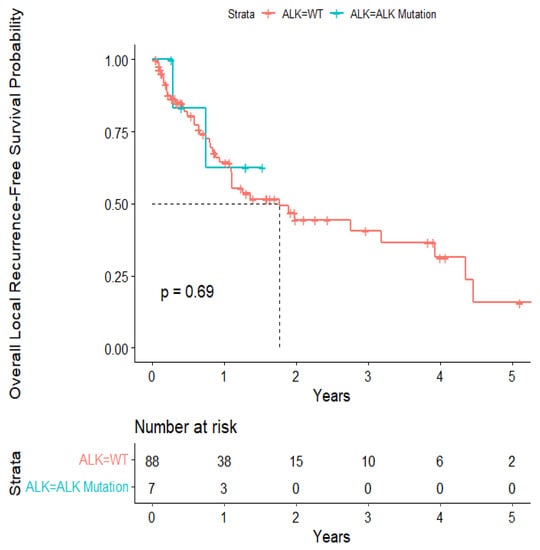
Figure 8.
Kaplan–Meir survival curve of ALK-rearranged mutational status predicting local recurrence-free survival.
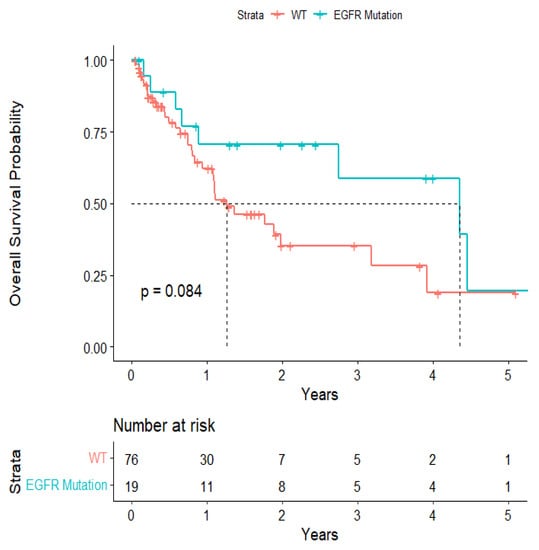
Figure 9.
Kaplan–Meir survival curve of EGFR-rearranged mutational status predicting local recurrence-free survival.
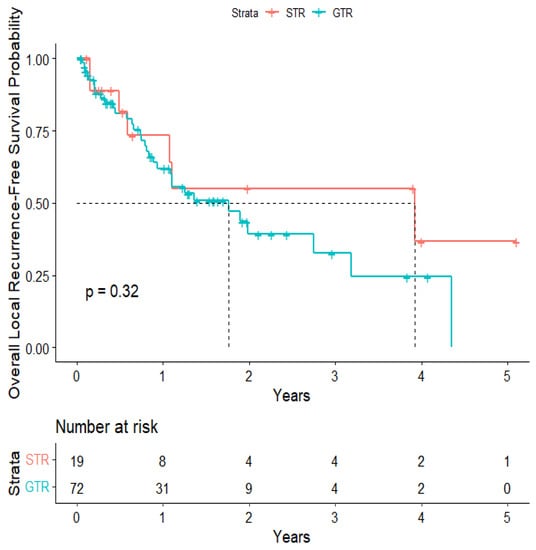
Figure 10.
Kaplan–Meier curve of extent of resection from 1st craniotomy predicting local recurrence-free survival.
3.3. Multivariate Associations
For OS, systemic progressive disease (HR: 4.74; 95% CI: 1.89–11.85; p-value ≤ 0.013), type of radiation therapy (SRS vs. standard fractionated) (HR: 0.30; 95% CI: 0.14–0.68; p-value = 0.008), new brain metastases within a year of surgery (HR: 1.12; 95% CI: 1.01–1.23; p-value = 0.010), and adjuvant chemotherapy (HR: 0.46; 95% CI: 0.21–1.02; p-value = 0.024) were prognostic factors for survival (Table 5).

Table 5.
Multivariate association with predictors of overall survival.
4. Discussion
With the growing importance of mutational marker analysis to target therapy, the multimodal management of BMs is poised for significant change. Consequently, multidisciplinary teams of neurosurgical, neuro-oncological, and radiation practitioners must be aware of the effects that mutations play on OS, systemic treatment response, and, potentially, regarding intracranial disease control as well. Our retrospective study evaluates the impact of ALK and EGFR mutational markers in the management, local control, and EOR of BMs as well as by discussing the potential role that newer second-line targeted therapies played for BM management.
Additionally, 30 patients without ALK or EGFR mutations in the biopsied brain tissue received targeted therapies, but they did not have ALK or EGFR mutations in the biopsied brain tissue. This may be attributed to ALK and EGFR mutations in the primary lung tumor sites that were not present in BM pathology. Such an incoherence between primary tumor and metastatic lesion mutational status is known as discordance. Discordance is common among metastatic lung cancer patients, with 30–50% of cases reporting this phenomenon [42,43]. As a result, outcomes of targeted therapy are further complicated, as discordant tumors could act as confounding factors on survival and the overall efficacy of treatments [44,45].
Our multivariate analysis showed that systemic progressive disease, type of radiation therapy, new BMs within a year of surgery, and adjuvant chemotherapy were prognostic factors for survival. These findings are externally validated by previous surgical series [46,47,48,49]. New BMs within a year of surgery is an especially interesting finding since current scientific literature does not provide a definitive time frame in which new BMs could yield a hazard to patient survival. Our analysis also showed that any new BMs within a year of resection can significantly reduce patient survival. Additionally, we also found the number of new BMs within a year of surgery and volume of tumor to be significant prognostic factors for LRFS. Tumor size has been a known predictor for local recurrence of BM and our results are externally validated by the literature [50,51]. The number of new BMs that occur within a year was another interesting prognostic factor. More new BMs meant a greater hazard for local recurrence of the disease, a finding that, although consistent with previous literature, is new and more specific [52,53].
Some of our results, however, had differing findings from sources in the external literature that studied the efficacy of BM mutational status and applied targeted therapy. Most notably, unlike the prior work by Colditz et al. detailing a relationship between BRAF mutational status predicting favorable outcomes in melanoma patients [41], we were not able to find significant associations between mutational status and EOR as well as OS in our lung cancer cohort. Their study cited heterogeneous treatment protocols, where first-line (single-agent immune checkpoint therapy) and second-line (anti-CTLA-4 and anti-PD-1) therapies were used at various time periods. Furthermore, their institution also had limitations on which patients could access BRAF-targeted therapy, requiring treatment with immune-checkpoint therapy first. Thus, varying administration protocols create an unstandardized basis for comparing our results to those of Colditz et al. Furthermore, the differences in our findings can also be attributed to potential deviations in the mechanisms of the pathophysiological disease progression to BM between metastatic lung cancer and melanoma.
Furthermore, our ALK and EGFR mutational patient cohort that received targeted therapy had a larger median OS and LRFS (6.01 and 5.75 years, respectively) than the group that did not have any mutations in biopsied brain tissue but did receive immunotherapy (4.08 and 1.89 years, respectively). These survival times, however, were greater than the median survival time for patients who did not receive any immunotherapy (1.70 and 1.76 years, respectively). The cost versus the benefit of improving OS by this much should be assessed in future studies. All three of our patient cohort groups had longer survival times than those published in the literature, especially for the groups that received targeted therapies, which tended to be 8–26 months OS [54,55,56,57,58]. In addition, literature findings showed 18–24 months to be the range for local recurrence free survival, externally validating our findings [59,60,61,62]. Furthermore, in our cohort, EGFR mutational status trended towards being significant in prolonging LRFS, which is in line with findings in the literature as well [63]. Thus, the combinations of targeted therapies in this series and their respective chronology of use seem to confer survival and local control benefits and will be studied further through future work from our group to create and evaluate formally assessable results.
Limitations
Our study was limited by its retrospective nature. Although targeted therapies for ALK-rearranged and EGFR-amplified mutations show promise in combination with surgical resection of cerebral lung cancer, additional analysis and further studies must elucidate these relationships to optimize their inclusion in treatment protocol. Larger sample sizes and randomized studies could yield better associations between surgical resection, mutation status, targeted therapy, and postoperative outcomes. Furthermore, our study spans 10 years; during this time, there has been an evolution of targeted therapies used for ALK-rearranged and EGFR-amplified mutations. Future work will focus on improved follow-up and increased numbers of patients via multi-institutional collaboration with complete genomic sequencing as well as classifying the patient mutational status and their respective therpies at a more granular level of detail. Given the time period of our studies, we also expect increasing utilization of cerebral-penetrant systemic therapies in future patients that will allow us to better evaluate the role of these therapies in surgical BM patients.
5. Conclusions
With our retrospective review of 95 patients undergoing surgical resection of cerebral lung cancer from January 2012 to May 2022, we identified key prognostic factors for OS and LRFS. Additionally, we assessed the role of ALK and EGFR mutations in local control of disease and OS. Patients with either ALK or EGFR mutation-related targeted therapies combined with immunotherapy showed the highest survival, though differences between groups were not statistically significant. Prognostic factors for OS included systemic progressive disease, type of radiation therapy used, new BMs within a year of surgery, and adjuvant chemotherapy.
In the evolving landscape of genomic medicine, our findings underscore the significance of directed-oncological therapies. We hope to continue building upon our work as we gather more patients who have received second line CNS-penetrant therapies better able to cross the blood–brain barrier and conduct follow-up studies comparing subsequent therapy cohorts to traditional systemic therapy. Furthermore, we hope to raise awareness for the administration of combination targeted therapies as well as their timing to inspire future scientific investigations for these questions. Gaining a better understanding of these second-line therapies will enable individualized therapies to potentially increase survival times and local control of BM.
Author Contributions
Conceptualization, K.B.H.; methodology, K.B.H.; software, S.S.M. and S.G.; validation, S.S.M., D.P.B., C.D.N. and S.G.; formal analysis, S.S.M. and S.G.; investigation, S.S.M., D.P.B. and C.D.N.; resources, D.P.B., J.Z., S.G., J.Z. and K.B.H.; data curation, S.S.M., D.P.B. and C.D.N.; writing—original draft preparation, S.S.M., D.P.B. and C.D.N.; writing—review and editing, S.S.M., D.P.B., C.D.N., J.Z., H.-K.S., B.E., L.S., S.G., C.D., E.K.N., J.O. and K.B.H.; visualization, S.S.M.; supervision, D.P.B. and K.B.H.; project administration, K.B.H.; funding acquisition, D.P.B., S.G. and K.B.H. All authors have read and agreed to the published version of the manuscript.
Funding
David P. Bray is partly supported by the Nell W. and William S. Elkin Research Fellowship in Oncology, Winship Cancer Institute, Emory University Hospital, Atlanta, GA, and supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 and TL1TR002382. Kimberly Hoang acknowledges funding from the Jordan Family Brain Tumor Initiative and Donaldson Research Synergy Fund. Research reported in this publication was supported in part by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Emory University School of Medicine (protocol code 117860 and date of approval: 1 April 2022).
Informed Consent Statement
The need for informed consent was waived given the retrospective nature of the study, as obtained via IRB approval.
Data Availability Statement
Due to privacy and ethical restrictions, the patient data supporting the reported results in this study cannot be shared publicly. Access to the anonymized data may be granted upon reasonable request and subject to appropriate ethical approval.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Doherty, G.J.; Petruzzelli, M.; Beddowes, E.; Ahmad, S.S.; Caldas, C.; Gilbertson, R.J. Cancer Treatment in the Genomic Era. Annu. Rev. Biochem. 2019, 88, 247–280. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.P.; Vazquez, F.; Tsherniak, A.; Hong, A.L.; Rinne, M.; Aguirre, A.J.; Boehm, J.S.; Hahn, W.C. Functional Genomic Characterization of Cancer Genomes. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, F.; Ng, C.K.Y.; Patsouris, A.; Droin, N.; Piscuoglio, S.; Carbuccia, N.; Soria, J.C.; Dien, A.T.; Adnani, Y.; Kamal, M.; et al. Genomic Characterization of Metastatic Breast Cancers. Nature 2019, 569, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, N.; Rzhetsky, A.; Gilliam, T.C. (Eds.) Systems Analysis of Human Multigene Disorders. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 799, ISBN 978-1-4614-8777-7. [Google Scholar]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of Brain Metastases According to Molecular Subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Fallah, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic Sites and Survival in Lung Cancer. Lung Cancer 2014, 86, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Popper, H.H. Progression and Metastasis of Lung Cancer. Cancer Metastasis Rev. 2016, 35, 75–91. [Google Scholar] [CrossRef]
- Carapella, C.M.; Gorgoglione, N.; Oppido, P.A. The Role of Surgical Resection in Patients with Brain Metastases. Curr. Opin. 2018, 30, 390–395. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, Q.; Dong, Y.; Li, F.; Li, W.; Wang, X. Microsurgery vs. Radiosurgery for the Treatment of Multiple Metastases in the Brain: A Retrospective Cohort Study. Cancer Biol. Med. 2021, 18, 884. [Google Scholar] [CrossRef]
- Schöggl, A.; Kitz, K.; Reddy, M.; Wolfsberger, S.; Schneider, B.; Dieckmann, K.; Ungersböck, K. Defining the Role of Stereotactic Radiosurgery Versus Microsurgery in the Treatment of Single Brain Metastases. Acta Neurochir. 2000, 142, 621–626. [Google Scholar] [CrossRef]
- Palmer, J.D.; Greenspoon, J.; Brown, P.D.; Johnson, D.R.; Roberge, D. Neuro-Oncology Practice Clinical Debate: Stereotactic Radiosurgery or Fractionated Stereotactic Radiotherapy Following Surgical Resection for Brain Metastasis. Neuro Oncol. Pract. 2020, 7, 263–267. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. Adjuvant Whole-Brain Radiotherapy Versus Observation After Radiosurgery or Surgical Resection of One to Three Cerebral Metastases: Results of the EORTC 22952-26001 Study. JCO 2011, 29, 134–141. [Google Scholar] [CrossRef]
- Eitz, K.A.; Lo, S.S.; Soliman, H.; Sahgal, A.; Theriault, A.; Pinkham, M.B.; Foote, M.C.; Song, A.J.; Shi, W.; Redmond, K.J.; et al. Multi-Institutional Analysis of Prognostic Factors and Outcomes After Hypofractionated Stereotactic Radiotherapy to the Resection Cavity in Patients with Brain Metastases. JAMA Oncol. 2020, 6, 1901. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H. Stereotactic Radiosurgery for the Management of Brain Metastases. New Engl. J. Med. 2010, 362, 1119–1127. [Google Scholar] [CrossRef]
- Harder, B.G.; Blomquist, M.R.; Wang, J.; Kim, A.J.; Woodworth, G.F.; Winkles, J.A.; Loftus, J.C.; Tran, N.L. Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma. Front. Oncol. 2018, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network® (NCCN®). NCCN Guidelines for Patients. In Anemia and Neutropenia Low Red and White Blood Cells; National Comprehensive Cancer Network® (NCCN®): Philadelphia, PA, USA, 2021; pp. 35–37. [Google Scholar]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR -Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-Line Ceritinib versus Platinum-Based Chemotherapy in Advanced ALK -Rearranged Non-Small-Cell Lung Cancer (ASCEND-4): A Randomised, Open-Label, Phase 3 Study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Sahebjam, S.; Le Rhun, E.; Bachelot, T.; Kabos, P.; Awada, A.; Yardley, D.; Chan, A.; Conte, P.; Diéras, V.; et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor–Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 5310–5319. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Tripathy, D.; Tolaney, S.M.; Seidman, A.D.; Anders, C.K.; Ibrahim, N.; Rugo, H.S.; Twelves, C.; Dieras, V.; Müller, V.; Tagliaferri, M.; et al. ATTAIN: Phase III Study of Etirinotecan Pegol versus Treatment of Physician’s Choice in Patients with Metastatic Breast Cancer and Brain Metastases. Future Oncol. 2019, 15, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.-J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus Trametinib in Patients with BRAFV600-Mutant Melanoma Brain Metastases (COMBI-MB): A Multicentre, Multicohort, Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination Nivolumab and Ipilimumab or Nivolumab Alone in Melanoma Brain Metastases: A Multicentre Randomised Phase 2 Study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Kluger, H.M.; Chiang, V.; Mahajan, A.; Zito, C.R.; Sznol, M.; Tran, T.; Weiss, S.A.; Cohen, J.V.; Yu, J.; Hegde, U.; et al. Long-Term Survival of Patients with Melanoma with Active Brain Metastases Treated with Pembrolizumab on a Phase II Trial. JCO 2019, 37, 52–60. [Google Scholar] [CrossRef]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of Brain Metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Shi, A.A.; Digumarthy, S.R.; Temel, J.S.; Halpern, E.F.; Kuester, L.B.; Aquino, S.L. Does Initial Staging or Tumor Histology Better Identify Asymptomatic Brain Metastases in Patients with Non–Small Cell Lung Cancer? J. Thorac. Oncol. 2006, 1, 205–210. [Google Scholar] [CrossRef]
- Hung, J.-J.; Jeng, W.-J.; Hsu, W.-H.; Wu, K.-J.; Chou, T.-Y.; Hsieh, C.-C.; Huang, M.-H.; Liu, J.-S.; Wu, Y.-C. Prognostic Factors of Postrecurrence Survival in Completely Resected Stage I Non-Small Cell Lung Cancer with Distant Metastasis. Thorax 2010, 65, 241–245. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Contessa, J.N.; Omay, S.B.; Chiang, V. Lung Cancer Brain Metastases. Cancer J. 2015, 21, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Rangachari, D.; Yamaguchi, N.; VanderLaan, P.A.; Folch, E.; Mahadevan, A.; Floyd, S.R.; Uhlmann, E.J.; Wong, E.T.; Dahlberg, S.E.; Huberman, M.S.; et al. Brain Metastases in Patients with EGFR -Mutated or ALK -Rearranged Non-Small-Cell Lung Cancers. Lung Cancer 2015, 88, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ballard, P.; Yates, J.W.T.; Yang, Z.; Kim, D.-W.; Yang, J.C.-H.; Cantarini, M.; Pickup, K.; Jordan, A.; Hickey, M.; Grist, M.; et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin. Cancer Res. 2016, 22, 5130–5140. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, S.; Lin, H.; Yang, H.; Chen, H.; Liao, Z.; Lin, W.; Zheng, W.; Xie, X. Correlation between EGFR Mutation Status and the Incidence of Brain Metastases in Patients with Non-Small Cell Lung Cancer. J. Thorac. Dis. 2017, 9, 2510–2520. [Google Scholar] [CrossRef]
- Aljohani, H.M.; Aittaleb, M.; Furgason, J.M.; Amaya, P.; Deeb, A.; Chalmers, J.J.; Bahassi, E.M. Genetic Mutations Associated with Lung Cancer Metastasis to the Brain. Mutagenesis 2018, 33, 137–145. [Google Scholar] [CrossRef]
- Griesinger, F.; Roeper, J.; Pöttgen, C.; Willborn, K.C.; Eberhardt, W.E.E. Brain Metastases in ALK-Positive NSCLC—Time to Adjust Current Treatment Algorithms. Oncotarget 2018, 9, 35181–35194. [Google Scholar] [CrossRef]
- Johung, K.L.; Yeh, N.; Desai, N.B.; Williams, T.M.; Lautenschlaeger, T.; Arvold, N.D.; Ning, M.S.; Attia, A.; Lovly, C.M.; Goldberg, S.; et al. Extended Survival and Prognostic Factors for Patients with ALK -Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. JCO 2016, 34, 123–129. [Google Scholar] [CrossRef]
- Hsu, F.; De Caluwe, A.; Anderson, D.; Nichol, A.; Toriumi, T.; Ho, C. EGFR Mutation Status on Brain Metastases from Non-Small Cell Lung Cancer. Lung Cancer 2016, 96, 101–107. [Google Scholar] [CrossRef]
- Colditz, M.J.; Lee, S.F.; Eastgate, M.; Elder, S.; Brandis, P.; Anderson, D.; Withers, T.; Jeffree, R.L.; Pinkham, M.B.; Olson, S. Surgical Series of Metastatic Cerebral Melanoma: Clinical Association of Resection, BRAF-Mutation Status, and Survival. Interdiscip. Neurosurg. 2021, 24, 101075. [Google Scholar] [CrossRef]
- Bozzetti, C.; Tiseo, M.; Lagrasta, C.; Nizzoli, R.; Guazzi, A.; Leonardi, F.; Gasparro, D.; Spiritelli, E.; Rusca, M.; Carbognani, P.; et al. Comparison Between Epidermal Growth Factor Receptor (EGFR) Gene Expression in Primary Non-Small Cell Lung Cancer (NSCLC) and in Fine-Needle Aspirates from Distant Metastatic Sites. J. Thorac. Oncol. 2008, 3, 18–22. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.A.; Riesenberg, H.; Bokemeyer, C.; Atanackovic, D. CNS Metastases in Non-Small-Cell Lung Cancer: Current Role of EGFR-TKI Therapy and Future Perspectives. Lung Cancer 2013, 80, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Soon, Y.Y.; Tan, C.L.; Koh, W.Y.; Leong, C.N.; Tey, J.C.S.; Tham, I.W.K. Discordance of Epidermal Growth Factor Receptor Mutation between Primary Lung Tumor and Paired Distant Metastases in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0218414. [Google Scholar] [CrossRef] [PubMed]
- El Rassy, E.; Botticella, A.; Kattan, J.; Le Péchoux, C.; Besse, B.; Hendriks, L. Non-Small Cell Lung Cancer Brain Metastases and the Immune System: From Brain Metastases Development to Treatment. Cancer Treat. Rev. 2018, 68, 69–79. [Google Scholar] [CrossRef]
- Li, B.; Yu, J.; Suntharalingam, M.; Kennedy, A.S.; Amin, P.P.; Chen, Z.; Yin, R.; Guo, S.; Han, T.; Wang, Y.; et al. Comparison of Three Treatment Options for Single Brain Metastasis from Lung Cancer. Int. J. Cancer 2000, 90, 37–45. [Google Scholar] [CrossRef]
- Jawahar, A.; Matthew, R.E.; Minagar, A.; Shukla, D.; Zhang, J.H.; Willis, B.K.; Ampil, F.; Nanda, A. Gamma Knife Surgery in the Management of Brain Metastases from Lung Carcinoma: A Retrospective Analysis of Survival, Local Tumor Control, and Freedom from New Brain Metastasis. J. Neurosurg. 2004, 100, 842–847. [Google Scholar] [CrossRef]
- Franciosi, V.; Cocconi, G.; Michiara, M.; Di Costanzo, F.; Fosser, V.; Tonato, M.; Carlini, P.; Boni, C.; Di Sarra, S. Front-Line Chemotherapy with Cisplatin and Etoposide for Patients with Brain Metastases from Breast Carcinoma, Nonsmall Cell Lung Carcinoma, or Malignant Melanoma. Cancer 1999, 85, 1599–1605. [Google Scholar] [CrossRef]
- Martini, N.; Bains, M.S.; Burt, M.E.; Zakowski, M.F.; McCormack, P.; Rusch, V.W.; Ginsberg, R.J. Incidence of Local Recurrence and Second Primary Tumors in Resected Stage I Lung Cancer. J. Thorac. Cardiovasc. Surg. 1995, 109, 120–129. [Google Scholar] [CrossRef]
- Harpole, D.H.; Herndon, J.E.; Young, W.G.; Wolfe, W.G.; Sabiston, D.C. Stage I Nonsmall Cell Lung Cancer. A Multivariate Analysis of Treatment Methods and Patterns of Recurrence. Cancer 1995, 76, 787–796. [Google Scholar] [CrossRef]
- Baykara, M.; Kurt, G.; Buyukberber, S.; Demirci, U.; Ceviker, N.; Algin, E.; Coskun, U.; Aykol, S.; Emmez, H.; Ozet, A.; et al. Management of Brain Metastases from Non-Small Cell Lung Cancer. J. Can. Res. Ther. 2014, 10, 915. [Google Scholar] [CrossRef]
- Arbit, E.; Wroński, M.; Burt, M.; Galicich, J.H. The Treatment of Patients with Recurrent Brain Metastases. A Retrospective Analysis of 109 Patients with Nonsmall Cell Lung Cancer. Cancer 1995, 76, 765–773. [Google Scholar] [CrossRef]
- Crinò, L. Nivolumab and Brain Metastases in Patients with Advanced Non-Squamous Non-Small Cell Lung Cancer. Lung Cancer 2019, 6, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk-Kasiuchnicz, A.; Ramlau, R.; Stencel, K. Treatment of Brain Metastases of Non-Small Cell Lung Carcinoma. Int. J. Mol. Sci. 2021, 21, 593. [Google Scholar] [CrossRef] [PubMed]
- Schapira, E.; Hubbeling, H.; Yeap, B.Y.; Mehan, W.A.; Shaw, A.T.; Oh, K.; Gainor, J.F.; Shih, H.A. Improved Overall Survival and Locoregional Disease Control with Concurrent PD-1 Pathway Inhibitors and Stereotactic Radiosurgery for Lung Cancer Patients with Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 624–629. [Google Scholar] [CrossRef]
- Hendriks, L.E.L. Outcome of Patients with Non-Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J. Thorac. Oncol. 2019, 14, 11. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating Survival in Patients with Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol. 2017, 3, 827. [Google Scholar] [CrossRef]
- Chaft, J.E.; Dagogo-Jack, I.; Santini, F.C.; Eng, J.; Yeap, B.Y.; Izar, B.; Chin, E.; Jones, D.R.; Kris, M.G.; Shaw, A.T.; et al. Clinical Outcomes of Patients with Resected, Early-Stage ALK-Positive Lung Cancer. Lung Cancer 2018, 122, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, P. Worse Disease-Free Survival in Never-Smokers with ALK+ Lung Adenocarcinoma. J. Thorac. Oncol. 2012, 7, 8. [Google Scholar] [CrossRef]
- Singh, R.; Lehrer, E.J.; Ko, S.; Peterson, J.; Lou, Y.; Porter, A.B.; Kotecha, R.; Brown, P.D.; Zaorsky, N.G.; Trifiletti, D.M. Brain Metastases from Non-Small Cell Lung Cancer with EGFR or ALK Mutations: A Systematic Review and Meta-Analysis of Multidisciplinary Approaches. Radiother. Oncol. 2020, 144, 165–179. [Google Scholar] [CrossRef]
- Weickhardt, A.J.; Scheier, B.; Burke, J.M.; Gan, G.; Lu, X.; Bunn, P.A.; Aisner, D.L.; Gaspar, L.E.; Kavanagh, B.D.; Doebele, R.C.; et al. Local Ablative Therapy of Oligoprogressive Disease Prolongs Disease Control by Tyrosine Kinase Inhibitors in Oncogene-Addicted Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 1807–1814. [Google Scholar] [CrossRef]
- Yagishita, S.; Horinouchi, H.; Katsui Taniyama, T.; Nakamichi, S.; Kitazono, S.; Mizugaki, H.; Kanda, S.; Fujiwara, Y.; Nokihara, H.; Yamamoto, N.; et al. Epidermal Growth Factor Receptor Mutation Is Associated with Longer Local Control After Definitive Chemoradiotherapy in Patients with Stage III Nonsquamous Non–Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 140–148. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).