Effect of Sarcopenia on Pneumonia after Endoscopic Submucosal Resection in Patients Aged ≥65 Years: A Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Measurements and Definitions of Sarcopenia
2.3. Data Collection
2.4. Statistical Analysis
2.5. Ethical Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oda, I.; Gotoda, T.; Hamanaka, H.; Eguchi, T.; Saito, Y.; Matsuda, T.; Bhandari, P.; Emura, F.; Saito, D.; Ono, H. Endoscopic submucosal dissection for early gastric cancer: Technical feasibility, operation time and complications from a large consecutive series. Dig. Endosc. 2005, 17, 54–58. [Google Scholar] [CrossRef]
- Isomoto, H.; Shikuwa, S.; Yamaguchi, N.; Fukuda, E.; Ikeda, K.; Nishiyama, H.; Ohnita, K.; Mizuta, Y.; Shiozawa, J.; Kohno, S. Endoscopic submucosal dissection for early gastric cancer: A large-scale feasibility study. Gut 2009, 58, 331–336. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, H.; Kim, D.W.; Chung, H.; Park, J.C.; Shin, S.K.; Hyung, W.J.; Lee, S.K.; Lee, Y.C.; Noh, S.H. Clinical safety of endoscopic submucosal dissection compared with surgery in elderly patients with early gastric cancer: A propensity-matched analysis. Gastrointest. Endosc. 2014, 80, 599–609. [Google Scholar] [CrossRef]
- Lin, J.P.; Zhang, Y.P.; Xue, M.; Chen, S.J.; Si, J.M. Endoscopic submucosal dissection for early gastric cancer in elderly patients: A meta-analysis. World J. Surg. Oncol. 2015, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H. Endoscopic submucosal dissection—Current success and future directions. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.; Golger, D.; Arnholdt, H.; Messmann, H. Endoscopic submucosal dissection of early cancers, flat adenomas, and submucosal tumors in the gastrointestinal tract. Clin. Gastroenterol. Hepatol. 2009, 7, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.H.; Ge, J.; Yang, C.M.; Liu, J.Y.; Zhao, S.L. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: A meta-analysis. World J. Gastroenterol. 2014, 20, 8282–8287. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Jung, H.Y.; Choi, K.D.; Choi, J.Y.; Kim, M.Y.; Lee, J.H.; Choi, K.S.; Kim, D.H.; Song, H.J.; Lee, G.H.; et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest. Endosc. 2011, 74, 485–493. [Google Scholar] [CrossRef]
- Saito, I.; Tsuji, Y.; Sakaguchi, Y.; Niimi, K.; Ono, S.; Kodashima, S.; Yamamichi, N.; Fujishiro, M.; Koike, K. Complications related to gastric endoscopic submucosal dissection and their managements. Clin. Endosc. 2014, 47, 398–403. [Google Scholar] [CrossRef]
- Oda, I.; Suzuki, H.; Nonaka, S.; Yoshinaga, S. Complications of gastric endoscopic submucosal dissection. Dig. Endosc. 2013, 25 (Suppl. S1), 71–78. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, H.; Kang, Y.A.; Cho, I.R.; Kim, B.; Heo, S.J.; Shin, S.; Lee, H.; Park, J.C.; Shin, S.K.; et al. Risk factors and prognosis of pulmonary complications after endoscopic submucosal dissection for gastric neoplasia. Dig. Dis. Sci. 2013, 58, 540–546. [Google Scholar] [CrossRef]
- Togo, M.; Akazawa, Y.; Akashi, T.; Yamashita, R.; Yoshitomi, I.; Ohba, K.; Hashimoto, S.; Iwashita, H.; Kurogi, T.; Osada, Y.; et al. Comprehensive prospective analysis of the factors contributing to aspiration pneumonia following endoscopic submucosal dissection in patients with early gastric neoplasms. Acta Med. Okayama 2020, 74, 407–413. [Google Scholar]
- Isomoto, H.; Ohnita, K.; Yamaguchi, N.; Fukuda, E.; Ikeda, K.; Nishiyama, H.; Akiyama, M.; Ozawa, E.; Nakao, K.; Kohno, S.; et al. Clinical outcomes of endoscopic submucosal dissection in elderly patients with early gastric cancer. Eur. J. Gastroenterol. Hepatol. 2010, 22, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Ebihara, S.; Mori, T.; Izumi, S.; Ebihara, T. Association between sarcopenia and pneumonia in older people. Geriatr. Gerontol. Int. 2020, 20, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Niederman, M.S.; Cilloniz, C. Aspiration pneumonia. Rev. Esp. Quimioter. 2022, 35 (Suppl. S1), 73–77. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Izumi, S.; Suzukamo, Y.; Okazaki, T.; Iketani, S. Ultrasonography to detect age-related changes in swallowing muscles. Eur. Geriatr. Med. 2019, 10, 753–760. [Google Scholar] [CrossRef]
- Elliott, J.E.; Greising, S.M.; Mantilla, C.B.; Sieck, G.C. Functional impact of sarcopenia in respiratory muscles. Respir. Physiol. Neurobiol. 2016, 226, 137–146. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised european consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef]
- Sheetz, T.; Lee, C.T. Frailty and geriatric assessment in urologic oncology. Curr. Opin. Urol. 2018, 28, 233–242. [Google Scholar] [CrossRef]
- Arao, M.; Mizutani, T.; Ozawa, N.; Hanai, T.; Takada, J.; Kubota, M.; Imai, K.; Ibuka, T.; Shiraki, M.; Araki, H.; et al. Skeletal muscle depletion: A risk factor for pneumonia following gastric endoscopic submucosal dissection in elderly patients. Dig. Dis. 2021, 39, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Choi, K.D.; Ko, Y.; Park, T.; Kim, K.W.; Park, S.Y.; Na, H.K.; Ahn, J.Y.; Lee, J.H.; Jung, K.W.; et al. Impact of comorbidities, sarcopenia, and nutritional status on the long-term outcomes after endoscopic submucosal dissection for early gastric cancer in elderly patients aged ≥80 years. Cancers 2021, 13, 3598. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Funasaka, K.; Miyahara, R.; Furukawa, K.; Yamamura, T.; Ishikawa, T.; Ohno, E.; Nakamura, M.; Kawashima, H.; Hirooka, Y.; et al. Relationship between psoas muscle index and long-term survival in older patients aged ≥80 years after endoscopic submucosal dissection for gastric cancer. Int. J. Clin. Oncol. 2022, 27, 729–738. [Google Scholar] [CrossRef]
- Bahat, G.; Turkmen, B.O.; Aliyev, S.; Catikkas, N.M.; Bakir, B.; Karan, M.A. Cut-off values of skeletal muscle index and psoas muscle index at l3 vertebra level by computerized tomography to assess low muscle mass. Clin. Nutr. 2021, 40, 4360–4365. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Iritani, S.; Imai, K.; Takai, K.; Hanai, T.; Ideta, T.; Miyazaki, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M.; Moriwaki, H. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol. 2015, 50, 323–332. [Google Scholar] [CrossRef]
- Miyata, H.; Sugimura, K.; Motoori, M.; Fujiwara, Y.; Omori, T.; Yanagimoto, Y.; Ohue, M.; Yasui, M.; Miyoshi, N.; Tomokuni, A.; et al. Clinical assessment of sarcopenia and changes in body composition during neoadjuvant chemotherapy for esophageal cancer. Anticancer Res. 2017, 37, 3053–3059. [Google Scholar]
- Mandell, L.A.; Niederman, M.S. Aspiration pneumonia. N. Engl. J. Med. 2019, 380, 651–663. [Google Scholar] [CrossRef]
- Dennison, E.M.; Sayer, A.A.; Cooper, C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yuan, F.; Ma, X.; Qu, H.; Li, Y.; Zhang, W.; Ma, H.; Liu, H.; Yang, Y.; Xu, L.; et al. Incidence rates, risk factors, and outcomes of aspiration pneumonia after gastric endoscopic submucosal dissection: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, R.; Choi, H.; Lee, S.J.; Bae, G.U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharm. Res. 2021, 44, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the prot-age study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Lee, J.Y.; O’Neill, E.A.; Meehan, A.G.; Kusek, J.W. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013, 16, 169–172. [Google Scholar] [CrossRef]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship between sarcopenia and cardiovascular diseases in the elderly: An overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Meunier, B.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Roche, F.; Feasson, L.; Barthelemy, J.C.; et al. Lower skeletal muscle capillarization in hypertensive elderly men. Exp. Gerontol. 2016, 76, 80–88. [Google Scholar] [CrossRef]



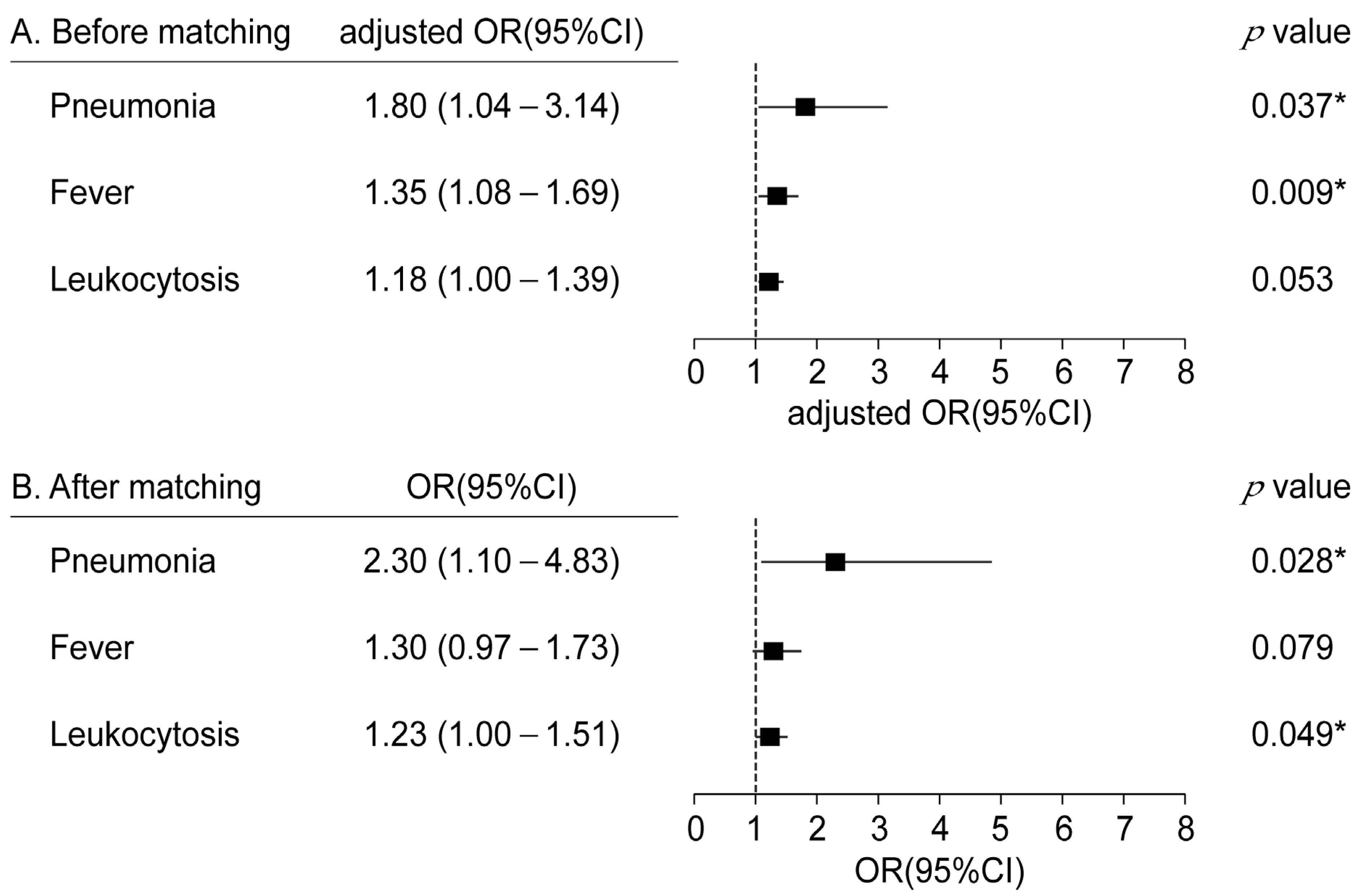
| Variable | No Pneumonia (n = 3218) | Pneumonia (n = 71) | p-Value |
|---|---|---|---|
| Age, years | 72 ± 5 | 74 ± 5 | 0.010 * |
| Male sex | 2301 (72) | 62 (87) | 0.003 * |
| ASA physical status | 0.316 | ||
| I | 668 (21) | 13 (18) | |
| II | 1801 (56) | 36 (51) | |
| III | 749 (23) | 22 (31) | |
| Current smoker | 1094 (34) | 27 (38) | 0.478 |
| Alcohol history | 1558 (48) | 41 (58) | 0.120 |
| Comorbidities | |||
| Hypertension | 1796 (56) | 40 (56) | 0.930 |
| Diabetes mellitus | 769 (24) | 17 (24) | 0.993 |
| Hepatitis | 95 (3) | 1 (1) | 0.723 |
| Asthma | 80 (2.5) | 1 (1.4) | >0.999 |
| Bronchiectasis | 7 (0.2) | 0 (0) | >0.999 |
| COPD | 24 (0.8) | 1 (1.4) | 0.422 |
| Old tuberculosis | 188 (5.8) | 4 (5.6) | >0.999 |
| Pneumoconiosis | 1 (0.03) | 0 (0) | >0.999 |
| Body mass index, kg/m2 | 24.1 ± 3.0 | 24.7 ± 3.3 | 0.107 |
| Skeletal muscle index, cm2/m2 | 48.9 ± 7.9 | 49.4 ± 7.9 | 0.582 |
| Procedure time, min | 43 ± 30 | 51 ± 44 | 0.155 |
| Recovery time, min | 25 ± 10 | 29 ± 18 | 0.084 |
| Tumor size, mm | 15.8 ± 9.7 | 17.9 ± 10.3 | 0.088 |
| Gross type of lesion | 0.812 | ||
| Elevated | 2432 (76) | 52 (73) | |
| Flat | 415 (13) | 11 (15) | |
| Depressed | 371 (12) | 8 (11) | |
| Location of lesion | 0.075 | ||
| Upper | 387 (12) | 3 (4) | |
| Middle | 738 (23) | 14 (20) | |
| Lower | 2093 (65) | 54 (76) | |
| En block resection | 3113 (97) | 68 (96) | 0.506 |
| Submucosal fibrosis | 817 (25) | 17 (24) | 0.782 |
| Sedation by anesthesiologist | 3014 (94) | 71 (100) | 0.021 * |
| Fever | 444 (14) | 60 (85) | <0.001 * |
| Leukocytosis | 1135 (35) | 65 (92) | <0.001 * |
| Antibiotics use | 821 (26) | 71 (100) | <0.001 * |
| Perforation | 44 (1) | 3 (4) | 0.080 |
| Pneumonia | |||
| Rt | 0 (0) | 4 (6) | <0.001 * |
| Lt | 0 (0) | 60 (85) | <0.001 * |
| Both | 0 (0) | 7 (10) | <0.001 * |
| Sarcopenia | 1527 (48) | 44 (62) | 0.015 * |
| Post-ESD hospital stay, days | 2.4 ± 1.0 | 7.8 ± 3.1 | <0.001 * |
| Variables | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| No Sarcopenia (n = 1718) | Sarcopenia (n = 1571) | p-Value | No Sarcopenia (n = 785) | Sarcopenia (n = 785) | p-Value | |
| Age | 71 ± 5 | 73 ± 5 | <0.001 * | 72 ± 5 | 72 ± 5 | 0.367 |
| Male sex | 1008 (59) | 1355 (86) | <0.001 * | 604 (77) | 602 (77) | 0.891 |
| ASA physical status | 0.485 | 0.704 | ||||
| I | 364 (21) | 317 (20) | 166 (21) | 158 (20) | ||
| II | 965 (56) | 872 (56) | 450 (57) | 439 (56) | ||
| III | 389 (23) | 382 (24) | 169 (22) | 188 (24) | ||
| Current smoker | 469 (27) | 652 (42) | <0.001 * | 281 (36) | 286 (36) | 0.790 |
| Alcohol history | 689 (40) | 910 (58) | <0.001 * | 397 (51) | 409 (52) | 0.537 |
| Comorbidities | ||||||
| Hypertension | 989 (58) | 847 (54) | 0.035 * | 418 (53) | 460 (59) | 0.032 * |
| Diabetes mellitus | 423 (25) | 363 (23) | 0.309 | 192 (24) | 186 (24) | 0.722 |
| Hepatitis | 55 (3) | 41 (3) | 0.314 | 24 (3) | 24 (3) | >0.999 |
| Asthma | 44 (3) | 37 (2) | 0.704 | 14 (2) | 13 (2) | 0.842 |
| Bronchiectasis | 5 (0.3) | 2 (0.1) | 0.456 | 2 (0.3) | 1 (0.1) | 0.564 |
| COPD | 8 (0.5) | 17 (1.1) | 0.042 * | 4 (0.5) | 7 (0.9) | 0.366 |
| Old tuberculosis | 69 (4.0) | 123 (7.8) | <0.001 * | 36 (4.6) | 38 (4.8) | 0.806 |
| Pneumoconiosis | 1 (0.1) | 0 (0) | >0.999 | 1 (0.1) | 0 (0) | 0.317 |
| BMI, kg/m2 | 25.6 ± 2.9 | 22.9 ± 2.6 | <0.001 * | 24.1 ± 2.4 | 24.1 ± 2.4 | 0.723 |
| SMI, cm2/m2 | 52.7 ± 7.8 | 44.8 ± 5.7 | <0.001 * | 54.0 ± 7.0 | 44.7 ± 6.1 | <0.001 * |
| Procedure time | 42 ± 31 | 45 ± 30 | 0.013 * | 44 ± 32 | 44 ± 30 | 0.628 |
| Recovery time | 24.9 ± 10.6 | 24.9 ± 9.7 | 0.982 | 24.4 ± 9.6 | 25.0 ± 9.4 | 0.246 |
| Tumor size, mm | 15.8 ± 10.0 | 15.8 ± 9.4 | 0.874 | 15.9 ± 9.5 | 15.2 ± 8.8 | 0.121 |
| Gross type of lesion | 0.029 * | 0.996 | ||||
| Elevated | 1321 (77) | 1163 (74) | 595 (76) | 596 (76) | ||
| Flat | 197 (11) | 229 (15) | 99 (13) | 98 (12) | ||
| Depressed | 200 (12) | 179 (11) | 91 (12) | 91 (12) | ||
| Location of lesion | 0.081 | 0.405 | ||||
| Upper | 194 (11) | 196 (12) | 101 (13) | 88 (11) | ||
| Middle | 372 (22) | 380 (24) | 187 (24) | 177 (23) | ||
| Lower | 1152 (67) | 995 (63) | 497 (63) | 520 (66) | ||
| En block resection | 1666 (97) | 1515 (96) | 0.387 | 760 (97) | 765 (97) | 0.446 |
| Submucosal fibrosis | 404 (24) | 430 (27) | 0.011 * | 204 (26) | 201 (26) | 0.863 |
| Sedation by anesthesiologist | 1613 (94) | 1472 (94) | 0.822 | 738 (94) | 737 (94) | 0.916 |
| Fever | 246 (14) | 258 (16) | 0.094 | 96 (12) | 120 (15) | 0.078 |
| Leukocytosis | 599 (35) | 601 (38) | 0.044 * | 265 (34) | 303 (39) | 0.048 * |
| Antibiotics use | 446 (26) | 446 (28) | 0.118 | 179 (23) | 217 (28) | 0.026 * |
| Perforation | 22 (1) | 25 (2) | 0.453 | 13 (2) | 14 (2) | 0.847 |
| Pneumonia | 27 (2) | 44 (3) | 0.015 * | 10 (1) | 23 (3) | 0.024 * |
| Rt | 1 (0) | 3 (0) | 0.354 | 1 (0) | 1 (0) | >0.999 |
| Lt | 24 (1) | 36 (2) | 0.556 | 7 (1) | 19 (2) | 0.019 * |
| Both | 2 (0) | 5 (0) | 0.270 | 2 (0) | 3 (0) | 0.655 |
| Post-ESD hospital stay | 2 ± 1 | 3 ± 2 | 0.011 * | 2 ± 1 | 3 ± 1 | 0.012 * |
| Total Patients, n | Pneumonia Incidence, n (%) | Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|
| No sarcopenia | 785 | 10 (1.3%) | 1 (ref) | ||
| Sarcopenia and age < 73 | 453 | 7 (1.6%) | 1.22 | [0.46–3.22] | 0.693 |
| Sarcopenia and age ≥73 | 332 | 16 (4.8%) | 3.92 | [1.79–8.74] | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-Y.; Kim, S.Y.; Shin, H.J.; Kweon, K.H.; Park, J.; Kim, N.Y. Effect of Sarcopenia on Pneumonia after Endoscopic Submucosal Resection in Patients Aged ≥65 Years: A Retrospective Study. Cancers 2023, 15, 4753. https://doi.org/10.3390/cancers15194753
Kim M-Y, Kim SY, Shin HJ, Kweon KH, Park J, Kim NY. Effect of Sarcopenia on Pneumonia after Endoscopic Submucosal Resection in Patients Aged ≥65 Years: A Retrospective Study. Cancers. 2023; 15(19):4753. https://doi.org/10.3390/cancers15194753
Chicago/Turabian StyleKim, Min-Yu, So Yeon Kim, Hye Jung Shin, Ki Hong Kweon, Jooeun Park, and Na Young Kim. 2023. "Effect of Sarcopenia on Pneumonia after Endoscopic Submucosal Resection in Patients Aged ≥65 Years: A Retrospective Study" Cancers 15, no. 19: 4753. https://doi.org/10.3390/cancers15194753
APA StyleKim, M.-Y., Kim, S. Y., Shin, H. J., Kweon, K. H., Park, J., & Kim, N. Y. (2023). Effect of Sarcopenia on Pneumonia after Endoscopic Submucosal Resection in Patients Aged ≥65 Years: A Retrospective Study. Cancers, 15(19), 4753. https://doi.org/10.3390/cancers15194753






