Evaluation of Prognostic Parameters to Identify Aggressive Penile Carcinomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort and Study Design
2.2. Statistical Analyses
3. Results
3.1. Patient Characteristics:
3.2. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bleeker, M.C.G.; Heideman, D.A.M.; Snijders, P.J.F.; Horenblas, S.; Dillner, J.; Meijer, C.J.L.M. Penile Cancer: Epidemiology, Pathogenesis and Prevention. World J. Urol. 2009, 27, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Tian, T.; Yao, K.; Chen, X.-F.; Luo, G.; Gao, Y.; Lin, Y.-F.; Wang, B.; Sun, Y.; Zheng, W.; et al. Global Pattern and Trends in Penile Cancer Incidence: Population-Based Study. JMIR Public Health Surveill. 2022, 8, e34874. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Necchi, A.; Muneer, A.; Tobias-Machado, M.; Tran, A.T.H.; Van Rompuy, A.-S.; Spiess, P.E.; Albersen, M. Penile Cancer. Nat. Rev. Dis. Primers 2021, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Christodoulidou, M.; Sahdev, V.; Houssein, S.; Muneer, A. Epidemiology of Penile Cancer. Curr. Probl. Cancer 2015, 39, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Kayes, O.; Ahmed, H.U.; Arya, M.; Minhas, S. Molecular and Genetic Pathways in Penile Cancer. Lancet Oncol. 2007, 8, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.B.; Sand, F.L.; Rasmussen, C.L.; Albieri, V.; Toft, B.G.; Norrild, B.; Munk, C.; Kjær, S.K. Prevalence of Human Papillomavirus DNA and P16(INK4a) in Penile Cancer and Penile Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis. Lancet Oncol. 2019, 20, 145–158. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The Molecular Landscape of Head and Neck Cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Bosch, F.X.; Broker, T.R.; Forman, D.; Moscicki, A.-B.; Gillison, M.L.; Doorbar, J.; Stern, P.L.; Stanley, M.; Arbyn, M.; Poljak, M.; et al. Comprehensive Control of Human Papillomavirus Infections and Related Diseases. Vaccine 2013, 31 (Suppl. S7), H1–H31. [Google Scholar] [CrossRef]
- Gil, J.; Peters, G. Regulation of the INK4b-ARF-INK4a Tumour Suppressor Locus: All for One or One for All. Nat. Rev. Mol. Cell Biol. 2006, 7, 667–677. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2019, 10, 3116. [Google Scholar] [CrossRef]
- Weinberg, R.A. The Retinoblastoma Protein and Cell Cycle Control. Cell 1995, 81, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Parza, K.; Mustasam, A.; Ionescu, F.; Paravathaneni, M.; Sandstrom, R.; Safa, H.; Grass, G.D.; Johnstone, P.A.; Eschrich, S.A.; Chadha, J.; et al. The Prognostic Role of Human Papillomavirus and P16 Status in Penile Squamous Cell Carcinoma-A Systematic Review. Cancers 2023, 15, 3713. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Cubilla, A.L.; Reuter, V.E.; Gregoire, L.; Ayala, G.; Ocampos, S.; Lancaster, W.D.; Fair, W. Basaloid Squamous Cell Carcinoma: A Distinctive Human Papilloma Virus-Related Penile Neoplasm: A Report of 20 Cases. Am. J. Surg. Pathol. 1998, 22, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Cubilla, A.L.; Velazques, E.F.; Reuter, V.E.; Oliva, E.; Mihm, M.C.J.; Young, R.H. Warty (Condylomatous) Squamous Cell Carcinoma of the Penis: A Report of 11 Cases and Proposed Classification of “verruciform” Penile Tumors. Am. J. Surg. Pathol. 2000, 24, 505–512. [Google Scholar] [CrossRef]
- Guidelines Program Oncology (German Cancer Society, German Cancer Aid, AWMF): Diagnostics, Therapy and Follow-up of Penile Carcinoma, Guideline Report. Version 1.0, 2020, AWMF Register Number: 043-042OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/peniskarzinom (accessed on 13 July 2023).
- Hölters, S.; Khalmurzaev, O.; Pryalukhin, A.; Loertzer, P.; Janssen, M.; Heinzelbecker, J.; Ueberdiek, S.; Pfuhl, T.; Smola, S.; Agaimy, A.; et al. Challenging the Prognostic Impact of the New WHO and TNM Classifications with Special Emphasis on HPV Status in Penile Carcinoma. Virchows Arch. Int. J. Pathol. 2019, 475, 211–221. [Google Scholar] [CrossRef]
- Jacobs, M.V.; de Roda Husman, A.M.; van den Brule, A.J.; Snijders, P.J.; Meijer, C.J.; Walboomers, J.M. Group-Specific Differentiation between High- and Low-Risk Human Papillomavirus Genotypes by General Primer-Mediated PCR and Two Cocktails of Oligonucleotide Probes. J. Clin. Microbiol. 1995, 33, 901–905. [Google Scholar] [CrossRef]
- Protzel, C.; Hakenberg, O.W. Penile cancer: Diagnosis and treatment. Urol. A 2020, 59, 209–218. [Google Scholar] [CrossRef]
- Stanley, M. HPV Vaccination in Boys and Men. Hum. Vaccines Immunother. 2014, 10, 2109–2111. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hisamatsu, K.; Suzui, N.; Hara, A.; Tomita, H.; Miyazaki, T. A Review of HPV-Related Head and Neck Cancer. J. Clin. Med. 2018, 7, 241. [Google Scholar] [CrossRef]
- Lindquist, D.; Romanitan, M.; Hammarstedt, L.; Näsman, A.; Dahlstrand, H.; Lindholm, J.; Onelöv, L.; Ramqvist, T.; Ye, W.; Munck-Wikland, E.; et al. Human Papillomavirus Is a Favourable Prognostic Factor in Tonsillar Cancer and Its Oncogenic Role Is Supported by the Expression of E6 and E7. Mol. Oncol. 2007, 1, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, A.L.; Lopes, A.; Santiago, G.H.; Ribeiro, K.C.; Latorre, M.R.; Villa, L.L. Human Papillomavirus as a Prognostic Factor in Carcinoma of the Penis: Analysis of 82 Patients Treated with Amputation and Bilateral Lymphadenectomy. Cancer 2001, 91, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, S.M.; Chaux, A.; Ball, M.W.; Faraj, S.F.; Munari, E.; Gonzalez-Roibon, N.; Sharma, R.; Bivalacqua, T.J.; Burnett, A.L.; Netto, G.J. Human Papillomavirus Infection and Immunohistochemical P16(INK4a) Expression as Predictors of Outcome in Penile Squamous Cell Carcinomas. Hum. Pathol. 2015, 46, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Chahoud, J.; Zacharias, N.M.; Pham, R.; Qiao, W.; Guo, M.; Lu, X.; Alaniz, A.; Segarra, L.; Martinez-Ferrer, M.; Gleber-Netto, F.O.; et al. Prognostic Significance of P16 and Its Relationship with Human Papillomavirus Status in Patients with Penile Squamous Cell Carcinoma: Results of 5 Years Follow-Up. Cancers 2022, 14, 6024. [Google Scholar] [CrossRef] [PubMed]
- Djajadiningrat, R.S.; Jordanova, E.S.; Kroon, B.K.; van Werkhoven, E.; de Jong, J.; Pronk, D.T.M.; Snijders, P.J.F.; Horenblas, S.; Heideman, D.A.M. Human Papillomavirus Prevalence in Invasive Penile Cancer and Association with Clinical Outcome. J. Urol. 2015, 193, 526–531. [Google Scholar] [CrossRef]
- Lont, A.P.; Kroon, B.K.; Horenblas, S.; Gallee, M.P.W.; Berkhof, J.; Meijer, C.J.L.M.; Snijders, P.J.F. Presence of High-Risk Human Papillomavirus DNA in Penile Carcinoma Predicts Favorable Outcome in Survival. Int. J. Cancer 2006, 119, 1078–1081. [Google Scholar] [CrossRef]
- Wiener, J.S.; Effert, P.J.; Humphrey, P.A.; Yu, L.; Liu, E.T.; Walther, P.J. Prevalence of Human Papillomavirus Types 16 and 18 in Squamous-Cell Carcinoma of the Penis: A Retrospective Analysis of Primary and Metastatic Lesions by Differential Polymerase Chain Reaction. Int. J. Cancer 1992, 50, 694–701. [Google Scholar] [CrossRef]
- Hakenberg, O.W.; Dräger, D.L.; Erbersdobler, A.; Naumann, C.M.; Jünemann, K.-P.; Protzel, C. The Diagnosis and Treatment of Penile Cancer. Dtsch. Arzteblatt Int. 2018, 115, 646–652. [Google Scholar] [CrossRef]
- De Vries, H.M.; Rafael, T.S.; Gil-Jimenez, A.; de Feijter, J.M.; Bekers, E.; van der Laan, E.; Lopez-Yurda, M.; Hooijberg, E.; Broeks, A.; Peters, D.; et al. Atezolizumab with or without Radiotherapy for Advanced Squamous Cell Carcinoma of the Penis (The PERICLES Study): A Phase II Trial. J. Clin. Oncol. 2023. [Google Scholar] [CrossRef]
- Chaux, A.; Caballero, C.; Soares, F.; Guimarães, G.C.; Cunha, I.W.; Reuter, V.; Barreto, J.; Rodríguez, I.; Cubilla, A.L. The Prognostic Index: A Useful Pathologic Guide for Prediction of Nodal Metastases and Survival in Penile Squamous Cell Carcinoma. Am. J. Surg. Pathol. 2009, 33, 1049–1057. [Google Scholar] [CrossRef]
- Qu, X.M.; Siemens, D.R.; Louie, A.V.; Yip, D.; Mahmud, A. Validation of Predictors for Lymph Node Status in Penile Cancer: Results from a Population-Based Cohort. Can. Urol. Assoc. J. 2018, 12, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Borchers, H.; Jakse, G. Lymphadenectomy for penile cancer. Diagnostic and prognostic significance as well as therapeutic benefit. Urol. A 2005, 44, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Zattoni, F.; Cunico, S.C.; Galetti, T.P.; Luciani, L.; Fandella, A.; Guazzieri, S.; Maruzzi, D.; Sava, T.; Siracusano, S.; et al. Lymphatic and Vascular Embolizations Are Independent Predictive Variables of Inguinal Lymph Node Involvement in Patients with Squamous Cell Carcinoma of the Penis: Gruppo Uro-Oncologico Del Nord Est (Northeast Uro-Oncological Group) Penile Cancer Data Base Data. Cancer 2005, 103, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Sun, J.; Wei, X.; Wu, G.; Wang, F.; Fan, C.; Yuan, H. Prognostic Value of Lymphovascular Invasion in Patients with Squamous Cell Carcinoma of the Penis Following Surgery. BMC Cancer 2019, 19, 476. [Google Scholar] [CrossRef]
- Lopes, A.; Hidalgo, G.S.; Kowalski, L.P.; Torloni, H.; Rossi, B.M.; Fonseca, F.P. Prognostic Factors in Carcinoma of the Penis: Multivariate Analysis of 145 Patients Treated with Amputation and Lymphadenectomy. J. Urol. 1996, 156, 1637–1642. [Google Scholar] [CrossRef]
- Wei, Z.; Yu, Z.; Li, H.; Peng, W.; Zhang, J.; Zhang, Y.; Song, W.; Liu, J.; Yang, W.; Wang, T. The Appropriate Number of Negative Lymph Nodes Dissection for Nonmetastatic Penile Cancer. Andrologia 2019, 51, e13154. [Google Scholar] [CrossRef]
- Sanchez, D.F.; Fernandez-Nestosa, M.J.; Canete-Portillo, S.; Rodriguez, I.; Cubilla, A.L. What Is New in the Pathologic Staging of Penile Carcinoma in the 8th Edition of AJCC TNM Model: Rationale for Changes with Practical Stage-by-Stage Category Diagnostic Considerations. Adv. Anat. Pathol. 2021, 28, 209–227. [Google Scholar] [CrossRef]
- Wang, B.; Gu, W.; Wan, F.; Wei, Y.; Xiao, W.; Lu, X.; Zhang, G.; Zhou, J.; Wang, Q.; Ding, X.; et al. Prognosis of the 8th TNM Staging System for Penile Cancer and Refinement of Prognostication by Incorporating High Risk Human Papillomavirus Status. J. Urol. 2020, 203, 562–569. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Lam, W.; Cao, Y.; Geng, J.; Ornellas, A.A.; Zhou, F.; Han, H. Corpora Cavernos Invasion vs. Corpus Spongiosum Invasion in Penile Cancer: A Systematic Review and Meta-Analysis. J. Cancer 2021, 12, 1960–1966. [Google Scholar] [CrossRef]

| Histological Subtype | HPV Negative | High-Risk HPV | Not Evaluable | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Intraepithelial neoplasia | 7 | 2.7 | 2 | 66.7 | 1 | 33.3 | 4 | |
| Non-HPV- related squamous cell carcinoma | Usual type | 140 | 53.3 | 101 | 78.9 | 27 | 21.1 | 12 |
| Pseudohyperplastic | 11 | 4.2 | 11 | 100 | 0 | 0.0 | - | |
| Pseudoglandular | 1 | 0.4 | 1 | 100 | 0 | 0.0 | - | |
| Pure verrucous | 12 | 4.6 | 9 | 81.8 | 2 | 18.2 | 1 | |
| Carcinoma cunilatum | 3 | 1.1 | 3 | 100 | 0 | 0.0 | - | |
| Papillary | 2 | 0.8 | 1 | 100 | 0 | 0.0 | 1 | |
| Sarcomatoid | 3 | 1.1 | 3 | 100 | 0 | 0.0 | - | |
| Mixed tumors | 1 | 0.4 | 1 | 100 | 0 | 0.0 | - | |
| HPV-related squamous cell carcinoma | Basaloid | 30 | 11.4 | 6 | 22.2 | 21 | 77.8 | 3 |
| Papillary-basaloid | 2 | 0.8 | 1 | 50.0 | 1 | 50.0 | - | |
| Warty | 15 | 5.7 | 12 | 80.0 | 3 | 20.0 | - | |
| Warty-basaloid | 35 | 13.3 | 12 | 34.3 | 23 | 65.7 | - | |
| Clear cell | 1 | 0.4 | 0 | 0.0 | 1 | 100 | - | |
| n = 297 | Seventh Edition | Eighth Edition | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Primary tumor | pTis | 3 | 1.2 | 3 | 1.2 |
| pT1a | 65 | 26.2 | 64 | 25.8 | |
| pT1b | 33 | 13.3 | 34 | 13.7 | |
| pT2 | 89 | 35.9 | 79 | 31.9 | |
| pT3 | 51 | 20.6 | 62 | 25.0 | |
| pT4 | 7 | 2.8 | 6 | 2.4 | |
| n/a | 49 | 49 | |||
| Regional lymph nodes | N0 | 167 | 68.7 | 167 | 68.8 |
| cN0 | 49 | 20.1 | 49 | 20.2 | |
| pN0 | 118 | 48.6 | 118 | 48.6 | |
| pN1 | 20 | 8.2 | 20 | 8.2 | |
| pN2 | 25 | 10.3 | 25 | 10.3 | |
| pN3 | 31 | 12.8 | 31 | 12.8 | |
| n/a | 54 | 54 | |||
| Distant metastasis | pM0 | 242 | 96.0 | 242 | 96.0 |
| pM1 | 10 | 4.0 | 10 | 4.0 | |
| n/a | 45 | 45 | |||
| Tumor characteristics | n | % | |||
| Grading | G1 | 38 | 13.6 | ||
| G2 | 150 | 53.6 | |||
| G3 | 92 | 32.8 | |||
| n/a | 17 | ||||
| Lymphovascular invasion (L1) | L0 | 220 | 82.1 | ||
| L1 | 48 | 17.9 | |||
| n/a | 29 | ||||
| Vascular invasion (V1) | V0 | 202 | 78.6 | ||
| V1 | 55 | 21.4 | |||
| n/a | 40 | ||||
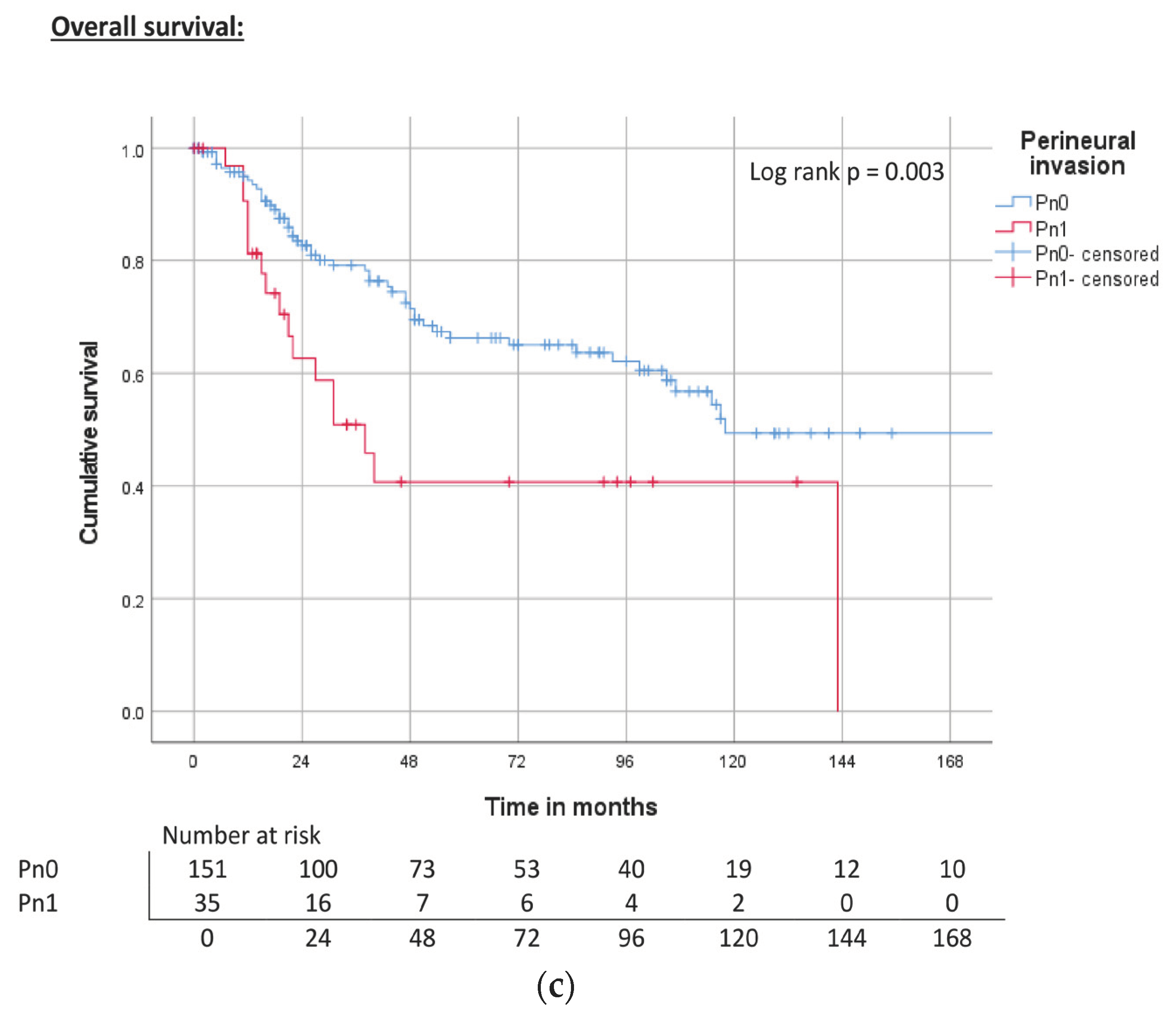
| Perineural invasion | Pn0 | 151 | 81.2 | ||
| Pn1 | 35 | 18.8 | |||
| n/a | 111 | ||||
| Tumor extension | |||||
| Corpus spongiosum | No | 113 | 47.5 | ||
| Yes | 125 | 52.5 | |||
| n/a | 59 | ||||
| Corpus cavernosum | No | 172 | 72.6 | ||
| Yes | 65 | 27.4 | |||
| n/a | 60 | ||||
| Urethra | No | 184 | 78.0 | ||
| Yes | 52 | 22.0 | |||
| n/a | 61 | ||||
| Adjacent structures | No | 229 | 96.6 | ||
| Yes | 8 | 3.4 | |||
| n/a | 60 | ||||
| Clinico-Pathological Parameters | Metastasis-Free Survival | Cancer-Specific Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median Survival Rate | 5-Year Survival Rate | Median Survival Rate | 5-Year Survival Rate | Median Survival Rate | 5-Year Survival Rate | |||||
| Month | p | Survival | Month | p | Survival | Month | p | Survival | ||
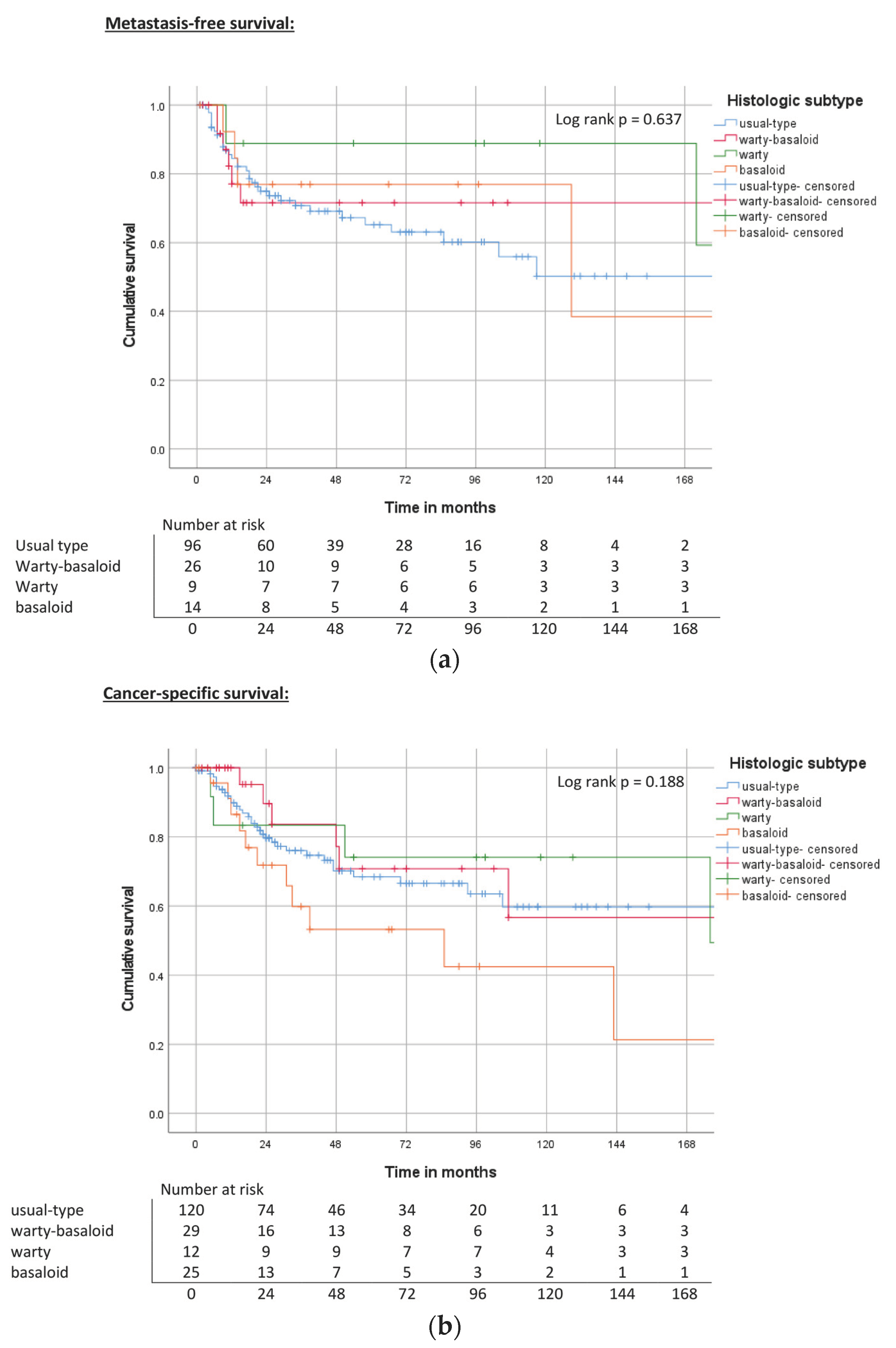
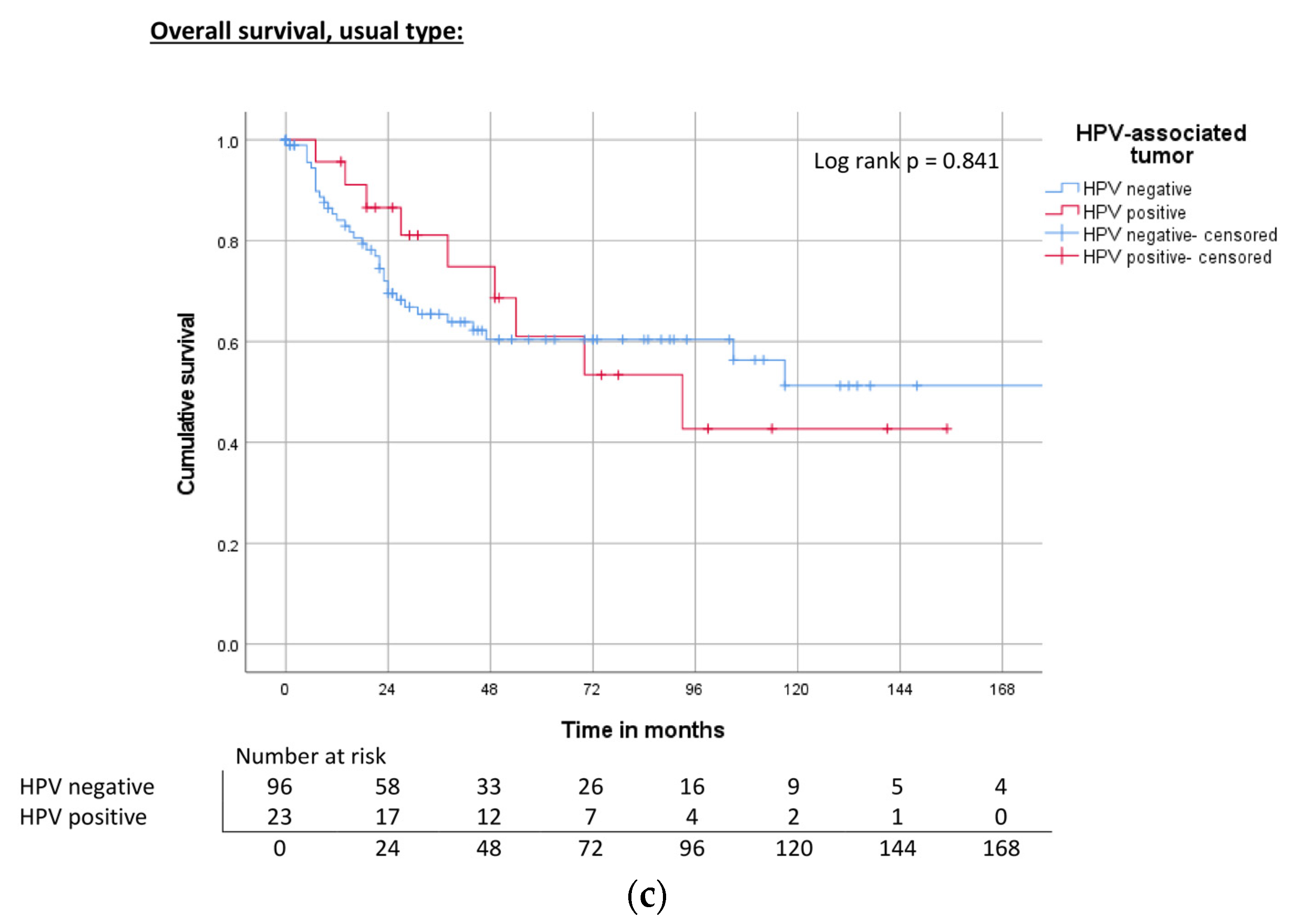
| Histologic subtype | Usual type | ND | 0.64 | 65% | ND | 0.19 | 69% | 117 | 0.46 | 59% |
| Basaloid | 129 | 76% | 85 | 54% | 39 | 45% | ||||
| Warty | ND | 89% | 176 | 74% | 176 | 74% | ||||
| Warty-basaloid | ND | 71% | ND | 70% | 57 | 49% | ||||
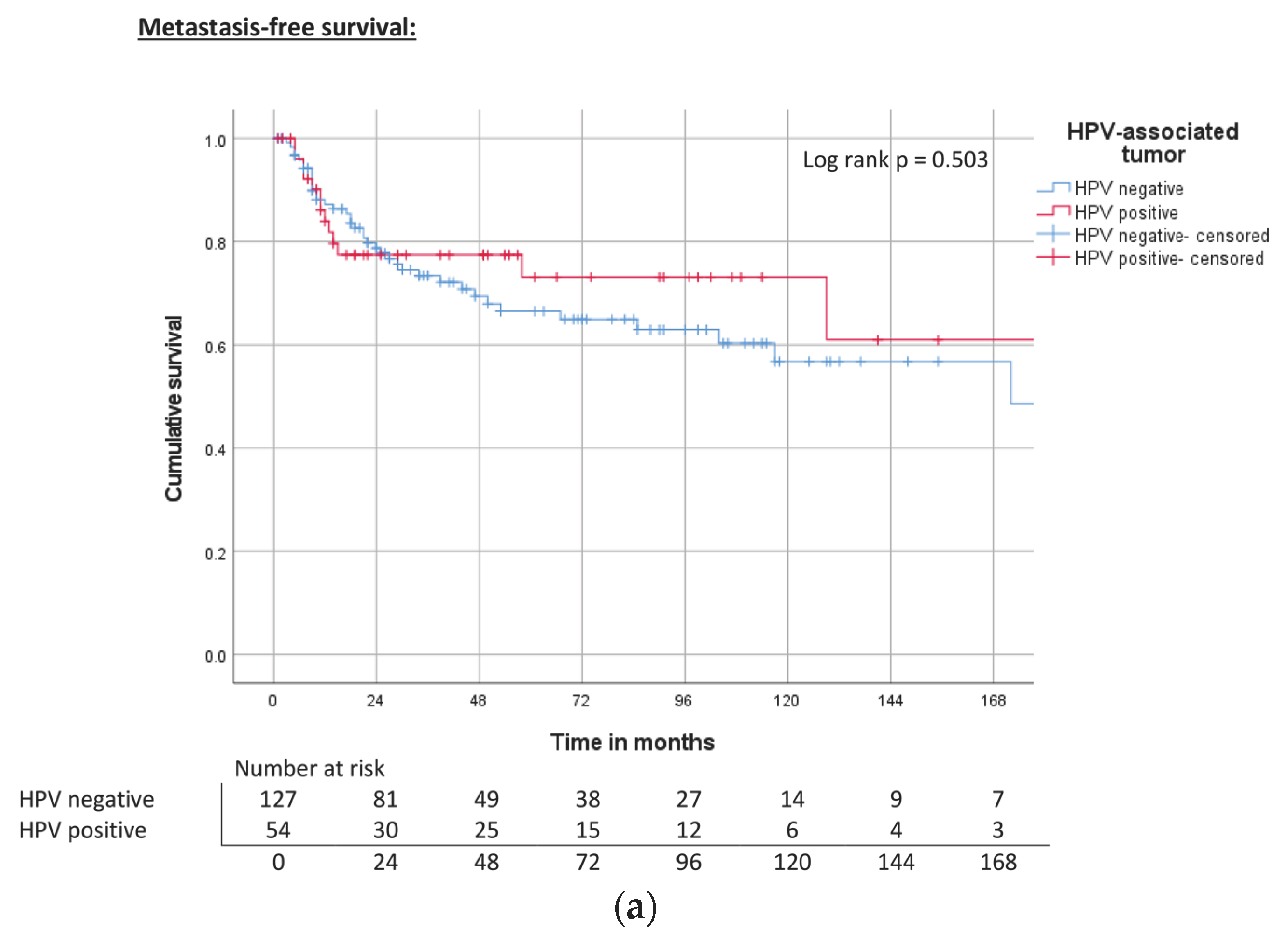
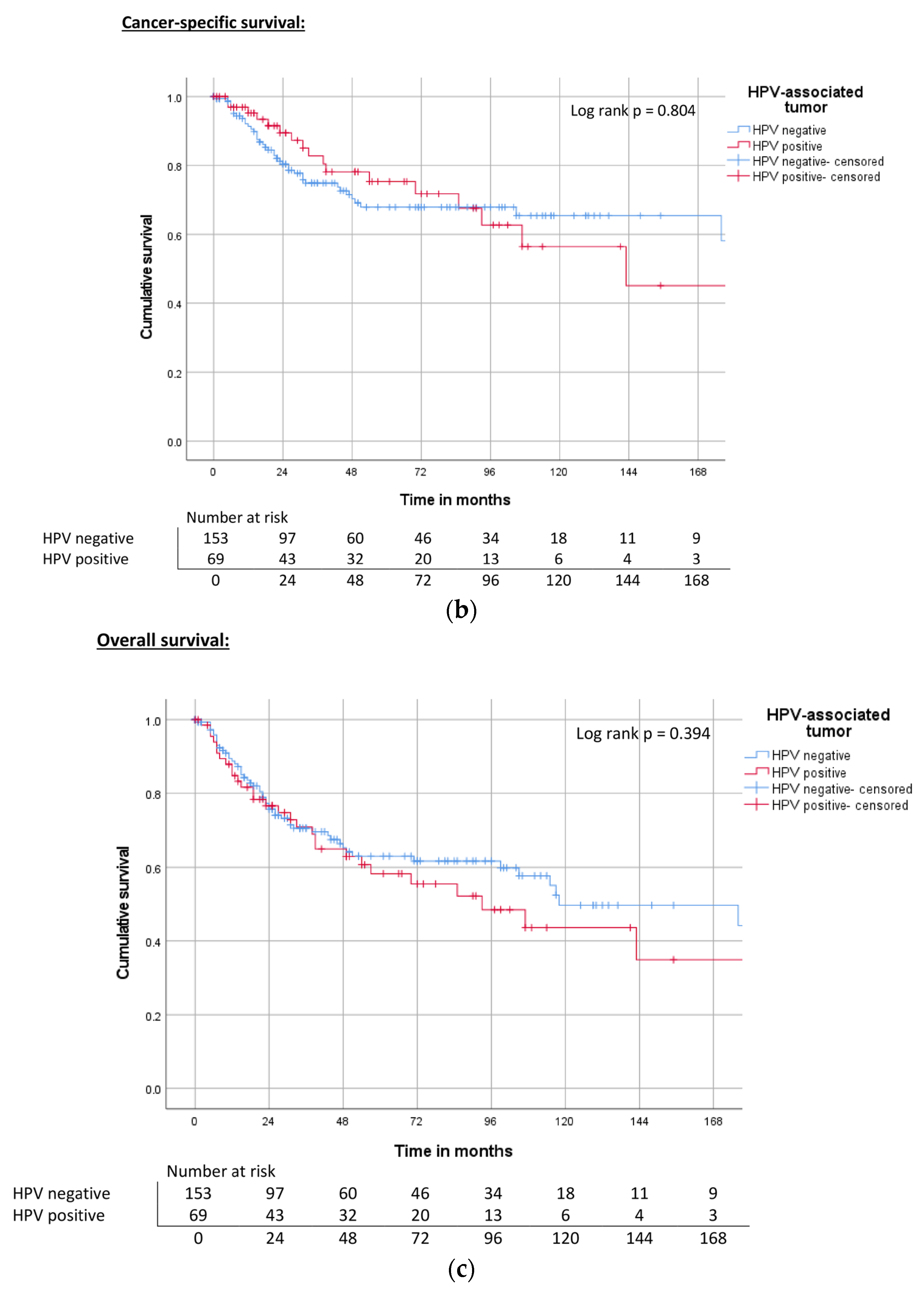
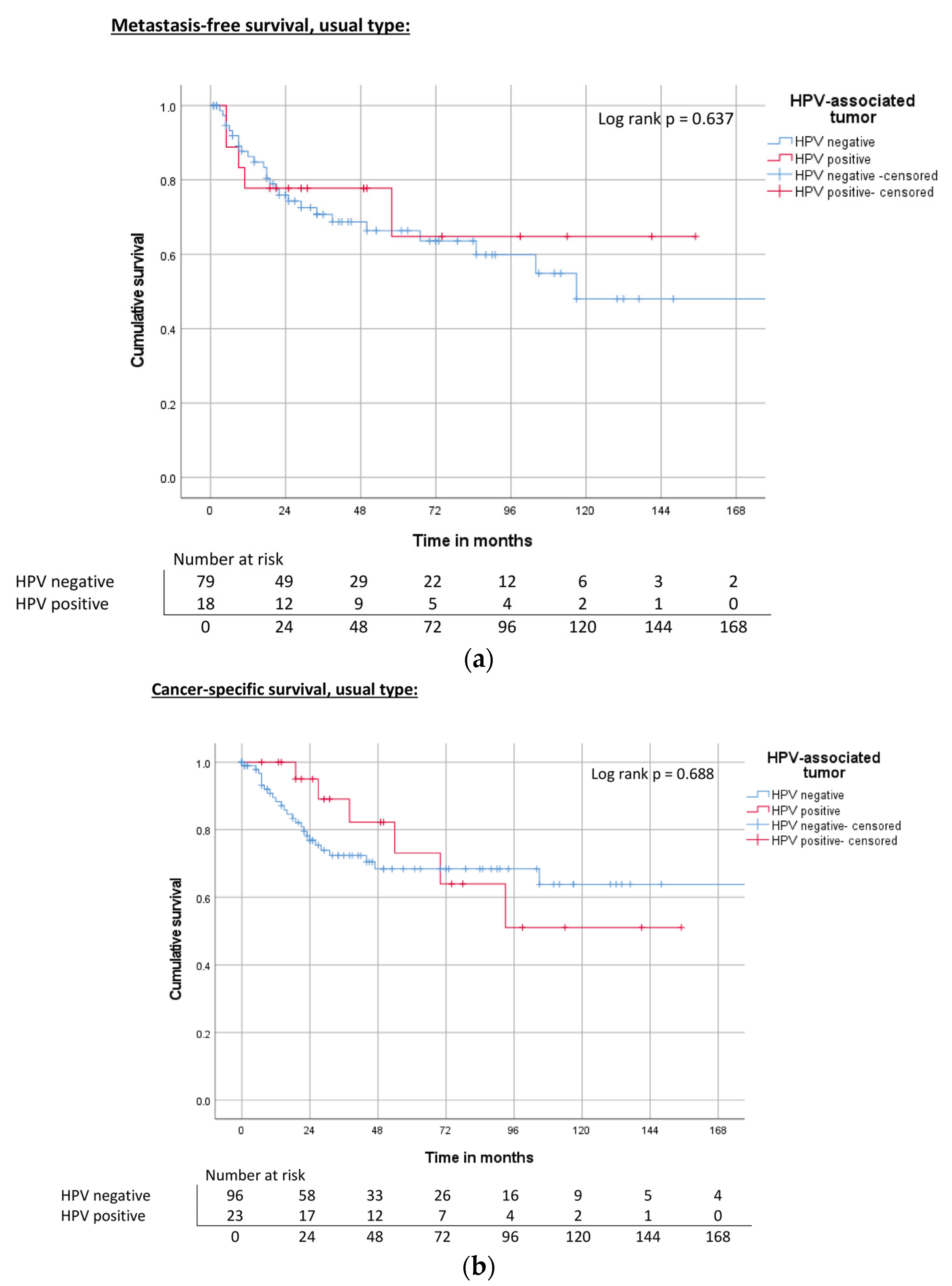
| HPV | Negative | 172 | 0.50 | 67% | ND | 0.80 | 68% | 118 | 0.394 | 63% |
| Hr-HPV positive | ND | 73% | 143 | 75% | 93 | 58% | ||||
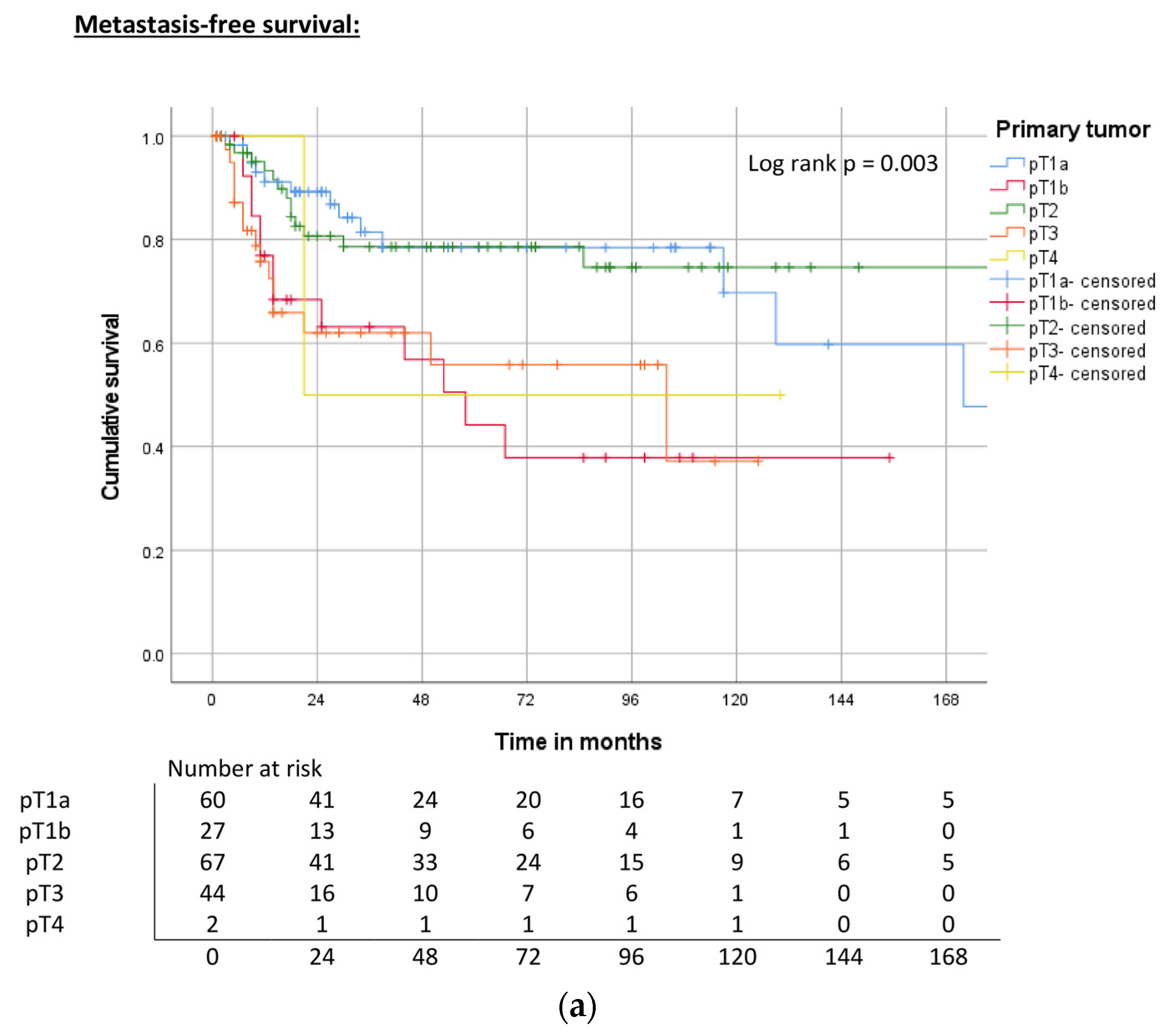
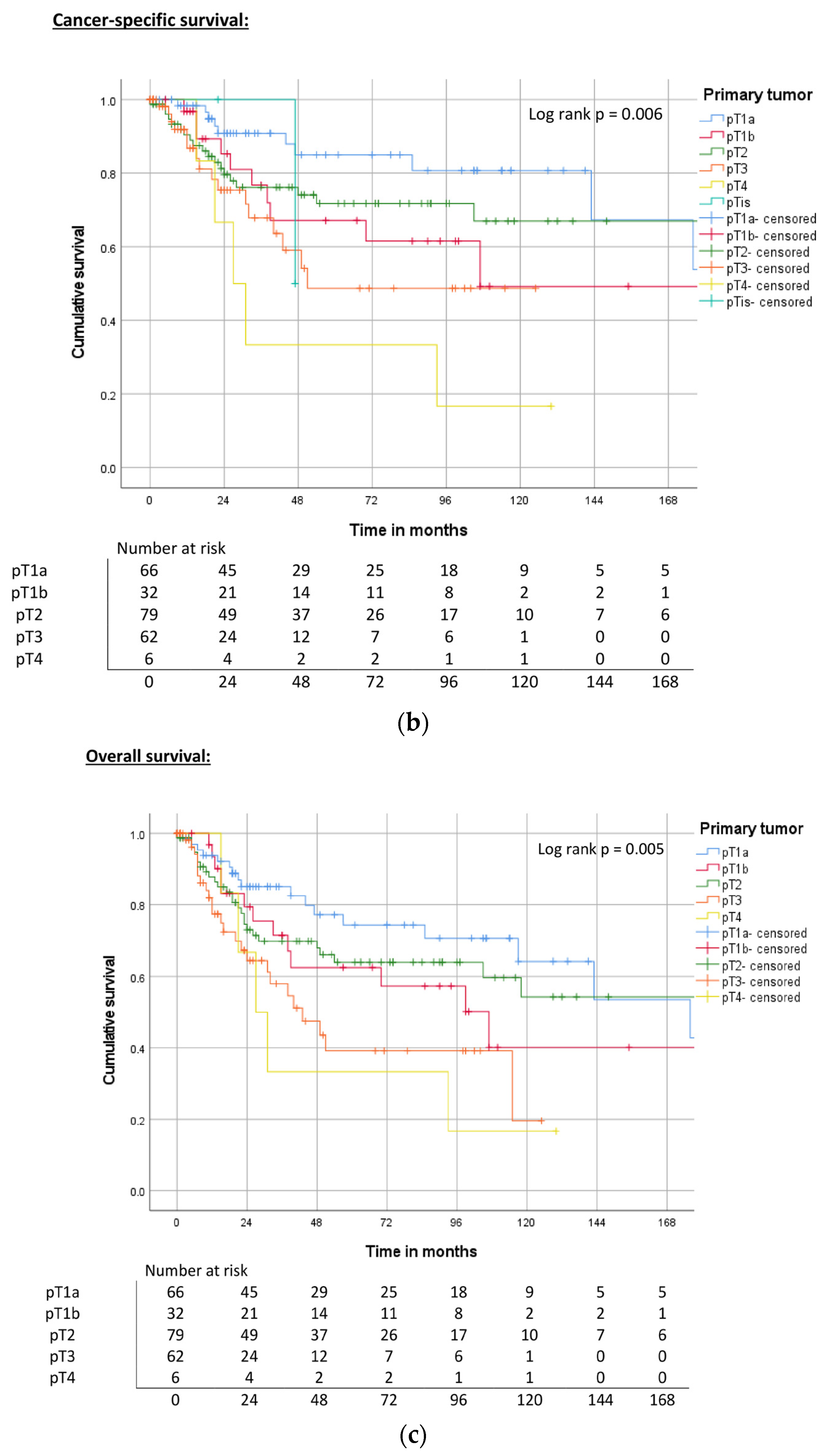
| Primary tumor | pT1a | 172 | 0.003 | 78% | ND | 0.006 | 85% | 176 | 0.010 | 74% |
| pT1b | 58 | 44% | 107 | 68% | 107 | 63% | ||||
| pT2 | ND | 78% | ND | 72% | 240 | 64% | ||||
| pT3 | 104 | 56% | 51 | 49% | 43 | 39% | ||||
| pT4 | 21 | 50% | 27 | 33% | 27 | 33% | ||||
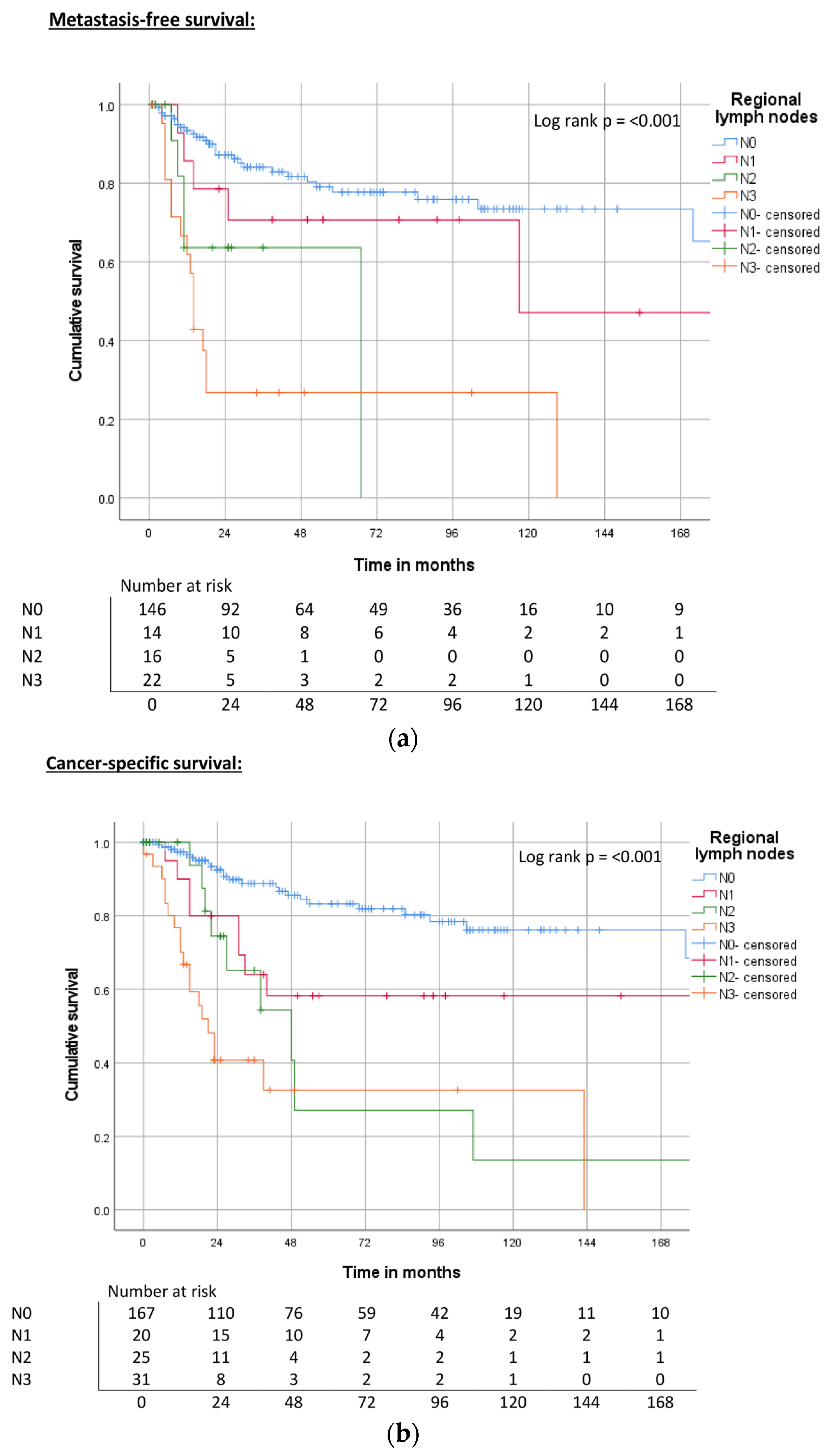
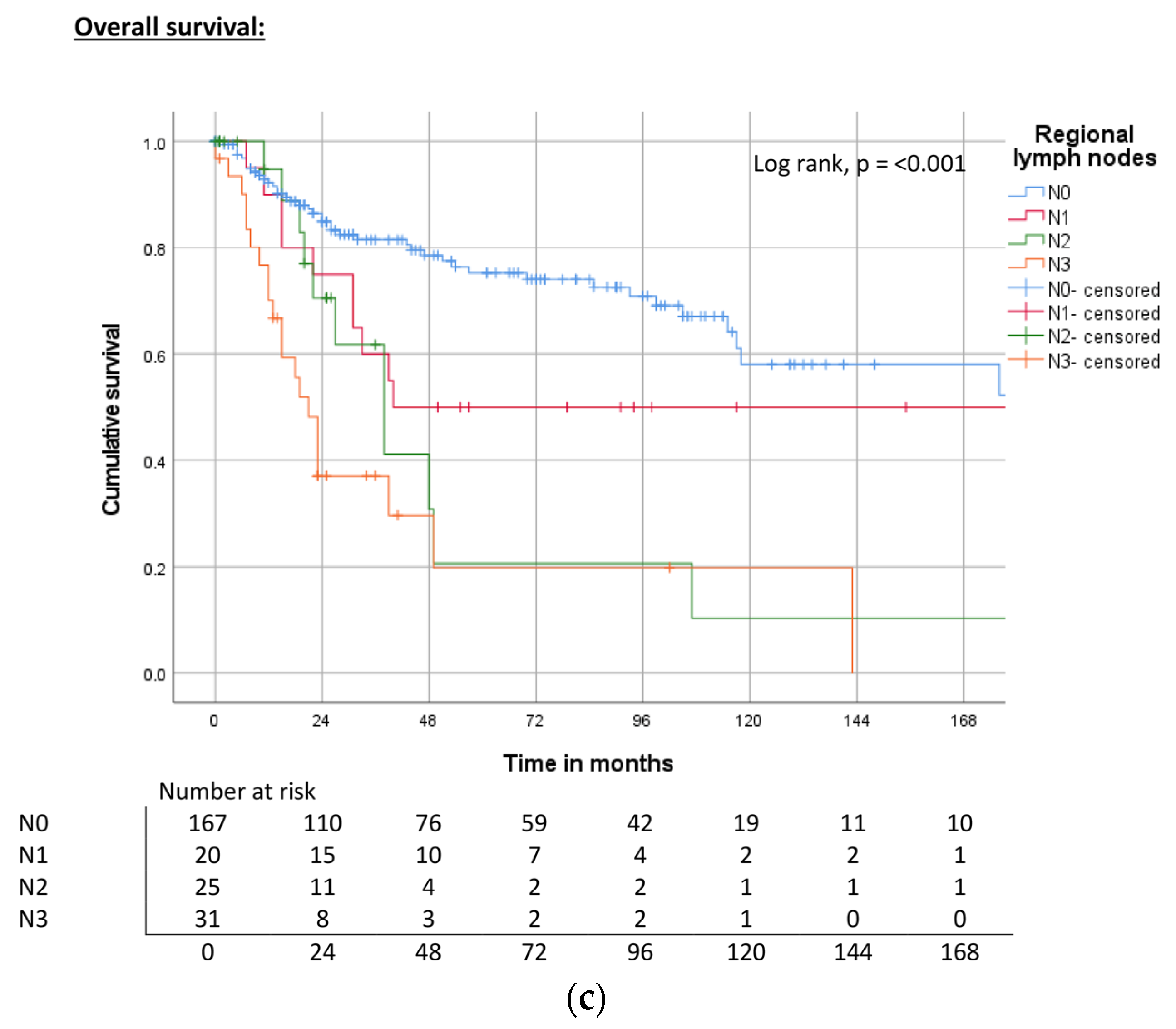
| Regional lymph nodes | pN0, cN0 | ND | <0.001 | 78% | 214 | <0.001 | 83% | 240 | <0.001 | 75% |
| pN1 | 117 | 70% | 178 | 58% | 40 | 50% | ||||
| pN2 | 67 | 69% | 100 | 28% | 38 | 22% | ||||
| pN3 | 14 | 27% | 83 | 32% | 21 | 20% | ||||
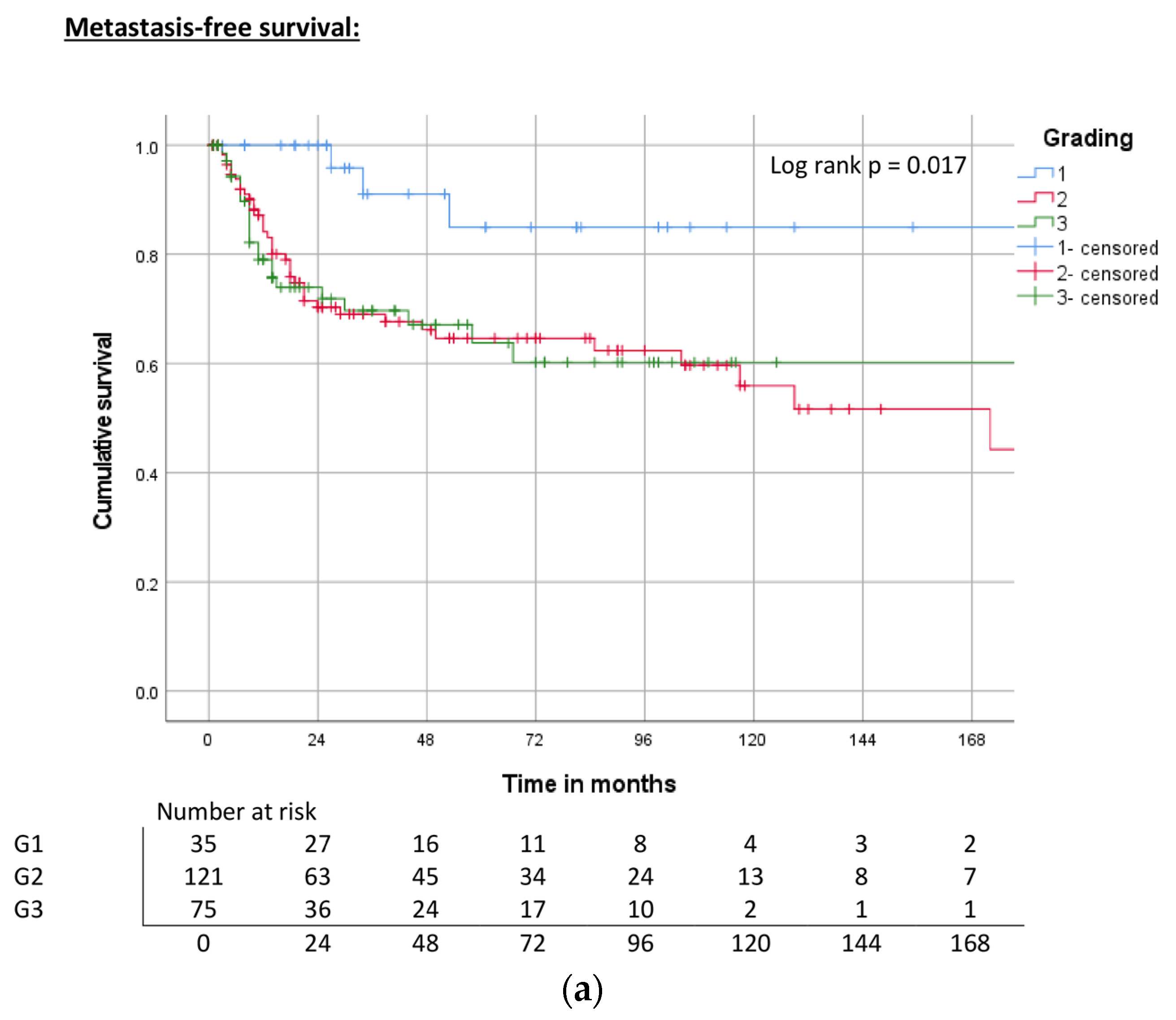
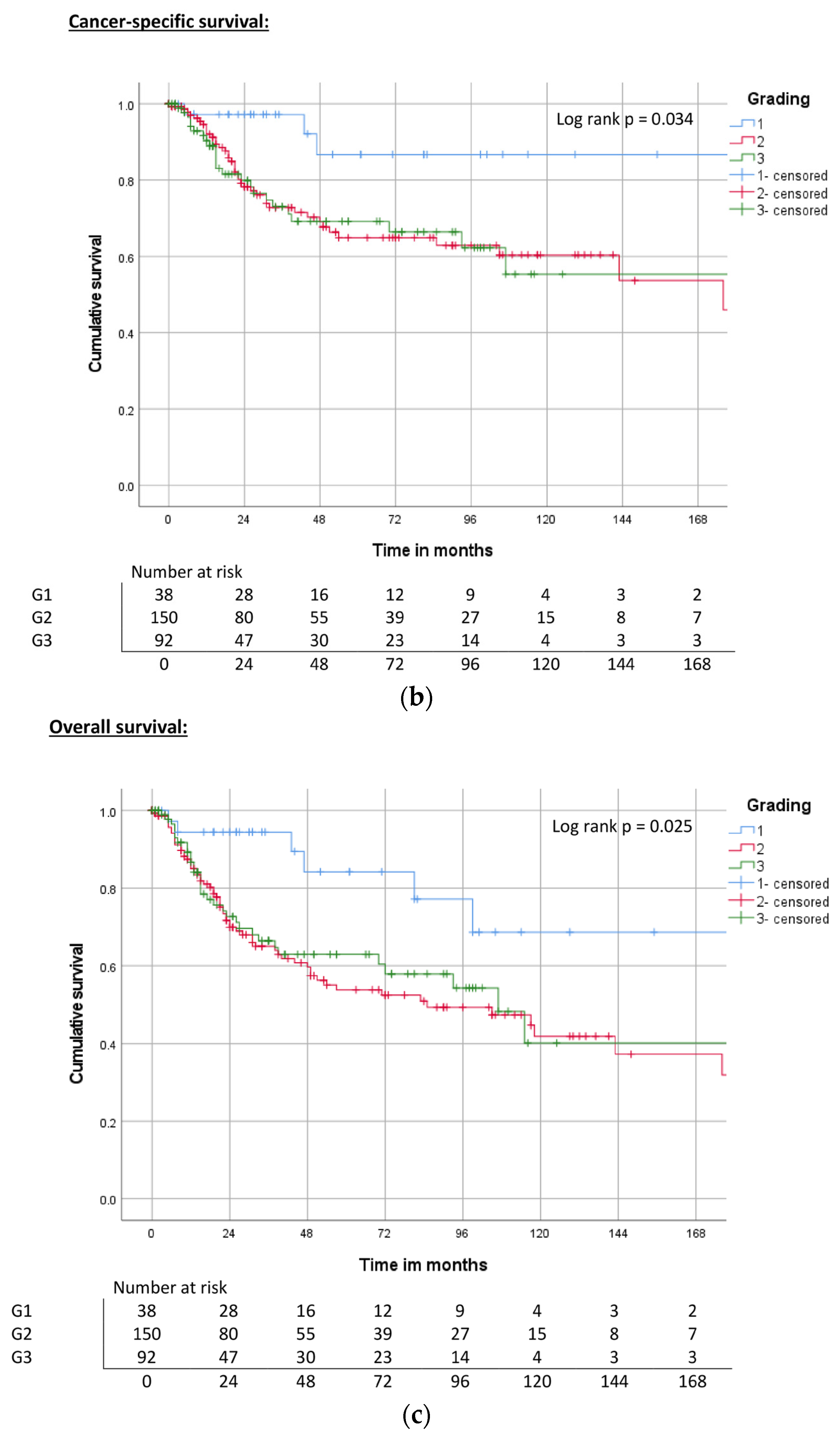
| Grading | G1 | ND | 0.017 | 84% | ND | 0.034 | 85% | ND | 0.025 | 83% |
| G2 | 172 | 65% | 176 | 65% | 85 | 53% | ||||
| G3 | ND | 60% | ND | 66% | 107 | 60% | ||||
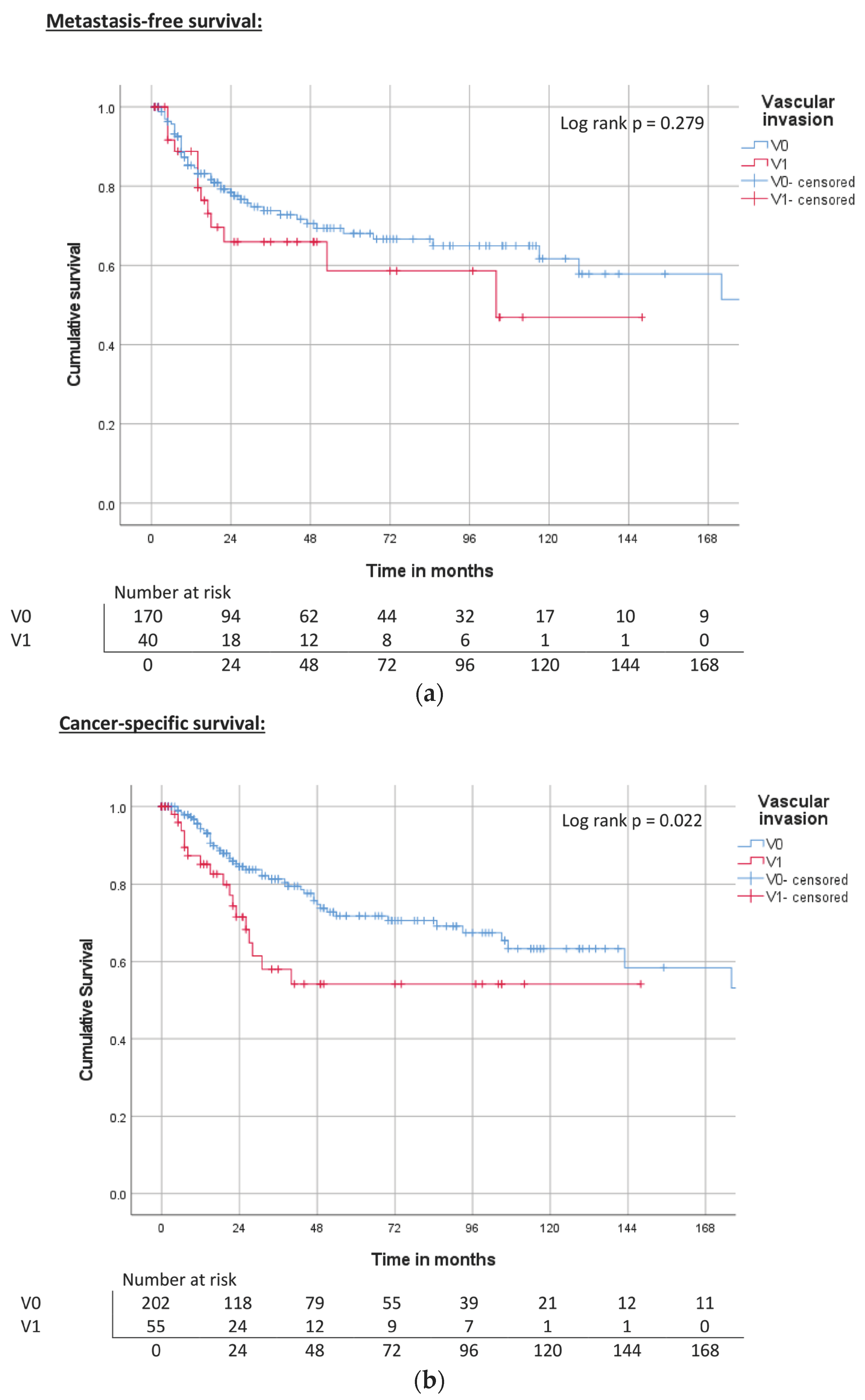
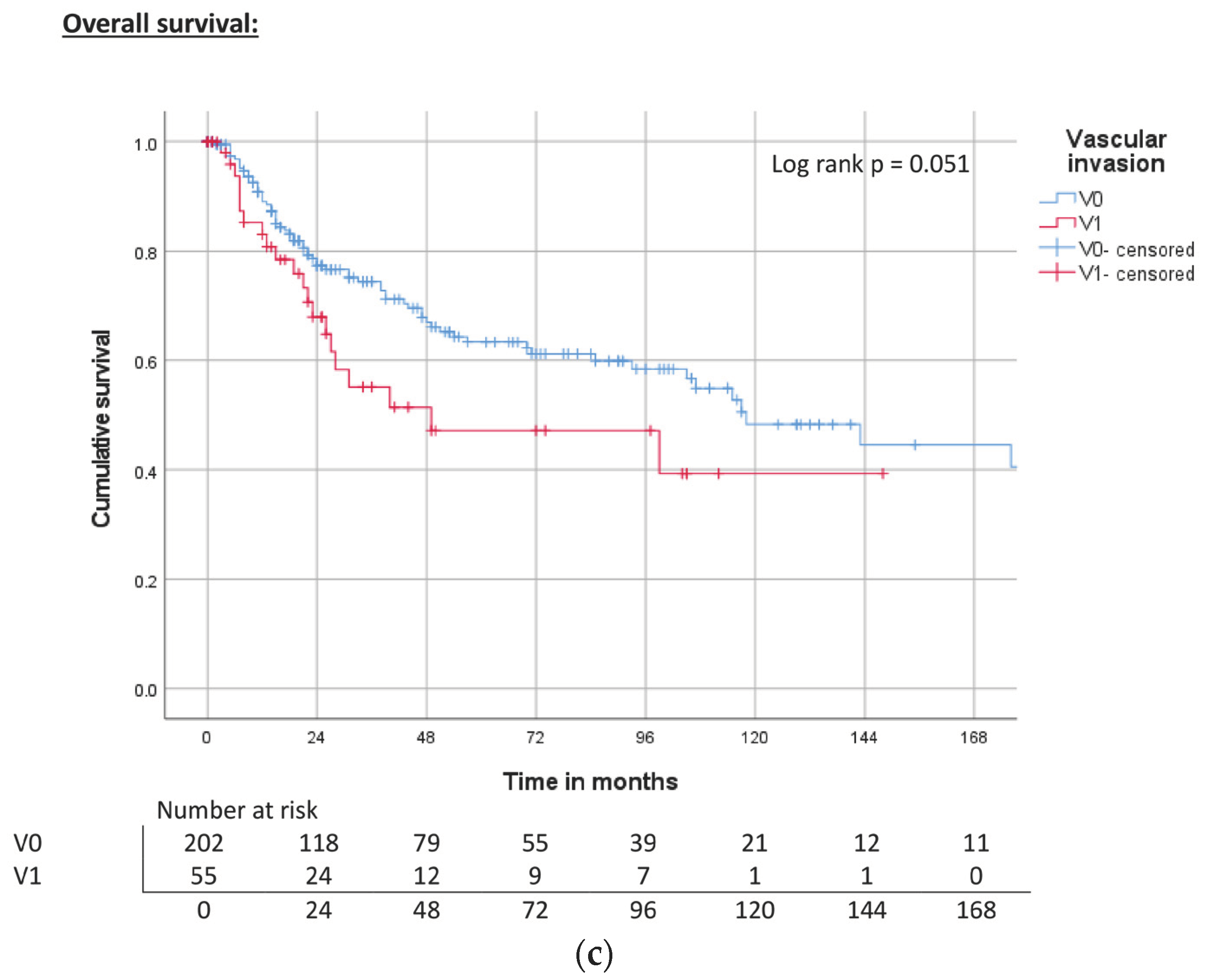
| Vascular invasion | V0 | ND | 0.279 | 68% | 240 | 0.109 | 72% | 143 | <0.001 | 64% |
| V1 | 104 | 60% | ND | 56% | 31 | 50% | ||||
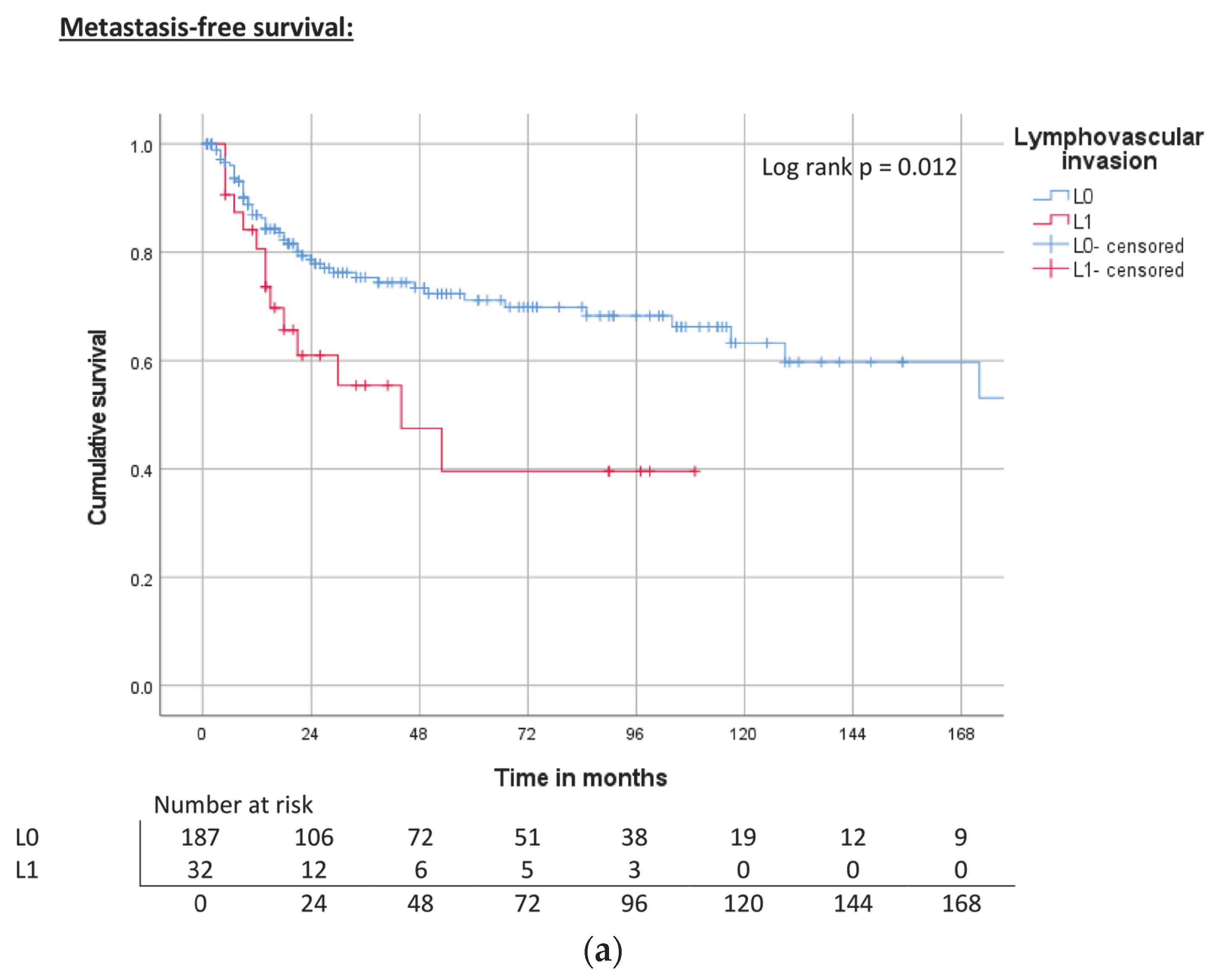
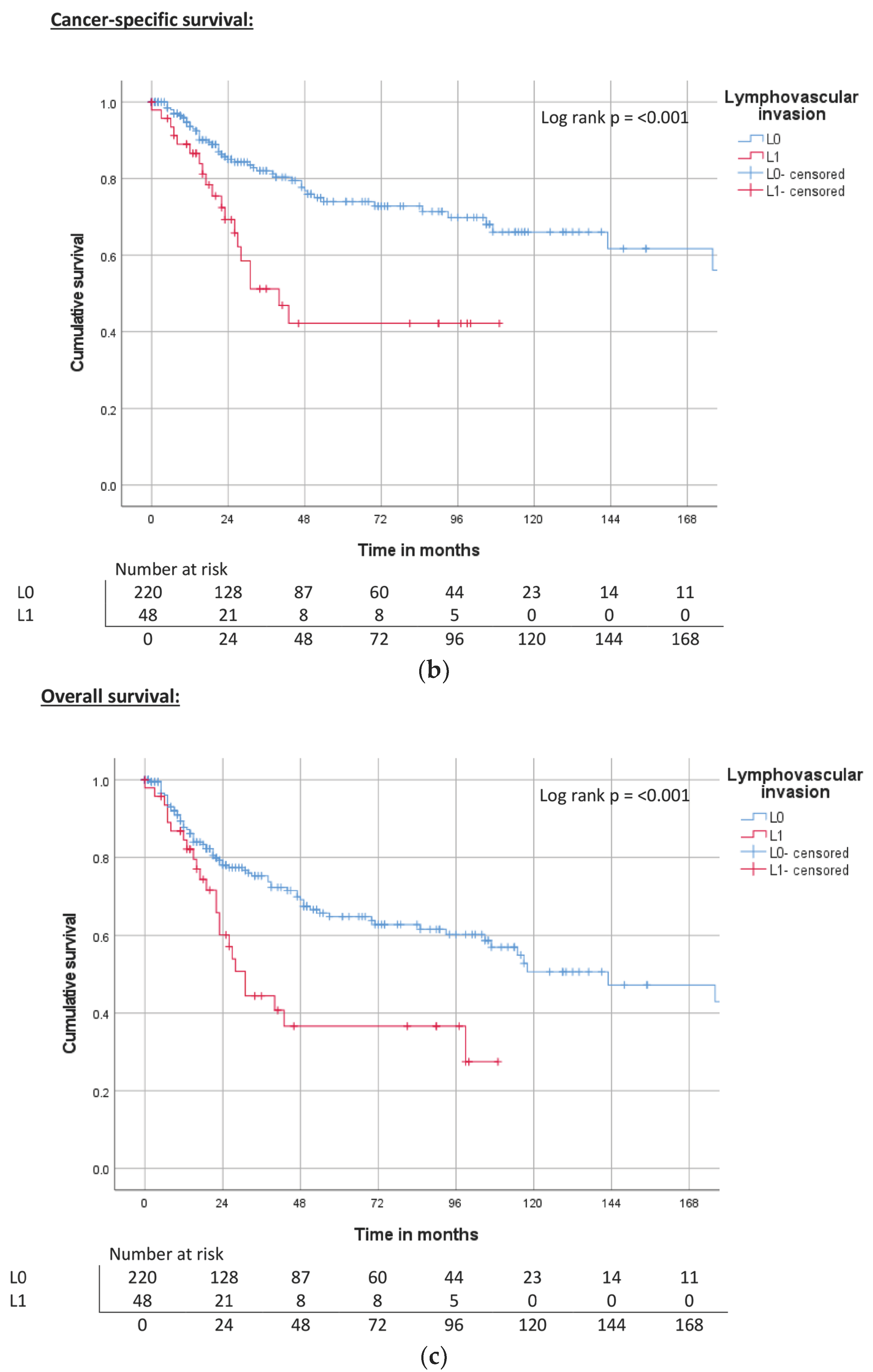
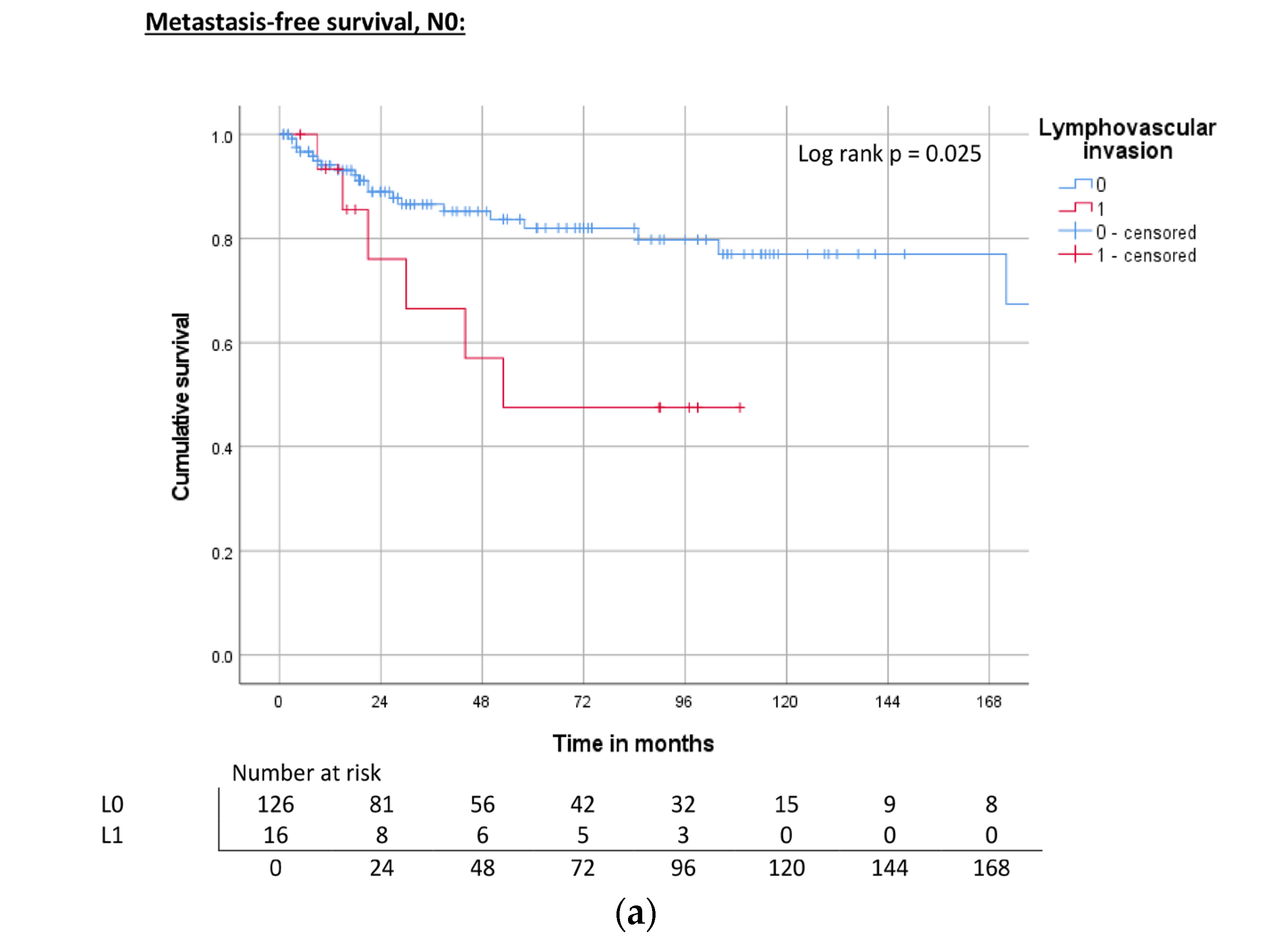
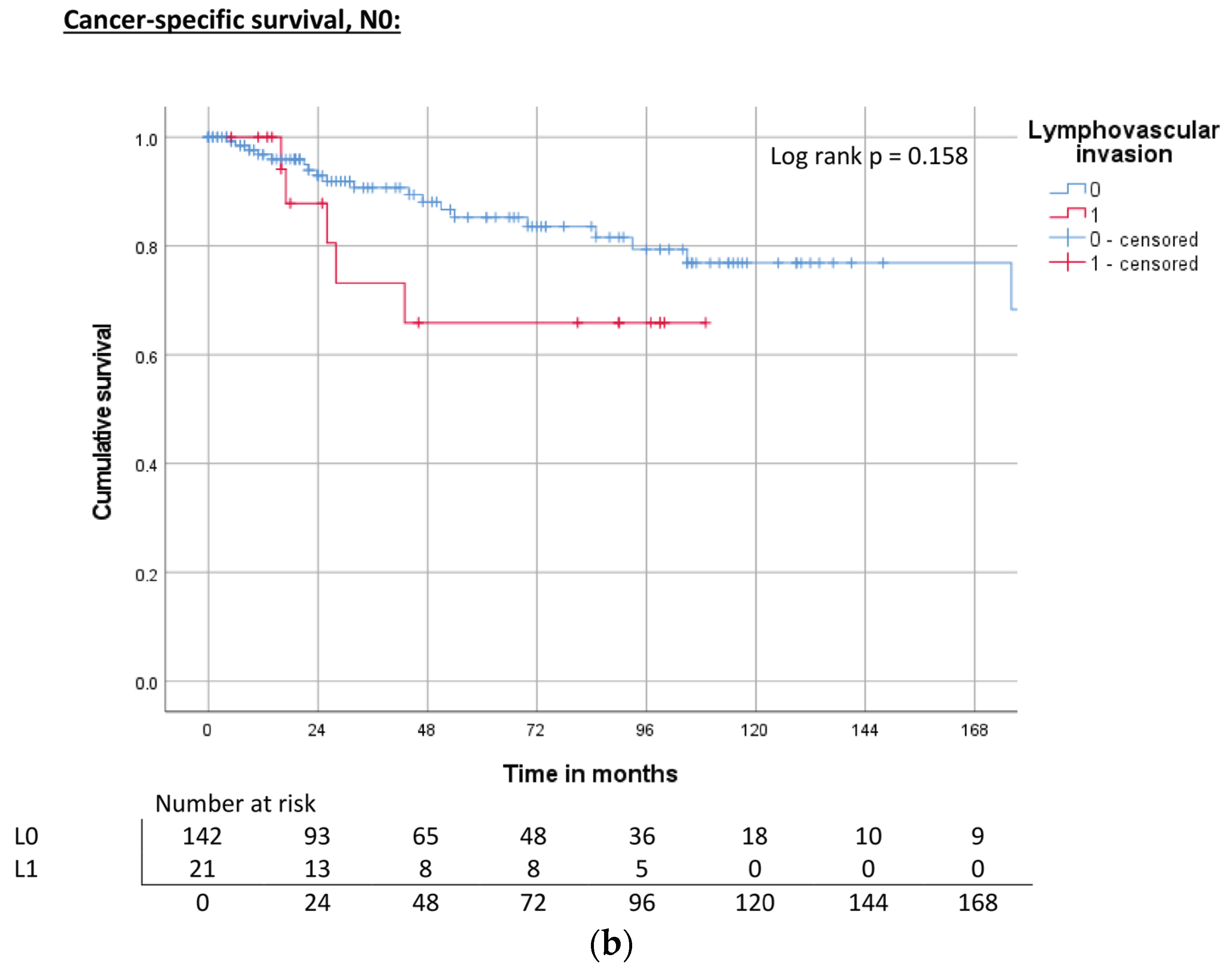
| Lymphovascular invasion | L0 | ND | 0.012 | 71% | 240 | 0.002 | 74% | 118 | 0.004 | 67% |
| L1 | 44 | 40% | 40 | 39% | 31 | 38% | ||||
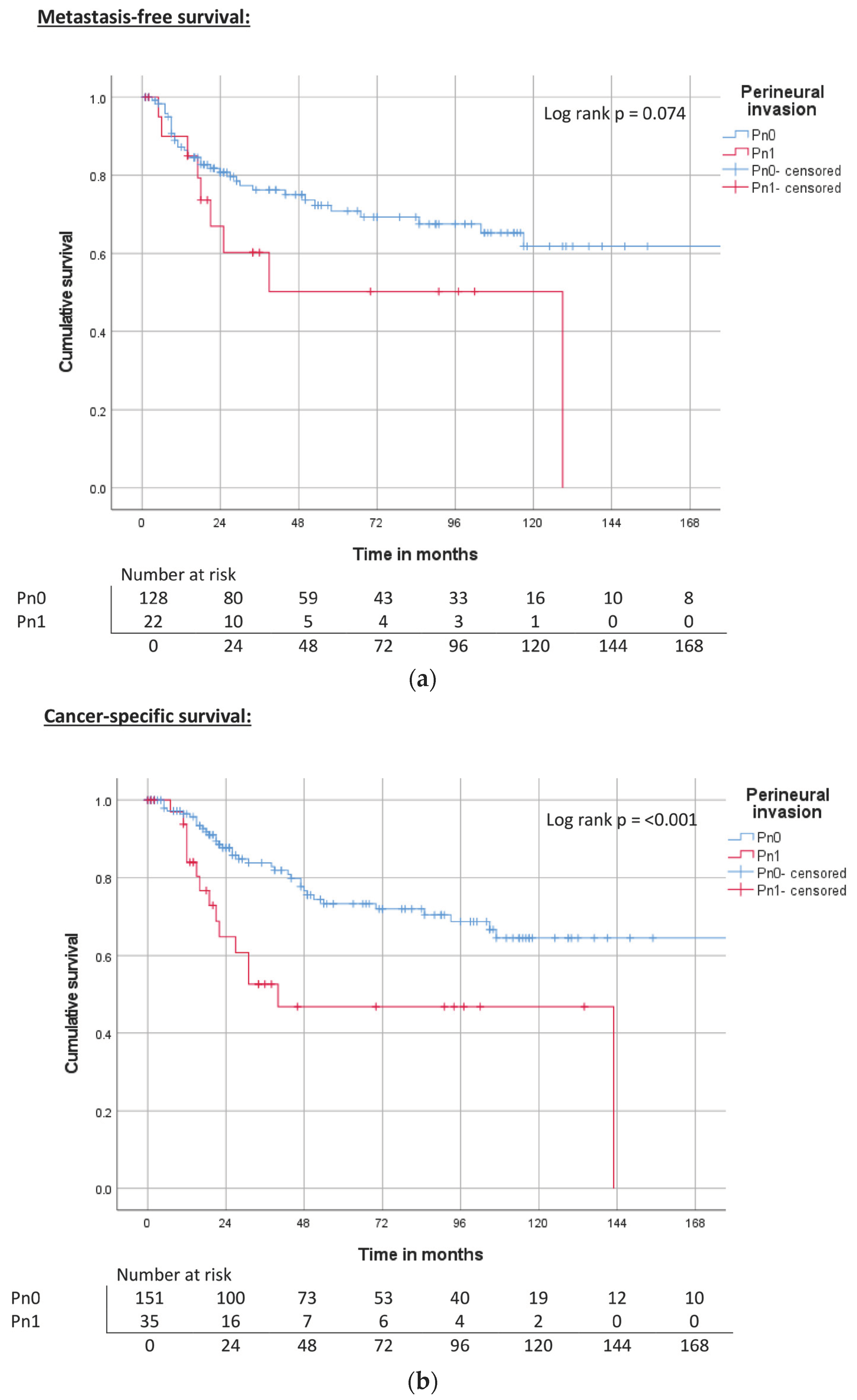
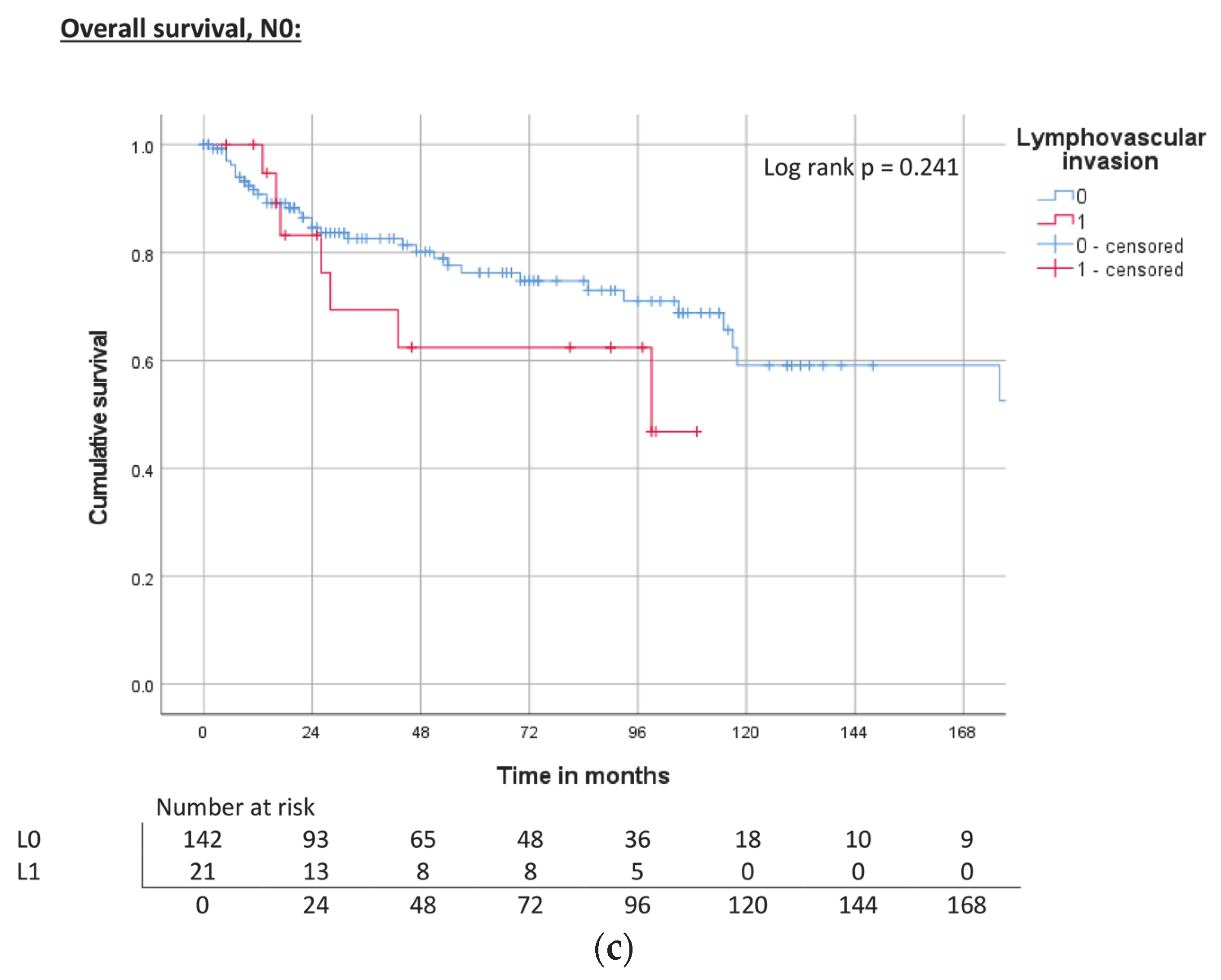
| Perineural invasion | Pn0 | ND | 0.074 | 71% | 240 | <0.001 | 74% | 143 | <0.001 | 67% |
| Pn1 | 129 | 51% | 40 | 47% | 22 | 41% | ||||
| Clinico-Pathological Parameters | Metastasis-Free Survival | Cancer-Specific Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | ||
| Primary tumor | pT1a | Reference | Reference | Reference | |||
| pT1b | 2.9 (1.3–6.2) | 0.008 | 2.2 (0.9–5.2) | 0.084 | 1.7 (0.8–3.5) | 0.158 | |
| pT2 | 0.92 (0.4–2.0) | 0.824 | 1.7 (0.8–3.6) | 0.178 | 1.4 (0.7–2.5) | 0.316 | |
| pT3 | 2.7 (1.3–5.8) | 0.009 | 3.2 (1.4–7.1) | 0.004 | 2.8 (1.5–5.2) | 0.001 | |
| pT4 | 1.8 (0.2–13.9) | 0.574 | 6.1 (2.1–17.8) | 0.001 | 3.5 (1.3–9.4) | 0.015 | |
| Regional lymph nodes | pN0, cN0 | Reference | Reference | Reference | |||
| pN1 | 1.7 (0.7–4.4) | 0.279 | 2.5 (1.1–5.6) | 0.023 | 1.9 (0.9–3.7) | 0.079 | |
| pN2 | 3.8 (1.5–10.1) | 0.007 | 4.3 (1.9–9.2) | <0.001 | 3.0 (1.5–5.9) | 0.001 | |
| pN3 | 7.1 (3.8–13.4) | <0.001 | 8.4 (4.5–15.7) | <0.001 | 5.2 (3.0–8.9) | <0.001 | |
| Grading | G1 | Reference | Reference | Reference | |||
| G2 | 4.5 (1.4–14.7) | 0.012 | 4.0 (1.2–13.0) | 0.02 | 3.0 (1.3–7.0) | 0.010 | |
| G3 | 4,7 (1,4–15,7) | 0.012 | 4.2 (1.3–14.0) | 0.019 | 2.7 (1.1–6.5) | 0.026 | |
| Vascular invasion | V0 | Reference | Reference | Reference | |||
| V1 | 1.4 (0.8–2.6) | 0.284 | 1.9 (1.1–3.3) | 0.025 | 1.6 (1.0–2.7) | 0.054 | |
| Lymphovascular invasion | L0 | Reference | Reference | Reference | |||
| L1 | 2.1 (1.2–3.9) | 0.015 | 2.6 (1.5–4.5) | 0.001 | 2.2 (1.4–3.5) | 0.001 | |
| Perineural invasion | Pn0 | Reference | Reference | Reference | |||
| Pn1 | 1.9 (0.9–4.1) | 0.081 | 2.8 (1.5–5.1) | 0.001 | 2.3 (1.3–4.0) | 0.004 | |
| Clinico-Pathological Parameters | Metastasis-Free Survival | Cancer-Specific Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | ||
| Primary tumor | pT1a | Reference | Reference | Reference | |||
| pT1b | 7.8 (1.6–13.9) | 0.017 | n.s | 0.939 | n.s | 0.703 | |
| pT2 | 0.8 (0.3–1.9) | 0.097 | n.s | 0.827 | n.s | 0.970 | |
| pT3 | 2.2 (0.9–5.5) | 0.324 | n.s | 0.675 | n.s | 0.895 | |
| pT4 | 1.9 (0.2–14.7) | 0.475 | 0.035 | n.s | 0.134 | ||
| Regional lymph nodes | pN0, cN0 | Reference | Reference | Reference | |||
| pN1 | 1.8 (0.7–4.8) | 0.216 | 2.2 (0.8–6.0) | 0.138 | 2.1 (0.9–5.0) | 0.082 | |
| pN2 | 3.9 (1.5–10.2) | 0.007 | 4.9 (2.1–11.6) | <0.001 | 4.2 (2.0–8.7) | <0.001 | |
| pN3 | 6.8 (3.5–13.0) | <0.001 | 5.9 (2.7–12.8) | <0.001 | 4.2 (2.1–8.4) | <0.001 | |
| Grading | G1 | Reference | Reference | Reference | |||
| G2 | n.s | 0.221 | n.s | 0.510 | n.s | 0.410 | |
| G3 | n.s | 0.959 | n.s | 0.864 | n.s | 0.891 | |
| Vascular invasion | V0 | Reference | Reference | Reference | |||
| V1 | n.s | n.s | n.s | 0.430 | n.s | n.s | |
| Lymphovascular invasion | L0 | Reference | Reference | Reference | |||
| L1 | 1.6 (0.8–3.3) | 0.346 | 2.7 (1.4–5.4) | 0.005 | 2.3 (1.3–4.4) | 0.007 | |
| Perineural invasion | Pn0 | Reference | Reference | Reference | |||
| Pn1 | n.s | n.s | n.s | 0.624 | n.s. | 0.628 | |
| Clinico-Pathological Parameters | Metastasis-Free Survival | Cancer-Specific Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | ||
| Primary tumor | pT1a | Reference | Reference | Reference | |||
| pT1b | 1.7 (0.5–5.7) | 0.367 | 2.2 (0.5–9.4) | 0.280 | 2.1 (0.8–5.8) | 0.141 | |
| pT2 | 1.0 (0.4–2.4) | 0.927 | 1.7 (0.6–5.1) | 0.337 | 1.0 (0.5–2.4) | 0.922 | |
| pT3 | 1.4 (0.4–4.6) | 0.577 | 2.7 (0.7–10.1) | 0.147 | 2.7 (1.1–6.5) | 0.031 | |
| pT4 | 2.5 (0.3–20.0) | 0.382 | 6.3 (1.2–32.5) | 0.030 | 2.7 (0.6–12.2) | 0.200 | |
| Grading | G1 | Reference | Reference | Reference | |||
| G2 | 3.5 (0.8–15.0) | 0.092 | 2.4 (0.6–10.6) | 0.244 | 2.8 (0.8–9.2) | 0.095 | |
| G3 | 2.5 (0.5–12.4) | 0.264 | 2.3 (0.5–11.4) | 0.292 | 2.8 (0.8–9.9) | 0.115 | |
| Vascular invasion | V0 | Reference | Reference | Reference | |||
| V1 | 1.0 (0.3–3.5) | 0.966 | 0.7 (0.2–3.0) | 0.645 | 0.9 (0.3–2.6) | 0.855 | |
| Lymphovascular invasion | L0 | Reference | Reference | Reference | |||
| L1 | 2.8 (1.1–6.9) | 0.032 | 2.0 (0.8–5.5) | 0.167 | 1.6 (0.7–3.7) | 0.247 | |
| Perineural invasion | Pn0 | Reference | Reference | Reference | |||
| Pn1 | 1.7 (0.4–7.3) | 0.491 | 0.8 (0.1–5.9) | 0.816 | 0.5 (0.1–3.8) | 0.523 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mink, J.N.; Khalmurzaev, O.; Pryalukhin, A.; Geppert, C.I.; Lohse, S.; Bende, K.; Lobo, J.; Henrique, R.; Loertzer, H.; Steffens, J.; et al. Evaluation of Prognostic Parameters to Identify Aggressive Penile Carcinomas. Cancers 2023, 15, 4748. https://doi.org/10.3390/cancers15194748
Mink JN, Khalmurzaev O, Pryalukhin A, Geppert CI, Lohse S, Bende K, Lobo J, Henrique R, Loertzer H, Steffens J, et al. Evaluation of Prognostic Parameters to Identify Aggressive Penile Carcinomas. Cancers. 2023; 15(19):4748. https://doi.org/10.3390/cancers15194748
Chicago/Turabian StyleMink, Jan Niklas, Oybek Khalmurzaev, Alexey Pryalukhin, Carol Immanuel Geppert, Stefan Lohse, Kristof Bende, João Lobo, Rui Henrique, Hagen Loertzer, Joachim Steffens, and et al. 2023. "Evaluation of Prognostic Parameters to Identify Aggressive Penile Carcinomas" Cancers 15, no. 19: 4748. https://doi.org/10.3390/cancers15194748
APA StyleMink, J. N., Khalmurzaev, O., Pryalukhin, A., Geppert, C. I., Lohse, S., Bende, K., Lobo, J., Henrique, R., Loertzer, H., Steffens, J., Jerónimo, C., Wunderlich, H., Heinzelbecker, J., Bohle, R. M., Stöckle, M., Matveev, V., Hartmann, A., & Junker, K. (2023). Evaluation of Prognostic Parameters to Identify Aggressive Penile Carcinomas. Cancers, 15(19), 4748. https://doi.org/10.3390/cancers15194748











