Simple Summary
The huge armamentarium of currently available theragnostic modalities allows a novel approach to prostate cancer from imaging to therapy. Clinical examination is the starting-point, then radiology and nuclear medicine are often needed to define the illness grading to set up the best therapeutic strategy. Prostate cancer care horizons are opening with the aid of nuclear medicine, which takes advantage of the technological ascendancy of prostate-specific membrane antigen-based imaging and therapy and is currently evolving with machine-learning approaches. We have focused our review on the current state, on the advancements, and on the future prospects of nuclear medicine modalities that could change prostate cancer’s standard of care.
Abstract
Prostate cancer is the most frequent epithelial neoplasia after skin cancer in men starting from 50 years and prostate-specific antigen (PSA) dosage can be used as an early screening tool. Prostate cancer imaging includes several radiological modalities, ranging from ultrasonography, computed tomography (CT), and magnetic resonance to nuclear medicine hybrid techniques such as single-photon emission computed tomography (SPECT)/CT and positron emission tomography (PET)/CT. Innovation in radiopharmaceutical compounds has introduced specific tracers with diagnostic and therapeutic indications, opening the horizons to targeted and very effective clinical care for patients with prostate cancer. The aim of the present review is to illustrate the current knowledge and future perspectives of nuclear medicine, including stand-alone diagnostic techniques and theragnostic approaches, in the clinical management of patients with prostate cancer from initial staging to advanced disease.
1. Introduction
Prostate cancer (PC) is the most frequent epithelial neoplasia after skin cancer in men starting from 50 years [1], but it is often not clinically evident at the early stage as prostatic intraepithelial neoplasia [2]. Not every intraepithelial neoplasia can progress to PC, even in high-grade cases. Prostate-specific antigen (PSA) dosage can be used as an early diagnostic tool, starting from the age of 50 years when the risk of PC increases. In the presence of specific risk factors such as family history, African ethnicity and BRCA1/2 carriers, screening should be brought forward to 45 years [3]. The standard approach to PC diagnosis comprises PSA screening and digital rectal examination. Multiparametric magnetic resonance (MR) and eventual prostate biopsy may be second-level tests [4,5,6]. Not every PC needs to be treated immediately, especially at early presentation, and it can be considered a chronic disease [7,8]. However, risk stratification (from very low to high risk) is the mainstream contention of modern guidelines to evaluate treatment options in more aggressive disease [8,9]. Different treatment options, ranging from early aggressive treatments, such as radical prostatectomy and radical radiotherapy, to deferred treatments (i.e., treating men when the disease progresses and becomes symptomatic), depend on parameters such as tumor grade and tumor stage. Imaging plays an important role both in the non-invasive detection, localization, grading, and staging of PC and in guiding histopathologic analysis by biopsies. In particular, magnetic resonance (MR) has become a powerful tool for achieving these goals [10,11,12,13,14,15]. Furthermore, the recent introduction of specific prostate ligands that can carry radioactive isotopes including β+ (18F, 68Ga), β-/γ(177Lu), and α (225Ac) emitters with diagnostic and therapeutic characteristics (Figure 1) may redefine the role of nuclear medicine in PC [16].
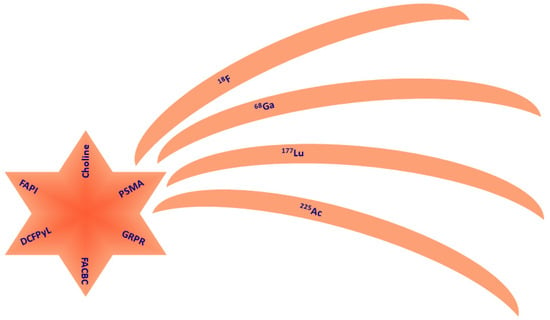
Figure 1.
Pictorial scheme of the available ligands (comet nucleus) and isotopes (comet tails) with application to prostate cancer management. PSMA is the only theragnostic compound.
The aim of the present review is to illustrate current knowledge and future perspectives of nuclear medicine, including stand-alone diagnostic techniques and theragnostic approaches, in the clinical management of patients with prostate cancer from initial staging to advanced disease.
2. Staging Prostate Cancer
The need for a reproducible description of cancer spread has been met by the creation of the staging system. The American Joint Committee on Cancer’s TNM system is widely used, but the most recent update dates from 2018 [17]. The TNM system for PC is based on the PSA level at the time of diagnosis; the extent of the primary tumor is described by the T parameter, the involvement of lymph nodes is described by the N parameter, and cancer extension to other regions of the body is described by the M parameter. However, the National Comprehensive Cancer Network (NCCN) Guidelines Version 1.2023 for Prostate Cancer treatment recommendations, released recently [18], are based on a risk stratification that includes TNM staging rather than on the American Joint Committee on Cancer prognostic grouping. The grading system for PC (based on the Gleason score) tries to measure how likely it is that the cancer will grow and spread quickly. This is determined by the results of the direct prostate examination by the pathologist.
However, the prognostic value of American Joint Committee on Cancer 8th edition staging is not applicable to some staged patients. Higher PSA levels or higher tumor grade are associated with a worse prognosis than that of patients with a higher stage but lower PSA level or lower tumor grade [19]. Several studies also showed that the high-grade group has a significantly worse prognosis than the lower-grade group and should be considered a distinct group [20,21,22,23]. Early-stage patients are commonly diagnosed with a localized, low-risk PC with excellent treatment outcomes [24,25,26]. Along with PSA, some other markers have been proposed. For instance, PC gene 3, an overexpressed long non-coding RNA, is detectable in urine sediments [27,28,29]. This gene correlates with cancer volume, but some data cast doubt on its correlation with grade [30]. A PC gene 3 test is now used to settle a second biopsy after a negative one [31]. The first attempt at risk categorization was made by D’Amico and co-workers that classified patients into low-, intermediate-, and high-risk groups according to PSA, tumor stage, and Gleason score at the moment of diagnosis [32]. Clinical therapeutic and technological upgrades have stimulated the need for a more precise risk assessment, because of the availability of a large variability of therapeutic choices and of a cost-effective option. The National Institute for Health and Care Excellence, European Association of Urology, Genito-Urinary Radiation Oncologists of Canada, American Urological Association, National Comprehensive Cancer Network, and Cambridge Prognostic Groups risk group systems have been created, which then evolved into the Cancer of the Prostate Risk Assessment score [33] and the Memorial Sloan Kettering Cancer Center nomogram [34]. Nomograms attempt to address the large amount of single risk factors and imaging features that may affect the prognosis and the therapeutic strategy of PC patients, with a simple readability of responses [35].
3. Radiological Imaging
3.1. Ultrasound
Following an abnormal PSA level or digital rectal examination, transrectal ultrasonography (TRUS) is frequently used as the first step in the diagnostic process to identify abnormalities and direct biopsies. Because of the high frequency of multiple localization, systematic sextant biopsies are indicated [36]. On ultrasonography, the primary PC can be hyper-echoic or isoechoic (30–40% of lesions), although it is typically detected as a hypoechoic lesion (60–70%) at the gland’s periphery [37]. The preferred technique for implanting brachytherapy seeds in the prostate is transrectal ultrasonography [38].
3.2. Magnetic Resonance
After ultrasound-guided prostate biopsy, MR plays a major role in the evaluation of known PC in order to determine if there is extracapsular extension [39,40,41]. Thus, MR can detect and localize cancer when the PSA is constantly elevated, but routine TRUS biopsy is negative. Both the American College of Radiology and the European Society of Uroradiology encourage the implementation of multiparametric MR for PC assessment consisting in a combination of T2-weighted imaging with functional techniques such as diffusion-weighted imaging, dynamic contrast-enhanced imaging, and spectroscopy [12]. MR can guide prostate biopsy, in the case of negative TRUS biopsy but high clinical suspicion due to elevated PSA levels [42,43]. MR has also has a role in PC patients surgically treated with radical prostatectomy. The use of multi-parametric MR is helpful in detecting and localizing a prostatic lesion [44]. T1 signal used as morphological examination can define prostate contour, neurovascular bundle incasement, and post-biopsy bleeding [45]. T2-weighted images acquired with an endorectal coil show PC usually appearing as a low-signal area within a normally high-signal peripheral zone [46,47]. Diffusion-weighted imaging (DWI) is the crucial sequence for peripheral zone tumor detection [48]. DWI/apparent diffusion coefficient sequences demonstrate restricted diffusion. Dynamic contrast imaging shows enhancement, but it is often a challenge in the central zone to distinguish lesions from prostatitis or benign prostatic hyperplasia. In addition, it has more specificity than T2 signal but extends the post-processing time [49,50]. MR findings can also be expressed by the Prostate Imaging Reporting and Data System (PI-RADS) score. PI-RADS has been released by a consensus of American College of Radiology, European Society of Urogenital Radiology, and AdMeTech Foundation experts to address and homologate the diagnosis likelihood of clinically significant cancer from MR findings. The latest revision (PI-RADS 2.1) was released in 2019 [51,52] and its clinical use is supported by the literature [53], but the necessity of a more precise assessment has led to the introduction of nomograms to integrate clinical, biological, and imaging data to improve diagnosis performance [54]. PI-RADS scoring has a good correlation with malignant prostate findings and thus with the Gleason score. This latter recognizes a primary and a secondary pattern, as well as five sub-patterns in each. The sum of the two patterns provides the Gleason score, which has prognostic significance. Patients with a low Gleason score do well clinically, whereas patients with a high score do poorly. Figure 2 provides a representative example of MR findings in a PC patient.
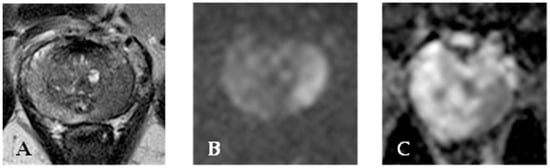
Figure 2.
MR of a peripheral left lobe PC. T2 weighted sequence (A) shows an area of hyperintensity in the left lobe. This finding is confirmed on apparent diffusion coefficient sequences (B). DWI sequence (C) reveals hyperintensity in the lesion area.
3.3. Magnetic Resonance Spectroscopy
Some metabolites such as choline citrate or choline creatine are increased in PC cells [55] and their levels can be evaluated by MR spectroscopy. The multiparametric techniques have been increasingly used in the assessment of prostate malignancy with MR but some issues have emerged with the use of powerful magnets, such as radiofrequency field dis-homogeneity and high local specific absorption rates, that may increase local heating into the body tissues and give rise to safety concerns [56,57]. While T1-weighted images can better describe lymphadenopathy, MR spectroscopy associated with fast T2-weighted imaging is a promising technology for the detection of primary disease [57]. The prostate physiologically produces citrate from the peripheral zone while PC cells do not [58]. Thus, citrate and polyamine levels are high and choline levels low in normal prostatic tissue while they have inverted concentrations in PC [59]. MR spectroscopy is definitely a powerful instrument and can be a game changer in border-line patients [60], but a standardization of findings is needed as inter-operator variability can affect medical report repeatability.
3.4. Computed Tomograhy
CT of the abdomen and pelvis and whole-body bone scans remain the standard of care for the detection of visceral, nodal, and bone metastasis. CT is not perfect at detecting in situ PC, and abdomen and pelvis scans are commonly used to finalize radiation therapy planning. In advanced PC, a CT is used for staging purpose, to detect metastatic lymph nodes in pelvis and the retroperitoneal space, hydronephrosis, and osteoblastic metastases [61].
4. Nuclear Medicine Imaging
4.1. Planar Scintigraphy and Single-Photon Emission Computed Tomography
Bone scintigraphy with planar and single-photon emission computed tomography (SPECT) imaging can detect high uptake of 99mTc-methylenediphosphonate (99mTc-MDP) as result of the bone metabolism. It is not a tumor-specific tracer; in fact, its uptake is higher at bone repair loci where bone metastasis can be located. PC bone metastases are osteoblastic; hence a bone scan can detect them as hot spots or localized accumulation [62]. Currently, bone scintigraphy is one of the first-line imaging techniques for staging and follow-up of PC bone metastasis, but the classical approach is only qualitative with low specificity. Some software-based indexes have been proposed to optimize bone scintigraphy medical reports [63,64,65,66]. The recent introduction of prostate-specific membrane antigen (PSMA) ligands has the potential to rapidly supersede bone scans with 99mTc-MDP [67] when they have been demonstrated to be a cost-efficient imaging modality.
4.2. Choline
In the 90s, choline was proposed as a positron emission tomography (PET) radiotracer for tumor detection with low urinary excretion [68]. Tumor cells need choline to make phosphatidylcholine and other choline-derived membrane constituents. Biochemical analyses have demonstrated that choline kinase activity is increased in tumor cells [69,70]. 18F-choline PET/CT can be a good tool in PC patients at a high risk of extracapsular disease and before surgery to exclude distant metastases [71] but it has demonstrated low sensitivity, despite good specificity, in the evaluation of nodal localization [72]. 18F-choline PET/CT is also characterized by high detection rate of local and distant recurrence post initial treatment of PC [73,74]. 11C-choline is a valuable radiotracer, especially in bone metastasis assessment. 11C has a higher positron energy than 18F (390 vs. 252 MeV) and longer positron range (1.27 vs. 0.66 mm), which is in theory a disadvantage for image quality [75], but probably can be useful in a dense stroma like bone. 11C-choline PET/CT PC detection performance varies, as reported in numerous studies [76,77,78,79]. This is probably due to the heterogeneity of patient samples regarding PSA level, staging, and castration therapy. As proposed by some authors, PSA measurement is involved in the detection rate of PC recurrence by 11C-choline PET/CT [76,80,81]; in particular, PSA doubling time and PSA velocity are predictors for pathological PET scan findings. Figure 3 and Figure 4 illustrate 18F-choline PET/CT and PET/MR imaging findings in patients with PC.
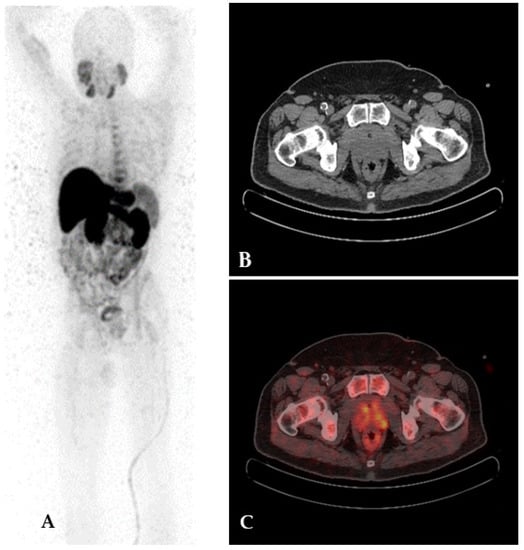
Figure 3.
18F-choline PET/CT imaging in a patient with prostate cancer. Maximum intensity projection (A), CT (B), and fused (C) axial slices show increased tracer uptake in the upper lobes of the prostate.
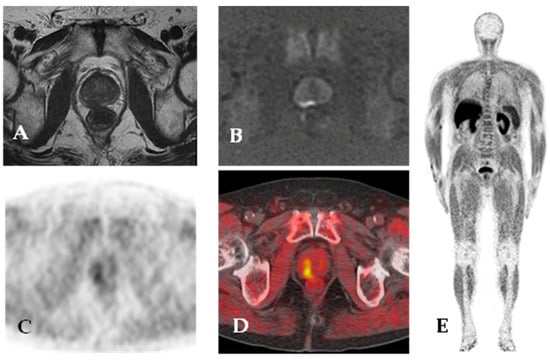
Figure 4.
18F-choline PET/MR. Staging MR imaging shows a large peripheral zone (right posterior mid-gland) lesion with low signal on T2-weighted images (A) and focal and marked hyperintensity on high b value DWI (B). 18F-choline PET/CT images (C,D) show increased tracer uptake in the right posterior area of the prostate gland. Maximum intensity projection (E) 18F-choline PET images.
4.3. Prostate-Specific Membrane Antigen
PSMA is a type II membrane glycoprotein that activates the protein kinase B pathway [82]. It has been proposed as PC marker because it is expressed at very high levels in PC cells but at lower levels also in normal prostate tissue, in the peripheral and central nervous system, in the bowel, and in the salivary glands [83]. Nevertheless, PSMA imaging has not demonstrated uptake in spinal cords without PC localization, and thanks to the blood–brain barrier, PSMA brain uptake has not been seen [84]. PSMA expression in the gastrointestinal tract is responsible for hydrolysis of poly-glutamate folates contained in foods. Folate is then transported into enterocytes and then to the liver [85]. PSMA is also known as folate hydrolase 1 and is involved in folate uptake; its substratum, however, can change in different tissues, for instance, malignant neo-vasculature [85,86,87]. The role of PSMA in carcinogenesis has been widely debated. Probably its overexpression can alter the G2/M cell cycle, eliciting aneuploidy [88]. Folates provide activation of the protein kinase B pathway in vitro and PSMA overexpression has been observed in more aggressive PC [82]. Given high PSMA expression in aggressive PC, patients probably must be careful about dietary habits [89]. The evolution of theragnostics from the first released cancer-specific antibodies to current tracers that can heal cancer with targeted radionuclide administration sparing normal tissues can be considered exceptional. Considering PSMA as a target for theragnostic purposes has been a natural consequence. 111In-capromab pendetide, a mouse monoclonal antibody marked with 111In, was the first tracer for PSMA SPECT imaging in PC patients; however, it was not perfect [90]. PSMA targeting has evolved with the introduction of a group of low-molecular-weight ligands (MIP-1095, MIP-1404, PSMA-11/617/1007, Piflufolastat, PSMA I&T) marked with 18F and 131I for imaging and therapy purposes, respectively, and has been studied in several US trials. In particular, PSMA can be labelled with 68Ga or 18F for PET/CT imaging. While 18F has better physics characteristics than 68Ga due to its lower positron range (2 mm vs. 3.5 mm), 68Ga and 18F have demonstrated similar performance in malignancy detection [91]. Nevertheless, 18F-PSMA seems to be sightly linked to potential false-positive findings, especially in bone tissue [92,93]. The role of PSMA-targeted imaging in initial staging and re-staging of PC has been proven as an effective option. CT, MR, and bone scans lack sensitivity and specificity for detecting occult metastatic disease, in particular in the case of low PSA values [11]. When compared to radio-labelled choline, PSMA imaging shows its strength in early PC recurrence detection, with PSA blood dosage lower than 1 ng/mL [94,95]. In addition, PSMA PET/CT imaging can better estimate PC tumor burden [96]. Figure 5 demonstrates the clear superiority of 68Ga-PSMA PET/CT over bone scan scintigraphy with 99mTc-MDP. Recently, the European Association of Nuclear Medicine and the Society of Nuclear Medicine and Molecular Imaging published the second release of their procedure guidelines for PC imaging [97]. This joint venture updated the specific PET/CT indications for PSMA ligands that include the initial staging of intermediate–high risk PC, the localization of metastasis in biochemical recurrent or persistent PC, especially when other imaging methods have failed, and staging and re-staging prior to and after radioligand therapy of PC [97]. This statement is confirmed by several PC-specific guidelines such as those of the European Association of Urology, European Society for Medical Oncology, National Comprehensive Cancer Network; and American Society of Clinical Oncology [3,15,38,98]. Their high sensitivity and specificity can be exploited by further improving MR-guided biopsy with multimodal imaging offered by PET/MR, as proposed by the study by Ferraro and coworkers [99]. PSMA ligand PET/TC needs some education in interpretation of uptake foci. Inflammation and infection, salivary glands, ganglia (stellate and celiac), gall bladder, and all kinds of prostate pathology can be associated with an increased PSMA uptake, whereby it can mimic a malignancy localization [100]. To address this issue, PSMA-RADS Version 1.0 [101] and Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) criteria have been developed and are currently evolving with clinical experience and technological advancement [102].
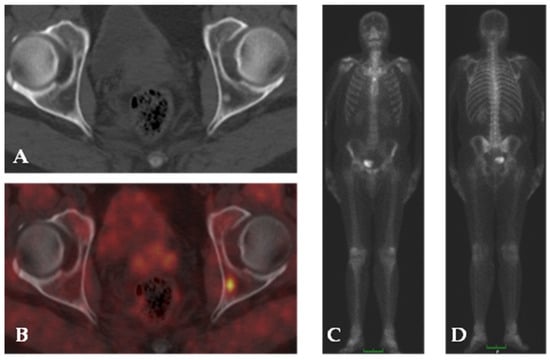
Figure 5.
68Ga-PSMA PET/CT imaging in a patient with PC. Co-registered CT (A) shows hyper-dense area in the left iliac bone with no evidence of focal uptake of 68Ga-PSMA on fused images (B). 99mTc-MDP bone scan anterior and posterior projection (C,D) shows normal tracer distribution.
4.4. Piflufolastat
Among PSMA ligands boasting high affinity for their extracellular domain, piflufolastat labeled with 18F (18F-DCFPyL) has been extensively investigated, demonstrating superior performance for the staging and re-staging of PC over traditional imaging modalities in the OSPREY and CONDOR clinical trials [103,104]. Szabo and coworkers [105] were the first to prospectively evaluate 18F-DCFPyL in nine hormone-naïve and castration-resistant PC (crPC) patients with metastatic evolution confirmed by histological examination. Dosimetric evaluation revealed that the kidneys adsorbed the highest dose, followed by the bladder wall, submandibular glands, and liver, with a distribution pattern similar to 18F-FDG, while physiological bio-distribution was seen in the liver, spleen, kidneys, the lacrimal and salivary glands, and the small bowel. Jansen et al. [106] reported high repeatability of both lesion detection rate and uptake in 12 patients with PC. Parameters such as volume and standardized uptake values showed better accuracy with 18F-DCFPyL, particularly for lymph node localization. If changes in semi-quantitative parameters are recorded between baseline and follow-up 18F-DCFPyL PET/CT, the reader is confident that such findings are not caused by uptake variability, suggesting that this compound may be a reliable image biomarker for response assessment [107]. Li et al. [108] also reported on variability in normal organ uptake using 18F-DCFPyL and demonstrated less variability in normal liver relative to other organs. Of note, the variability was even lower when compared to liver uptake using 18F-FDG (coefficient of variation, 18F-DCFPyL, 13.8–14.5% vs. 18F-FDG, 21–23%) [108,109]. In addition, a recent study investigated whether uptake in normal organs correlates with higher tumor burden [106]. Of note, in patients with high 18F-DCFPyL uptake metastatic volume, the tumor sink effect was minimal. However, it should be considered that inter-patient and intra-patient factors may impact the intrinsic organ variability [110]. However, dosimetry for PSMA-targeted radioligand therapy could be further improved and PET protocols could be better refined to enhance tracer uptake in putative sites of disease. A recent randomized phase 2 research clinical trial (ORIOLE) underlined the potential role of PSMA-targeted radioligand PET in directing and enhancing the therapeutic efficiency of metastatic-directed therapy administered by radionuclide therapy, and demonstrated that individuals with total consolidation of disease detectable by PSMA-targeted PET-CT were associated with lower risk of new metastases at 6 months [111]. This work serves as an excellent example of the crucial function that PSMA imaging plays.
4.5. Fluciclovine
18F-fluciclovine is an amino-acid analogue that takes advantage of the increased energy demand of PC and acts as a radiotracer when labeled with 18F. It is taken in to the cells by facilitated transport, in particular by alanine-serine-cysteine transporter and L-type amino acid 1 transporter [112,113,114]. The US Food and Drug Administration and the European Medicines Agency approved 18F-fluciclovine as a PET radiotracer in men with PC with biochemical recurrence after radical prostatectomy or radiotherapy [113]. Uptake is normally high in the liver and pancreas, while the salivary glands, pituitary, lymphoid tissue of Waldeyer’s ring, thyroid, breast parenchyma, esophagus, stomach, adrenal glands, bowel, and renal parenchyma show lower distribution. Although it has been shown that urinary excretion is low, and it offers high contrast for primary PC detection, some studies have demonstrated that 18F-fluciclovine PET cannot independently characterize primary lesions requiring integration with multiparametric MR findings [115,116], so 18F-fluciclovine cannot be used for PC staging. PC recurrence identification, especially in the pelvis, is the most promising field of use of 18F-fluciclovine in the near future [117].The role of 18F-fluciclovine PET/CT in PC patients with biochemical recurrence after curative-intent primary therapy has been studied by the LOCATE (NCT02680041) [118] and FALCON (NCT02578940) [119] trials. They found that this tracer could lead to the most appropriate treatment approach by determining the tumor burden and location linked to biochemical recurrence. In addition,18F-fluciclovine has demonstrated a superior recurrence detection performance over 18F-choline PET/CT, especially in patients with blood PSA level inferior to 1 ng/mL [120,121,122,123]. So, this radiolabeled compound can be considered to assess earlier PC recurrency, with a possible advantage in clinical management and prognosis. However, 18F-choline PET/CT performance does not seems to be superior to the more accessible radiolabeled PSMA PET/CT in recurrence detection far from the bladder [124,125].
4.6. Fluorodeoxyglucose
18F-fluorodeoxyglucose (FDG) is the first radiotracer utilized for human brain PET and still is the radiotracer of choice in several PET/CT oncologic applications. 18F-FDG is rapidly captured from plasma by cells, and then the 18F-FDG takes advantage of the Warburg effect and is phosphorylated to prevent further metabolism and blood recirculation [126]. 18F-FDG is excreted by the kidneys, concentrated in urine and the bladder. Prostate proximity to the bladder limits the utility of 18F-FDG PET/TC in primary PC definition [127]. It should be also be taken into consideration that bone scan scintigraphy can be superior to 18F-FDG PET/TC for the definition of bone metastasis [128,129,130]. A potential role of 18F-FDG PET/TC is as a technique of evaluating mismatches in 18F-FDG and prostate-specific tracers PET/TC as an index of cancer de-differentiation with negative prognostic connotation [131,132]. In particular, when PC has become castration-resistant, PSA cannot be considered the best indicator of response to therapy, due to the increase in cancer cell heterogeneity with the presence of PSA-producing and nonproducing cells and the presence of flare phenomena as a result of therapy response. When PC progresses, despite therapy, PSMA uptake may decrease along with an increase in 18F-FDG uptake, according to the “flip flop” phenomenon [133]. In this scenario, 18F-FDG can be used parallel to PSMA PET/TC and therapy plans can be changed according to PET findings, in order to monitor and treat tumor de-differentiation, if possible [134].
4.7. Hetero-Bivalent Agents Targeting Gastrin-Releasing Peptide Receptor or Fibroblast Activation Protein Inhibitor and PSMA
Gastrin-releasing peptide receptor (GRPR) belongs to the bombesin family, and its concentration is highest in the pancreas. Low levels of GRPR are expressed in the bowel and, interestingly, in benign prostate tissue [135]. GRPR is involved in intestinal smooth muscle contraction and increases cancer cell proliferation [136,137]. Over-expression has been observed in PC, but also in breast, lung, head and neck, pancreatic cancers, and malignant brain tumors [138,139]. Radiopharmaceutical engineering has investigated ligands that can target either PSMA or GRPR to improve PC theragnostics. Clinical application is linked to single target expression homogeneity. Heterogeneity of expression of the two targets has been explored by studies of the binding of 68Ga-Ga-RM2 and 68Ga-Ga-PSMA-11 to their ligands in biochemically recurrent PC [140]. Large areas of negative PSMA expression can be found in primary tumors or metastases, independently of the Gleason score, histological class, and metastasis sites [141]. So, targeting both GRPR and PSMA could be an advantage. On the other hand, PET/CT imaging with radiolabeled fibroblast activation protein (FAP) inhibitor has been proposed in various diseases, including PC. This protein is a serine peptidase expressed in the cell membrane that can be upregulated in activated fibroblasts at wounds, inflammatory sites, and in cancer tissue. Quinoline-based PET tracers acting as FAP inhibitors can accurately detect cancer-activated fibroblasts, demonstrating clear tumor imaging when labeled with 68Ga [142,143,144,145].
Table 1 summarizes the available tracers for PET/TC imaging in patients with PC.

Table 1.
Available tracers for PET/TC prostate cancer imaging.
5. Radioactive Therapy of PC Bone Metastasis
PC can metastasize to bones, especially in advanced stages. Several targeted bone radioactive isotopes, such as 186Re, 89Sr, and 153Sm, were firstly introduced into clinical use for treatment of bone metastasis; then they were superseded by 223Ra, an α-emitter, and reserved for palliation therapy in selected patients. The ALSYMPCA trial [113,114,146] was a phase 3 trial that demonstrated that 223Ra, an α-emitter, can improve crPC patients’ overall survival compared with placebo and it is well-tolerated. The National Comprehensive Cancer Network also showed first-line use of 223Ra for symptomatic bone PC metastasis or bone-predominant disease after chemotherapy in the case of absence of visceral metastases [147]. Bone scintigraphy and 18F-fluorocholine PET/CT can help physicians to predict 223Ra treatment response. A moderate burden of disease is a good predictor of good response to 223Ra [148]. Nevertheless, 223Ra has inherent limits because of its restriction to metastatic bone disease uptake. The therapeutic performance of 223Ra treatment has been proved by several studies that demonstrate an increase in overall survival and quality of life, along with an excellent safety profile, especially when introduced earlier into the treatment iteration [149,150,151].
6. Radioligand Therapy of Advanced Prostate Cancer
The main actors on the ground for a promising novel approach to treat PC are 177Lu and 225Ac [152]. 177Lu is a widely used radioisotope for targeted cancer radiotherapy with a half-life of 6.7 days, emitting both beta particles with an energy of 490 KeV and a therapeutic range of 0.7 to 2.1 mm and gamma rays (112.9 and 208.4 KeV peaks) [153,154,155,156]. 225Ac is an alpha emitter (energies ranging from 5.8 to 8.4 MeV) with a half-life of 9.9 days and a very short tissue range (47 to 85 µm) [157]. 225Ac can be considered the natural alpha-emitting counterpart to 177Lu because of its similar radiolabeling characteristics. Alpha emitters have a clear advantage over beta emitters in cancer cell destruction. They can provoke very efficient double-strand DNA damage, cell cycle arrest; and micronuclei formation, leading ultimately to cell death. Current research suggests that treating the primary tumor or metastases with radionuclides may improve survival in carefully chosen patients with low-volume metastatic PC [158]. However, visceral localization and high alkaline phosphatase are negative predictors of response to 177Lu ligand therapy. On the other hand, the onset of high-grade hematotoxicity (anemia, thrombocytopenia, and leukopenia) should be taken into account [159,160]. Although 177Lu-PSMA treatment produced an impressive response for pre-treated patients, 40% of PC patients did not respond [161]. Kratochwil et al. [161] tested 225Ac-PSMA therapy in strongly pre-treated crPC patients and realized that α-emitters can be more effective than β-emitters. Moreover, α-emitters can have an edge over β-emitters in the case of bone diffused disease, acting as a super-scan for nuclear medicine imaging and sparing bone marrow thanks to low tissue penetration. The VISION phase 3 trial of targeted radioligand therapy including 831 patients demonstrated that radionuclide therapy with 177Lu-PSMA extended imaging-based progression-free survival (8.7 vs. 3.4 months) and overall survival (15.3 vs. 11.3 months) when added to standard care in patients with metastatic crPC [162]. TheraP, a phase 2 study, compared 177Lu-PSMA to chemotherapy in two hundred men [163]. Prostate-specific antigen (PSA) levels, which usually increase with cancer growth, were monitored. 177Lu-PSMA treatment made PSA levels fall by half and, according to the VISION trial, a longer delay of cancer progression was found, confirmed by conventional imaging. 177Lu-PSMA was generally well-tolerated, but it also had side effects including fatigue, nausea, kidney complications, and bone marrow suppression. The safety, kinetics, and dosimetry of 177Lu-PSMA have been evaluated on a large cohort of patients, demonstrating favorable safety in metastatic crPC patients [164]. The highest absorbed doses among healthy organs were observed for the lacrimal and parotid glands, still not resulting in any significant clinical side effects. Given the encouraging clinical trial results, the European Association of Nuclear Medicine guidelines stated that 177Lu-PSMA therapy can be administered when chemotherapy or new androgen-axis compounds therapies have failed, in metastatic crPC with documented PSMA uptake, still with therapeutic purposes rather than with the aim of palliation [165]. However, the safety and efficacy of 177Lu-PSMA have suggested potential earlier use in PC [166,167]. Some studies have suggested the possibility of early adoption of 177Lu-PSMA therapy, taking advantage of better initial conditions of crPC patients, naïve to chemotherapy, or prior to surgery. [168,169]. However, resistance can occur sometimes. In the LuPIN trial [170], the combination of 177Lu-PSMA with NOX66, a flavonoid derivate that activates the mitochondrial caspase system, has been evaluated in end-stage patients, while a 177Lu-PSMA combination with abiraterone has been tested in a different sub-trial [171]. 177Lu-PSMA can prolong PC patients’ survival, but cannot always inhibit progression, usually to bone but also to liver [155]. In the AlphaBet trial, 223Ra has been combined with 177Lu-PSMA in order to treat pre-clinical bone micrometastasis and possibly prevent progression to bone, also taking into account that low bone marrow irradiation is a challenge [172]. Drug combinations can be considered as salvage therapies when a single drug fails, but a multimodal approach can also be used. External beam radiation and 177Lu-PSMA combined therapy can be considered as an option, but criticality may emerge from the dosimetric point of view [173]. 177Lu-PSMA therapy is a reliable option for men with metastatic crPC, but should also have utility in less advanced PC. For now, 177Lu-PSMA and 225Ac-labeled PSMA are reserved to PC patients after failure of all approved therapies, and generally a limited life expectancy is anticipated; this can affect the duration of possible side effects as well. Some authors have investigated the possible synergic therapeutic effect of 225Ac-PSMA after previous administration of 177Lu-PSMA. A recent meta-analysis highlights the possible development of cancer resistance as a drawback of a previous exposure to 177Lu [174]. Other authors tried a simultaneous administration of 177Lu-PSMA and 225Ac-PSMA in late-stage crPC patients to enhance the response rate [175,176].The PSMA determinant is physiologically expressed in the salivary glands by acinar and ductal cells. A risk of salivary gland toxicity in the case of administration of alpha-emitter radiolabeled PSMA is possible [177]. A risk-to-reward ratio should be evaluated to determine the eligibility criteria of PC patients, the isotope of choice, and the optimal therapy to be administered. Earlier α-therapy administration should be carried out in the context of prospective studies to reduce the treatment administered according to optimal therapeutic efficacy. Therapeutic window optimization will be a strong argument for a more controlled and systematic assessment of radioligand therapy.
7. Conclusions and Future Perspectives
Promising compounds labelling radionuclides are ready to deal with the limitations of the first released theragnostic radioligands. Salivary gland toxicity is a major challenge because it is frequent and can be irreversible. The PSMA uptake mechanism in the salivary glands is not clear, but some strategies to decrease accumulation are currently available. Nevertheless, long-term salivary gland toxicity needs to be investigated. A recent pilot study investigated the use of cold DCFPyL instillation into the salivary glands in order to decrease salivary uptake, mitigating xerostomia in patients scheduled for radioligand therapy [178]. Numerous studies have recently evaluated the application of Cu as PET/TC radiotracer in oncology because of its capacity to act as diagnostic and theragnostic radiotracer. It can produce β+ emissions and high-resolution PET images, while β and Auger electrons emission are suitable for targeted radiotherapy. The production of 64Cu is convenient: due to its long half-life, it can be produced by a single center and distributed to several PET centers, even if far away. Cu is essential for multiple biological functions, such as cellular respiration, redox reactions, cellular adhesion, and connective tissue synthesis. High serum Cu levels have been found in some tumors, and a correlation with disease stage has been demonstrated [179]. 64CuCl2 as a PET tracer in PC has been explored in few studies. Capasso et al. [180] compared 64CuCl2 PET/CT with multiparametric MR for staging purpose. The detection rate for primary tumors was similar, and the radiotracer had no urinary excretion and no side effects. Piccardo et al. [181] studied 50 patients with biochemical relapse with 64CuCl2 PET/CT, 18F-Choline PET/CT, and MR. They found that 64CuCl2 PET/CT was better than 18F-Choline PET/CT and multiparametric MR in metastatic detection. A high liver uptake was observed, but no organ-specific toxicity was detected. From a theragnostic point of view, the potential of 64CuCl2 in PC has only been evaluated in an in vitro setting. PSMA-directed molecular imaging is currently evolving through machine-learning approaches. Leung et al. described an automated deep-learning method comparing conventional and semi-automated thresholding-based methods for 18F-DCFPyL PET/CT evaluation in 207 patients [182]. They found that a deep-learning approach can help with more accurate segmentation and can lead to better therapy monitoring and future care planning. Machine learning and radiomics are emerging topics in clinical imaging. It can extract multiple features from pathological findings and potentially define new markers of disease [183,184,185]. Radiomics has the potential to further increase the value of imaging in PC management; nevertheless, its introduction into current clinical practice is full of questions, as emphasized by several radiomic studies [186,187,188,189]. Several approaches have been proposed and standardization is the major issue.
Recently, a new beta emitter, 161Tb (half-life = 6.89 days; Eβ͞av = 154 keV), has been proposed as a potential player in the radio-theragnostic playground in various types of cancer, including PC. Labeled with PSMA, it seems to have a better emission profile than 177Lu regarding adsorbed dose, because of its rich Auger electron emission rate [190,191]. Among alpha emitters, 212Pb and 213Bi with a short half-life (10.64 h and 1 h, respectively) [192], 227Th (half-life 18.7 days), and 211At (half-life 7.2 h) are promising radioisotopes. In particular, 211At provides a better biodistribution and strong labelling of PSMA, decreasing the dose delivered to non-targeted tissue, especially in regard to the salivary glands [193]. Furthermore, 211At production cost is considerably lower than other alpha emitters, resulting in a clear advantage for future application [194]. However, dosimetry remains the main challenge, requiring a robust body of investigation to optimize the potential of PSMA theragnostics.
Author Contributions
Conceptualization, F.V. and C.N.; methodology, F.V., C.N., M.K., C.G.M., E.Z., A.P., L.P. and A.C.; validation, A.C., M.K. and C.N.; formal analysis, A.C.; investigation, F.V.; resources, F.V., M.I., A.P. and C.G.M.; writing—original draft preparation, F.V.; writing—review and editing, C.N.; visualization, M.K.; supervision, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved on 8 November 2022 by the Institutional Review Committee of the Department of Advanced Biomedical Sciences of the University of Naples Federico II (24984/22).
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M. High-Grade Prostatic Intraepithelial Neoplasia, PIN-like Carcinoma, Ductal Carcinoma, and Intraductal Carcinoma of the Prostate. Mod. Pathol. 2018, 31, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of Prostate Systematic and Targeted Biopsy on the Basis of Multiparametric MRI in Biopsy-Naive Patients (MRI-FIRST): A Prospective, Multicentre, Paired Diagnostic Study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic Accuracy of Multi-Parametric MRI and TRUS Biopsy in Prostate Cancer (PROMIS): A Paired Validating Confirmatory Study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Valbekmo, A.G.; Mo, L.; Gjøsund, G.; Håland, E.; Melby, L. Exploring Wait Time Variations in a Prostate Cancer Patient Pathway-A Qualitative Study. Int. J. Health Plann. Manag. 2022, 37, 2122–2134. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2022, 20, 1288–1298. [Google Scholar]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- Li, H.; Sugimura, K.; Kaji, Y.; Kitamura, Y.; Fujii, M.; Hara, I.; Tachibana, M. Conventional MRI Capabilities in the Diagnosis of Prostate Cancer in the Transition Zone. Am. J. Roentgenol. 2006, 186, 729–742. [Google Scholar] [CrossRef]
- Hövels, A.M.; Heesakkers, R.A.M.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The Diagnostic Accuracy of CT and MRI in the Staging of Pelvic Lymph Nodes in Patients with Prostate Cancer: A Meta-Analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. ESUR Prostate MR Guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Tamada, T.; Prabhu, V.; Li, J.; Babb, J.S.; Taneja, S.S.; Rosenkrantz, A.B. Prostate Cancer: Diffusion-Weighted MR Imaging for Detection and Assessment of Aggressiveness Comparison between Conventional and Kurtosis Models. Radiology 2017, 284, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.C.; Yildirim, O.; Woo, S.; Vargas, H.A.; Hricak, H. The Role of MRI in Prostate Cancer: Current and Future Directions. Magn. Reson. Mater. Phys. Biol. Med. 2022, 35, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, E.; Bodei, L.; Morris, M.J. The Role of Theranostics in Prostate Cancer. Semin. Radiat. Oncol. 2021, 31, 71–82. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network, Prostate Cancer Guidelines Version 1. 2023. Available online: https://www.nccn.org/ (accessed on 18 May 2023).
- Xiao, W.-J.; Zhu, Y.; Dai, B.; Ye, D.-W. Evaluation of the Major Changes in Eighth Edition of the American Joint Committee on Cancer Pathological Staging for Prostate Cancer Treated with Prostatectomy. PLoS ONE 2017, 12, e0187887. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Pierorazio, P.M.; Walsh, P.C.; Partin, A.W.; Epstein, J.I. Prognostic Gleason Grade Grouping: Data Based on the Modified Gleason Scoring System. BJU Int. 2013, 111, 753–760. [Google Scholar] [CrossRef]
- Tsao, C.-K.; Gray, K.P.; Nakabayashi, M.; Evan, C.; Kantoff, P.W.; Huang, J.; Galsky, M.D.; Pomerantz, M.; Oh, W.K. Patients with Biopsy Gleason 9 and 10 Prostate Cancer Have Significantly Worse Outcomes Compared to Patients with Gleason 8 Disease. J. Urol. 2015, 194, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Bhindi, B.; Karnes, R.J.; Rangel, L.J.; Mason, R.J.; Gettman, M.T.; Frank, I.; Tollefson, M.K.; Lin, D.W.; Thompson, R.H.; Boorjian, S.A. Independent Validation of the American Joint Committee on Cancer 8th Edition Prostate Cancer Staging Classification. J. Urol. 2017, 198, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Quality of Life in Active Surveillance for Early Prostate Cancer—Kato—2020—International Journal of Urology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/iju.14202 (accessed on 12 January 2023).
- Lardas, M.; Liew, M.; van den Bergh, R.C.; De Santis, M.; Bellmunt, J.; Van den Broeck, T.; Cornford, P.; Cumberbatch, M.G.; Fossati, N.; Gross, T.; et al. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Moul, J.W.; Anderson, J.; Penson, D.F.; Klotz, L.H.; Soloway, M.S.; Schulman, C.C. Early Prostate Cancer: Prevention, Treatment Modalities, and Quality of Life Issues. Eur. Urol. 2003, 44, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Deras, I.L.; Aubin, S.M.J.; Blase, A.; Day, J.R.; Koo, S.; Partin, A.W.; Ellis, W.J.; Marks, L.S.; Fradet, Y.; Rittenhouse, H.; et al. PCA3: A Molecular Urine Assay for Predicting Prostate Biopsy Outcome. J. Urol. 2008, 179, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Groskopf, J.; Fritsche, H.A.; Bhadkamkar, V.; Blase, A.; Kumar, S.V.; Davis, J.W.; Troncoso, P.; Rittenhouse, H.; Babaian, R.J. PCA3 Molecular Urine Assay Correlates With Prostate Cancer Tumor Volume: Implication in Selecting Candidates for Active Surveillance. J. Urol. 2008, 179, 1804–1810. [Google Scholar] [CrossRef]
- Hessels, D.; van Gils, M.P.M.Q.; van Hooij, O.; Jannink, S.A.; Witjes, J.A.; Verhaegh, G.W.; Schalken, J.A. Predictive Value of PCA3 in Urinary Sediments in Determining Clinico-Pathological Characteristics of Prostate Cancer. Prostate 2010, 70, 10–16. [Google Scholar] [CrossRef]
- Auprich, M.; Bjartell, A.; Chun, F.K.-H.; de la Taille, A.; Freedland, S.J.; Haese, A.; Schalken, J.; Stenzl, A.; Tombal, B.; van der Poel, H. Contemporary Role of Prostate Cancer Antigen 3 in the Management of Prostate Cancer. Eur. Urol. 2011, 60, 1045–1054. [Google Scholar] [CrossRef]
- Nicholson, A.; Mahon, J.; Boland, A.; Beale, S.; Dwan, K.; Fleeman, N.; Hockenhull, J.; Dundar, Y. The Clinical Effectiveness and Cost-Effectiveness of the PROGENSA® Prostate Cancer Antigen 3 Assay and the Prostate Health Index in the Diagnosis of Prostate Cancer: A Systematic Review and Economic Evaluation. Health Technol. Assess. 2015, 19, i–xxxi. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Fondurulia, J.; Chen, M.-H.; Tomaszewski, J.E.; Renshaw, A.A.; Wein, A.; Richie, J.P. Clinical Utility of the Percentage of Positive Prostate Biopsies in Defining Biochemical Outcome After Radical Prostatectomy for Patients With Clinically Localized Prostate Cancer. J. Clin. Oncol. 2000, 18, 1164–1172. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Pasta, D.J.; Elkin, E.P.; Litwin, M.S.; Latini, D.M.; Duchane, J.; Carroll, P.R. The University of California, San Francisco Cancer of the Prostate Risk Assessment Score: A Straightforward and Reliable Preoperative Predictor of Disease Recurrence after Radical Prostatectomy. J. Urol. 2005, 173, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Vis, A.N.; Meijer, D.; Roberts, M.J.; Siriwardana, A.R.; Morton, A.; Yaxley, J.W.; Samaratunga, H.; Emmett, L.; van de Ven, P.M.; Heymans, M.W.; et al. Development and External Validation of a Novel Nomogram to Predict the Probability of Pelvic Lymph-Node Metastases in Prostate Cancer Patients Using Magnetic Resonance Imaging and Molecular Imaging with Prostate-Specific Membrane Antigen Positron Emission Tomography. Eur. Urol. Oncol. 2023, S2588-9311(23)00075-5. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Scardino, P.T.; Eastham, J.A.; Bianco, F.J., Jr.; Dotan, Z.A.; Fearn, P.A.; Kattan, M.W. Preoperative Nomogram Predicting the 10-Year Probability of Prostate Cancer Recurrence after Radical Prostatectomy. J. Natl. Cancer Inst. 2006, 98, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Streicher, J.; Meyerson, B.L.; Karivedu, V.; Sidana, A. A Review of Optimal Prostate Biopsy: Indications and Techniques. Ther. Adv. Urol. 2019, 11, 1756287219870074. [Google Scholar] [CrossRef] [PubMed]
- Kawa, S.M.; Stroomberg, H.V.; Larsen, S.B.; Helgstrand, J.T.; Toft, B.G.; Brasso, K.; Røder, M.A. Detection of Clinically Significant Prostate Cancer by Systematic TRUS-Biopsies in a Population-Based Setting Over a 20 Year Period. Urology 2021, 155, 20–25. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, X.; Fu, C.; Wei, X.; Bao, J.; Ji, X.; Bai, H.; Xia, W.; Gao, X.; Huang, Y.; et al. MRI-Based Radiomics Models to Assess Prostate Cancer, Extracapsular Extension and Positive Surgical Margins. Cancer Imaging 2021, 21, 46. [Google Scholar] [CrossRef]
- Michael, J.; Neuzil, K.; Altun, E.; Bjurlin, M.A. Current Opinion on the Use of Magnetic Resonance Imaging in Staging Prostate Cancer: A Narrative Review. Cancer Manag. Res. 2022, 14, 937–951. [Google Scholar] [CrossRef]
- Ellis, E.E.; Frye, T.P. Role of Multi-Parametric Magnetic Resonance Imaging Fusion Biopsy in Active Surveillance of Prostate Cancer: A Systematic Review. Ther. Adv. Urol. 2022, 14, 17562872221106884. [Google Scholar] [CrossRef]
- Sala, E.; Eberhardt, S.C.; Akin, O.; Moskowitz, C.S.; Onyebuchi, C.N.; Kuroiwa, K.; Ishill, N.; Zelefsky, M.J.; Eastham, J.A.; Hricak, H. Endorectal MR Imaging before Salvage Prostatectomy: Tumor Localization and Staging. Radiology 2006, 238, 176–183. [Google Scholar] [CrossRef]
- van der Poel, H.; Grivas, N.; van Leeuwen, P.; Heijmink, S.; Schoots, I. The Role of MRI for Detection and Staging of Radio- and Focal Therapy-Recurrent Prostate Cancer. World J. Urol. 2019, 37, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Cuocolo, R.; Verde, F.; Ponsiglione, A.; Romeo, V.; Petretta, M.; Imbriaco, M.; Stanzione, A. Clinically Significant Prostate Cancer Detection With Biparametric MRI: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2021, 216, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, M.; Ahmed, H.; Nam, R.; Schaeffer, E.; Schiavina, R.; Taneja, S.; Weidner, W.; Loeb, S. Complications After Systematic, Random, and Image-Guided Prostate Biopsy. Eur. Urol. 2017, 71, 353–365. [Google Scholar] [CrossRef]
- Hricak, H.; Choyke, P.L.; Eberhardt, S.C.; Leibel, S.A.; Scardino, P.T. Imaging Prostate Cancer: A Multidisciplinary Perspective. Radiology 2007, 243, 28–53. [Google Scholar] [CrossRef]
- Verma, S.; Rajesh, A. A Clinically Relevant Approach to Imaging Prostate Cancer: Review. Am. J. Roentgenol. 2011, 196, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.A.; van der Kwast, T.H.; Tanguay, J.; Evans, A.J.; Hashmi, A.-T.; Lockwood, G.; Trachtenberg, J. Combined T2-Weighted and Diffusion-Weighted MRI for Localization of Prostate Cancer. Am. J. Roentgenol. 2007, 189, 323–328. [Google Scholar] [CrossRef]
- Ocak, I.; Bernardo, M.; Metzger, G.; Barrett, T.; Pinto, P.; Albert, P.S.; Choyke, P.L. Dynamic Contrast-Enhanced MRI of Prostate Cancer at 3 T: A Study of Pharmacokinetic Parameters. Am. J. Roentgenol. 2007, 189, W192–W201. [Google Scholar] [CrossRef]
- Alonzi, R.; Padhani, A.R.; Allen, C. Dynamic Contrast Enhanced MRI in Prostate Cancer. Eur. J. Radiol. 2007, 63, 335–350. [Google Scholar] [CrossRef]
- American College of Radiology. PI-RADS: Prostate Imaging—Reporting and Data System. Version 2.1. Available online: https://www.acr.org/-/media/ACR/Files/RADS/PI-RADS/PIRADS-V2-1.pdf (accessed on 27 February 2023).
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Cuocolo, R.; Stanzione, A.; Ponsiglione, A.; Verde, F.; Ventimiglia, A.; Romeo, V.; Petretta, M.; Imbriaco, M. Prostate MRI Technical Parameters Standardization: A Systematic Review on Adherence to PI-RADSv2 Acquisition Protocol. Eur. J. Radiol. 2019, 120, 108662. [Google Scholar] [CrossRef]
- Hiremath, A.; Shiradkar, R.; Fu, P.; Mahran, A.; Rastinehad, A.R.; Tewari, A.; Tirumani, S.H.; Purysko, A.; Ponsky, L.; Madabhushi, A. An Integrated Nomogram Combining Deep Learning, Prostate Imaging–Reporting and Data System (PI-RADS) Scoring, and Clinical Variables for Identification of Clinically Significant Prostate Cancer on Biparametric MRI: A Retrospective Multicentre Study. Lancet Digit. Health 2021, 3, e445–e454. [Google Scholar] [CrossRef] [PubMed]
- Bonekamp, D.; Jacobs, M.A.; El-Khouli, R.; Stoianovici, D.; Macura, K.J. Advancements in MR Imaging of the Prostate: From Diagnosis to Interventions. Radiographics 2011, 31, 677–703. [Google Scholar] [CrossRef] [PubMed]
- Tenbergen, C.J.A.; Metzger, G.J.; Scheenen, T.W.J. Ultra-High-Field MR in Prostate Cancer: Feasibility and Potential. MAGMA 2022, 35, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Asuncion, A.; Walker, P.M.; Bertaut, A.; Blanc, J.; Labarre, M.; Martin, E.; Bardet, F.; Cassin, J.; Cormier, L.; Crehange, G.; et al. Prediction of Prostate Cancer Recurrence after Radiation Therapy Using Multiparametric Magnetic Resonance Imaging and Spectroscopy: Assessment of Prognostic Factors on Pretreatment Imaging. Quant. Imaging Med. Surg. 2022, 12, 5309–5325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Jagannathan, N.R. Metabolism of Prostate Cancer by Magnetic Resonance Spectroscopy (MRS). Biophys. Rev. 2020, 12, 1163–1173. [Google Scholar] [CrossRef]
- Sanchez-Dahl Gonzalez, M.; Muti, I.H.; Cheng, L.L. High Resolution Magic Angle Spinning MRS in Prostate Cancer. Magn. Reason. Mater. Phy. 2022, 35, 695–705. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Chen, M. Patients With “Gray Zone” PSA Levels: Application of Prostate MRI and MRS in the Diagnosis of Prostate Cancer. J. Magn. Reason. Imaging 2022. [Google Scholar] [CrossRef]
- Mohsen, N. Role of MRI, Ultrasound, and Computed Tomography in the Management of Prostate Cancer. PET Clin. 2022, 17, 565–583. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M.; et al. The EANM Practice Guidelines for Bone Scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef]
- Nakajima, K.; Edenbrandt, L.; Mizokami, A. Bone Scan Index: A New Biomarker of Bone Metastasis in Patients with Prostate Cancer. Int. J. Urol. 2017, 24, 668–673. [Google Scholar] [CrossRef]
- Mota, J.M.; Armstrong, A.J.; Larson, S.M.; Fox, J.J.; Morris, M.J. Measuring the Unmeasurable: Automated Bone Scan Index as a Quantitative Endpoint in Prostate Cancer Clinical Trials. Prostate Cancer Prostatic. Dis. 2019, 22, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, T.; Hadebe, B.; Aldous, C.; Tinarwo, P.; Nyakale, N. Segmented Linear Correlations between Bone Scan Index and Prostate Cancer Biomarkers, Alkaline Phosphatase, and Prostate Specific Antigen in Patients with a Gleason Score ≥7. Medicine 2022, 101, e29515. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, S.; Yoshida, A.; Kawabe, J. Predicting the Prognosis of Prostate Cancer Bone Metastasis Using the Bone Scan Index and Hot Spots Calculated Using VSBONE® Bone Scan Index from Tc-99m-Hydroxymethylene Diphosphonate Bone Scintigraphy. Urol. Int. 2022, 106, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Liepe, K.; Baehr, M. Tc-99m-PSMA-SPECT/CT Is Superior to Tc-99m-MDP-SPECT/CT in the Staging of Prostatic Cancer with Osseous Metastases after External Beam Radiotherapy. World J. Nucl. Med. 2022, 21, 62–64. [Google Scholar] [CrossRef]
- Castellucci, P.; Ceci, F.; Fanti, S. Imaging of Prostate Cancer Using 11C-Choline PET/Computed Tomography. Urol. Clin. N. Am. 2018, 45, 481–487. [Google Scholar] [CrossRef]
- Pelosi, E.; Arena, V.; Skanjeti, A.; Pirro, V.; Douroukas, A.; Pupi, A.; Mancini, M. Role of Whole-Body 18F-Choline PET/CT in Disease Detection in Patients with Biochemical Relapse after Radical Treatment for Prostate Cancer. Radiol. Med. 2008, 113, 895–904. [Google Scholar] [CrossRef]
- Ackerstaff, E.; Pflug, B.R.; Nelson, J.B.; Bhujwalla, Z.M. Detection of Increased Choline Compounds with Proton Nuclear Magnetic Resonance Spectroscopy Subsequent to Malignant Transformation of Human Prostatic Epithelial Cells. Cancer Res. 2001, 61, 3599–3603. [Google Scholar]
- Beheshti, M.; Imamovic, L.; Broinger, G.; Vali, R.; Waldenberger, P.; Stoiber, F.; Nader, M.; Gruy, B.; Janetschek, G.; Langsteger, W. 18F Choline PET/CT in the Preoperative Staging of Prostate Cancer in Patients with Intermediate or High Risk of Extracapsular Disease: A Prospective Study of 130 Patients. Radiology 2010, 254, 925–933. [Google Scholar] [CrossRef]
- Zanoni, L.; Bianchi, L.; Nanni, C.; Pultrone, C.; Giunchi, F.; Bossert, I.; Matti, A.; Schiavina, R.; Fiorentino, M.; Romagnoli, D.; et al. [18F]-Fluciclovine PET/CT for Preoperative Nodal Staging in High-Risk Primary Prostate Cancer: Final Results of a Prospective Trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 390–409. [Google Scholar] [CrossRef]
- Detti, B.; Scoccianti, S.; Franceschini, D.; Cipressi, S.; Cassani, S.; Villari, D.; Gacci, M.; Pupi, A.; Vaggelli, L.; Saieva, C.; et al. Predictive Factors of [18F]-Choline PET/CT in 170 Patients with Increasing PSA after Primary Radical Treatment. J. Cancer Res. Clin. Oncol. 2013, 139, 521–528. [Google Scholar] [CrossRef]
- Gauvin, S.; Cerantola, Y.; Haberer, E.; Pelsser, V.; Probst, S.; Bladou, F.; Anidjar, M. Initial Single-Centre Canadian Experience with 18F-Fluoromethylcholine Positron Emission Tomography-Computed Tomography (18F-FCH PET/ CT) for Biochemical Recurrence in Prostate Cancer Patients Initially Treated with Curative Intent. Can. Urol. Assoc. J. 2017, 11, 47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conti, M.; Eriksson, L. Physics of Pure and Non-Pure Positron Emitters for PET: A Review and a Discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Schilling, D.; Schlemmer, H.P.; Wagner, P.H.; Böttcher, P.; Merseburger, A.S.; Aschoff, P.; Bares, R.; Pfannenberg, C.; Ganswindt, U.; Corvin, S.; et al. Histological Verification of 11C-Choline-Positron Emission/Computed Tomography-Positive Lymph Nodes in Patients with Biochemical Failure after Treatment for Localized Prostate Cancer. BJU Int. 2008, 102, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Scattoni, V.; Picchio, M.; Suardi, N.; Messa, C.; Freschi, M.; Roscigno, M.; Pozzo, L.D.; Bocciardi, A.; Rigatti, P.; Fazio, F. Detection of Lymph-Node Metastases with Integrated [11C]Choline PET/CT in Patients with PSA Failure after Radical Retropubic Prostatectomy: Results Confirmed by Open Pelvic-Retroperitoneal Lymphadenectomy. Eur. Urol. 2007, 52, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Murphy, R.C.; Nathan, M.A.; Froemming, A.T.; Hagen, C.E.; Takahashi, N.; Kawashima, A. Detection of Recurrent Prostate Cancer after Radical Prostatectomy: Comparison of 11C-Choline PET/CT with Pelvic Multiparametric MR Imaging with Endorectal Coil. J. Nucl. Med. 2014, 55, 223–232. [Google Scholar] [CrossRef]
- Fuccio, C.; Castellucci, P.; Schiavina, R.; Santi, I.; Allegri, V.; Pettinato, V.; Boschi, S.; Martorana, G.; Al-Nahhas, A.; Rubello, D.; et al. Role of 11C-Choline PET/CT in the Restaging of Prostate Cancer Patients Showing a Single Lesion on Bone Scintigraphy. Ann. Nucl. Med. 2010, 24, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Breeuwsma, A.J.; Pruim, J.; van den Bergh, A.C.M.; Leliveld, A.M.; Nijman, R.J.M.; Dierckx, R.A.J.O.; de Jong, I.J. Detection of Local, Regional, and Distant Recurrence in Patients With PSA Relapse After External-Beam Radiotherapy Using 11C-Choline Positron Emission Tomography. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Rinnab, L.; Mottaghy, F.M.; Blumstein, N.M.; Reske, S.N.; Hautmann, R.E.; Hohl, K.; Möller, P.; Wiegel, T.; Kuefer, R.; Gschwend, J.E. Evaluation of [11C]-Choline Positron-Emission/Computed Tomography in Patients with Increasing Prostate-Specific Antigen Levels after Primary Treatment for Prostate Cancer. BJU Int. 2007, 100, 786–793. [Google Scholar] [CrossRef]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-Specific Membrane Antigen Cleavage of Vitamin B9 Stimulates Oncogenic Signaling through Metabotropic Glutamate Receptors. J. Exp. Med. 2018, 215, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L. Side Effects of Therapy with Radiolabelled Prostate Specific Membrane Antigen (PSMA). In Nuclear Medicine and Molecular Imaging; Signore, A., Ed.; Elsevier: Oxford, UK, 2022; pp. 214–219. ISBN 978-0-12-822980-4. [Google Scholar]
- de Galiza Barbosa, F.; Queiroz, M.A.; Nunes, R.F.; Costa, L.B.; Zaniboni, E.C.; Marin, J.F.G.; Cerri, G.G.; Buchpiguel, C.A. Nonprostatic Diseases on PSMA PET Imaging: A Spectrum of Benign and Malignant Findings. Cancer Imaging 2020, 20, 23. [Google Scholar] [CrossRef]
- Pinto, J.T.; Suffoletto, B.P.; Berzin, T.M.; Qiao, C.H.; Lin, S.; Tong, W.P.; May, F.; Mukherjee, B.; Heston, W.D. Prostate-Specific Membrane Antigen: A Novel Folate Hydrolase in Human Prostatic Carcinoma Cells. Clin. Cancer Res. 1996, 2, 1445–1451. [Google Scholar] [PubMed]
- Samplaski, M.K.; Heston, W.; Elson, P.; Magi-Galluzzi, C.; Hansel, D.E. Folate Hydrolase (Prostate-Specific Antigen) 1 Expression in Bladder Cancer Subtypes and Associated Tumor Neovasculature. Mod. Pathol. 2011, 24, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-Specific Membrane Antigen Expression in Normal and Malignant Human Tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Rajasekaran, S.A.; Christiansen, J.J.; Schmid, I.; Oshima, E.; Sakamoto, K.; Weinstein, J.; Rao, N.P.; Rajasekaran, A.K. Prostate-Specific Membrane Antigen Associates with Anaphase-Promoting Complex and Induces Chromosomal Instability. Mol. Cancer Ther. 2008, 7, 2142–2151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bacich, D.J.; Wozniak, K.M.; Lu, X.-C.M.; O’Keefe, D.S.; Callizot, N.; Heston, W.D.W.; Slusher, B.S. Mice Lacking Glutamate Carboxypeptidase II Are Protected from Peripheral Neuropathy and Ischemic Brain Injury. J. Neurochem. 2005, 95, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.M.; Savir-Baruch, B.; Nieh, P.T.; Master, V.A.; Halkar, R.K.; Rossi, P.J.; Lewis, M.M.; Nye, J.A.; Yu, W.; Bowman, F.D. Detection of Recurrent Prostate Carcinoma with Anti-1-Amino-3-18F-Fluorocyclobutane-1-Carboxylic Acid PET/CT and 111In–Capromab Pendetide SPECT/CT. Radiology 2011, 259, 852. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; von Eyben, F.E.; Fischer, N.; Rosar, F.; Müller-Hübenthal, J.; Buchholz, H.-G.; Wieler, H.J.; Schreckenberger, M. Comparison of [18F]PSMA-1007 with [68Ga]Ga-PSMA-11 PET/CT in Restaging of Prostate Cancer Patients with PSA Relapse. Cancers 2022, 14, 1479. [Google Scholar] [CrossRef]
- Evangelista, L.; Maurer, T.; van der Poel, H.; Alongi, F.; Kunikowska, J.; Laudicella, R.; Fanti, S.; Hofman, M.S. [68Ga]Ga-PSMA Versus [18F]PSMA Positron Emission Tomography/Computed Tomography in the Staging of Primary and Recurrent Prostate Cancer. A Systematic Review of the Literature. Eur. Urol. Oncol. 2022, 5, 273–282. [Google Scholar] [CrossRef]
- Rauscher, I.; Krönke, M.; König, M.; Gafita, A.; Maurer, T.; Horn, T.; Schiller, K.; Weber, W.; Eiber, M. Matched-Pair Comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: Frequency of Pitfalls and Detection Efficacy in Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. 2020, 61, 51–57. [Google Scholar] [CrossRef]
- Treglia, G.; Pereira Mestre, R.; Ferrari, M.; Bosetti, D.G.; Pascale, M.; Oikonomou, E.; De Dosso, S.; Jermini, F.; Prior, J.O.; Roggero, E.; et al. Radiolabelled Choline versus PSMA PET/CT in Prostate Cancer Restaging: A Meta-Analysis. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 127–139. [Google Scholar]
- Metz, R.; Rauscher, A.; Vaugier, L.; Supiot, S.; Drouet, F.; Campion, L.; Rousseau, C. Comparison of Hormone-Sensitive Oligorecurrent Prostate Cancer Patients Based on Routine Use of Choline and/or PSMA PET/CT to Guide Metastasis-Directed Therapy. Cancers 2023, 15, 1898. [Google Scholar] [CrossRef] [PubMed]
- Fossati, N.; Scarcella, S.; Gandaglia, G.; Suardi, N.; Robesti, D.; Boeri, L.; Karnes, R.J.; Heidenreich, A.; Pfister, D.; Kretschmer, A.; et al. Underestimation of Positron Emission Tomography/Computerized Tomography in Assessing Tumor Burden in Prostate Cancer Nodal Recurrence: Head-to-Head Comparison of 68Ga-PSMA and 11C-Choline in a Large, Multi-Institutional Series of Extended Salvage Lymph Node Dissections. J. Urol. 2020, 204, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Calais, J.; Ceci, F.; Cho, S.Y.; Fanti, S.; Giesel, F.L.; Goffin, K.; et al. PSMA PET/CT: Joint EANM Procedure Guideline/SNMMI Procedure Standard for Prostate Cancer Imaging 2.0. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, E.J.; Rumble, R.B.; Jadvar, H.; Hope, T.; Pomper, M.; Turkbey, B.; Rosenkrantz, A.B.; Verma, S.; Margolis, D.J.; Froemming, A.; et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 30, 1963. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.A.; Becker, A.S.; Kranzbühler, B.; Mebert, I.; Baltensperger, A.; Zeimpekis, K.G.; Grünig, H.; Messerli, M.; Rupp, N.J.; Rueschoff, J.H.; et al. Diagnostic Performance of 68Ga-PSMA-11 PET/MRI-Guided Biopsy in Patients with Suspected Prostate Cancer: A Prospective Single-Center Study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3315–3324. [Google Scholar] [CrossRef]
- Shetty, D.; Patel, D.; Le, K.; Bui, C.; Mansberg, R. Pitfalls in Gallium-68 PSMA PET/CT Interpretation—A Pictorial Review. Tomography 2018, 4, 182–193. [Google Scholar] [CrossRef]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. PSMA-RADS Version 1.0: A Step Towards Standardizing the Interpretation and Reporting of PSMA–Targeted PET Imaging Studies. Eur. Urol. 2018, 73, 485–487. [Google Scholar] [CrossRef]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed MiTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.C.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef]
- Szabo, Z.; Mena, E.; Rowe, S.P.; Plyku, D.; Nidal, R.; Eisenberger, M.A.; Antonarakis, E.S.; Fan, H.; Dannals, R.F.; Chen, Y.; et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Mol. Imaging Biol. 2015, 17, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.H.E.; Cysouw, M.C.F.; Vis, A.N.; Van Moorselaar, R.J.A.; Voortman, J.; Bodar, Y.J.L.; Schober, P.R.; Hendrikse, N.H.; Hoekstra, O.S.; Boellaard, R.; et al. Repeatability of Quantitative 18F-DCFPyL PET/CT Measurements in Metastatic Prostate Cancer. J. Nucl. Med. 2020, 61, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Balagurunathan, Y.; Kumar, V.; Gu, Y.; Kim, J.; Wang, H.; Liu, Y.; Goldgof, D.B.; Hall, L.O.; Korn, R.; Zhao, B.; et al. Test-Retest Reproducibility Analysis of Lung CT Image Features. J. Digit. Imaging 2014, 27, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rowe, S.P.; Leal, J.P.; Gorin, M.A.; Allaf, M.E.; Ross, A.E.; Pienta, K.J.; Lodge, M.A.; Pomper, M.G. Semiquantitative Parameters in PSMA-Targeted PET Imaging with 18F-DCFPyL: Variability in Normal-Organ Uptake. J. Nucl. Med. 2017, 58, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Viner, M.; Mercier, G.; Hao, F.; Malladi, A.; Subramaniam, R.M. Liver SULmean at FDG PET/CT: Interreader Agreement and Impact of Placement of Volume of Interest. Radiology 2013, 267, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, K.; Li, X.; Lodge, M.A.; Werner, R.A.; Bundschuh, R.A.; Bundschuh, L.; Kulkarni, H.R.; Schuchardt, C.; Baum, R.P.; Pienta, K.J.; et al. Semiquantitative Parameters in PSMA-Targeted PET Imaging with [18F]DCFPyL: Intrapatient and Interpatient Variability of Normal Organ Uptake. Mol. Imaging Biol. 2020, 22, 181–189. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef]
- Huang, C.; McConathy, J. Radiolabeled Amino Acids for Oncologic Imaging. J. Nucl. Med. 2013, 54, 1007–1010. [Google Scholar] [CrossRef]
- Nanni, C.; Zanoni, L.; Bach-Gansmo, T.; Minn, H.; Willoch, F.; Bogsrud, T.V.; Edward, E.P.; Savir-Baruch, B.; Teoh, E.; Ingram, F.; et al. [18F]Fluciclovine PET/CT: Joint EANM and SNMMI Procedure Guideline for Prostate Cancer Imaging—Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 579–591. [Google Scholar] [CrossRef]
- Schuster, D.M.; Taleghani, P.A.; Nieh, P.T.; Master, V.A.; Amzat, R.; Savir-Baruch, B.; Halkar, R.K.; Fox, T.; Osunkoya, A.O.; Moreno, C.S.; et al. Characterization of Primary Prostate Carcinoma by Anti-1-Amino-2-[(18)F]-Fluorocyclobutane-1-Carboxylic Acid (Anti-3-[(18)F] FACBC) Uptake. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 85–96. [Google Scholar]
- Hole, K.H.; Tulipan, A.J.; Reijnen, J.S.; Hernes, E.; Vlatkovic, L.; Lie, A.K.; Revheim, M.-E.; Seierstad, T. Localization of Primary Prostate Cancer: FACBC PET/CT Compared with Multiparametric MRI Using Histopathology as Reference Standard. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 387–394. [Google Scholar] [PubMed]
- Jambor, I.; Kuisma, A.; Kähkönen, E.; Kemppainen, J.; Merisaari, H.; Eskola, O.; Teuho, J.; Perez, I.M.; Pesola, M.; Aronen, H.J.; et al. Prospective Evaluation of 18F-FACBC PET/CT and PET/MRI versus Multiparametric MRI in Intermediate- to High-Risk Prostate Cancer Patients (FLUCIPRO Trial). Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Scarsbrook, A.F.; Savir-Baruch, B. Impact of 18F-Fluciclovine PET/CT on Plans for Androgen Deprivation Therapy in Patients with Biochemical Recurrence of Prostate Cancer: Data Analysis from Two Prospective Clinical Trials. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 293.e1–293.e7. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Kostakoglu, L.; Chau, A.; Duan, F.; Mahmood, U.; Mankoff, D.A.; Schuster, D.M.; Siegel, B.A. LOCATE Study Group The Impact of Positron Emission Tomography with 18F-Fluciclovine on the Treatment of Biochemical Recurrence of Prostate Cancer: Results from the LOCATE Trial. J. Urol. 2019, 201, 322–331. [Google Scholar] [CrossRef]
- Scarsbrook, A.F.; Bottomley, D.; Teoh, E.J.; Bradley, K.M.; Payne, H.; Afaq, A.; Bomanji, J.; van As, N.; Chua, S.; Hoskin, P.; et al. Effect of 18F-Fluciclovine Positron Emission Tomography on the Management of Patients With Recurrence of Prostate Cancer: Results From the FALCON Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 316–324. [Google Scholar] [CrossRef]
- Ferrari, C.; Mammucci, P.; Lavelli, V.; Pisani, A.R.; Nappi, A.G.; Rubini, D.; Sardaro, A.; Rubini, G. [18F]Fluciclovine vs. [18F]Fluorocholine Positron Emission Tomography/Computed Tomography: A Head-to-Head Comparison for Early Detection of Biochemical Recurrence in Prostate Cancer Patients. Tomography 2022, 8, 2709–2722. [Google Scholar] [CrossRef]
- Nappi, A.G.; Ferrari, C.; Mammucci, P.; Rubini, D.; Lavelli, V.; Sardaro, A.; Pisani, A.R.; Rubini, G. [18F]Fluciclovine PET/CT Improves the Clinical Management of Early Recurrence Prostate Cancer Patients. Cancers 2022, 14, 1461. [Google Scholar] [CrossRef]
- Bach-Gansmo, T.; Nanni, C.; Nieh, P.T.; Zanoni, L.; Bogsrud, T.V.; Sletten, H.; Korsan, K.A.; Kieboom, J.; Tade, F.I.; Odewole, O.; et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine (18F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J. Urol. 2017, 197, 676–683. [Google Scholar] [CrossRef]
- Marcus, C.; Abiodun-Ojo, O.A.; Jani, A.B.; Schuster, D.M. Clinical Utility of 18F-Fluciclovine PET/CT in Recurrent Prostate Cancer with Very Low (≤0.3 Ng/ML) Prostate-Specific Antigen Levels. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 406–414. [Google Scholar]
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. 18F-Fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in Patients with Early Biochemical Recurrence after Prostatectomy: A Prospective, Single-Centre, Single-Arm, Comparative Imaging Trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Pernthaler, B.; Kulnik, R.; Gstettner, C.; Salamon, S.; Aigner, R.M.; Kvaternik, H. A Prospective Head-to-Head Comparison of 18F-Fluciclovine With 68Ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT. Clin. Nucl. Med. 2019, 44, e566–e573. [Google Scholar] [CrossRef] [PubMed]
- Cochran, B.J.; Ryder, W.J.; Parmar, A.; Klaeser, K.; Reilhac, A.; Angelis, G.I.; Meikle, S.R.; Barter, P.J.; Rye, K.-A. Determining Glucose Metabolism Kinetics Using 18F-FDG Micro-PET/CT. J. Vis. Exp. 2017, 123, 55184. [Google Scholar] [CrossRef]
- Bauckneht, M.; Bertagna, F.; Donegani, M.I.; Durmo, R.; Miceli, A.; De Biasi, V.; Laudicella, R.; Fornarini, G.; Berruti, A.; Baldari, S.; et al. The Prognostic Power of 18F-FDG PET/CT Extends to Estimating Systemic Treatment Response Duration in Metastatic Castration-Resistant Prostate Cancer (MCRPC) Patients. Prostate Cancer Prostatic. Dis. 2021, 24, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.D.; Imbriaco, M.; Larson, S.M.; Garza, D.; Zhang, J.J.; Kalaigian, H.; Finn, R.D.; Reddy, D.; Horowitz, S.M.; Goldsmith, S.J.; et al. Detection of Bony Metastases of Androgen-Independent Prostate Cancer by PET-FDG. Nucl. Med. Biol. 1996, 23, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.J.R.; Azad, G.; Padhani, A.R. Bone Imaging in Prostate Cancer: The Evolving Roles of Nuclear Medicine and Radiology. Clin. Transl. Imaging 2016, 4, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bjurlin, M.A.; Rosenkrantz, A.B.; Beltran, L.S.; Raad, R.A.; Taneja, S.S. Imaging and Evaluation of Patients with High-Risk Prostate Cancer. Nat. Rev. Urol. 2015, 12, 617–628. [Google Scholar] [CrossRef]
- Gu, J.; Lu, Y.; Xu, G. Mismatched Lesions on 18F-FDG PET and 18F-Fluciclovine PET Images in a Patient With Metastatic Prostate Small Cell Carcinoma. Clin. Nucl. Med. 2022, 47, 255–257. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Zhu, Y.; Shi, Y.; Xu, L.; Huang, G.; Liu, J. The Added Value of 18F-FDG PET/CT Compared with 68Ga-PSMA PET/CT in Patients with Castration-Resistant Prostate Cancer. J. Nucl. Med. 2022, 63, 69–75. [Google Scholar] [CrossRef]
- Thang, S.P.; Violet, J.; Sandhu, S.; Iravani, A.; Akhurst, T.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; Williams, S.G.; Hicks, R.J.; et al. Poor Outcomes for Patients with Metastatic Castration-Resistant Prostate Cancer with Low Prostate-Specific Membrane Antigen (PSMA) Expression Deemed Ineligible for 177Lu-Labelled PSMA Radioligand Therapy. Eur. Urol. Oncol. 2019, 2, 670–676. [Google Scholar] [CrossRef]
- Kepenek, F.; Can, C.; Kömek, H.; Kaplan, İ.; Gündoğan, C.; Ebinç, S.; Güzel, Y.; Agüloglu, N.; Karaoglan, H.; Taşdemir, B. Combination of [68Ga]Ga-PSMA PET/CT and [18F]FDG PET/CT in Demonstrating Dedifferentiation in Castration-Resistant Prostate Cancer. Médecine Nucléaire 2023, 47, 193–199. [Google Scholar] [CrossRef]
- Markwalder, R.; Reubi, J.C. Gastrin-Releasing Peptide Receptors in the Human Prostate: Relation to Neoplastic Transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar] [PubMed]
- Elshafae, S.M.; Hassan, B.B.; Supsavhad, W.; Dirksen, W.P.; Camiener, R.Y.; Ding, H.; Tweedle, M.F.; Rosol, T.J. Gastrin-Releasing Peptide Receptor (GRPr) Promotes EMT, Growth, and Invasion in Canine Prostate Cancer. Prostate 2016, 76, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef] [PubMed]
- Ananias, H.J.K.; van den Heuvel, M.C.; Helfrich, W.; de Jong, I.J. Expression of the Gastrin-Releasing Peptide Receptor, the Prostate Stem Cell Antigen and the Prostate-Specific Membrane Antigen in Lymph Node and Bone Metastases of Prostate Cancer. Prostate 2009, 69, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Montani, M.; Gerhardt, J.; Wild, P.J.; Hany, T.F.; Hermanns, T.; Müntener, M.; Kristiansen, G. Profiling Gastrin-Releasing Peptide Receptor in Prostate Tissues: Clinical Implications and Molecular Correlates. Prostate 2012, 72, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot Comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2016, 57, 557. [Google Scholar] [CrossRef]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of Prostate-Specific Membrane Antigen (PSMA) Expression in Prostate Carcinoma with Distant Metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Armstrong, A.J.; Bahnson, R.R.; D’Amico, A.V.; Davis, B.J.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; Horwitz, E.M.; et al. Prostate Cancer, Version 1.2016. J. Natl. Compr. Cancer Netw. 2016, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Klain, M.; Gaudieri, V.; Petretta, M.; Zampella, E.; Storto, G.; Nappi, C.; Buonerba, C.; Crocetto, F.; Gallicchio, R.; Volpe, F.; et al. Combined Bone Scintigraphy and Fluorocholine PET/Computed Tomography Predicts Response to Radium-223 Therapy in Patients with Prostate Cancer. Future Sci. OA 2021, 7, FSO719. [Google Scholar] [CrossRef] [PubMed]
- Frantellizzi, V.; Monari, F.; Mascia, M.; Costa, R.P.; Rubini, G.; Spanu, A.; Farcomeni, A.; Lodi Rizzini, E.; Cindolo, L.; Tripoli, V.; et al. Radium-223 in MCPRC Patients: A Large Real-Life Italian Multicenter Study. Minerva Urol. Nephrol. 2022, 74, 21–28. [Google Scholar] [CrossRef]
- Buscombe, J.; Gillett, D.; Bird, N.; Powell, A.; Heard, S.; Aloj, L. Quantifying the Survival Benefit of Completing All the Six Cycles of Radium-223 Therapy in Patients with Castrate-Resistant Prostate Cancer with Predominant Bone Metastases. World J. Nucl. Med. 2020, 20, 139–144. [Google Scholar] [CrossRef]
- Jarvis, P.; Ho, A.; Sundram, F. Radium-223 Therapy for Metastatic Castration-Resistant Prostate Cancer: Survival Benefit When Used Earlier in the Treatment Pathway. Nucl. Med. Commun. 2021, 42, 332–336. [Google Scholar] [CrossRef]
- Ling, S.W.; de Blois, E.; Hooijman, E.; van der Veldt, A.; Brabander, T. Advances in 177Lu-PSMA and 225Ac-PSMA Radionuclide Therapy for Metastatic Castration-Resistant Prostate Cancer. Pharmaceutics 2022, 14, 2166. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Essler, M.; Biersack, H.J. PSMA-Based Theranostics: A Step-by-Step Practical Approach to Diagnosis and Therapy for MCRPC Patients. Semin. Nucl. Med. 2020, 50, 98–109. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hofman, M.S. Prostate-Specific Membrane Antigen Theranostics: Therapy with Lutetium-177. Curr. Opin. Urol. 2018, 28, 197–204. [Google Scholar] [CrossRef]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Hofman, M.S.; Hicks, R.J.; Scalzo, M.; Violet, J. Radiation Dosimetry in 177Lu-PSMA-617 Therapy Using a Single Posttreatment SPECT/CT Scan: A Novel Methodology to Generate Time- and Tissue-Specific Dose Factors. J. Nucl. Med. 2020, 61, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Broughman, J.R.; Fleming, C.W.; Mian, O.Y.; Stephans, K.L.; Tendulkar, R.D. Management of Oligometastatic Prostate Cancer. Appl. Radiat. Oncol. 2020, 9, 6. [Google Scholar] [CrossRef]
- Fendler, W.P.; Rahbar, K.; Herrmann, K.; Kratochwil, C.; Eiber, M. 177Lu-PSMA Radioligand Therapy for Prostate Cancer. J. Nucl. Med. 2017, 58, 1196–1200. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.-J. 177 Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef]
- Rohith, G. VISION Trial: 177Lu-PSMA-617 for Progressive Metastatic Castration-Resistant Prostate Cancer. Indian J. Urol. 2021, 37, 372–373. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Violet, J.; Y Zhang, A.; Lawrence, N.J.; Stockler, M.; Francis, R.J.; Iravani, A.; Williams, S.; Azad, A.; et al. TheraP: A Randomized Phase 2 Trial of 177 Lu-PSMA-617 Theranostic Treatment vs Cabazitaxel in Progressive Metastatic Castration-Resistant Prostate Cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019, 124 (Suppl. S1), 5–13. [Google Scholar] [CrossRef]
- Schuchardt, C.; Zhang, J.; Kulkarni, H.R.; Chen, X.; Müller, D.; Baum, R.P. Prostate-Specific Membrane Antigen Radioligand Therapy Using 177Lu-PSMA I&T and 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: Comparison of Safety, Biodistribution, and Dosimetry. J. Nucl. Med. 2022, 63, 1199–1207. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L.; et al. Joint EANM/SNMMI Procedure Guideline for the Use of 177Lu-Labeled PSMA-Targeted Radioligand-Therapy (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2830–2845. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.G.; Hofman, M.S.; Azad, A.; Violet, J.; Hicks, R.J.; Lawrentschuk, N. Going Nuclear: It Is Time to Embed the Nuclear Medicine Physician in the Prostate Cancer Multidisciplinary Team. BJU Int. 2019, 124, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.G.; Sathianathen, N.; Hofman, M.S.; Azad, A.; Lawrentschuk, N. Where to Next for Theranostics in Prostate Cancer? Eur. Urol. Oncol. 2019, 2, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, W.J.; McBean, R.; Wong, D.; Grimes, D.; Vasey, P.; Frydenberg, M.; Yaxley, J.W. Should Lutetium-Prostate Specific Membrane Antigen Radioligand Therapy for Metastatic Prostate Cancer Be Used Earlier in Men with Lymph Node Only Metastatic Prostate Cancer? Investig. Clin. Urol. 2021, 62, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Golan, S.; Frumer, M.; Zohar, Y.; Rosenbaum, E.; Yakimov, M.; Kedar, D.; Margel, D.; Baniel, J.; Steinmetz, A.P.; Groshar, D.; et al. Neoadjuvant 177Lu-PSMA-I&T Radionuclide Treatment in Patients with High-Risk Prostate Cancer Before Radical Prostatectomy: A Single-Arm Phase 1 Trial. Eur. Urol. Oncol. 2022, 6, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Pathmanandavel, S.; Crumbaker, M.; Nguyen, A.; Yam, A.O.W.; Wilson, P.; Niman, R.; Ayers, M.; Sharma, S.; Eu, P.; Martin, A.J.; et al. The Prognostic Value of Post-Treatment PSMA and FDG PET/CT in Metastatic, Castration-Resistant Prostate Cancer Treated with 177LuPSMA-617 and NOX66 in a Phase I/II Trial (LuPIN). J. Nucl. Med. 2022, 64, 69–74. [Google Scholar] [CrossRef]
- Suman, S.; Parghane, R.V.; Joshi, A.; Prabhash, K.; Talole, S.; Basu, S. Combined 177Lu-PSMA-617 PRLT and Abiraterone Acetate versus 177Lu-PSMA-617 PRLT Monotherapy in Metastatic Castration-Resistant Prostate Cancer: An Observational Study Comparing the Response and Durability. Prostate 2021, 81, 1225–1234. [Google Scholar] [CrossRef]
- Kostos, L.; Buteau, J.P.; Yeung, T.; Iulio, J.D.; Xie, J.; Cardin, A.; Chin, K.Y.; Emmerson, B.; Owen, K.L.; Parker, B.S.; et al. AlphaBet: Combination of Radium-223 and [177Lu]Lu-PSMA-I&T in Men with Metastatic Castration-Resistant Prostate Cancer (Clinical Trial Protocol). Front. Med. 2022, 9, 1059122. [Google Scholar] [CrossRef]
- Abbott, E.M.; Falzone, N.; Lenzo, N.; Vallis, K.A. Combining External Beam Radiation and Radionuclide Therapies: Rationale, Radiobiology, Results and Roadblocks. Clin. Oncol. 2021, 33, 735–743. [Google Scholar] [CrossRef]
- Stangl-Kremser, J.; Ricaurte-Fajardo, A.; Subramanian, K.; Osborne, J.R.; Sun, M.; Tagawa, S.T.; Bander, N.H. Response to RL-225Ac in Prostate Cancer: Effect of Prior Treatment with RL-177Lu: A Systematic Review of the Literature. Prostate 2023, 83, 901–911. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholomä, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 Tandem Therapy of Metastatic Castration-Resistant Prostate Cancer: Pilot Experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef]
- Rosar, F.; Krause, J.; Bartholomä, M.; Maus, S.; Stemler, T.; Hierlmeier, I.; Linxweiler, J.; Ezziddin, S.; Khreish, F. Efficacy and Safety of [225Ac]Ac-PSMA-617 Augmented [177Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Highly Advanced MCRPC with Poor Prognosis. Pharmaceutics 2021, 13, 722. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; Valstar, M.H.; de Keizer, B.; Willems, S.M.; Smit, L.A.; Al-Mamgani, A.; Smeele, L.E.; van Es, R.J.J.; de Bree, R.; Vogel, W.V. Physiologic Distribution of PSMA-Ligand in Salivary Glands and Seromucous Glands of the Head and Neck on PET/CT. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 478–486. [Google Scholar] [CrossRef]
- Roy, J.; Warner, B.M.; Basuli, F.; Zhang, X.; Zheng, C.; Goldsmith, C.; Phelps, T.; Wong, K.; Ton, A.T.; Pieschl, R.; et al. Competitive Blocking of Salivary Gland [18F]DCFPyL Uptake via Localized, Retrograde Ductal Injection of Non-Radioactive DCFPyL: A Preclinical Study. EJNMMI Res. 2021, 11, 66. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated Copper and Oxidative Stress in Cancer Cells as a Target for Cancer Treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Capasso, E.; Durzu, S.; Piras, S.; Zandieh, S.; Knoll, P.; Haug, A.; Hacker, M.; Meleddu, C.; Mirzaei, S. Role of 64CuCl2 PET/CT in Staging of Prostate Cancer. Ann. Nucl. Med. 2015, 29, 482–488. [Google Scholar] [CrossRef]
- Piccardo, A.; Paparo, F.; Puntoni, M.; Righi, S.; Bottoni, G.; Bacigalupo, L.; Zanardi, S.; DeCensi, A.; Ferrarazzo, G.; Gambaro, M.; et al. 64CuCl2 PET/CT in Prostate Cancer Relapse. J. Nucl. Med. 2018, 59, 444–451. [Google Scholar] [CrossRef]
- Leung, K.H.; Rowe, S.P.; Leal, J.P.; Ashrafinia, S.; Sadaghiani, M.S.; Chung, H.W.; Dalaie, P.; Tulbah, R.; Yin, Y.; VanDenBerg, R.; et al. Deep Learning and Radiomics Framework for PSMA-RADS Classification of Prostate Cancer on PSMA PET. EJNMMI Res. 2022, 12, 76. [Google Scholar] [CrossRef]
- Yip, S.S.F.; Aerts, H.J.W.L. Applications and Limitations of Radiomics. Phys. Med. Biol. 2016, 61, R150–R166. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The Facts and the Challenges of Image Analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Stanzione, A.; Gambardella, M.; Cuocolo, R.; Ponsiglione, A.; Romeo, V.; Imbriaco, M. Prostate MRI Radiomics: A Systematic Review and Radiomic Quality Score Assessment. Eur. J. Radiol. 2020, 129, 109095. [Google Scholar] [CrossRef]
- Solari, E.L.; Gafita, A.; Schachoff, S.; Bogdanović, B.; Villagrán Asiares, A.; Amiel, T.; Hui, W.; Rauscher, I.; Visvikis, D.; Maurer, T.; et al. The Added Value of PSMA PET/MR Radiomics for Prostate Cancer Staging. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 527–538. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zheng, A.; Gao, J.; Yuan, W.; Shen, C.; Bai, L.; Duan, X. Evaluation of a Radiomics Nomogram Derived from Fluoride-18 PSMA-1007 PET/CT for Risk Stratification in Newly Diagnosed Prostate Cancer. Front. Oncol. 2022, 12, 1018833. [Google Scholar] [CrossRef]
- Gravina, M.; Spirito, L.; Celentano, G.; Capece, M.; Creta, M.; Califano, G.; Collà Ruvolo, C.; Morra, S.; Imbriaco, M.; Di Bello, F.; et al. Machine Learning and Clinical-Radiological Characteristics for the Classification of Prostate Cancer in PI-RADS 3 Lesions. Diagnostics 2022, 12, 1565. [Google Scholar] [CrossRef]
- Müller, C.; Umbricht, C.A.; Gracheva, N.; Tschan, V.J.; Pellegrini, G.; Bernhardt, P.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P. Terbium-161 for PSMA-Targeted Radionuclide Therapy of Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1919–1930. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Scott, A.M. 161Tb-PSMA Unleashed: A Promising New Player in the Theranostics of Prostate Cancer. Nucl. Med. Mol. Imaging 2023, 57, 168–171. [Google Scholar] [CrossRef]
- Kokov, K.V.; Egorova, B.V.; German, M.N.; Klabukov, I.D.; Krasheninnikov, M.E.; Larkin-Kondrov, A.A.; Makoveeva, K.A.; Ovchinnikov, M.V.; Sidorova, M.V.; Chuvilin, D.Y. 212Pb: Production Approaches and Targeted Therapy Applications. Pharmaceutics 2022, 14, 189. [Google Scholar] [CrossRef]
- Mease, R.C.; Kang, C.M.; Kumar, V.; Banerjee, S.R.; Minn, I.; Brummet, M.; Gabrielson, K.L.; Feng, Y.; Park, A.; Kiess, A.P.; et al. An Improved 211At-Labeled Agent for PSMA-Targeted α-Therapy. J. Nucl. Med. 2022, 63, 259–267. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Pruszynski, M. Astatine-211: Production and Availability. Curr. Radiopharm. 2011, 4, 177–185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).