Simple Summary
We evaluated the outcomes of salvage hepatectomy for local recurrence of hepatocellular carcinoma after radiofrequency ablation. Short-term outcomes including operation time, intraoperative blood loss, postoperative hospital stay, and postoperative complication rates were similar in salvage hepatectomy patients and a propensity score-matched control group who underwent liver resection as primary treatment. Recurrent tumors after radiofrequency ablation showed poorer differentiation, more aggressive behavior, and higher recurrence rates. Less extensive resection compared to the initial plan, negative but close (<0.1 cm) resection margin, and R1 resection were significant predictors for recurrence after salvage hepatectomy. These results suggest that salvage hepatectomy could be a rescue therapy for local recurrence after ablation, and wide resection margins are essential to prevent recurrence after surgery.
Abstract
Background and Objectives: Although radiofrequency ablation (RFA) is a well-established locoregional treatment modality for hepatocellular carcinoma (HCC), the optimal strategy to handle local recurrence after ablation is still debated. This study aims to investigate the role of salvage hepatectomy (SH) as a rescue therapy for recurrent HCC after RFA. Materials and Methods: Between January 2004 and December 2020, 1161 patients were subject to surgical resection for HCC. Among them, 47 patients who underwent SH for local recurrence after ablation were retrospectively analyzed and compared to a propensity score-matched group of controls (n = 47) who received primary hepatectomy (PH). Short-term and long-term outcomes were analyzed between the two groups. Results: After matching, operation time, intraoperative blood loss, postoperative hospital stay, and postoperative morbidity rates showed no statistically significant difference. Tumors in the SH group were associated with poor differentiation (SH 9 (19.1%) vs. PH 1 (2.1%), p < 0.001). The 5-year disease-free survival rates (31.6% vs. 73.4%, p < 0.001) and overall survival rates (80.3% vs. 94.2%, p = 0.047) were significantly lower in the SH group. In multivariable analysis, less extensive resection compared to the initial plan (hazard ratio (HR) 4.68, p = 0.024), higher grade (HR 5.38, P < 0.001), negative but close (<0.1 cm) resection margin (HR 22.14, p = 0.007), and R1 resection (HR 3.13, p = 0.006) were significant predictors for recurrence. Conclusions: SH for recurrent tumors after ablation showed safety and effectiveness equivalent to primary resection. As recurrent tumors show a higher grade and more aggressive behavior, more extensive resections with wide surgical margins are necessary to prevent recurrence.
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer worldwide, and its observed incidence rates are higher in East Asian countries, including Korea [1,2,3]. Therapeutic options are chosen based on several factors, including tumor number and size, liver functional reserve, and general performance status [4]. The Barcelona Clinic Liver Cancer staging system defines curative treatment options for early HCC as surgical resection, liver transplantation, and thermal ablation [5,6]. Local ablation techniques, including a percutaneous ethanol injection, microwave ablation, and radiofrequency ablation (RFA), are widely used in clinical practice and accepted as first-line therapy for early HCC cases [7,8,9]. Yet, HCC shows a limited long-term prognosis due to frequent recurrence, and local tumor progression rates after RFA have also been reported as high [4,10,11]. It is generally known that recurrence directly affects long-term survival in patients undergoing RFA [12].
Local recurrence after ablation is known to be associated with several risk factors, including tumor size, tumor number, and location near the liver surface [13]. When the complete ablation of the tumor is not achieved, patients are more likely to experience tumor progression; therefore, successful ablation without a residual tumor after ablation significantly improves survival [14,15]. Previous studies have also suggested that tumors exhibit more aggressive features after recurrence, possibly due to higher vascular invasion rates and dedifferentiation of the tumor via a heat shock effect during the thermal ablation procedure [10,16,17,18].
Repeated local ablation or transcatheter arterial chemoembolization (TACE) is mostly advocated as second-line treatment for local recurrence after initial ablation [19]. As recurrence after RFA is usually associated with advanced tumors, surgical resection could have a role as salvage therapy in selected patients. Yet, the existing literature on the role of surgical treatment after local recurrence is scarce, with most studies based on retrospective analyses with small sample sizes. This study aims to compare both the short-term and long-term outcomes of salvage hepatectomy for local recurrence after RFA with primary hepatic resection.
2. Materials and Methods
2.1. Study Population
We analyzed patients who underwent liver resection for HCC at a tertiary referral center in Korea (Seoul National University Bundang Hospital) between January 2004 and December 2020. Among the 1161 patients, those with repeated hepatic resection for recurrence after initial surgery (n = 214) and hepatectomy for recurrence after TACE (n = 353) were excluded from the analysis. As a result, a total of 594 patients were included, of whom 47 consecutive patients received salvage hepatic resection for local recurrence after RFA (SH group), and 547 patients underwent primary hepatectomy as their initial treatment (PH group). To adjust for clinicopathological differences between these two groups and reduce the potential effect of selection bias, 1:1 propensity score matching (PSM) was performed. Matching factors included patient demographics (age, sex), liver function (Child–Pugh class, liver cirrhosis), and tumor characteristics (number, size). Finally, 47 patients from the SH group and 47 patients from the PH group were successfully matched. This study was approved by the institutional review board of SNUBH and conducted in compliance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies [20].
2.2. Data Collection and Definitions
Patient data, including baseline demographics, operative information, pathologic reports, and survival outcomes, were retrospectively collected from medical records. Hepatectomy procedures were categorized as described by the Brisbane classification [21]. Major liver resection was defined as the resection of three or more adjacent liver segments, such as hemihepatectomy or trisectionectomy. Minor liver resection included non-anatomical liver wedge resection, segmentectomy, or sectionectomy. Postoperative complications were graded according to the Clavien–Dindo system [22]. Mortality at any time during the patient’s postoperative hospital stay was defined as in-hospital death.
2.3. Surgical Procedures
Indications of salvage hepatectomy included a technical difficulty in repeating RFA due to tumor location or size, the presence of tumor thrombus, or individual patient preference. Whether major or minor hepatic resection could be performed was decided the same in the SH and PH groups based on preoperative imaging studies and liver functional reserve markers. In all study participants, macroscopically curative resection was planned. In the SH group, the initial computed tomography (CT) scans before RFA were additionally reviewed for the extent of resection necessary. This initial plan was compared to the actual hepatic resection performed after recurrence.
2.4. Follow-Up
All patients were assigned follow-up visits in the outpatient clinic at regular intervals. They underwent clinical examinations and screening for recurrence, including blood tests for the tumor markers α-fetoprotein (AFP) and des-γ-carboxyprothrombin (DCP) and imaging studies such as CT or magnetic resonance imaging. Recurrence was diagnosed when a hepatic lesion with radiologic features typical of HCC or distant metastasis was newly detected. The interval between the time of operation and the date of first recurrence was defined as disease-free survival (DFS). The interval between the time of operation and the date of cancer-related death or the last follow-up visit was defined as overall survival (OS). The median follow-up duration was 34 months.
2.5. Statistical Analysis
The Statistical Package for the Social Sciences (SPSS version 25.0, IBM Inc., Armonk, NY, USA) was used for statistical analyses. Normally distributed continuous variables were expressed as the mean (standard deviation), and non-normally distributed variables were presented as the median (interquartile range). Student’s t-test or the Mann–Whitney U test was used for comparisons. Categorical variables were indicated as the frequency (percentage); they were compared using Pearson’s chi-square test or Fisher’s exact test. Survival outcomes were compared using Kaplan–Meier analysis with lthe og-rank test. Risk factors for recurrence and cancer-related death were analyzed through Cox regression. All p-values were two-sided, and p < 0.05 was considered statistically significant.
3. Results
3.1. Study Population
Before matching, the SH and PH groups differed significantly in sex, tumor number, and tumor size. After matching, differences in baseline characteristics disappeared. The baseline characteristics of the patients before and after matching are summarized in Table 1.

Table 1.
Baseline characteristics of study participants before and after propensity score matching.
3.2. Operative Parameters and Pathologic Features
After PSM, a comparison of operative parameters between the two groups showed similar tumor locations (Table 2). An operative approach, major hepatectomy and anatomical resection rates, operation time, and intraoperative blood loss showed no difference between the SH and PH groups. In the SH group, 31.9% of patients underwent a more extensive resection after recurrence compared to the initial plan; in 6.4%, a less extensive resection was performed.

Table 2.
Operative parameters between patients undergoing salvage hepatectomy and primary hepatectomy.
A comparison of pathological features between the two groups showed no difference in tumor size and number (Table 3). The Edmonson–Steiner (ES) grade of the tumors was higher after salvage hepatectomy (grade III: SH 20 (42.6%) vs. PH 11 (23.4%), grade IV: SH 9 (19.1%) vs. PH 1 (2.1%), p < 0.001). There was no difference in either macrovascular or microvascular invasion rates. R1 resection rates were similar between the SH and PH groups (SH 1 [2.1%] vs. PH 3 [6.4%], p = 0.617).

Table 3.
Postoperative outcomes after salvage hepatectomy and primary hepatectomy.
3.3. Postoperative Outcomes
When postoperative outcomes were analyzed, postoperative morbidity rates showed no statistically significant difference between the two groups (Table 3). There was no mortality during hospitalization in either group. The duration of postoperative hospital stay was similar between the two groups (SH 7 [5–9] days vs. PH 7 [5–8] days, p = 0.683).
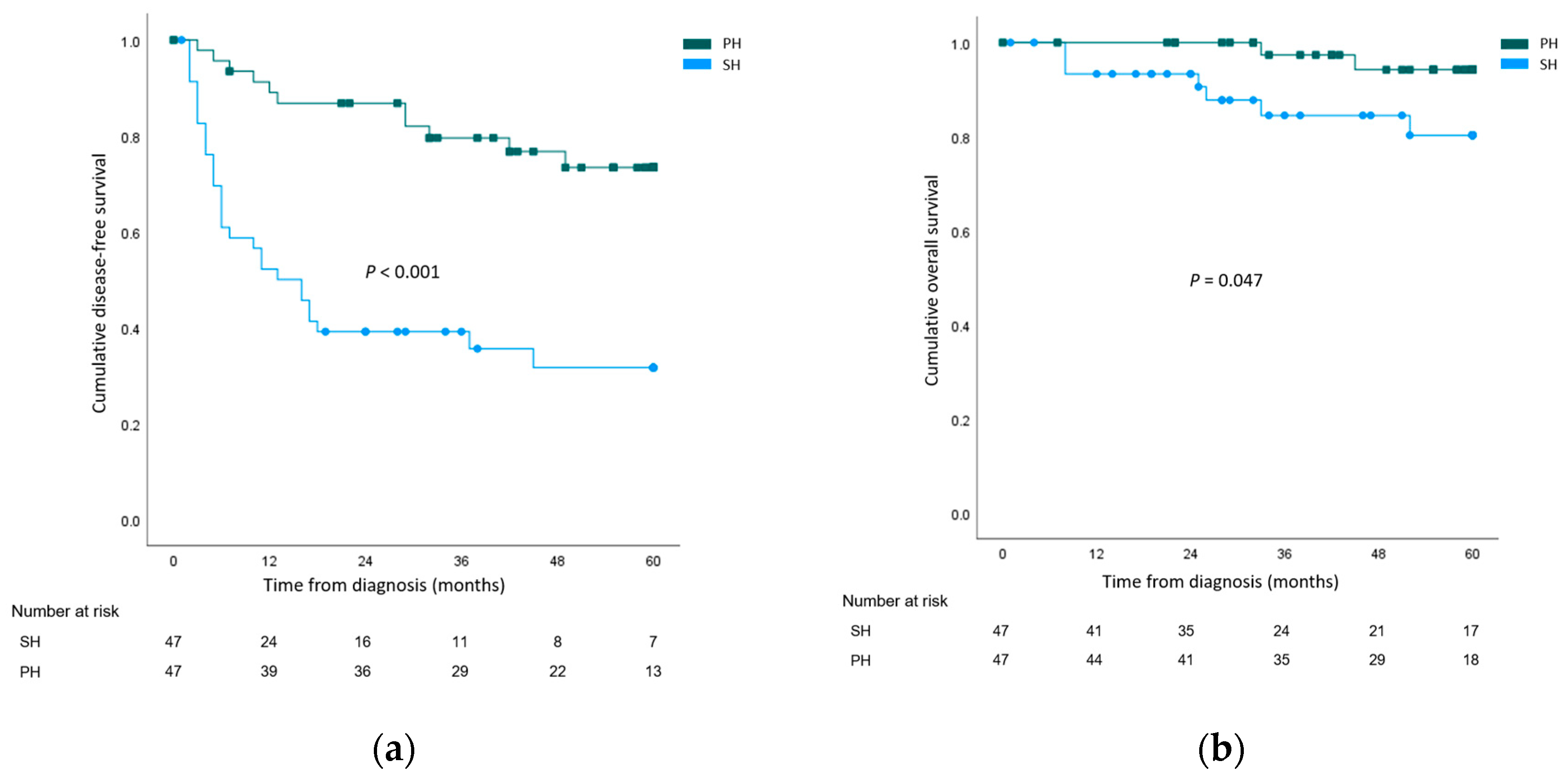
The cumulative overall 1-, 3-, and 5-year DFS rates of the whole study population were 70.6%, 62.9%, and 52.6%, respectively. Recurrence rates were higher after salvage hepatetomy for both local recurrence (SH 29 (61.7%) vs. PH 9 (19.1%), p < 0.001) and systemic recurrence (SH 17 (36.2%) vs. PH 3 (6.4%), p = 0.001). When 5-year DFS rates were compared, the outcomes were significantly worse for the SH group (SH 31.6% vs. PH 73.4%, p < 0.001). The cumulative overall 1-, 3-, and 5-year OS rates of the whole study population were 96.7%, 91.2%, and 87.7%, respectively. Cancer-related mortality rates were higher in the SH group (SH 12 (25.5%) vs. PH 3 (6.4%), p = 0.024). The 5-year OS rate was significantly lower in the SH group (SH 80.3% vs. PH 94.2%, p = 0.047). The survival curves are illustrated in Figure 1.
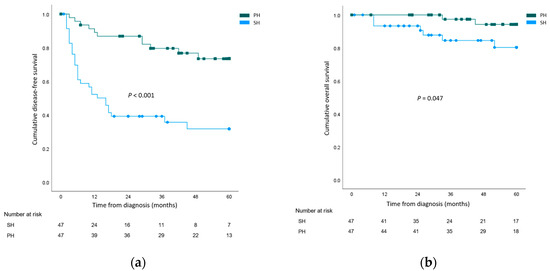
Figure 1.
Survival analysis comparing the salvage hepatectomy (SH) group and the primary hepatectomy (PH) group. (a) Disease-free survival; (b) Overall survival.
3.4. Regression Analysis for Risk Factors of Recurrence and Cancer-Related Death
Univariable and multivariable Cox regression analyses were performed for risk factors of recurrence in the SH group. In univariable analysis, less extensive resection compared to the initial plan, high tumor grade, negative but very close (less than 0.1 cm) surgical margin, and R1 resection were significant predictors (Table 4). In multivariable analysis, a less extensive resection compared to the initial plan (hazard ratio (HR) 4.68, p = 0.024), ES grade IV (HR 5.38, p < 0.001), negative but close surgical margin (HR 22.14, p = 0.007), and R1 resection (HR 3.13, p = 0.006) were all found to be significant. Subgroup analysis was performed for local and systemic recurrence. Risk factors for local recurrence included ES grade IV (HR 3.02, p = 0.010) and R1 resection (HR 6.20, p = 0.022). For systemic recurrence, only the poor differentiation of the tumor reached marginal significance as a predictor (HR 2.80, p = 0.057).

Table 4.
Univariable and multivariable regression analyses for recurrence after salvage hepatectomy.
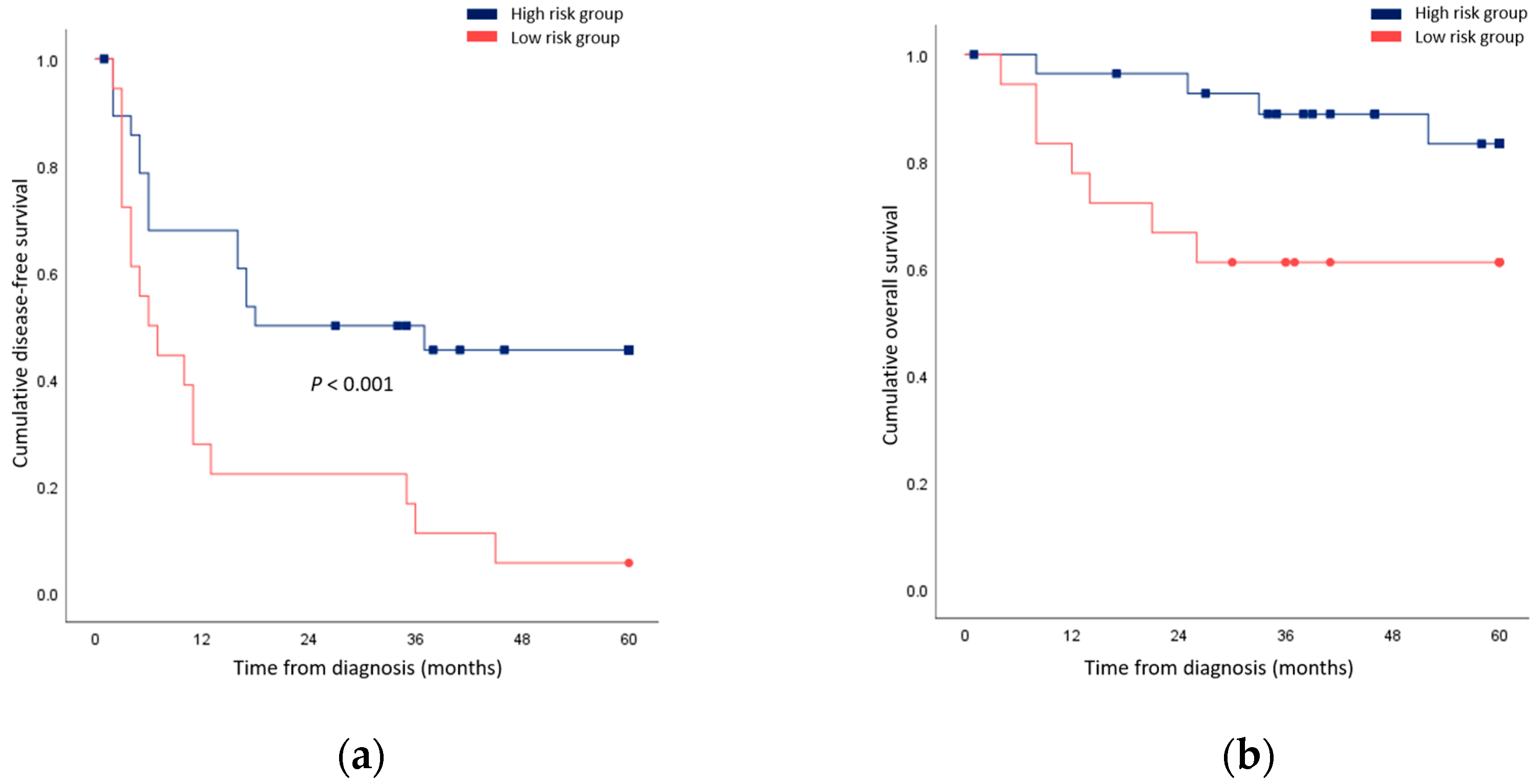
Univariable and multivariable regression was additionally performed for cancer-related death. In univariable analysis, the tumor grade and close resection margin predicted outcomes (Table 5). In multivariable analysis, both factors were found to be prognostic (ES grade IV: HR 10.97, p = 0.009; close resection margin: HR 68.53, p = 0.004). When patients underwent less extensive resection compared to the initial plan, surgical resection with a negative but close margin, or R1 resection were grouped together; their survival outcomes were significantly worse compared to patients who possessed none of these risk factors (Figure 2).

Table 5.
Univariable and multivariable regression analyses for cancer-related death after salvage hepatectomy.
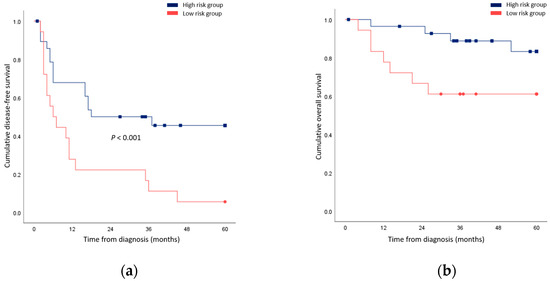
Figure 2.
Comparison of survival rates between patients who underwent less extensive resection compared to the initial plan, a surgical resection with negative but close margin, or R1 resection (high risk group) and patients who possessed none of these risk factors (low risk group). (a) Disease-free survival; (b) Overall survival.
4. Discussion
RFA is considered a curative treatment option for early HCC, and it is actively considered when the functional reserve of a patient’s liver is limited [17,23]. Yet, recurrent HCC after RFA is associated with a higher malignant potential than primary tumors, and the treatment strategies for local recurrence and their long-term outcomes remain unexplored [13,24,25]. In the current study, we compared patients who received salvage resection for local recurrence after RFA with a control group undergoing primary liver resection for HCC. Operative parameters, including operation time, blood loss, and postoperative morbidities, showed no difference between the groups, which proved the safety and feasibility of salvage hepatectomy after RFA. However, the SH group showed significantly higher recurrence rates, which reflected the aggressive nature of recurrent tumors after RFA.
In certain cases, RFA procedures might render subsequent surgical procedures extremely difficult due to adhesion formation between the diaphragm, abdominal wall, and liver. For this reason, previous studies have questioned if salvage hepatectomy after RFA is technically feasible. Two studies have shown that salvage resection after RFA results in prolonged operation time, more bleeding, and higher concomitant extrahepatic resection rates [10,26]. Yamashita et al. also reported that they had to change the operative plan during surgery mainly due to extensive adhesions, which are secondary to RFA in the majority of salvage hepatectomy patients [27]. In our study, operation time, intraoperative blood loss, and postoperative morbidity rates showed no statistically significant difference. We found that 35.8% of patients undergoing salvage hepatectomy required resections with a wider extent compared to the estimated resection extent required before ablation. However, the minimally invasive approach, anatomical resection, and major hepatectomy rates were comparable to the PH group. In accordance with previous studies, there was no case of mortality during hospitalization in the SH group. According to these findings, we concluded salvage resection to be safe and feasible in local recurrence after RFA.
Only a few studies have reported on the long-term results of salvage hepatectomy after ablation. A Japanese study comparing salvage hepatectomy to primary hepatectomy reported no significant difference in OS; the survival rate of salvage hepatectomy was 67% at five years, which indicated a clear survival benefit [10]. However, this study reported high recurrence rates, with inferior DFS outcomes in the salvage group. Imai et al. reported an overall 5-year survival rate of 58.3% in the salvage hepatectomy group [28]. This survival outcome was comparable to the nationwide survey results for the surgical treatment of HCC in Japan [28]. By contrast, another study from Japan reported that salvage hepatectomy reached 5-year cumulative survival rates of only 9.5% [29]. Torzilli et al. found both disease-free and overall survival rates to be worse after salvage hepatectomy, with a 2-year OS of 44.4% [26]. This study included both primary liver tumors, such as HCC, and secondary metastatic liver lesions. In our study, OS rates after salvage and primary hepatectomy were equivalent. Long-term survival outcomes in the salvage group were superior to previous studies, with a 5-year OS of 78.0%. However, recurrence rates were significantly higher after salvage hepatectomy and systemic recurrence rates were especially high.
There is still ongoing debate on the pathophysiological mechanism underlying local recurrence after RFA. Three major hypotheses have been proposed regarding the recurrence mechanism after HCC local control therapy [30,31]. First, primary treatment failure with incomplete ablation might lead to early recurrence [13]. Recent studies have suggested that incomplete RFA might induce changes in the molecular phenotype, resulting in higher invasive and metastatic potential [32,33]. Sub-lethal heat shock conferred during ablation might lead to higher proliferation rates and increased chemoresistance [34]. Second, recurrence might arise from a preexisting microscopic tumor that is undetectable via imaging methods [35,36]. Lastly, HCC might recur due to RFA procedure-related causes, including the dissemination of malignant cells due to RFA needle-induced direct seeding, the transvenous spread from incompletely ablated lesions, or the microrupture of the tumor due to increased intratumoral pressure during ablation [37,38,39]. Previous studies have found that recurrent tumors show higher vascular invasion rates compared to primary tumors undergoing resection, which might be explained by the third hypothesis [10].
Yamamoto et al. found high proliferation, poor histological grade, and portal venous invasion as notable characteristics in recurrent HCC after RFA [29]. The findings of the current study were in accordance with the existing literature, as we found that tumors in the SH group showed a significantly higher ES grade. In multivariable analysis, a high tumor grade was an independent predictor for both recurrence and cancer-related death. These results support that tumor biology plays an essential role in the aggressive recurrence pattern of the SH group. On a molecular level, it was recently reported that tumors receiving RFA showed a higher expression of epithelial-mesenchymal transition-related genes and markers of tumor angiogenesis [40,41]. One study comparing the needle biopsy results before RFA and after ablation also found that the dedifferentiation of the tumor was observed after the procedure [16]. Ahmed et al. also reported that RFA stimulated extrahepatic tumor growth through the activation of a hepatocyte growth factor and vascular endothelial growth factor [42]. This could provide a potential explanation for the high rate of systemic recurrence in the SH group in the current study. Further prospective studies focusing on the alterations between molecular markers before and after ablation could further elucidate the underlying mechanism of such aggressive behavior.
Resection margin status is a known risk factor related to recurrence and cancer-related death after surgery for HCC [43]. Some studies have proved that wide surgical margins could decrease tumor recurrence; on the other hand, several others failed to show the survival benefit of more extensive resections [36,44,45,46,47]. As the oncologic significance of the resection margin width remains controversial, previous studies have proposed that certain HCC patient subgroups might benefit from wider margins, including those with high AFP levels, those undergoing non-anatomical resections, and those with microvascular invasion [43,48,49]. In the current study, negative but close (less than 0.1 cm) margins significantly impacted both DFS and OS in patients undergoing salvage hepatectomy. Recurrent tumors after RFA showed poor differentiation and more aggressive behavior, and previous studies have also reported higher vascular invasion rates. Therefore, it might be necessary to perform liver resection with wide resection margins for oncological safety in these cases.
Another finding of our study was that less extensive resection compared to the initial plan before RFA significantly increased the risk of recurrence. It was suggested by previous studies that viable tumor cells might be hidden in ablated lesions that seem like complete necrosis in imaging studies [50]. In a previous study, Portolani et al. suggested that salvage hepatectomy for local recurrence after RFA should encompass extensive resections to cover the necrotic area, which might hide active tumor cells [51]. Careful preoperative planning to a surgical extent with the consultation of imaging studies for the initial tumor before ablation is necessary to perform salvage hepatectomy effectively. Major hepatectomies encompassing both the recurrent lesion and the previously ablated area should be preferred if possible, considering the underlying liver condition of the patient.
This study has limitations. First, this was a single-center study conducted in a retrospective manner. Therefore, estimations for operative parameters and postoperative survival outcomes could only be made by comparing the study population to a matched control group. Future large-scale prospective studies might be helpful to further validate the outcomes of salvage hepatectomy. Second, the SH group included only those patients who were referred for surgical resection. They represented a limited proportion of all patients who experienced local recurrence after RFA, and it is highly possible that they are associated with less advanced tumors and better liver condition compared to patients who were found unsuitable for operative treatment. As a clear selection bias existed, our results might have underestimated the aggressive nature of recurrent HCC after RFA.
5. Conclusions
As short-term outcomes, including operation time, intraoperative blood loss, and postoperative complication rates, are comparable to primary hepatic resection, salvage hepatectomy is a viable rescue therapy for local recurrence after ablation. Recurrent HCC, after RFA, shows poor differentiation and exhibits more aggressive behavior, and wide resection margins are essential in salvage hepatectomy to prevent recurrence after surgery.
Author Contributions
Conceptualization, Y.-S.Y. and H.W.L.; methodology, B.L.; formal analysis, Y.P.; resources, C.J.Y.; data curation, M.K. and J.K.; writing—original draft preparation, Y.P.; writing—review and editing, J.Y.C.; supervision, H.-S.H.; project administration, J.Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No.: B-2306-836-103, approved on 3 June 2023.)
Informed Consent Statement
Patient consent was waived because of the retrospective nature of the study and the analysis using only anonymous clinical data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Chon, Y.E.; Park, S.Y.; Hong, H.P.; Son, D.; Lee, J.; Yoon, E.; Kim, S.S.; Ahn, S.B.; Jeong, S.W.; Jun, D.W. Hepatocellular carcinoma incidence is decreasing in Korea but increasing in the very elderly. Clin. Mol. Hepatol. 2023, 29, 120–134. [Google Scholar] [CrossRef]
- Hong, S.Y.; Kang, M.J.; Kim, T.; Jung, K.W.; Kim, B.W. Incidence, mortality, and survival of liver cancer using Korea central cancer registry database: 1999–2019. Ann. Hepatobil. Pancreat. Surg. 2022, 26, 211–219. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 583–705. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Coombs, A.W.; Jordan, C.; Hussain, S.A.; Ghandour, O. Scoring systems for the management of oncological hepato-pancreato-biliary patients. Ann. Hepatobil. Pancreat. Surg. 2022, 26, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.Y.; Lai, E.C. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: A systematic review. Ann. Surg. 2009, 249, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Wang, Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007, 72, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577. [Google Scholar] [CrossRef]
- Sugo, H.; Ishizaki, Y.; Yoshimoto, J.; Imamura, H.; Kawasaki, S. Salvage hepatectomy for local recurrent hepatocellular carcinoma after ablation therapy. Ann. Surg. Oncol. 2012, 19, 2238–2245. [Google Scholar] [CrossRef]
- Tateishi, R.; Shiina, S.; Yoshida, H.; Teratani, T.; Obi, S.; Yamashiki, N.; Yoshida, H.; Akamatsu, M.; Kawabe, T.; Omata, M. Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology 2006, 44, 1518–1527. [Google Scholar] [CrossRef]
- Nault, J.C.; Sutter, O.; Nahon, P.; Ganne-Carrié, N.; Séror, O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J. Hepatol. 2018, 68, 783–797. [Google Scholar] [CrossRef]
- Lam, V.W.; Ng, K.K.; Chok, K.S.; Cheung, T.T.; Yuen, J.; Tung, H.; Tso, W.K.; Fan, S.T.; Poon, R.T. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J. Am. Coll. Surg. 2008, 207, 20–29. [Google Scholar] [CrossRef]
- Fernandes, M.L.; Lin, C.C.; Lin, C.J.; Chen, W.T.; Lin, S.M. Risk of tumour progression in early-stage hepatocellular carcinoma after radiofrequency ablation. Br. J. Surg. 2009, 96, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Llovet, J.M.; Vilana, R.; Bianchi, L.; Solé, M.; Ayuso, C.; Brú, C.; Bruix, J.; Barcelona Clínic Liver Cancer Group. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004, 40, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Tajima, H.; Ohta, T.; Okamoto, K.; Nakanuma, S.; Hayashi, H.; Nakagawara, H.; Onishi, I.; Takamura, H.; Kitagawa, H.; Fushida, S.; et al. Radiofrequency ablation induces dedifferentiation of hepatocellular carcinoma. Oncol. Lett. 2010, 1, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Kim, J.K.; Kim, W.T.; Chung, J.W. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: A Markov model analysis. Hepatology 2010, 51, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.W.; Lim, H.K.; Cha, D.I. Aggressive tumor recurrence after radiofrequency ablation for hepatocellular carcinoma. Clin. Mol. Hepatol. 2017, 23, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Jiang, C.; Peng, Z.; Liu, B.; Hu, W.; Wang, Y.; Lin, M.; Lu, M.; Kuang, M. Local Recurrence after Radiofrequency Ablation of Hepatocellular Carcinoma: Treatment Choice and Outcome. J. Gastrointest. Surg. 2015, 19, 1466–1475. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Belghiti, J.; Clavien, P.A.; Gadzijev, E.; Garden, J.O.; Lau, W.Y.; Makuuchi, M.; Strong, R.W. The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB 2000, 2, 333–339. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Ogihara, M.; Wong, L.L.; Machi, J. Radiofrequency ablation versus surgical resection for single nodule hepatocellular carcinoma: Long-term outcomes. HPB 2005, 7, 214–221. [Google Scholar] [CrossRef]
- Mulier, S.; Ni, Y.; Jamart, J.; Ruers, T.; Marchal, G.; Michel, L. Local recurrence after hepatic radiofrequency coagulation: Multivariate meta-analysis and review of contributing factors. Ann. Surg. 2005, 242, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jin, Y.J.; Shin, S.K.; Kwon, J.H.; Kim, S.G.; Suh, Y.J.; Jeong, Y.; Yu, J.H.; Lee, J.W.; Kwon, O.S.; et al. Surgery versus radiofrequency ablation in patients with Child- Pugh class-A/single small (≤3 cm) hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Del Fabbro, D.; Palmisano, A.; Marconi, M.; Makuuchi, M.; Montorsi, M. Salvage hepatic resection after incomplete interstitial therapy for primary and secondary liver tumours. Br. J. Surg. 2007, 94, 208–213. [Google Scholar] [CrossRef]
- Yamashita, S.; Aoki, T.; Inoue, Y.; Kaneko, J.; Sakamoto, Y.; Sugawara, Y.; Hasegawa, K.; Kokudo, N. Outcome of salvage hepatic resection for recurrent hepatocellular carcinoma after radiofrequency ablation therapy. Surgery 2015, 157, 463–472. [Google Scholar] [CrossRef]
- Imai, K.; Beppu, T.; Chikamoto, A.; Mima, K.; Okabe, H.; Hayashi, H.; Nitta, H.; Ishiko, T.; Baba, H. Salvage treatment for local recurrence of hepatocellular carcinoma after local ablation therapy. Hepatol. Res. 2014, 44, e335–e345. [Google Scholar] [CrossRef]
- Yamamoto, N.; Okano, K.; Kushida, Y.; Deguchi, A.; Yachida, S.; Suzuki, Y. Clinicopathology of recurrent hepatocellular carcinomas after radiofrequency ablation treated with salvage surgery. Hepatol. Res. 2014, 44, 1062–1071. [Google Scholar] [CrossRef]
- Ikemoto, T.; Shimada, M.; Yamada, S. Pathophysiology of recurrent hepatocellular carcinoma after radiofrequency ablation. Hepatol. Res. 2017, 47, 23–30. [Google Scholar] [CrossRef]
- Cha, D.I.; Lee, M.W.; Ahn, S.H.; Song, K.D.; Kang, T.W.; Sinn, D.H.; Rhim, H. Rescue therapy for local tumor progression after radiofrequency ablation of small hepatocellular carcinoma: A comparison between repeated ablation and transcatheter arterial chemoembolization. Br. J. Radiol. 2023, 96, 20211037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Chai, Z.T.; Zhu, Z.M.; Zhu, X.D.; Ma, D.N.; Zhang, Q.B.; Zhao, Y.M.; Wang, M.; Ao, J.Y.; et al. Incomplete radiofrequency ablation enhances invasiveness and metastasis of residual cancer of hepatocellular carcinoma cell HCCLM3 via activating β-catenin signaling. PLoS ONE 2014, 9, e115949. [Google Scholar] [CrossRef]
- Yoshida, S.; Kornek, M.; Ikenaga, N.; Schmelzle, M.; Masuzaki, R.; Csizmadia, E.; Wu, Y.; Robson, S.C.; Schuppan, D. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology 2013, 58, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, C.; Li, G.; Lv, P.; Gu, J. Incomplete radiofrequency ablation induced chemoresistance by up-regulating heat shock protein 70 in hepatocellular carcinoma. Exp. Cell Res. 2021, 409, 112910. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Osaki, Y.; Iguchi, E.; Takeda, H.; Matsuda, F.; Nakajima, J.; Sakamoto, A.; Hatamaru, K.; Saito, S.; Nasu, A.; et al. Radiofrequency ablation for hepatocellular carcinoma: The relationship between a new grading system for the ablative margin and clinical outcomes. J. Gastroenterol. 2013, 48, 951–965. [Google Scholar] [CrossRef]
- Shi, M.; Guo, R.P.; Lin, X.J.; Zhang, Y.Q.; Chen, M.S.; Zhang, C.Q.; Lau, W.Y.; Li, J.Q. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: A prospective randomized trial. Ann. Surg. 2007, 245, 36–43. [Google Scholar] [CrossRef]
- Seki, T.; Tamai, T.; Ikeda, K.; Imamura, M.; Nishimura, A.; Yamashiki, N.; Nakagawa, T.; Inoue, K. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur. J. Gastroenterol. Hepatol. 2001, 13, 291–294. [Google Scholar] [CrossRef]
- Nicoli, N.; Casaril, A.; Abu Hilal, M.; Mangiante, G.; Marchiori, L.; Ciola, M.; Invernizzi, L.; Campagnaro, T.; Mansueto, G. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am. J. Surg. 2004, 188, 165–167. [Google Scholar] [CrossRef]
- Ruzzenente, A.; Manzoni, G.D.; Molfetta, M.; Pachera, S.; Genco, B.; Donataccio, M.; Guglielmi, A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J. Gastroenterol. 2004, 10, 1137–1140. [Google Scholar] [CrossRef]
- Iwahashi, S.; Shimada, M.; Utsunomiya, T.; Imura, S.; Morine, Y.; Ikemoto, T.; Takasu, C.; Saito, Y.; Yamada, S. Epithelial-mesenchymal transition-related genes are linked to aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablation. Cancer Lett. 2016, 375, 47–50. [Google Scholar] [CrossRef]
- Yamada, S.; Utsunomiya, T.; Morine, Y.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Kanamoto, M.; Iwahashi, S.; Saito, Y.; Takasu, C.; et al. Expressions of hypoxia-inducible factor-1 and epithelial cell adhesion molecule are linked with aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablation therapy. Ann. Surg. Oncol. 2014, 21 (Suppl. S3), S436–S442. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Kumar, G.; Moussa, M.; Wang, Y.; Rozenblum, N.; Galun, E.; Goldberg, S.N. Hepatic radiofrequency ablation-induced stimulation of distant tumor growth is suppressed by c-Met inhibition. Radiology 2016, 279, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Munir, M.M.; Woldesenbet, S.; Katayama, E.; Ratti, F.; Marques, H.P.; Cauchy, F.; Lam, V.; Poultsides, G.A.; Kitago, M.; et al. Impact of Surgical Margin Width on Prognosis Following Resection of Hepatocellular Carcinoma Varies on the Basis of Preoperative Alpha-Feto Protein and Tumor Burden Score. Ann. Surg. Oncol. 2023, 30, 6581–6589. [Google Scholar] [CrossRef]
- Lee, K.T.; Wang, S.N.; Su, R.W.; Chen, H.Y.; Shi, H.Y.; Ker, C.G.; Chiu, H.C. Is wider surgical margin justified for better clinical outcomes in patients with resectable hepatocellular carcinoma? J. Formos. Med. Assoc. 2012, 111, 160–170. [Google Scholar] [CrossRef]
- Poon, R.T.; Fan, S.T.; Ng, I.O.; Wong, J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann. Surg. 2000, 231, 544–551. [Google Scholar] [CrossRef]
- 46. Field, W.B.S.; Rostas, J.W.; Philps, P.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C.G., 2nd. Wide versus narrow margins after partial hepatectomy for hepatocellular carcinoma: Balancing recurrence risk and liver function. Am. J. Surg. 2017, 214, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Blanc, J.F.; Nobili, S.; Sa Cunha, A.; le Bail, B.; Bioulac-Sage, P.; Balabaud, C.; Capdepont, M.; Saric, J. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J. Am. Coll. Surg. 2005, 201, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kubota, K.; Hasegawa, K.; Kubo, S.; Izumi, N.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Nakashima, O.; et al. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br. J. Surg. 2020, 107, 113–120. [Google Scholar] [CrossRef]
- Yang, P.; Si, A.; Yang, J.; Cheng, Z.; Wang, K.; Li, J.; Xia, Y.; Zhang, B.; Pawlik, T.M.; Lau, W.Y.; et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery 2019, 165, 721–730. [Google Scholar] [CrossRef]
- Kim, Y.S.; Rhim, H.; Lim, H.K.; Park, C.K.; Lee, W.J.; Do, Y.S.; Cho, J.W. Completeness of treatment in hepatocellular carcinomas treated with image-guided tumor therapies: Evaluation of positive predictive value of contrast-enhanced CT with histopathologic correlation in the explanted liver specimen. J. Comput. Assist. Tomogr. 2006, 30, 578–582. [Google Scholar] [CrossRef]
- Portolani, N.; Baiocchi, G.L.; Coniglio, A.; Grazioli, L.; Frassi, E.; Gheza, F.; Giulini, S.M. Sequential multidisciplinary treatment of hepatocellular carcinoma: The role of surgery as rescue therapy for failure of percutaneous ablation therapies. J. Surg. Oncol. 2009, 100, 580–584. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).