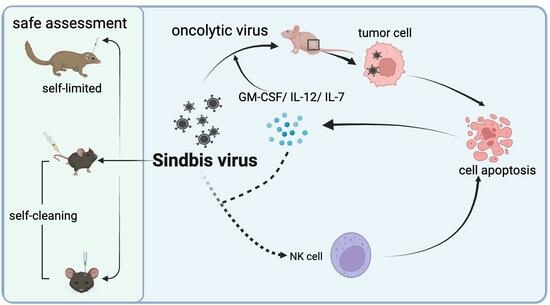
Oncolytic Viral Therapy for Glioma by Recombinant Sindbis Virus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Viruses and Titration
2.3. Cell Viability Assay
2.4. Safety Assessment of SINV
2.5. Animal Models
2.6. IVIS Imaging
2.7. Flow Cytometry Analysis
2.8. Statistical Analysis
3. Results
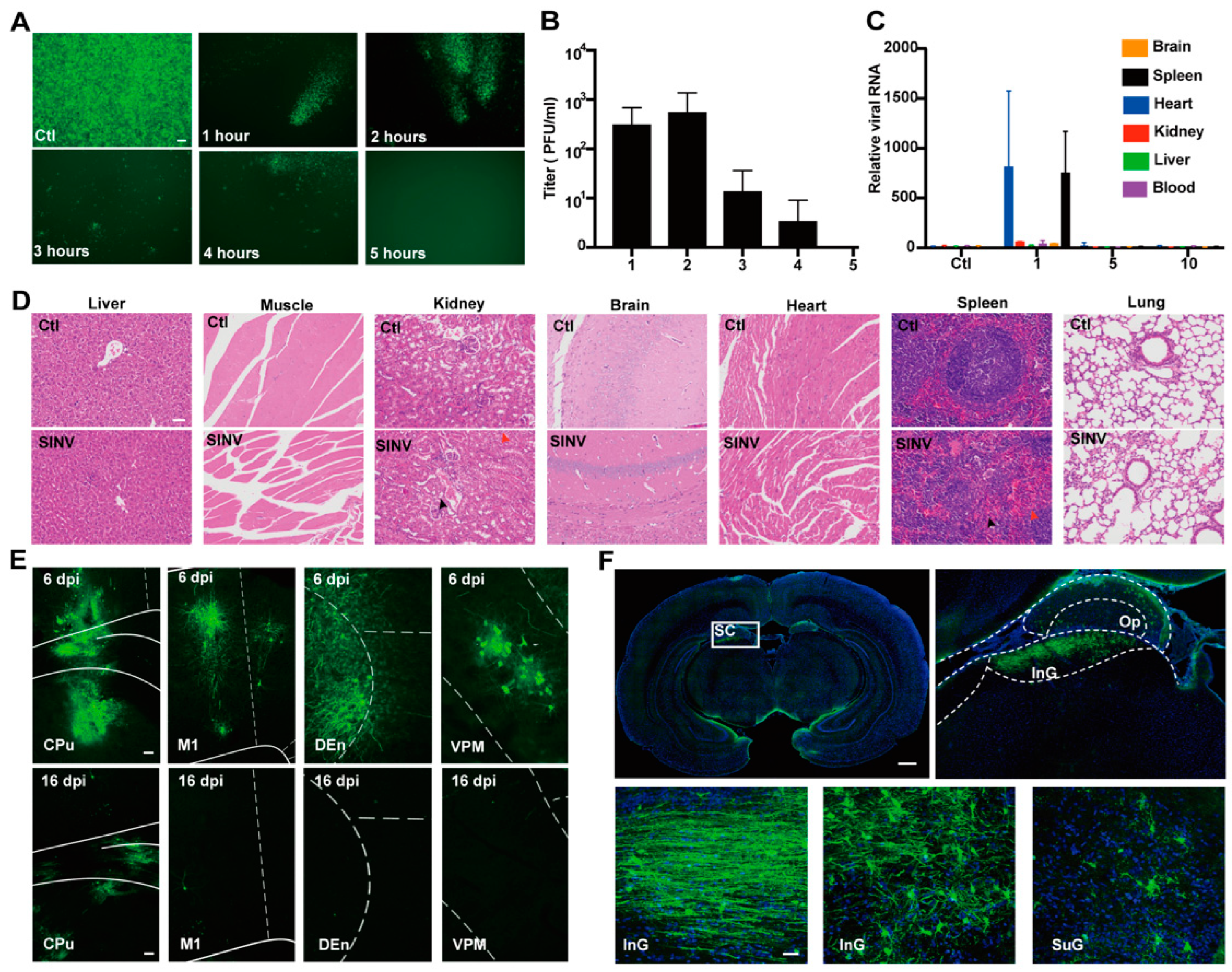
3.1. SINV Is Safe for Mice
3.2. SINV Can Kill GBM Cells In Vitro
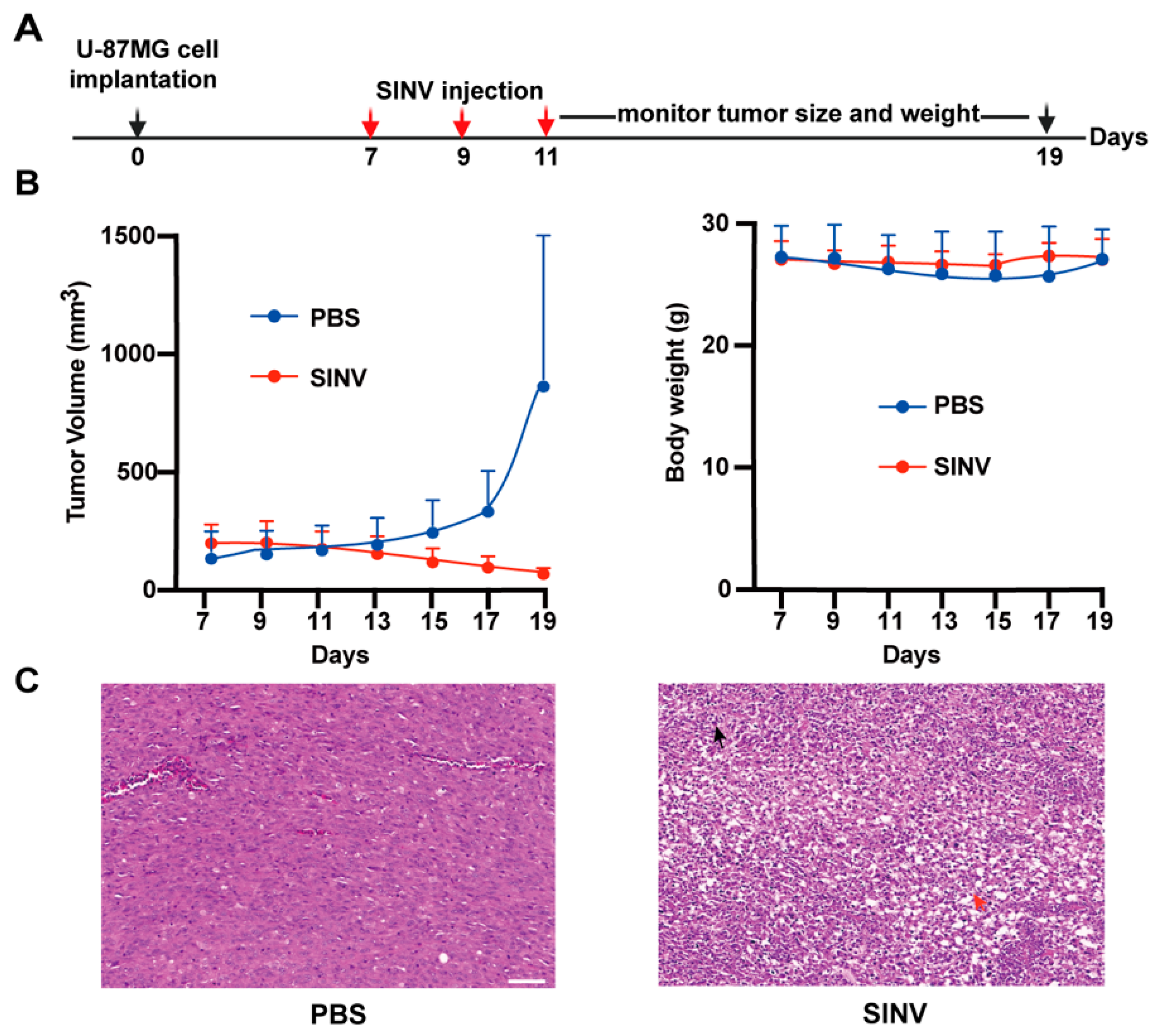
3.3. SINV Effectively Kills U-87MG Subcutaneous Tumors
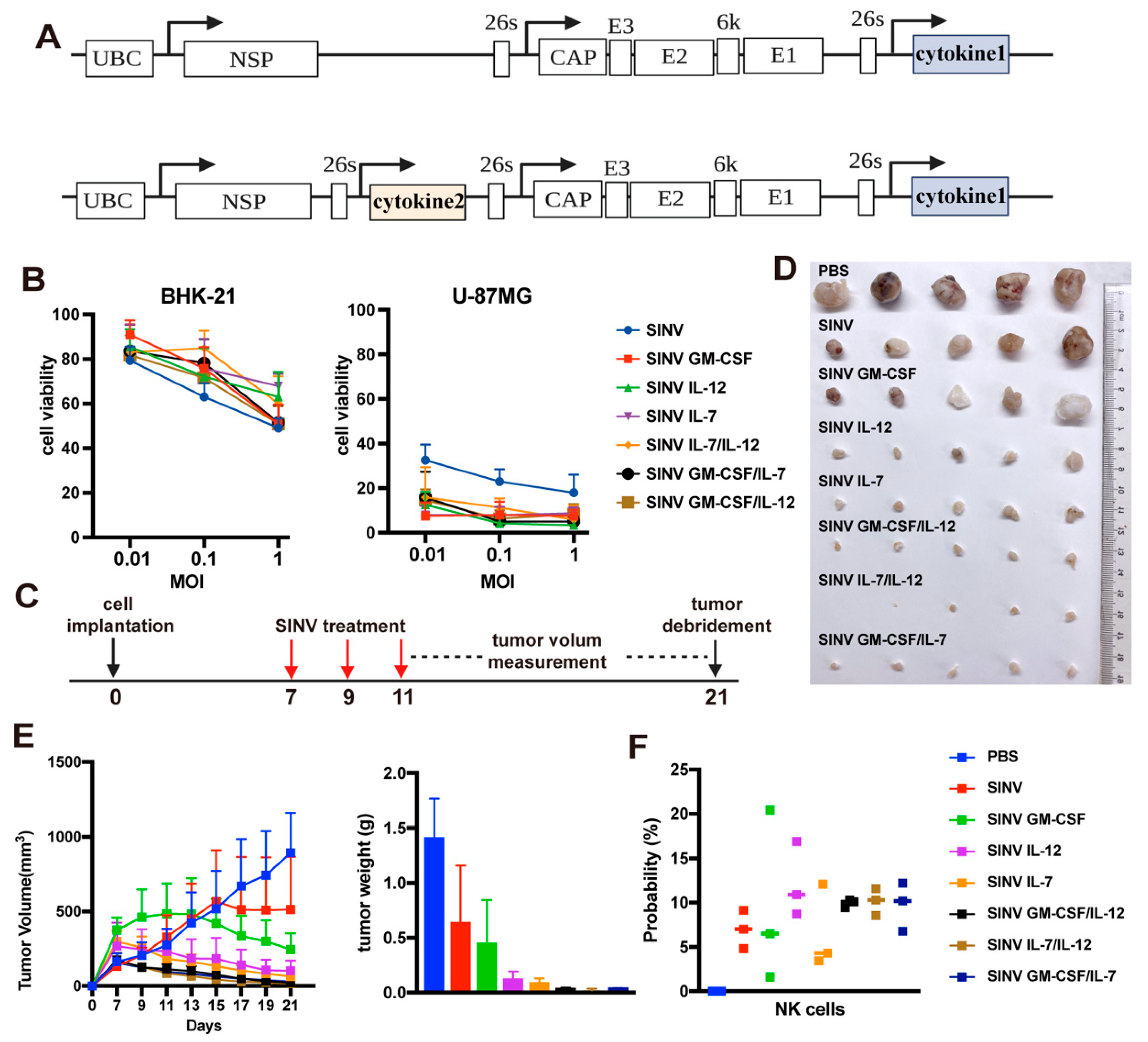
3.4. Cytokines Improve the Tumor-Killing Ability of SINV
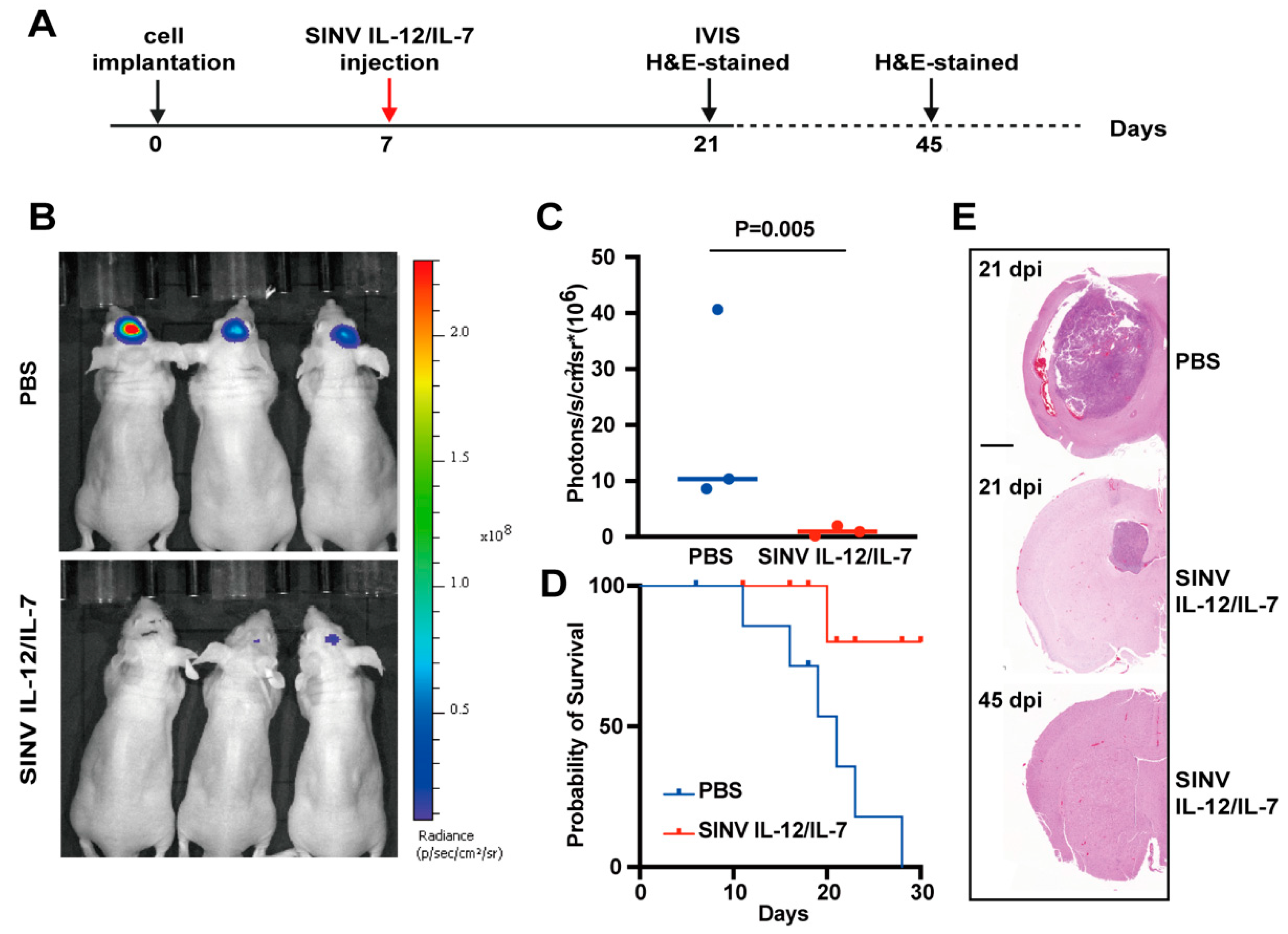
3.5. SINV IL-12/IL-7 Treatment Prolongs Survival in an Intracranial Glioblastoma Model
3.6. Systemic Administration of SINV Can Kill GMB Transplanted in Peripheral Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blair, H.A. Sotorasib: First approval. Drugs 2021, 81, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; Ng, L.F.; Hall, R.A.; Smith, D.W.; Johansen, C.A. Arbovirus infection. In Manson’s Tropical Diseases, 23rd ed.; Farrar, J., Hotez, P.J., Junghanss, T., Kang, G., Lalloo, D., White, N.J., Eds.; Saunders Ltd.: London, UK, 2013; pp. 129–161. [Google Scholar]
- Laine, M.; Luukkainen, R.; Toivanen, A. Sindbis viruses and other alphaviruses as cause of human arthritic disease. J. Intern. Med. 2004, 256, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Adouchief, S.; Smura, T.; Sane, J.; Vapalahti, O.; Kurkela, S. Sindbis virus as a human pathogen—Epidemiology, clinical picture and pathogenesis. Rev. Med. Virol. 2016, 26, 221–241. [Google Scholar] [CrossRef]
- Tucker, P.; Griffin, D. Mechanism of altered Sindbis virus neurovirulence associated with a single-amino-acid change in the E2 glycoprotein. J. Virol. 1991, 65, 1551–1557. [Google Scholar] [CrossRef]
- Lustig, S.; Jackson, A.C.; Hahn, C.S.; Griffin, D.E.; Strauss, E.G.; Strauss, J.H. Molecular basis of Sindbis virus neurovirulence in mice. J. Virol. 1988, 62, 2329–2336. [Google Scholar] [CrossRef]
- Jackson, A.C.; Moench, T.; Trapp, B.; Griffin, D. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab. Investig. J. Tech. Methods Pathol. 1988, 58, 503–509. [Google Scholar]
- Yamanaka, R.; Xanthopoulos, K.G. Development of improved Sindbis virus-based DNA expression vector. DNA Cell Biol. 2004, 23, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dittgen, T.; Nimmerjahn, A.; Waters, J.; Pawlak, V.; Helmchen, F.; Schlesinger, S.; Seeburg, P.H.; Osten, P. Sindbis vector SINrep (nsP2S726): A tool for rapid heterologous expression with attenuated cytotoxicity in neurons. J. Neurosci. Methods 2004, 133, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Xiong, C.; Levis, R.; Shen, P.; Schlesinger, S.; Rice, C.M.; Huang, H.V. Sindbis virus: An efficient, broad host range vector for gene expression in animal cells. Science 1989, 243, 1188–1191. [Google Scholar] [CrossRef]
- Jan, J.-T.; Griffin, D.E. Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J. Virol. 1999, 73, 10296–10302. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.-T.; Chatterjee, S.; Griffin, D.E. Sindbis virus entry into cells triggers apoptosis by activating sphingomyelinase, leading to the release of ceramide. J. Virol. 2000, 74, 6425–6432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, K.-S.; Kuhn, R.J.; Strauss, E.G.; Ou, S.; Strauss, J.H. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 1992, 66, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, X.; Fernández, P.L.; Miquel, R.; Muñoz, J.; Castronovo, V.; Ménard, S.; Palacín, A.; Cardesa, A.; Campo, E. Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. J. Pathol. 1996, 179, 376–380. [Google Scholar] [CrossRef]
- Ozaki, I.; Yamamoto, K.; Mizuta, T.; Kajihara, S.; Fukushima, N.; Setoguchi, Y.; Morito, F.; Sakai, T. Differential expression of laminin receptors in human hepatocellular carcinoma. Gut 1998, 43, 837–842. [Google Scholar] [CrossRef]
- Ménard, S.; Tagliabue, E.; Colnaghi, M.I. The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res. Treat. 1998, 52, 137–145. [Google Scholar] [CrossRef]
- Granot, T.; Yamanashi, Y.; Meruelo, D. Sindbis viral vectors transiently deliver tumor-associated antigens to lymph nodes and elicit diversified antitumor CD8+ T-cell immunity. Mol. Ther. 2014, 22, 112–122. [Google Scholar] [CrossRef]
- Granot, T.; Venticinque, L.; Tseng, J.-C.; Meruelo, D. Activation of cytotoxic and regulatory functions of NK cells by Sindbis viral vectors. PLoS ONE 2011, 6, e20598. [Google Scholar] [CrossRef]
- Tseng, J.-C.; Levin, B.; Hurtado, A.; Yee, H.; De Castro, I.P.; Jimenez, M.; Shamamian, P.; Jin, R.; Novick, R.P.; Pellicer, A. Systemic tumor targeting and killing by Sindbis viral vectors. Nat. Biotechnol. 2004, 22, 70–77. [Google Scholar] [CrossRef]
- Takenouchi, A.; Saito, K.; Saito, E.; Saito, T.; Hishiki, T.; Matsunaga, T.; Isegawa, N.; Yoshida, H.; Ohnuma, N.; Shirasawa, H. Oncolytic viral therapy for neuroblastoma cells with Sindbis virus AR339 strain. Pediatr. Surg. Int. 2015, 31, 1151–1159. [Google Scholar] [CrossRef]
- Unno, Y.; Shino, Y.; Kondo, F.; Igarashi, N.; Wang, G.; Shimura, R.; Yamaguchi, T.; Asano, T.; Saisho, H.; Sekiya, S. Oncolytic viral therapy for cervical and ovarian cancer cells by Sindbis virus AR339 strain. Clin. Cancer Res. 2005, 11, 4553–4560. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Markert, J.M.; Liechty, P.G.; Wang, W.; Gaston, S.; Braz, E.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Lakeman, A.D.; Palmer, C.A. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol. Ther. 2009, 17, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Wollmann, G.; Rogulin, V.; Simon, I.; Rose, J.K.; van den Pol, A.N. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J. Virol. 2010, 84, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Wildner, O. Comparison of herpes simplex virus-and conditionally replicative adenovirus-based vectors for glioblastoma treatment. Cancer Gene Ther. 2007, 14, 627–639. [Google Scholar] [CrossRef][Green Version]
- Misra, U.K.; Tan, C.T.; Kalita, J. Viral encephalitis and epilepsy. Epilepsia 2008, 49, 13–18. [Google Scholar] [CrossRef]
- Quiroz, E.; Moreno, N.; Peralta, P.H.; Tesh, R.B. A human case of encephalitis associated with vesicular stomatitis virus (Indiana serotype) infection. Am. J. Trop. Med. Hyg. 1988, 39, 312–314. [Google Scholar] [CrossRef]
- Papanastassiou, V.; Rampling, R.; Fraser, M.; Petty, R.; Hadley, D.; Nicoll, J.; Harland, J.; Mabbs, R.; Brown, M. The potential for efficacy of the modified (ICP 34.5−) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: A proof of principle study. Gene Ther. 2002, 9, 398–406. [Google Scholar] [CrossRef]
- Lun, X.; Senger, D.L.; Alain, T.; Oprea, A.; Parato, K.; Stojdl, D.; Lichty, B.; Power, A.; Johnston, R.N.; Hamilton, M. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV ΔM51) on multifocal and invasive gliomas. J. Natl. Cancer Inst. 2006, 98, 1546–1557. [Google Scholar] [CrossRef]
- Shi, X.-W.; Jia, F.; Lyu, P.; Xu, F.-Q. A new anterograde trans-synaptic tracer based on Sindbis virus. Neural Regen. Res. 2022, 17, 2761. [Google Scholar]
- Nakao, S.; Arai, Y.; Tasaki, M.; Yamashita, M.; Murakami, R.; Kawase, T.; Amino, N.; Nakatake, M.; Kurosaki, H.; Mori, M. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci. Transl. Med. 2020, 12, eaax7992. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.J.; Linette, G.P.; Hamid, O.; Agarwala, S.S.; Starodub, A.; Sun, L.; Lebel, F.; Barrett, J.A.; Lewis, J. Regulated intratumoral expression of IL-12 as a basis for combination therapy in melanoma. J. Transl. Med. 2014, 12, O11. [Google Scholar] [CrossRef]
- Strong, M.J.; Koduri, S.; Allison, J.A.; Pesavento, C.M.; Ogunsola, S.; Ogunsola, O.; Yee, T.J.; Khalsa, S.S.S.; Saadeh, Y.S.; Joseph, J.R. Bone metastasis from glioblastoma: A systematic review. J. Neuro-Oncol. 2022, 158, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.; Lok, E.; Gautam, S.; Wu, E.; Wong, E.T. The natural history of extracranial metastasis from glioblastoma multiforme. J. Neuro-Oncol. 2011, 105, 261–273. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Wang, Y.; Kong, Z.; Chen, W.; Li, J.; Chen, W.; Tong, Y.; Ma, W.; Wang, Y. Effects of oncolytic viruses and viral vectors on immunity in glioblastoma. Gene Ther. 2022, 29, 115–126. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Tseng, J.-C.; Levin, B.; Hirano, T.; Yee, H.; Pampeno, C.; Meruelo, D. In vivo antitumor activity of Sindbis viral vectors. J. Natl. Cancer Inst. 2002, 94, 1790–1802. [Google Scholar] [CrossRef]
- Opp, S.; Hurtado, A.; Pampeno, C.; Lin, Z.; Meruelo, D. Potent and Targeted Sindbis Virus Platform for Immunotherapy of Ovarian Cancer. Cells 2023, 12, 77. [Google Scholar] [CrossRef]
- Byrnes, A.P.; Griffin, D.E. Large-plaque mutants of Sindbis virus show reduced binding to heparan sulfate, heightened viremia, and slower clearance from the circulation. J. Virol. 2000, 74, 644–651. [Google Scholar] [CrossRef]
- Metcalf, T.U.; Griffin, D.E. Alphavirus-induced encephalomyelitis: Antibody-secreting cells and viral clearance from the nervous system. J. Virol. 2011, 85, 11490–11501. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Gorelkin, L. Selective clearance of a benign clone of Venezuelan equine encephalitis virus from hamster plasma by hepatic reticuloendothelial cells. J. Infect. Dis. 1975, 132, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, A.P.; Griffin, D.E. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 1998, 72, 7349–7356. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, W.B.; Ryman, K.D.; Johnston, R.E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 1998, 72, 7357–7366. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Deakin, J.A.; Gallagher, J.T. Liver heparan sulfate structure. A novel molecular design. J. Biol. Chem. 1994, 269, 11208–11215. [Google Scholar] [CrossRef]
- Wells, M.J.; Blajchman, M.A. In vivo clearance of ternary complexes of vitronectin-thrombin-antithrombin is mediated by hepatic heparan sulfate proteoglycans. J. Biol. Chem. 1998, 273, 23440–23447. [Google Scholar] [CrossRef]
- Yuge, T.; Furukawa, A.; Nakamura, K.; Nagashima, Y.; Shinozaki, K.; Nakamura, T.; Kimura, R. Metabolism of the intravenously administered recombinant human basic fibroblast growth factor, trafermin, in liver and kidney: Degradation implicated in its selective localization to the fenestrated type microvasculatures. Biol. Pharm. Bull. 1997, 20, 786–793. [Google Scholar] [CrossRef][Green Version]
- Postic, B.; Schleupner, C.J.; Armstrong, J.A.; Ho, M. Two variants of Sindbis virus which differ in interferon induction and serum clearance. I. The phenomenon. J. Infect. Dis. 1969, 120, 339–347. [Google Scholar] [CrossRef]
- Levine, B.; Hardwick, J.M.; Trapp, B.D.; Crawford, T.O.; Bollinger, R.C.; Griffin, D.E. Antibody-mediated clearance of alphavirus infection from neurons. Science 1991, 254, 856–860. [Google Scholar] [CrossRef]
- Russell, S.J. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002, 9, 961–966. [Google Scholar] [CrossRef]
- Masemann, D.; Boergeling, Y.; Ludwig, S. Employing RNA viruses to fight cancer: Novel insights into oncolytic virotherapy. Biol. Chem. 2017, 398, 891–909. [Google Scholar] [CrossRef]
- Domingo-Gil, E.; Toribio, R.; Nájera, J.L.; Esteban, M.; Ventoso, I. Diversity in viral anti-PKR mechanisms: A remarkable case of evolutionary convergence. PLoS ONE 2011, 6, e16711. [Google Scholar] [CrossRef]
- Sanada, T.; Tsukiyama-Kohara, K.; Shin-I, T.; Yamamoto, N.; Kayesh, M.E.H.; Yamane, D.; Takano, J.-i.; Shiogama, Y.; Yasutomi, Y.; Ikeo, K. Construction of complete Tupaia belangeri transcriptome database by whole-genome and comprehensive RNA sequencing. Sci. Rep. 2019, 9, 12372. [Google Scholar] [CrossRef]
- Meng, X.; Shen, F.; Li, C.; Li, Y.; Wang, X. Depression-like behaviors in tree shrews and comparison of the effects of treatment with fluoxetine and carbetocin. Pharmacol. Biochem. Behav. 2016, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hai-Ying, C.; Tanaka, Y.; Hifumi, T.; Shoji, K.; Kayesh, M.E.H.; Hashem, M.A.; Kitab, B.; Sanada, T.; Fujiyuki, T.; Yoneda, M. Pathological and genetic aspects of spontaneous mammary gland tumor in Tupaia belangeri (tree shrew). PLoS ONE 2020, 15, e0233232. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, M.E.H.; Hashem, M.A.; Kitab, B.; Tsukiyama-Kohara, K. Pathogenesis and immune response caused by vector-borne and other viral infections in a Tupaia model. Microorganisms 2019, 7, 686. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Miao, H.; Zhu, X.; Xu, F. Pseudo-typed Semliki Forest virus delivers EGFP into neurons. J. Neurovirol. 2017, 23, 205–215. [Google Scholar] [CrossRef]
- Lundstrom, K. Oncolytic alphaviruses in cancer immunotherapy. Vaccines 2017, 5, 9. [Google Scholar] [CrossRef]
- Sportès, C.; Babb, R.R.; Krumlauf, M.C.; Hakim, F.T.; Steinberg, S.M.; Chow, C.K.; Brown, M.R.; Fleisher, T.A.; Noel, P.; Maric, I. Phase I Study of Recombinant Human Interleukin-7 Administration in Subjects with Refractory Malignancy. Clin. Cancer Res. 2010, 16, 727–735. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Shi, X.; Li, L.; Nie, X.; Xu, L.; Jia, F.; Xu, F. Oncolytic Viral Therapy for Glioma by Recombinant Sindbis Virus. Cancers 2023, 15, 4738. https://doi.org/10.3390/cancers15194738
Sun K, Shi X, Li L, Nie X, Xu L, Jia F, Xu F. Oncolytic Viral Therapy for Glioma by Recombinant Sindbis Virus. Cancers. 2023; 15(19):4738. https://doi.org/10.3390/cancers15194738
Chicago/Turabian StyleSun, Kangyixin, Xiangwei Shi, Li Li, Xiupeng Nie, Lin Xu, Fan Jia, and Fuqiang Xu. 2023. "Oncolytic Viral Therapy for Glioma by Recombinant Sindbis Virus" Cancers 15, no. 19: 4738. https://doi.org/10.3390/cancers15194738
APA StyleSun, K., Shi, X., Li, L., Nie, X., Xu, L., Jia, F., & Xu, F. (2023). Oncolytic Viral Therapy for Glioma by Recombinant Sindbis Virus. Cancers, 15(19), 4738. https://doi.org/10.3390/cancers15194738