Dual-Armed Oncolytic Myxoma Virus Encoding IFN-γ and CD47 Promotes Lymphocyte Infiltration and Tumor Suppression of Syngeneic Murine Melanoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Development of Armed MyxV
2.3. Virus Production and TCID50
2.4. Animal Experiment
2.5. qRT-PCR
2.6. IFN-γ ELISA
2.7. IHC and Immunofluorescence Staining
2.8. TIL Isolation and FACS Analysis
2.9. Statistics
3. Results
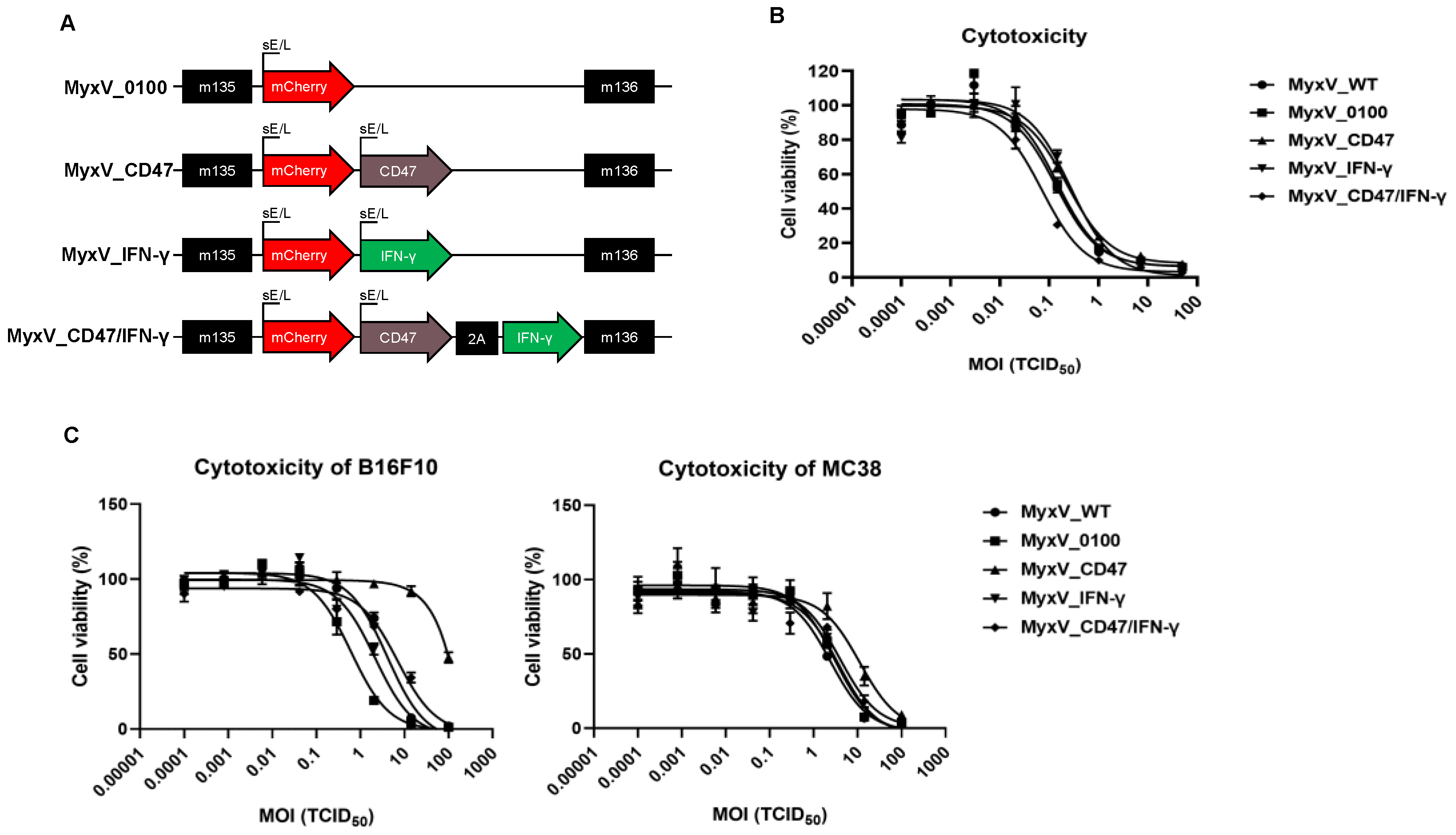
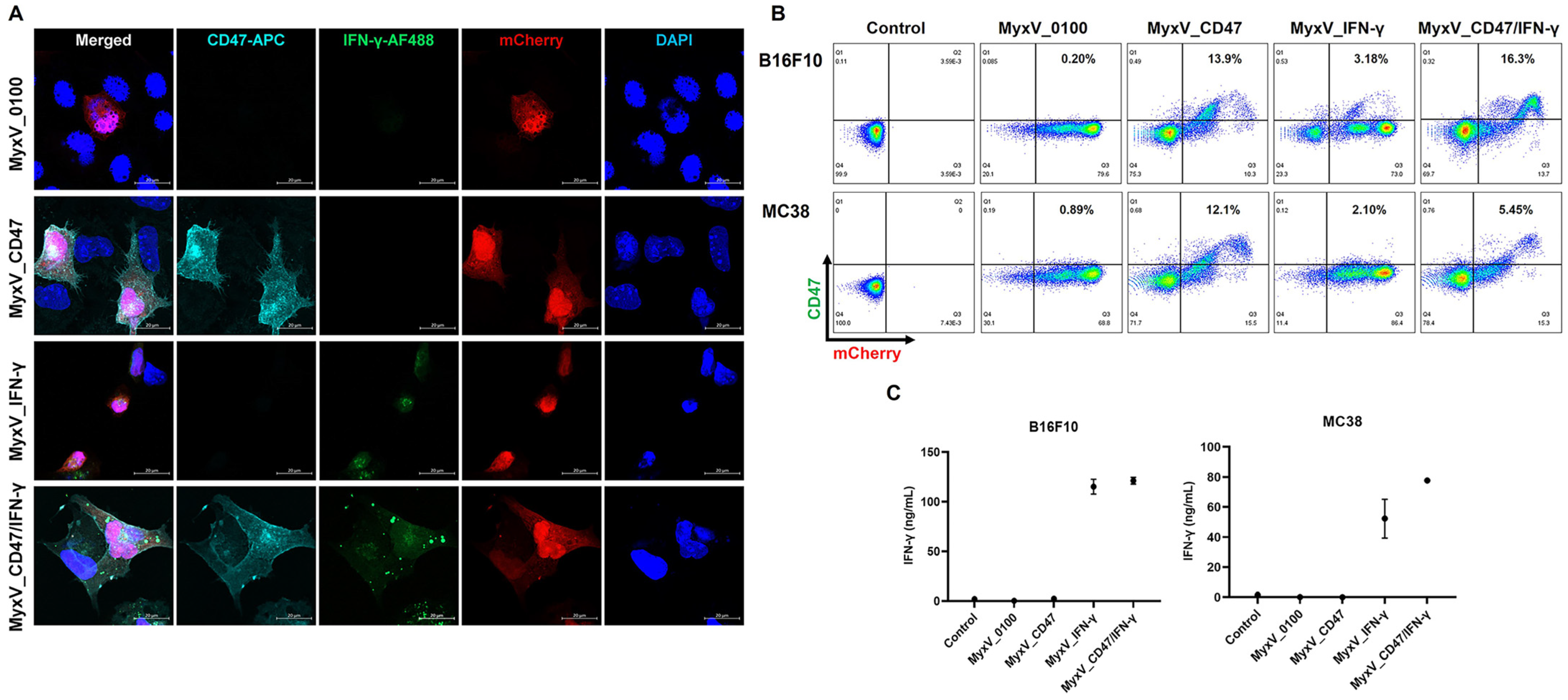
3.1. Characterization of Recombinant CD47 and IFN-γ Encoding Dual-Armed MyxV
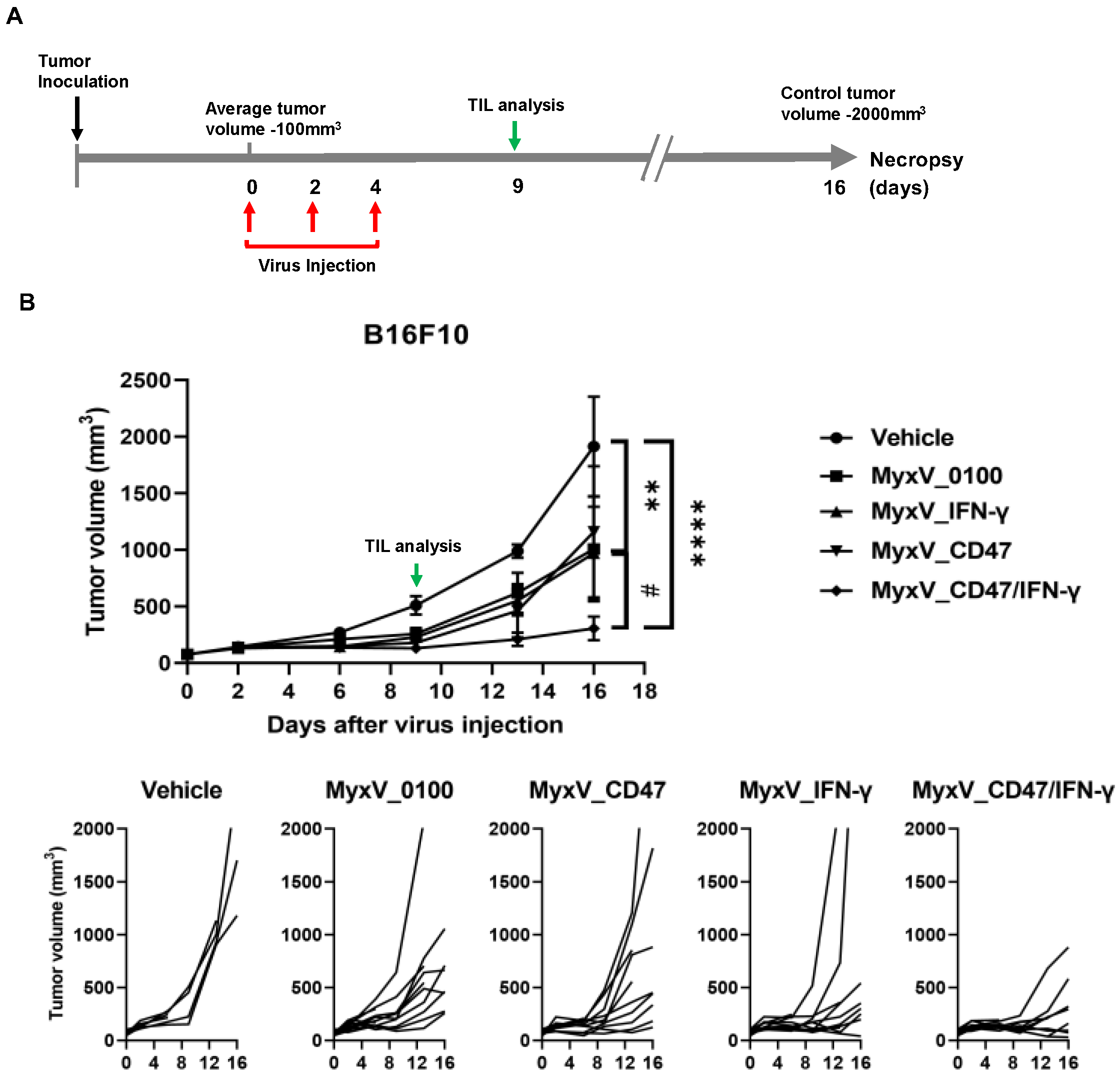
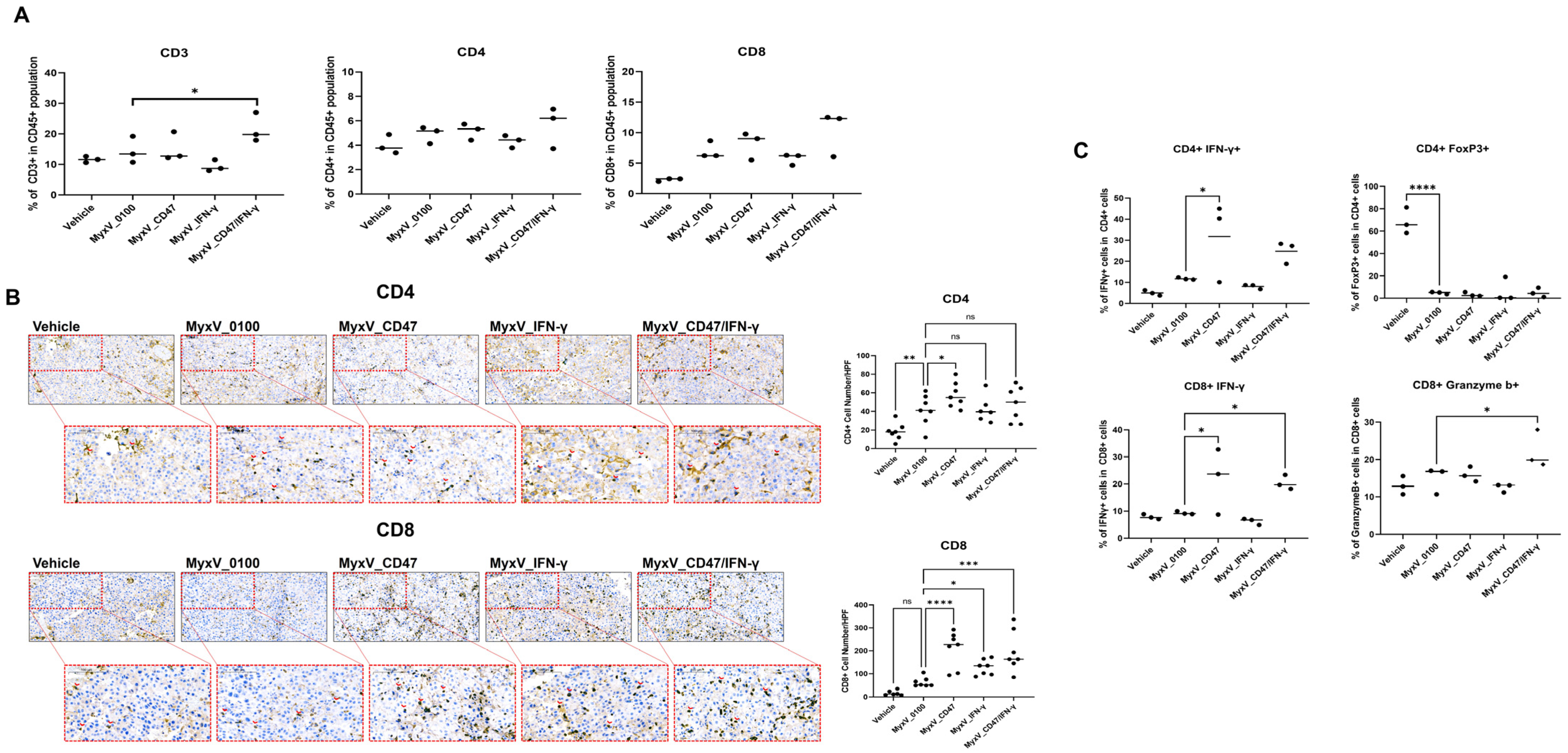
3.2. Validation of the Anticancer and Immune Stimulation Activity of Dual-Armed MyxV against Murine Melanoma
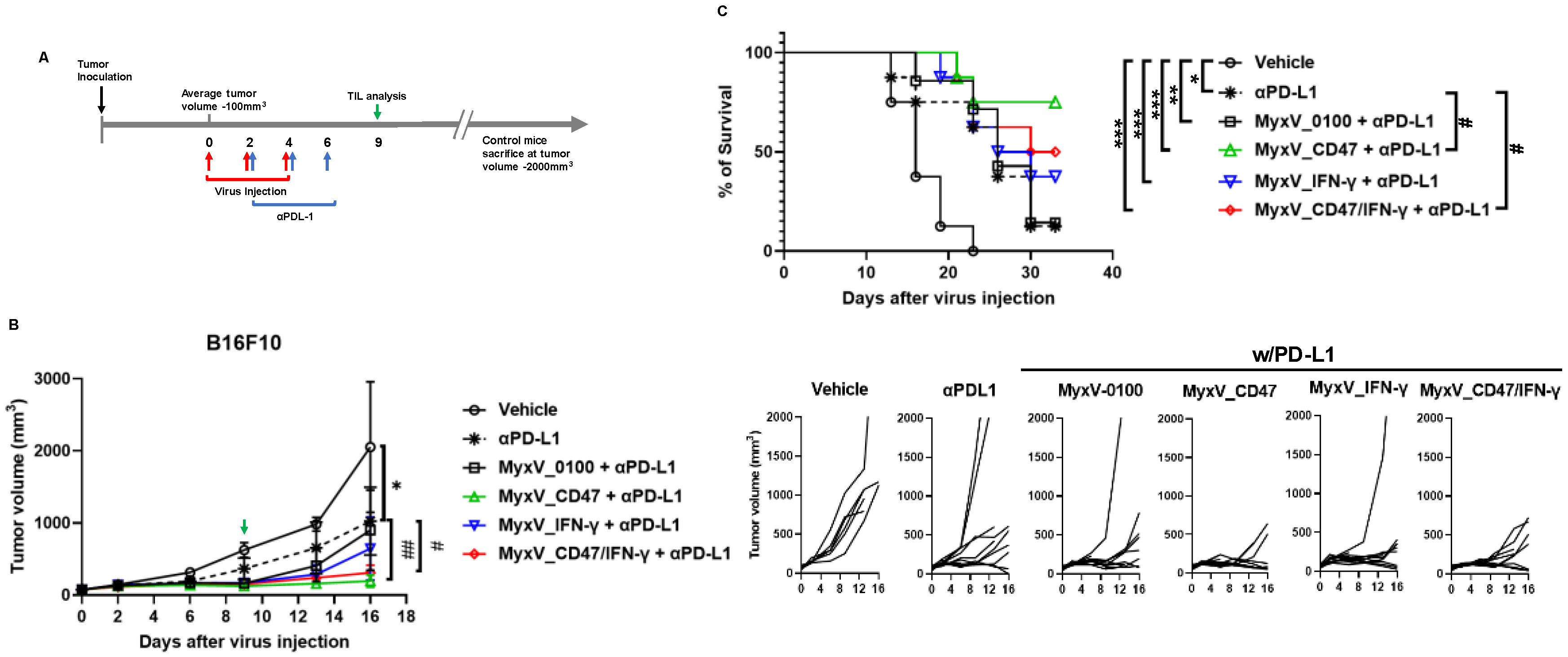
3.3. Anticancer Efficacy and Prolonged Survival of B16F10 Mouse Melanoma Administered Armed MyxV in Combination with Immune Checkpoint Inhibitor αPD-L1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Araki, H.; Tazawa, H.; Kanaya, N.; Kajiwara, Y.; Yamada, M.; Hashimoto, M.; Kikuchi, S.; Kuroda, S.; Yoshida, R.; Umeda, Y.; et al. Oncolytic virus-mediated p53 overexpression promotes immunogenic cell death and efficacy of PD-1 blockade in pancreatic cancer. Mol. Ther. Oncolytics 2022, 27, 3–13. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Wang, L.; Chard Dunmall, L.S.; Cheng, Z.; Wang, Y. Remodeling the tumor microenvironment by oncolytic viruses: Beyond oncolysis of tumor cells for cancer treatment. J. Immunother. Cancer 2022, 10, e004167. [Google Scholar] [CrossRef]
- Flores, E.B.; Aksoy, B.A.; Bartee, E. Initial dose of oncolytic myxoma virus programs durable antitumor immunity independent of in vivo viral replication. J. Immunother. Cancer 2020, 8, e000804. [Google Scholar] [CrossRef]
- Kumar, V.; Giacomantonio, M.A.; Gujar, S. Role of Myeloid Cells in Oncolytic Reovirus-Based Cancer Therapy. Viruses 2021, 13, 654. [Google Scholar] [CrossRef]
- Chon, H.J.; Lee, W.S.; Yang, H.; Kong, S.J.; Lee, N.K.; Moon, E.S.; Choi, J.; Han, E.C.; Kim, J.H.; Ahn, J.B.; et al. Tumor Microenvironment Remodeling by Intratumoral Oncolytic Vaccinia Virus Enhances the Efficacy of Immune-Checkpoint Blockade. Clin. Cancer Res. 2019, 25, 1612–1623. [Google Scholar] [CrossRef]
- Rahman, M.M.; McFadden, G. Oncolytic Virotherapy with Myxoma Virus. J. Clin. Med. 2020, 9, 171. [Google Scholar] [CrossRef]
- Deng, L.; Fan, J.; Ding, Y.; Zhang, J.; Zhou, B.; Zhang, Y.; Huang, B.; Hu, Z. Oncolytic cancer therapy with a vaccinia virus strain. Oncol. Rep. 2019, 41, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Sachdev, E.; Mita, A.C.; Mita, M.M. Clinical development of reovirus for cancer therapy: An oncolytic virus with immune-mediated antitumor activity. World J. Methodol. 2016, 6, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Kerr, P.J.; Liu, J.; Cattadori, I.; Ghedin, E.; Read, A.F.; Holmes, E.C. Myxoma virus and the Leporipoxviruses: An evolutionary paradigm. Viruses 2015, 7, 1020–1061. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Appel, N.; Zhang, L.; Lowe, K.; Kilbourne, J.; Daggett-Vondras, J.; Elliott, N.; Lucas, A.R.; Blattman, J.N.; Rahman, M.M.; et al. Systemic Delivery of mLIGHT-Armed Myxoma Virus Is Therapeutic for Later-Stage Syngeneic Murine Lung Metastatic Osteosarcoma. Cancers 2022, 14, 337. [Google Scholar] [CrossRef]
- Torres-Dominguez, L.E.; de Matos, A.L.; Rahman, M.M.; McFadden, G. Methods for the Construction of Recombinant Oncolytic Myxoma Viruses. Methods Mol. Biol. 2021, 2225, 63–75. [Google Scholar] [CrossRef]
- Christie, J.D.; Appel, N.; Canter, H.; Achi, J.G.; Elliott, N.M.; de Matos, A.L.; Franco, L.; Kilbourne, J.; Lowe, K.; Rahman, M.M.; et al. Systemic delivery of TNF-armed myxoma virus plus immune checkpoint inhibitor eliminates lung metastatic mouse osteosarcoma. Mol. Ther. Oncolytics 2021, 22, 539–554. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, J.; Tong, A.; Yang, H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther. Oncolytics 2019, 15, 234–247. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, H.; Yu, J.; Tian, W.; Song, Y. Targeting CD47 for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 180. [Google Scholar] [CrossRef]
- Takimoto, C.H.; Chao, M.P.; Gibbs, C.; McCamish, M.A.; Liu, J.; Chen, J.Y.; Majeti, R.; Weissman, I.L. The Macrophage ‘Do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann. Oncol. 2019, 30, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Cham, L.B.; Adomati, T.; Li, F.; Ali, M.; Lang, K.S. CD47 as a Potential Target to Therapy for Infectious Diseases. Antibodies 2020, 9, 44. [Google Scholar] [CrossRef]
- Cham, L.B.; Torrez Dulgeroff, L.B.; Tal, M.C.; Adomati, T.; Li, F.; Bhat, H.; Huang, A.; Lang, P.A.; Moreno, M.E.; Rivera, J.M.; et al. Immunotherapeutic Blockade of CD47 Inhibitory Signaling Enhances Innate and Adaptive Immune Responses to Viral Infection. Cell Rep. 2020, 31, 107494. [Google Scholar] [CrossRef]
- Walter, M.R. The Role of Structure in the Biology of Interferon Signaling. Front. Immunol. 2020, 11, 606489. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Fenton, S.E.; Saleiro, D.; Platanias, L.C. Type I and II Interferons in the Anti-Tumor Immune Response. Cancers 2021, 13, 1037. [Google Scholar] [CrossRef] [PubMed]
- Lortat-Jacob, H.; Baltzer, F.; Grimaud, J.A. Heparin decreases the blood clearance of interferon-gamma and increases its activity by limiting the processing of its carboxyl-terminal sequence. J. Biol. Chem. 1996, 271, 16139–16143. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Takahashi, Y.; Yamashita, T.; Fujimoto, M.; Nishikawa, M.; Watanabe, Y.; Takakura, Y. Prevention of adverse events of interferon gamma gene therapy by gene delivery of interferon gamma-heparin-binding domain fusion protein in mice. Mol. Ther. Methods Clin. Dev. 2014, 1, 14023. [Google Scholar] [CrossRef]
- Kremer, M.; Volz, A.; Kreijtz, J.H.; Fux, R.; Lehmann, M.H.; Sutter, G. Easy and efficient protocols for working with recombinant vaccinia virus MVA. Methods Mol. Biol. 2012, 890, 59–92. [Google Scholar] [CrossRef]
- Valenzuela-Cardenas, M.; Gowan, C.; Dryja, P.; Bartee, M.Y.; Bartee, E. TNF blockade enhances the efficacy of myxoma virus-based oncolytic virotherapy. J. Immunother. Cancer 2022, 10, e00477. [Google Scholar] [CrossRef]
- Cristi, F.; Gutierrez, T.; Hitt, M.M.; Shmulevitz, M. Genetic Modifications That Expand Oncolytic Virus Potency. Front. Mol. Biosci. 2022, 9, 831091. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.M.; Rahman, M.M.; McFadden, G. Oncolytic myxoma virus: The path to clinic. Vaccine 2013, 31, 4252–4258. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Tao, L.; Zhang, X. Genetically coating oncolytic herpes simplex virus with CD47 allows efficient systemic delivery and prolongs virus persistence at tumor site. Oncotarget 2018, 9, 34543–34553. [Google Scholar] [CrossRef]
- Xu, B.; Tian, L.; Chen, J.; Wang, J.; Ma, R.; Dong, W.; Li, A.; Zhang, J.; Antonio Chiocca, E.; Kaur, B.; et al. An oncolytic virus expressing a full-length antibody enhances antitumor innate immune response to glioblastoma. Nat. Commun. 2021, 12, 5908. [Google Scholar] [CrossRef] [PubMed]
- Kondelkova, K.; Vokurkova, D.; Krejsek, J.; Borska, L.; Fiala, Z.; Ctirad, A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove) 2010, 53, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Chiec, L.; Mohindra, N.A.; Munshi, H.G. Regulatory T-Cells as an Emerging Barrier to Immune Checkpoint Inhibition in Lung Cancer. Front. Oncol. 2021, 11, 684098. [Google Scholar] [CrossRef]
- Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors in cancer therapy: A focus on T-regulatory cells. Immunol. Cell Biol. 2018, 96, 21–33. [Google Scholar] [CrossRef]
- Petersen, R.P.; Campa, M.J.; Sperlazza, J.; Conlon, D.; Joshi, M.B.; Harpole, D.H., Jr.; Patz, E.F., Jr. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 2006, 107, 2866–2872. [Google Scholar] [CrossRef]
- Wang, W.; Hodkinson, P.; McLaren, F.; MacKinnon, A.; Wallace, W.; Howie, S.; Sethi, T. Small cell lung cancer tumour cells induce regulatory T lymphocytes, and patient survival correlates negatively with FOXP3+ cells in tumour infiltrate. Int. J. Cancer 2012, 131, E928–E937. [Google Scholar] [CrossRef]
- Gowan, C.C.; Bartee, M.Y.; Flores, E.; Aksoy, B.A.; Templeton, C.; Baillie, K.; Happe, M.; Bartee, E. The Combination of TIM3-Based Checkpoint Blockade and Oncolytic Virotherapy Regresses Established Solid Tumors. J. Immunother. 2023, 46, 1–4. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, J.; Xue, G.; Wang, Z.; Li, X.; Zhou, M.; Jin, G.; Rahman, M.M.; McFadden, G.; Lu, Y. Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance. Cancer Cell 2022, 40, 973–985.e977. [Google Scholar] [CrossRef]
- Tang, B.; Guo, Z.S.; Bartlett, D.L.; Yan, D.Z.; Schane, C.P.; Thomas, D.L.; Liu, J.; McFadden, G.; Shisler, J.L.; Roy, E.J. Synergistic Combination of Oncolytic Virotherapy and Immunotherapy for Glioma. Clin. Cancer Res. 2020, 26, 2216–2230. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, J.K.; Kim, T.-G.; Im, N.Y.; Son, K.-Y.; Cho, M.; Jeong, Y.J.; Hong, J.-I.; Kang, B.; Enkhtaivan, G.; Cho, N.-H.; et al. Dual-Armed Oncolytic Myxoma Virus Encoding IFN-γ and CD47 Promotes Lymphocyte Infiltration and Tumor Suppression of Syngeneic Murine Melanoma. Cancers 2023, 15, 4703. https://doi.org/10.3390/cancers15194703
Woo JK, Kim T-G, Im NY, Son K-Y, Cho M, Jeong YJ, Hong J-I, Kang B, Enkhtaivan G, Cho N-H, et al. Dual-Armed Oncolytic Myxoma Virus Encoding IFN-γ and CD47 Promotes Lymphocyte Infiltration and Tumor Suppression of Syngeneic Murine Melanoma. Cancers. 2023; 15(19):4703. https://doi.org/10.3390/cancers15194703
Chicago/Turabian StyleWoo, Jong Kyu, Tae-Geuk Kim, Na Yeon Im, Ka-Yeon Son, Minhyeon Cho, Yeo Jin Jeong, Jeong-Im Hong, BoRim Kang, Gansukh Enkhtaivan, Nam-Hyuk Cho, and et al. 2023. "Dual-Armed Oncolytic Myxoma Virus Encoding IFN-γ and CD47 Promotes Lymphocyte Infiltration and Tumor Suppression of Syngeneic Murine Melanoma" Cancers 15, no. 19: 4703. https://doi.org/10.3390/cancers15194703
APA StyleWoo, J. K., Kim, T.-G., Im, N. Y., Son, K.-Y., Cho, M., Jeong, Y. J., Hong, J.-I., Kang, B., Enkhtaivan, G., Cho, N.-H., Alain, T., Park, D. G., & Lee, Y.-S. (2023). Dual-Armed Oncolytic Myxoma Virus Encoding IFN-γ and CD47 Promotes Lymphocyte Infiltration and Tumor Suppression of Syngeneic Murine Melanoma. Cancers, 15(19), 4703. https://doi.org/10.3390/cancers15194703






