Proximal Tibia Tumour Location and Curettage Are Major Risk Factors of Local Recurrence in Giant Cell Tumour of Bone
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Characteristics
2.2. Evaluation of Functional Results
2.3. Statistical Analysis
3. Results
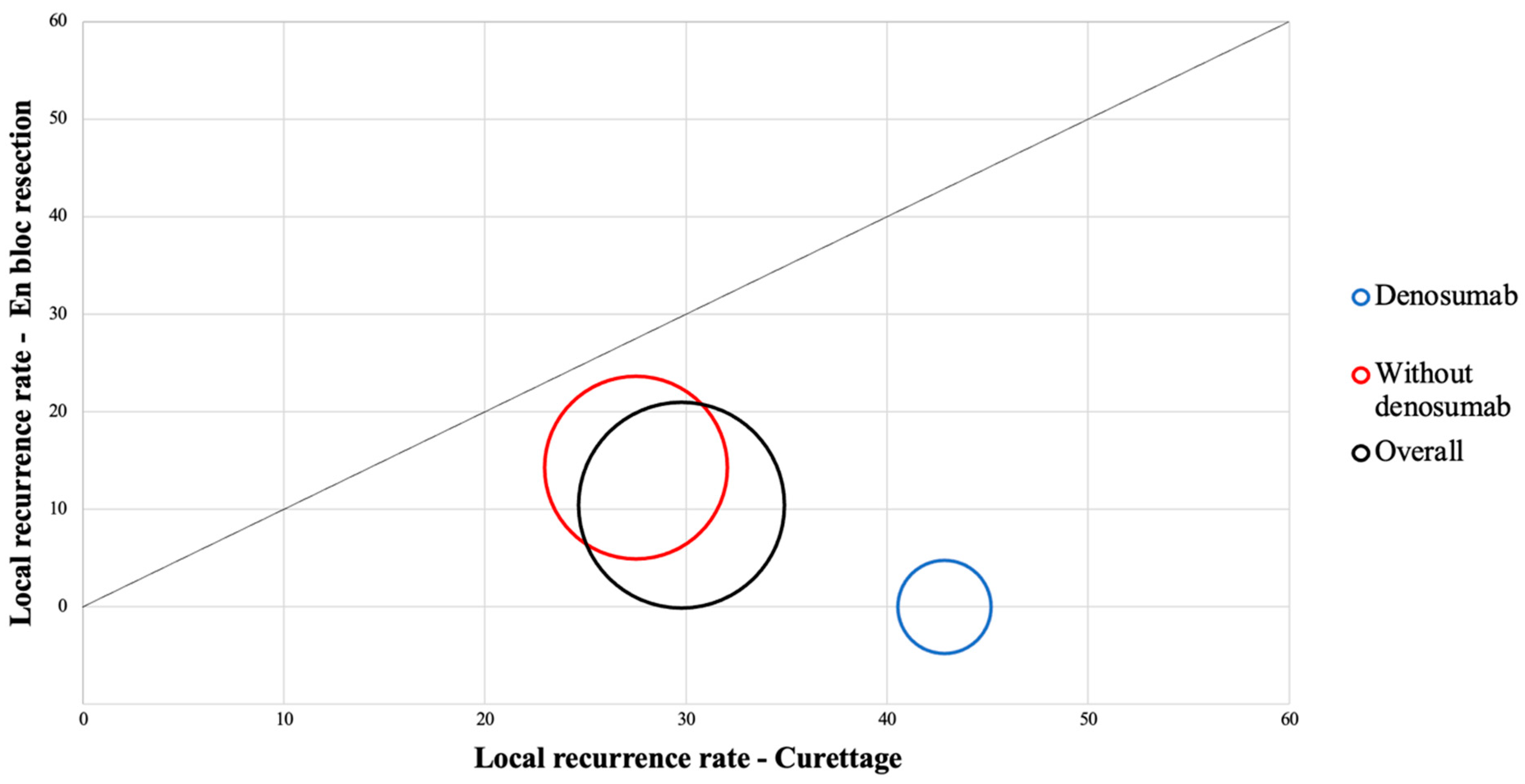
3.1. Identification of the Main Risk Factors for Local Recurrence
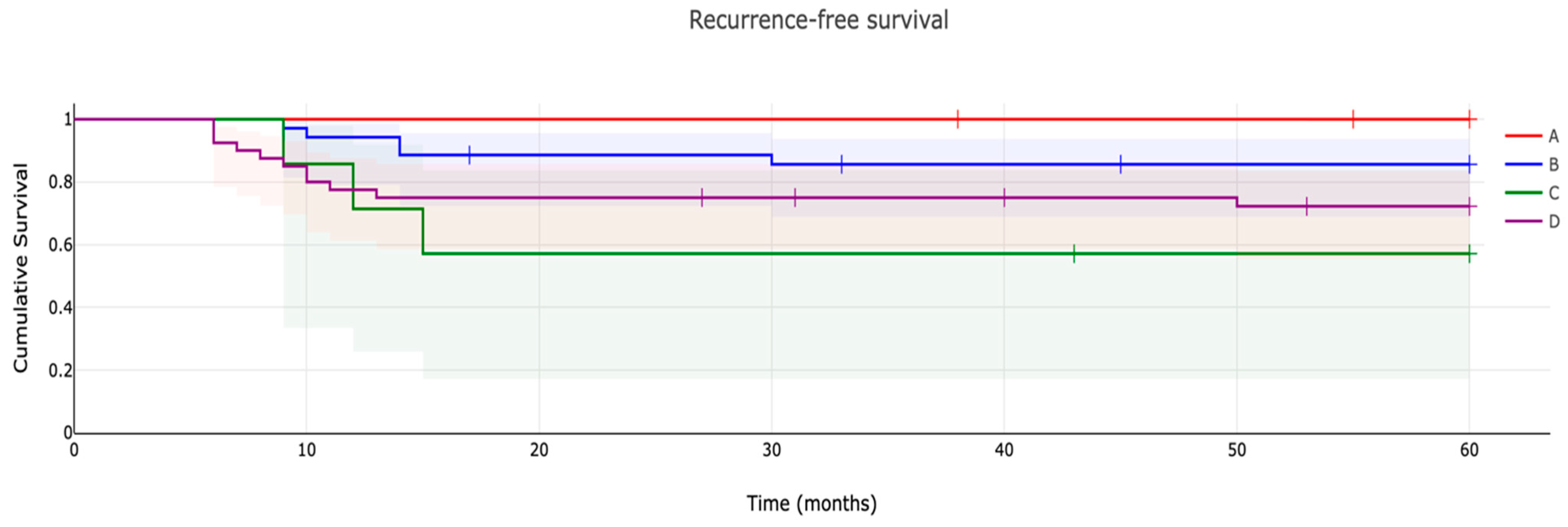
3.2. Recurrence-Free Survival Evaluation
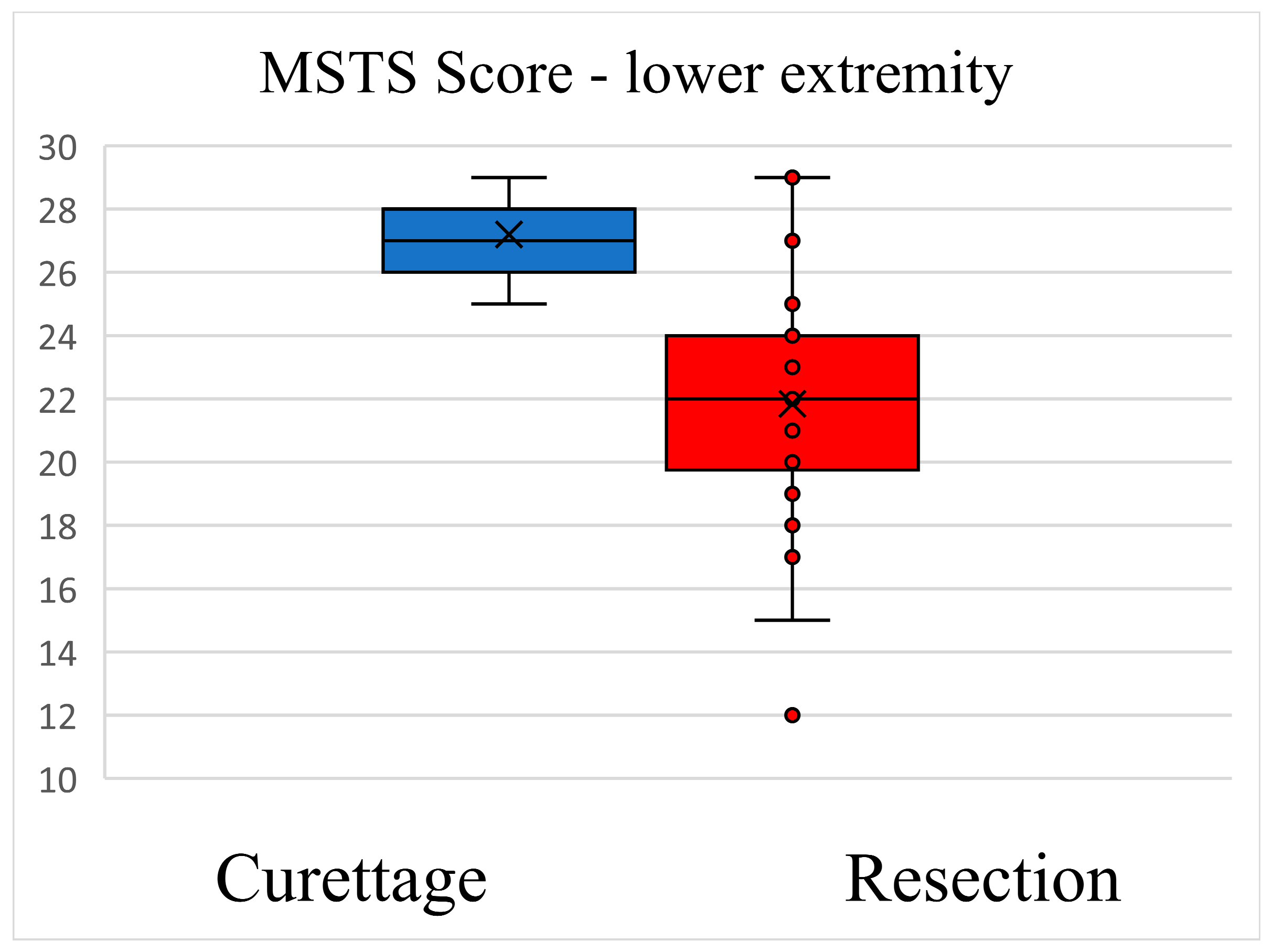
3.3. Functional Outcome Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campanacci, M.; Baldini, N.; Boriani, S.; Sudanese, A. Giant-Cell Tumor of Bone. J. Bone Jt. Surg. Am. 1987, 69, 106–114. [Google Scholar] [CrossRef]
- Thomas, D.M.; Skubitz, K.M. Giant Cell Tumour of Bone. Curr. Opin. Oncol. 2009, 21, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, L.; Dijkstra, P.D.S.; van de Sande, M.A.J.; Kroep, J.R.; Nout, R.A.; van Rijswijk, C.S.P.; Bovée, J.V.M.G.; Hogendoorn, P.C.W.; Gelderblom, H. The Clinical Approach Toward Giant Cell Tumor of Bone. Oncologist 2014, 19, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Balke, M.; Schremper, L.; Gebert, C.; Ahrens, H.; Streitbuerger, A.; Koehler, G.; Hardes, J.; Gosheger, G. Giant Cell Tumor of Bone: Treatment and Outcome of 214 Cases. J. Cancer Res. Clin. Oncol. 2008, 134, 969–978. [Google Scholar] [CrossRef]
- Errani, C.; Ruggieri, P.; Asenzio, M.A.N.; Toscano, A.; Colangeli, S.; Rimondi, E.; Rossi, G.; Longhi, A.; Mercuri, M. Giant Cell Tumor of the Extremity: A Review of 349 Cases from a Single Institution. Cancer Treat. Rev. 2010, 36, 1–7. [Google Scholar] [CrossRef]
- Klenke, F.M.; Wenger, D.E.; Inwards, C.Y.; Rose, P.S.; Sim, F.H. Giant Cell Tumor of Bone: Risk Factors for Recurrence. Clin. Orthop. Relat. Res. 2011, 469, 591–599. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, L.; Zhang, H.; Yu, X.; Wang, Z.; Ye, Z.; Wu, S.; Guo, S.; Zhang, G.; Wang, J.; et al. Recurrence Rates and Risk Factors for Primary Giant Cell Tumors around the Knee: A Multicentre Retrospective Study in China. Sci. Rep. 2016, 6, 36332. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Seng, C.; Tan, M.H. Risk Factors for Recurrence of Giant Cell Tumours of Bone. J. Orthop. Surg. 2014, 22, 108–110. [Google Scholar] [CrossRef]
- Lin, X.; Liu, J.; Xu, M. The Prognosis of Giant Cell Tumor of Bone and the Vital Risk Factors That Affect Its Postoperative Recurrence: A Meta-Analysis. Transl. Cancer Res. TCR 2021, 10, 1712–1722. [Google Scholar] [CrossRef]
- Li, Z.; Müller, R.; Ruffoni, D. Bone Remodeling and Mechanobiology around Implants: Insights from Small Animal Imaging: Bone remodeling and mechanobiology around implants. J. Orthop. Res. 2017, 36, 584–593. [Google Scholar] [CrossRef]
- Errani, C.; Tsukamoto, S.; Mavrogenis, A.F. How Safe and Effective Is Denosumab for Bone Giant Cell Tumour? Int. Orthop. (SICOT) 2017, 41, 2397–2400. [Google Scholar] [CrossRef] [PubMed]
- Kivioja, A.H.; Blomqvist, C.; Hietaniemi, K.; Trovik, C.; Walloe, A.; Bauer, H.C.F.; Jorgensen, P.H.; Bergh, P.; Follerås, G. Cement Is Recommended in Intralesional Surgery of Giant Cell Tumors: A Scandinavian Sarcoma Group Study of 294 Patients Followed for a Median Time of 5 Years. Acta Orthop. 2008, 79, 86–93. [Google Scholar] [CrossRef]
- Su, Y.-P.; Chen, W.-M.; Chen, T.-H. Giant-Cell Tumors of Bone: An Analysis of 87 Cases. Int. Orthop. (SICOT) 2004, 28, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Saito, M.; Kobayashi, E.; Morii, T.; Kikuta, K.; Watanabe, I.; Anazawa, U.; Takeuchi, K.; Suzuki, Y.; Susa, M.; et al. Preoperative Denosumab Therapy Against Giant Cell Tumor of Bone Is Associated with an Increased Risk of Local Recurrence After Curettage Surgery. Ann. Surg. Oncol. 2022, 29, 3992–4000. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Suehara, Y.; Okubo, T.; Sasa, K.; Kurihara, T.; Akaike, K.; Kubota, D.; Torigoe, T.; Hasegawa, N.; Ishii, M.; et al. Preoperative Denosumab Treatment with Curettage May Be a Risk Factor for Recurrence of Giant Cell Tumor of Bone. J. Orthop. Surg. 2020, 28, 230949902092978. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Hu, T.; Zhang, H.; Huang, J.; Yang, Q. Factors Affecting the Recurrence of Giant Cell Tumor of Bone After Surgery: A Clinicopathological Study of 80 Cases from a Single Center. Cell Physiol. Biochem. 2015, 36, 1961–1970. [Google Scholar] [CrossRef]
- Van der Heijden, L.; Sander Dijkstra, P.D.; Campanacci, D.A.; Gibbons, C.L.M.H.; van de Sande, M.A.J. Giant Cell Tumor with Pathologic Fracture: Should We Curette or Resect? Clin. Orthop. Relat. Res. 2013, 471, 820–829, Erratum in Clin. Orthop. Relat. Res. 2012, 470, 3626. [Google Scholar] [CrossRef]
- Chawla, S.; Blay, J.-Y.; Rutkowski, P.; Le Cesne, A.; Reichardt, P.; Gelderblom, H.; Grimer, R.J.; Choy, E.; Skubitz, K.; Seeger, L.; et al. Denosumab in Patients with Giant-Cell Tumour of Bone: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2019, 20, 1719–1729. [Google Scholar] [CrossRef]
- Van der Heijden, L.; Dijkstra, P.D.S.; Blay, J.-Y.; Gelderblom, H. Giant Cell Tumour of Bone in the Denosumab Era. Eur. J. Cancer 2017, 77, 75–83. [Google Scholar] [CrossRef]
- Unni, K.K.; Inwards, C.Y. Dahlin’s Bone Tumors: General Aspects and Data on 10,165 Cases, 6th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Chinder, P.S.; Hindiskere, S.; Doddarangappa, S.; Pal, U. Evaluation of Local Recurrence in Giant-Cell Tumor of Bone Treated by Neoadjuvant Denosumab. Clin. Orthop. Surg. 2019, 11, 352. [Google Scholar] [CrossRef]
- Palmerini, E.; Staals, E.L.; Jones, L.B.; Donati, D.M.; Longhi, A.; Randall, R.L. Role of (Neo)Adjuvant Denosumab for Giant Cell Tumor of Bone. Curr. Treat. Options Oncol. 2020, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Mahdal, M.; Neradil, J.; Mudry, P.; Paukovcekova, S.; Staniczkova Zambo, I.; Urban, J.; Macsek, P.; Pazourek, L.; Tomas, T.; Veselska, R. New Target for Precision Medicine Treatment of Giant-Cell Tumor of Bone: Sunitinib Is Effective in the Treatment of Neoplastic Stromal Cells with Activated PDGFRβ Signaling. Cancers 2021, 13, 3543. [Google Scholar] [CrossRef] [PubMed]
- Streitbürger, A.; Hardes, J.; Nottrott, M.; Guder, W.K. Reconstruction Survival of Segmental Megaendoprostheses: A Retrospective Analysis of 28 Patients Treated for Intercalary Bone Defects after Musculoskeletal Tumor Resections. Arch. Orthop. Trauma Surg. 2022, 142, 41–56. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A System for the Functional Evaluation of Reconstructive Procedures after Surgical Treatment of Tumors of the Musculoskeletal System. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- Thomas, D.M. RANKL, Denosumab, and Giant Cell Tumor of Bone. Curr. Opin. Oncol. 2012, 24, 397–403. [Google Scholar] [CrossRef]
- Turcotte, R.E. Giant Cell Tumor of Bone. Orthop. Clin. N. Am. 2006, 37, 35–51. [Google Scholar] [CrossRef]
- Errani, C.; Tsukamoto, S.; Ciani, G.; Donati, D.M. Present Day Controversies and Consensus in Curettage for Giant Cell Tumor of Bone. J. Clin. Orthop. Trauma 2019, 10, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Machak, G.N.; Snetkov, A.I. The Impact of Curettage Technique on Local Control in Giant Cell Tumour of Bone. Int. Orthop. 2021, 45, 779–789. [Google Scholar] [CrossRef]
- Errani, C.; Tsukamoto, S.; Leone, G.; Akahane, M.; Cevolani, L.; Tanzi, P.; Kido, A.; Honoki, K.; Tanaka, Y.; Donati, D.M. Higher Local Recurrence Rates after Intralesional Surgery for Giant Cell Tumor of the Proximal Femur Compared to Other Sites. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 813–819. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.J.; Springfield, D.S.; Motwani, H.K.; Ready, J.E.; Gebhardt, M.C.; Mankin, H.J. Recurrence of Giant-Cell Tumors of the Long Bones after Curettage and Packing with Cement. J. Bone Jt. Surg. Am. 1994, 76, 1827–1833. [Google Scholar] [CrossRef]
- Sheth, D.S.; Healey, J.H.; Sobel, M.; Lane, J.M.; Marcove, R.C. Giant Cell Tumor of the Distal Radius. J. Hand Surg. 1995, 20, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Puchner, S.E.; Kutscha-Lissberg, P.; Kaider, A.; Panotopoulos, J.; Puchner, R.; Böhler, C.; Hobusch, G.; Windhager, R.; Funovics, P.T. Outcome after Reconstruction of the Proximal Tibia--Complications and Competing Risk Analysis. PLoS ONE 2015, 10, e0135736. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Zhu, S.; Wang, Y.; Qian, W. Pre-Operative Denosumab Is Associated with Higher Risk of Local Recurrence in Giant Cell Tumor of Bone: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2020, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- Branstetter, D.G.; Nelson, S.D.; Manivel, J.C.; Blay, J.-Y.; Chawla, S.; Thomas, D.M.; Jun, S.; Jacobs, I. Denosumab Induces Tumor Reduction and Bone Formation in Patients with Giant-Cell Tumor of Bone. Clin. Cancer Res. 2012, 18, 4415–4424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Liu, W.; Xu, H.; Niu, X. A Nonrandomized Controlled Study of Sacral Giant Cell Tumors with Preoperative Treatment of Denosumab. Medicine 2018, 97, e13139. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, O.D.; Bolia, I.K.; Chloros, G.D.; Papanastasiou, J.; Koutsouradis, P.; Papagelopoulos, P.J. Denosumab: Current Use in the Treatment of Primary Bone Tumors. Orthopedics 2017, 40, 204–210. [Google Scholar] [CrossRef]
- Puri, A.; Gulia, A.; Hegde, P.; Verma, V.; Rekhi, B. Neoadjuvant Denosumab: Its Role and Results in Operable Cases of Giant Cell Tumour of Bone. Bone Jt. J. 2019, 101, 170–177. [Google Scholar] [CrossRef]
- Rutkowski, P.; Gaston, L.; Borkowska, A.; Stacchiotti, S.; Gelderblom, H.; Baldi, G.G.; Palmerini, E.; Casali, P.; Gronchi, A.; Parry, M.; et al. Denosumab Treatment of Inoperable or Locally Advanced Giant Cell Tumor of Bone—Multicenter Analysis Outside Clinical Trial. Eur. J. Surg. Oncol. 2018, 44, 1384–1390. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Ma, T.-X.; Qi, D.-W.; Zhao, M.; Hu, T.; Zhang, G.-C. Short-Term Preoperative Denosumab with Surgery in Unresectable or Recurrent Giant Cell Tumor of Bone. Orthop. Surg. 2019, 11, 1101–1108. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanzi, P.; Leone, G.; Righi, A.; Akahane, M.; Kido, A.; Honoki, K.; Tanaka, Y.; Donati, D.M.; et al. Similar Local Recurrence but Better Function with Curettage versus Resection for Bone Giant Cell Tumor and Pathological Fracture at Presentation. J. Surg. Oncol. 2019, 119, 864–872. [Google Scholar] [CrossRef]
- Chen, T.H.; Chen, W.M.; Huang, C.K. Reconstruction after Intercalary Resection of Malignant Bone Tumours: Comparison between segmental allograft and extracorporeally-irradiated autograft. J. Bone Jt. Surgery. Br. Vol. 2005, 87, 704–709. [Google Scholar] [CrossRef] [PubMed]

| Features | Overall | Resection | Curettage | |
|---|---|---|---|---|
| Number of patients | 95 | 48 (50.5%) | 47 (49.5%) | |
| Age at inclusion (years) | 34.4 ± 13.3 | 33.9 ± 14.2 | 35.0 ± 13.2 | p = 0.659 |
| Sex | ||||
| Female | 43 (45.3%) | 20 (46.5%) | 23 (53.5%) | |
| Male | 52 (54.7%) | 28 (53.8%) | 24 (46.2%) | |
| Follow-up (years) | 8.53 ± 4.91 | 8.84 ± 4.88 | 8.22 ± 4.98 | p = 0.522 |
| Grading | ||||
| C1 | 5 | 0 | 5 | |
| C2 | 51 | 14 | 37 | |
| C3 | 39 | 34 | 5 | |
| Pathological Fracture | 12 | 11 | 1 | |
| Tumour size | 5.46 ± 2.71 | 5.14 ± 2.51 | 5.77 ± 2.88 | p = 0.258 |
| Neoadjuvant therapy | ||||
| Denosumab | 20 | 13 | 7 | |
| None | 75 | 35 | 40 | |
| Anatomical location | ||||
| Distal femur | 23 | 12 | 11 | |
| Proximal tibia | 22 | 6 | 16 | |
| Forearm | 18 | 11 | 7 | |
| Foot | 10 | 5 | 5 | |
| Proximal femur | 6 | 4 | 2 | |
| Hand | 5 | 3 | 2 | |
| Fibula | 4 | 3 | 1 | |
| Other | 7 | 4 | 3 |
| Risk Factor | Local Recurrence Incidence Exposed Group/Number at Risk | Local Recurrence Incidence Unexposed Group/Number at Risk | Odds Ratio | 95% CI | p Value |
|---|---|---|---|---|---|
| Female gender | 8/43 | 11/52 | 0.85 | 0.30–2.35 | 0.757 |
| Curettage | 14/47 | 5/48 | 3.64 | 1.19–11.15 | 0.023 |
| Denosumab | 3/20 | 16/75 | 0.65 | 0.16–2.50 | 0.531 |
| Campanacci G3 | 10/39 | 9/56 | 1.80 | 0.65–4.95 | 0.254 |
| Age < 30 years | 7/40 | 12/55 | 0.89 | 0.31–2.52 | 0.833 |
| Size > 5 cm | 11/38 | 8/57 | 2.06 | 0.75–5.60 | 0.155 |
| Treatment | Resection Local Recurrence/Number at Risk | Curettage Local Recurrence/Number at Risk | Total Local Recurrence/Number at Risk | Odds Ratio | 95% CI | p Value | |
|---|---|---|---|---|---|---|---|
| Location | |||||||
| Proximal tibia | 2/6 | 6/16 | 8/22 | 3.22 | 1.09–9.48 | 0.033 | |
| Forearm | 1/11 | 2/7 | 3/18 | 0.76 | 0.19–2.96 | 0.695 | |
| Foot and hand | 1/8 | 1/7 | 2/15 | 0.69 | 0.11–2.77 | 0.486 | |
| Distal femur | 0/12 | 2/11 | 2/23 | 0.30 | 0.06–1.45 | 0.136 | |
| Other | 1/4 | 3/3 | 4/7 | 1.29 | 0.36–4.52 | 0.688 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdal, M.; Tomáš, T.; Apostolopoulos, V.; Adámková, D.; Múdry, P.; Staniczková Zambo, I.; Pazourek, L. Proximal Tibia Tumour Location and Curettage Are Major Risk Factors of Local Recurrence in Giant Cell Tumour of Bone. Cancers 2023, 15, 4664. https://doi.org/10.3390/cancers15184664
Mahdal M, Tomáš T, Apostolopoulos V, Adámková D, Múdry P, Staniczková Zambo I, Pazourek L. Proximal Tibia Tumour Location and Curettage Are Major Risk Factors of Local Recurrence in Giant Cell Tumour of Bone. Cancers. 2023; 15(18):4664. https://doi.org/10.3390/cancers15184664
Chicago/Turabian StyleMahdal, Michal, Tomáš Tomáš, Vasileios Apostolopoulos, Dagmar Adámková, Peter Múdry, Iva Staniczková Zambo, and Lukáš Pazourek. 2023. "Proximal Tibia Tumour Location and Curettage Are Major Risk Factors of Local Recurrence in Giant Cell Tumour of Bone" Cancers 15, no. 18: 4664. https://doi.org/10.3390/cancers15184664
APA StyleMahdal, M., Tomáš, T., Apostolopoulos, V., Adámková, D., Múdry, P., Staniczková Zambo, I., & Pazourek, L. (2023). Proximal Tibia Tumour Location and Curettage Are Major Risk Factors of Local Recurrence in Giant Cell Tumour of Bone. Cancers, 15(18), 4664. https://doi.org/10.3390/cancers15184664









