Nuclear Estrogen Receptors in Prostate Cancer: From Genes to Function
Abstract
Simple Summary
Abstract
1. Introduction
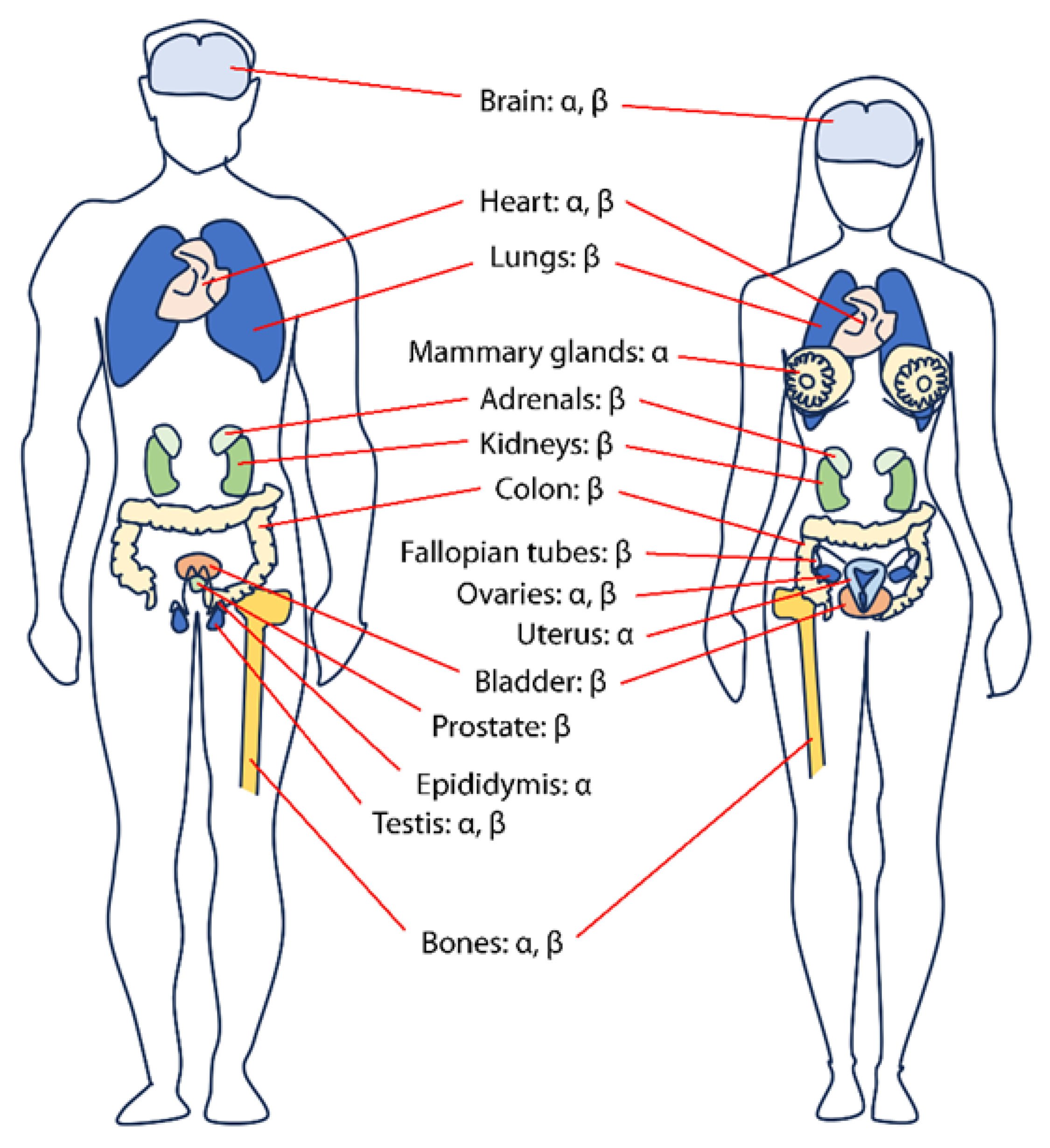
2. Physiology of Estrogens and Their Roles
2.1. Estrogen Biosynthesis and Physiological Functions
2.2. Role of Estrogens in the Prostate
3. Nuclear Estrogen Receptors
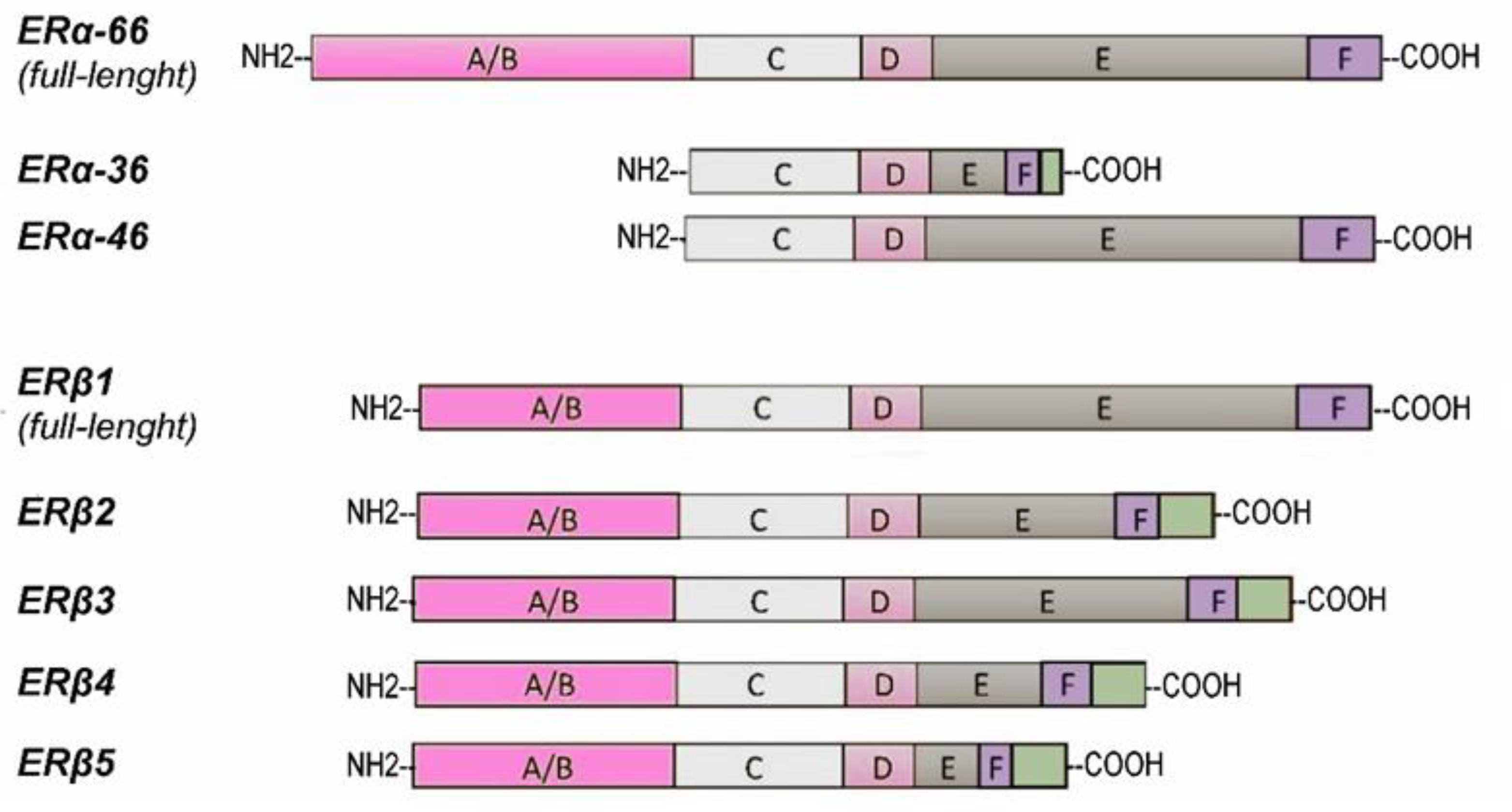
4. Expression and Splice Isoforms of Estrogen Receptors in Prostate Cancer
4.1. Estrogen Receptor Expression in Prostate Cancer
4.2. ER Isoforms in Prostate Cancer
5. Functions of Estrogen Receptors in Prostate Cancer Cells
5.1. Transcriptional Activity of ERs
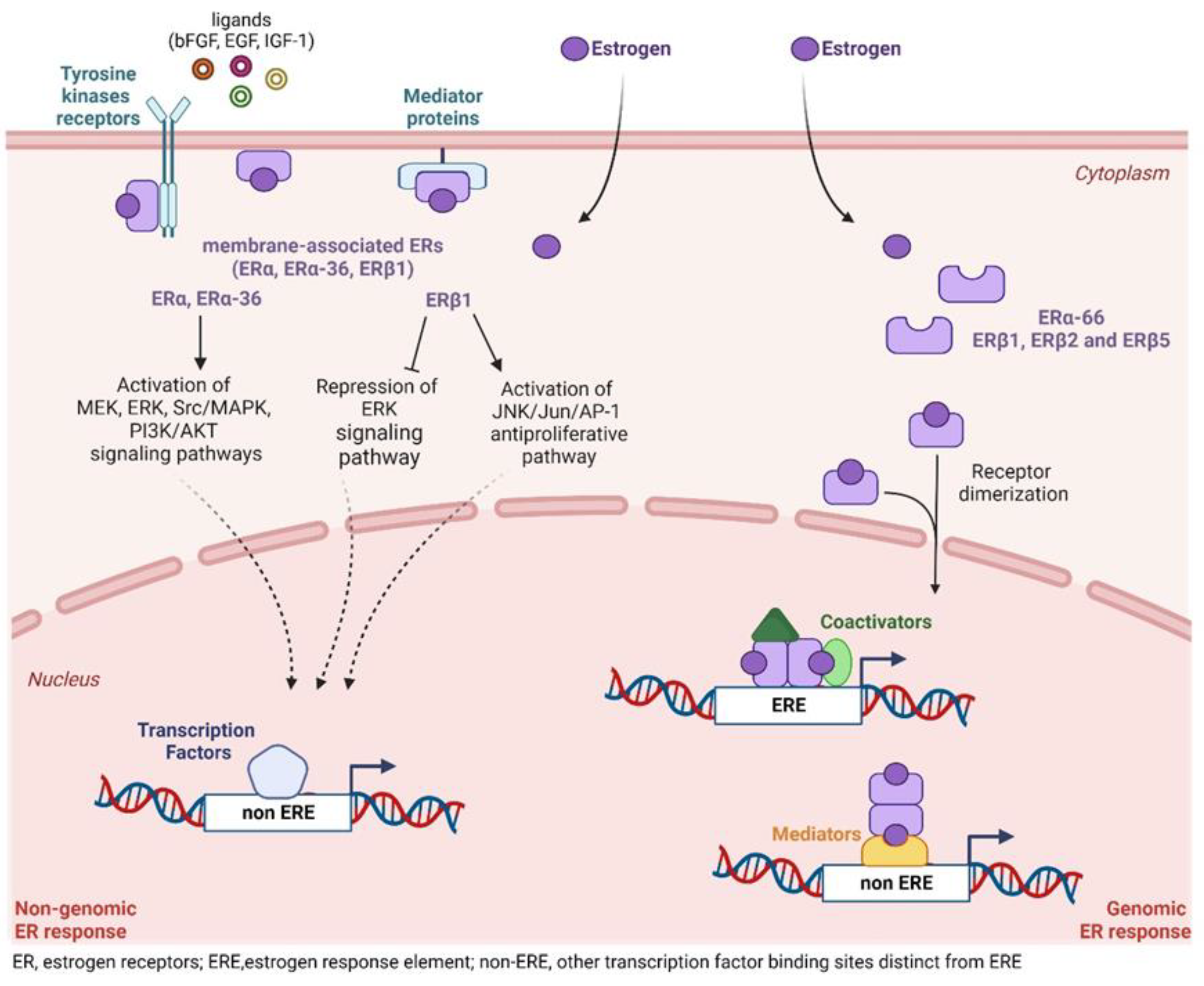
5.2. Non-Transcriptional ER Signaling
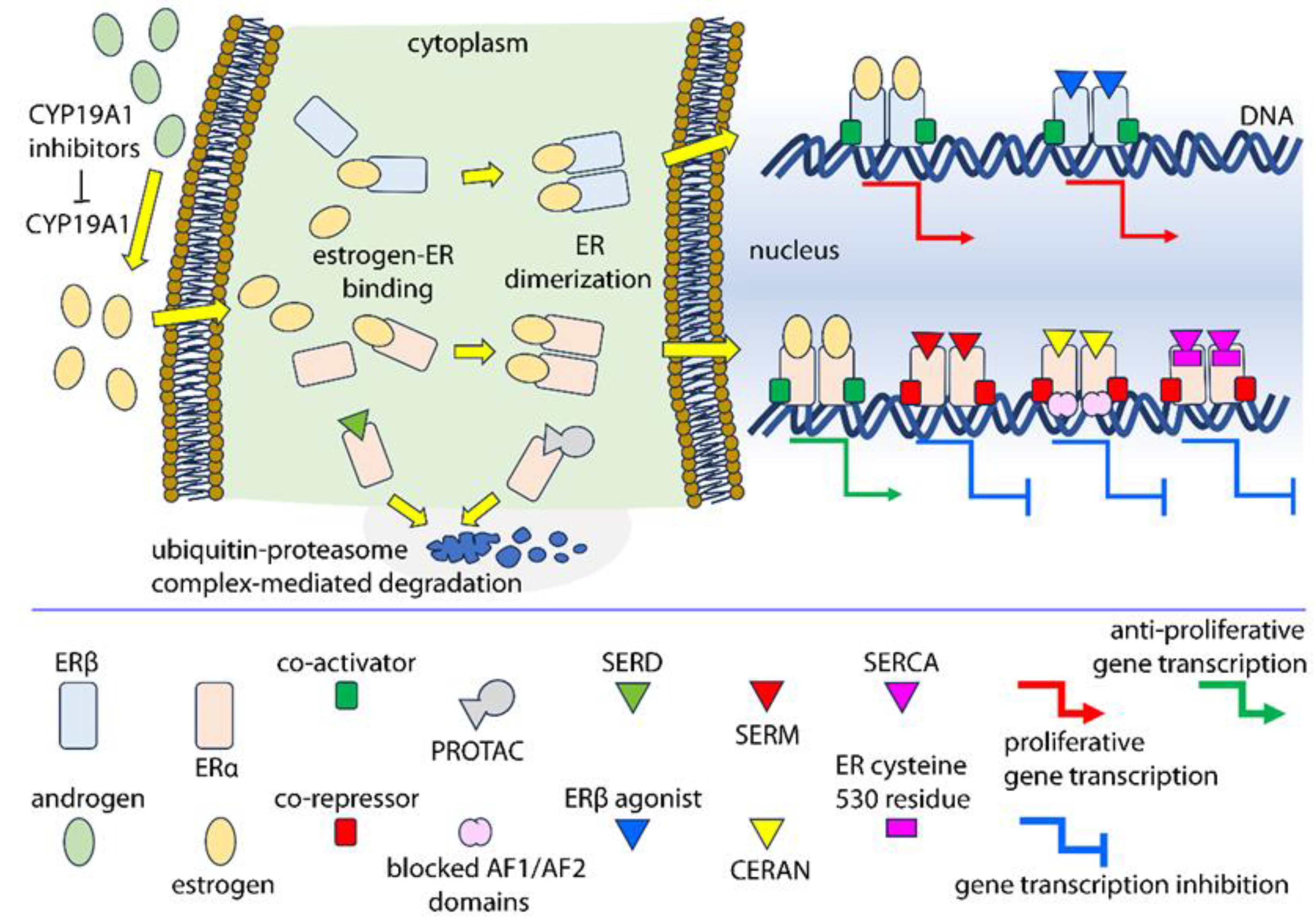
6. Molecules Targeting Nuclear Estrogen Receptors
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rochira, V.; Fabbi, M.; Valassi, E.; Madeo, B.; Carani, C. Estrogens, Male Reproduction and Beyond. Andrologie 2000, 13, 51–61. [Google Scholar] [CrossRef]
- Selye, H. The General Adaptation Syndrome and the Diseases of Adaptation. J. Clin. Endocrinol. Metab. 1946, 6, 117–230. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-Lorence, S.; Amarneh, B.; Ito, Y.; Fisher, C.R.; Michael, M.D.; et al. Aromatase Cytochrome P450, the Enzyme Responsible for Estrogen Biosynthesis. Endocr. Rev. 1994, 15, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Mahendroo, M.S.; Means, G.D.; Mendelson, C.R.; Simpson, E.R. Tissue-Specific Expression of Human P-450AROM. The Promoter Responsible for Expression in Adipose Tissue Is Different from That Utilized in Placenta. J. Biol. Chem. 1991, 266, 11276–11281. [Google Scholar] [CrossRef] [PubMed]
- Mahendroo, M.S.; Mendelson, C.R.; Simpson, E.R. Tissue-Specific and Hormonally Controlled Alternative Promoters Regulate Aromatase Cytochrome P450 Gene Expression in Human Adipose Tissue. J. Biol. Chem. 1993, 268, 19463–19470. [Google Scholar] [CrossRef]
- Stocco, C. Tissue Physiology and Pathology of Aromatase. Steroids 2012, 77, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.M.J. Extra-Gonadal Sites of Estrogen Biosynthesis and Function. BMB Rep. 2016, 49, 488–496. [Google Scholar] [CrossRef]
- Bose, H.S.; Pescovitz, O.H.; Miller, W.L. Spontaneous Feminization in a 46,XX Female Patient with Congenital Lipoid Adrenal Hyperplasia Due to a Homozygous Frameshift Mutation in the Steroidogenic Acute Regulatory Protein. J. Clin. Endocrinol. Metab. 1997, 82, 1511–1515. [Google Scholar] [CrossRef]
- Casarini, L.; Lazzaretti, C.; Paradiso, E.; Limoncella, S.; Riccetti, L.; Sperduti, S.; Melli, B.; Marcozzi, S.; Anzivino, C.; Sayers, N.S.; et al. Membrane Estrogen Receptor (GPER) and Follicle-Stimulating Hormone Receptor (FSHR) Heteromeric Complexes Promote Human Ovarian Follicle Survival. iScience 2020, 23, 101812. [Google Scholar] [CrossRef]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two Hormones for One Receptor: Evolution, Biochemistry, Actions, and Pathophysiology of LH and HCG. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.A.; Millena, A.C.; Reddy, S.; Finlay, S.; Vizcarra, J.; Khan, S.A.; Davis, J.S. Follicle-Stimulating Hormone-Induced Aromatase in Immature Rat Sertoli Cells Requires an Active Phosphatidylinositol 3-Kinase Pathway and Is Inhibited via the Mitogen-Activated Protein Kinase Signaling Pathway. Mol. Endocrinol. 2006, 20, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.F.; Hsueh, A.J.W. Stimulation of Aromatase Activity by Follicle Stimulating Hormone in Rat Granulosa Cells In Vivo and In Vitro. Endocrinology 1978, 102, 1275–1282. [Google Scholar] [CrossRef]
- Liu, Y.X.; Hsueh, A.J.W. Synergism between Granulosa and Theca-Interstitial Cells in Estrogen Biosynthesis by Gonadotropin-Treated Rat Ovaries: Studies on the Two-Cell, Two-Gonadotropin Hypothesis Using Steroid Antisera. Biol. Reprod. 1986, 35, 27–36. [Google Scholar] [CrossRef]
- Wada, Y.; Tsuiki, A.; Fukaya, T.; Shinkawa, O.; Satoh, S.; Horiguchi, M.; Hoshiai, H.; Yajima, A. Effects of Androgen on 17 Beta-Estradiol Production by Cultured Human Granulosa Cells. Tohoku J. Exp. Med. 1988, 154, 253–260. [Google Scholar] [CrossRef]
- Millier, S.G.; Whitelaw, P.F.; Smyth, C.D. Follicular Oestrogen Synthesis: The “two-Cell, Two-Gonadotrophin” Model Revisited. Mol. Cell Endocrinol. 1994, 100, 51–54. [Google Scholar] [CrossRef]
- Miller, W.L. Steroid Hormone Biosynthesis and Actions in the Materno-Feto-Placental Unit. Clin. Perinatol. 1998, 25, 799–817. [Google Scholar] [CrossRef]
- Ishimoto, H.; Jaffe, R.B. Development and Function of the Human Fetal Adrenal Cortex: A Key Component in the Feto-Placental Unit. Endocr. Rev. 2011, 32, 317–355. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.L.; Torbati, T.; Dutra, E. Hypothalamic Amenorrhea and the Long-Term Health Consequences. Semin. Reprod. Med. 2017, 35, 256–262. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, H.; Zhang, C. Selected Genetic Factors Associated with Primary Ovarian Insufficiency. Int. J. Mol. Sci. 2023, 24, 4423. [Google Scholar] [CrossRef]
- Stuenkel, C.A.; Gompel, A.; Davis, S.R.; Pinkerton, J.A.V.; Lumsden, M.A.; Santen, R.J. Approach to the Patient With New-Onset Secondary Amenorrhea: Is This Primary Ovarian Insufficiency? J. Clin. Endocrinol. Metab. 2022, 107, 825–835. [Google Scholar] [CrossRef]
- Kaplan, J.R.; Manuck, S.B. Ovarian Dysfunction, Stress, and Disease: A Primate Continuum. ILAR J. 2004, 45, 89–115. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H.; Carlson, J.L. The Pathophysiology of Amenorrhea in the Adolescent. Ann. N. Y. Acad. Sci. 2008, 1135, 163–178. [Google Scholar] [CrossRef]
- Riggs, B.L.; Khosla, S.; Melton, L.J. A Unitary Model for Involutional Osteoporosis: Estrogen Deficiency Causes Both Type I and Type II Osteoporosis in Postmenopausal Women and Contributes to Bone Loss in Aging Men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L. The Mechanisms of Estrogen Regulation of Bone Resorption. J. Clin. Investig. 2000, 106, 1203–1204. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.M. Osteocytes and Estrogen Deficiency. Curr. Osteoporos. Rep. 2021, 19, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Carani, C.; Qin, K.; Simoni, M.; Faustini-Fustini, M.; Serpente, S.; Boyd, J.; Korach, K.S.; Simpson, E.R. Effect of Testosterone and Estradiol in a Man with Aromatase Deficiency. N. Engl. J. Med. 1997, 337, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wasada, T.; Akamine, Y.; Kato, K.I.; Ibayashi, H.; Nomura, Y. Adrenal Contribution to Circulating Estrogens in Woman. Endocrinol. Jpn. 1978, 25, 123–128. [Google Scholar] [CrossRef]
- Almeida-Pereira, G.; Rorato, R.; Reis, L.C.; Elias, L.L.K.; Antunes-Rodrigues, J. The Role of Estradiol in Adrenal Insufficiency and Its Interaction with Corticosterone on Hydromineral Balance. Horm. Behav. 2013, 64, 847–855. [Google Scholar] [CrossRef]
- Caroccia, B.; Seccia, T.M.; Barton, M.; Rossi, G.P. Estrogen Signaling in the Adrenal Cortex: Implications for Blood Pressure Sex Differences. Hypertension 2016, 68, 840–848. [Google Scholar] [CrossRef]
- Kaludjerovic, J.; Ward, W.E. The Interplay between Estrogen and Fetal Adrenal Cortex. J. Nutr. Metab. 2012, 2012, 837901. [Google Scholar] [CrossRef]
- Meseguer, A.; Puche, C.; Cabero, A. Sex Steroid Biosynthesis in White Adipose Tissue. Horm. Metab. Res. 2002, 34, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Merrill, J.C.; Hollub, A.J.; Graham-Lorence, S.; Mendelson, C.R. Regulation of Estrogen Biosynthesis by Human Adipose Cells. Endocr. Rev. 1989, 10, 136–148. [Google Scholar] [CrossRef]
- Mendelson, C.R.; Simpson, E.R. Regulation of Estrogen Biosynthesis by Human Adipose Cells in Vitro. Mol. Cell. Endocrinol. 1987, 52, 169–176. [Google Scholar] [CrossRef]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Jo Corbin, C.; Mendelson, C.R. Tissue-Specific Promoters Regulate Aromatase Cytochrome P450 Expression. J. Steroid Biochem. Mol. Biol. 1993, 44, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mair, K.M.; Gaw, R.; MacLean, M.R. Obesity, Estrogens and Adipose Tissue Dysfunction—Implications for Pulmonary Arterial Hypertension. Pulm. Circ. 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Steiner, B.M.; Berry, D.C. The Regulation of Adipose Tissue Health by Estrogens. Front. Endocrinol. 2022, 13, 889923. [Google Scholar] [CrossRef]
- Cooke, P.S.; Heine, P.A.; Taylor, J.A.; Lubahn, D.B. The Role of Estrogen and Estrogen Receptor-α in Male Adipose Tissue. Mol. Cell. Endocrinol. 2001, 178, 147–154. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, H.T.; Kim, Y.J. The Role of Estrogen in Adipose Tissue Metabolism: Insights into Glucose Homeostasis Regulation. Endocr. J. 2014, 61, 1055–1067. [Google Scholar] [CrossRef]
- Simpson, E.R.; Mahendroo, M.S.; Nichols, J.E.; Bulun, S.E. Aromatase Gene Expression in Adipose Tissue: Relationship to Breast Cancer. Int. J. Fertil. Menopausal Stud. 1994, 39 (Suppl. 2), 75–83. [Google Scholar] [PubMed]
- Simpson, E.R. Sources of Estrogen and Their Importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, L.; Shangguan, A.J.; Bulun, S.E. Aromatase Expression and Regulation in Breast and Endometrial Cancer. J. Mol. Endocrinol. 2016, 57, R19–R33. [Google Scholar] [CrossRef]
- Bulun, S.E.; Price, T.M.; Aitkens, J.; Mahendroos, M.S.; Simpso, E.R. A Link between Breast Cancer and Local Estrogen Biosynthesis Suggested by Quantification of Breast Adipose Tissue Aromatase Cytochrome P450 Transcripts Using Competitive Polymerase Chain Reaction after Reverse Transcription. J. Clin. Endocrinol. Metab. 1993, 77, 1622–1628. [Google Scholar] [CrossRef]
- Wang, X.; Simpson, E.R.; Brown, K.A. Aromatase Overexpression in Dysfunctional Adipose Tissue Links Obesity to Postmenopausal Breast Cancer. J. Steroid Biochem. Mol. Biol. 2015, 153, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R. Biology of Aromatase in the Mammary Gland. J. Mammary Gland Biol. Neoplasia 2000, 5, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef]
- Azcoitia, I.; Yague, J.G.; Garcia-Segura, L.M. Estradiol Synthesis within the Human Brain. Neuroscience 2011, 191, 139–147. [Google Scholar] [CrossRef]
- Azcoitia, I.; Mendez, P.; Garcia-Segura, L.M. Aromatase in the Human Brain. Androg. Clin. Res. Ther. 2021, 2, 189–202. [Google Scholar] [CrossRef]
- Duncan, K.A.; Saldanha, C.J. Central Aromatization: A Dramatic and Responsive Defense against Threat and Trauma to the Vertebrate Brain. Front. Neuroendocrinol. 2020, 56, 100816. [Google Scholar] [CrossRef]
- Brann, D.W.; Lu, Y.; Wang, J.; Zhang, Q.; Thakkar, R.; Sareddy, G.R.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K. Brain-Derived Estrogen and Neural Function. Neurosci. Biobehav. Rev. 2022, 132, 793–817. [Google Scholar] [CrossRef]
- Spool, J.A.; Bergan, J.F.; Remage-Healey, L. A Neural Circuit Perspective on Brain Aromatase. Front. Neuroendocrinol. 2022, 65, 100973. [Google Scholar] [CrossRef]
- Loucks, T.L.; Berga, S.L. Does Postmenopausal Estrogen Use Confer Neuroprotection? Semin. Reprod. Med. 2009, 27, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, M.; Livonen, S.; Soininen, H. Aromatase Enzyme and Alzheimer’s Disease. Minerva Endocrinol. 2006, 31, 61–73. [Google Scholar] [PubMed]
- Hess, R.A. Estrogen in the Adult Male Reproductive Tract: A Review. Reprod. Biol. Endocrinol. 2003, 1, 52. [Google Scholar] [CrossRef]
- Adamopoulos, D.; Lawrence, D.M.; Vassilopoulos, P.; Kapolla, N.; Kontogeorgos, L.; McGarrigle, H.H.G. Hormone Levels in the Reproductive System of Normospermic Men and Patients with Oligospermia and Varicocele. J. Clin. Endocrinol. Metab. 1984, 59, 447–452. [Google Scholar] [CrossRef]
- O’Donnell, L.; Robertson, K.M.; Jones, M.E.; Simpson, E.R. Estrogen and Spermatogenesis. Endocr. Rev. 2001, 22, 289–318. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen Resistance Caused by a Mutation in the Estrogen-Receptor Gene in a Man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Fukami, M.; Ogata, T. Congenital Disorders of Estrogen Biosynthesis and Action. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 3, 101580. [Google Scholar] [CrossRef]
- Morishima, A.; Grumbach, M.M.; Simpson, E.R.; Fisher, C.; Qin, K. Aromatase Deficiency in Male and Female Siblings Caused by a Novel Mutation and the Physiological Role of Estrogens. J. Clin. Endocrinol. Metab. 1995, 80, 3689–3698. [Google Scholar] [CrossRef]
- Huijben, M.; Huijsmans, R.L.N.; Lock, M.T.W.T.; de Kemp, V.F.; de Kort, L.M.O.; van Breda, J.H.M.K. Clomiphene Citrate for Male Infertility: A Systematic Review and Meta-Analysis. Andrology 2023, 11, 987–996. [Google Scholar] [CrossRef]
- Balló, A.; Busznyákné Székvári, K.; Czétány, P.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. Estrogenic and Non-Estrogenic Disruptor Effect of Zearalenone on Male Reproduction: A Review. Int. J. Mol. Sci. 2023, 24, 1578. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Walker, W.H. Nonclassical Androgen and Estrogen Signaling Is Essential for Normal Spermatogenesis. Semin. Cell Dev. Biol. 2022, 121, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.; Grossmann, M. Mechanisms in Endocrinology: Estradiol as a Male Hormone. Eur. J. Endocrinol. 2019, 181, R23–R43. [Google Scholar] [CrossRef] [PubMed]
- Ellem, S.J.; Risbridger, G.P. The Dual, Opposing Roles of Estrogen in the Prostate. Ann. N. Y. Acad. Sci. 2009, 1155, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Nelles, J.L.; Hu, W.Y.; Prins, G.S. Estrogen Action and Prostate Cancer. Expert Rev. Endocrinol. Metab. 2011, 6, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Jarred, R.A.; McPherson, S.J.; Bianco, J.J.; Couse, J.F.; Korach, K.S.; Risbridger, G.P. Prostate Phenotypes in Estrogen-Modulated Transgenic Mice. Trends Endocrinol. Metab. 2002, 13, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ellem, S.J.; Schmitt, J.F.; Pedersen, J.S.; Frydenberg, M.; Risbridger, G.P. Local Aromatase Expression in Human Prostate Is Altered in Malignancy. J. Clin. Endocrinol. Metab. 2004, 89, 2434–2441. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, Y.; Chen, L.; Shi, J.; Wang, C.Y.; Miao, L.; Klocker, H.; Park, I.; Lee, C.; Zhang, J. Benign Prostatic Hyperplasia (BPH) Epithelial Cell Line BPH-1 Induces Aromatase Expression in Prostatic Stromal Cells via Prostaglandin E2. J. Endocrinol. 2007, 195, 89–94. [Google Scholar] [CrossRef]
- Lafront, C.; Germain, L.; Weidmann, C.; Audet-Walsh, É. A Systematic Study of the Impact of Estrogens and Selective Estrogen Receptor Modulators on Prostate Cancer Cell Proliferation. Sci. Rep. 2020, 10, 4024. [Google Scholar] [CrossRef]
- Sciarra, F. Anti-Estrogens and Aromatase Inhibitors: Tamoxifen and Testolactone. J. Endocrinol. Investig. 1988, 11, 755–762. [Google Scholar] [CrossRef]
- Greene, G.L.; Gilna, P.; Waterfield, M.; Baker, A.; Hort, Y.; Shine, J. Sequence and Expression of Human Estrogen Receptor Complementary DNA. Science 1986, 231, 1150–1154. [Google Scholar] [CrossRef]
- Walter, P.; Green, S.; Greene, G.; Krust, A.; Bornert, J.M.; Jeltsch, J.M.; Staub, A.; Jensen, E.; Scrace, G.; Waterfield, M. Cloning of the Human Estrogen Receptor CDNA. Proc. Natl. Acad. Sci. USA 1985, 82, 7889–7893. [Google Scholar] [CrossRef] [PubMed]
- Mosselman, S.; Polman, J.; Dijkema, R. ER Beta: Identification and Characterization of a Novel Human Estrogen Receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Jiang, C. Signal Crosstalk and the Role of Estrogen Receptor Beta (ERβ) in Prostate Cancer. Med. Sci. Monit. 2022, 28, e935599-1. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Zakharov, M.N.; Khan, S.H.; Miki, R.; Jang, H.; Toraldo, G.; Singh, R.; Bhasin, S.; Jasuja, R. The dynamic structure of the estrogen receptor. Res. J. Amino Acids 2011, 2011, 812540. [Google Scholar] [CrossRef] [PubMed]
- Barkhem, T.; Carlsson, B.; Nilsson, Y.; Enmark, E.; Gustafsson, J.Å.; Nilsson, S. Differential Response of Estrogen Receptor Alpha and Estrogen Receptor Beta to Partial Estrogen Agonists/Antagonists. Mol. Pharmacol. 1998, 54, 105–112. [Google Scholar] [CrossRef]
- Yi, P.; Wang, Z.; Feng, Q.; Pintilie, G.D.; Foulds, C.E.; Lanz, R.B.; Ludtke, S.J.; Schmid, M.F.; Chiu, W.; O’Malley, B.W. Structure of a Biologically Active Estrogen Receptor-Coactivator Complex on DNA. Mol. Cell 2015, 57, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonism of This Interaction by Tamoxifen. Cell 1998, 95, 927–937. [Google Scholar] [CrossRef]
- Harms, M.J.; Eick, G.N.; Goswami, D.; Colucci, J.K.; Griffin, P.R.; Ortlund, E.A.; Thornton, J.W. Biophysical Mechanisms for Large-Effect Mutations in the Evolution of Steroid Hormone Receptors. Proc. Natl. Acad. Sci. USA 2013, 110, 11475–11480. [Google Scholar] [CrossRef]
- Eick, G.N.; Colucci, J.K.; Harms, M.J.; Ortlund, E.A.; Thornton, J.W. Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors. PLoS Genet. 2012, 8, e1003072. [Google Scholar] [CrossRef]
- D’arrigo, G.; Gianquinto, E.; Rossetti, G.; Cruciani, G.; Lorenzetti, S.; Spyrakis, F. Binding of Androgen- and Estrogen-Like Flavonoids to Their Cognate (Non)Nuclear Receptors: A Comparison by Computational Prediction. Molecules 2021, 26, 1613. [Google Scholar] [CrossRef] [PubMed]
- McInerney, E.M.; Ince, B.A.; Shapiro, D.J.; Katzenellenbogen, B.S. A Transcriptionally Active Estrogen Receptor Mutant Is a Novel Type of Dominant Negative Inhibitor of Estrogen Action. Mol. Endocrinol. 1996, 10, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. ESR1 Ligand-Binding Domain Mutations in Hormone-Resistant Breast Cancer. Nat. Genet. 2013, 45, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, M.H.; Thompson, E.A.; Bishop, D.T.; Cannon, L.A. Possible Linkage of a Breast Cancer-Susceptibility Locus to the ABO Locus: Sensitivity of LOD Scores to a Single New Recombinant Observation. Genet. Epidemiol. 1984, 1, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Styrkarsdottir, U.; Halldorsson, B.V.; Gretarsdottir, S.; Gudbjartsson, D.F.; Walters, G.B.; Ingvarsson, T.; Jonsdottir, T.; Saemundsdottir, J.; Center, J.R.; Nguyen, T.V.; et al. Multiple Genetic Loci for Bone Mineral Density and Fractures. N. Engl. J. Med. 2008, 358, 2355–2365. [Google Scholar] [CrossRef]
- Herrington, D.M.; Howard, T.D.; Hawkins, G.A.; Reboussin, D.M.; Xu, J.; Zheng, S.L.; Brosnihan, K.B.; Meyers, D.A.; Bleecker, E.R. Estrogen-Receptor Polymorphisms and Effects of Estrogen Replacement on High-Density Lipoprotein Cholesterol in Women with Coronary Disease. N. Engl. J. Med. 2002, 346, 967–974. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Inoue, S.; Hosoi, T.; Orimo, H.; Emi, M. Isolation and Radiation Hybrid Mapping of Dinucleotide Repeat Polymorphism at the Human Estrogen Receptor Beta Locus. J. Hum. Genet. 1998, 43, 73–74. [Google Scholar] [CrossRef]
- Ogawa, S.; Emi, M.; Shiraki, M.; Hosoi, T.; Ouchi, Y.; Inoue, S. Association of Estrogen Receptor Beta (ESR2) Gene Polymorphism with Blood Pressure. J. Hum. Genet. 2000, 45, 327–330. [Google Scholar] [CrossRef]
- Forsell, C.; Enmark, E.; Axelman, K.; Blomberg, M.; Wahlund, L.O.; Gustafsson, J.Å.; Lannfelt, L. Investigations of a CA Repeat in the Oestrogen Receptor Beta Gene in Patients with Alzheimer’s Disease. Eur. J. Hum. Genet. 2001, 9, 802–804. [Google Scholar] [CrossRef]
- Beleza-Meireles, A.; Kockum, I.; Lundberg, F.; Söderhäll, C.; Nordenskjöld, A. Risk Factors for Hypospadias in the Estrogen Receptor 2 Gene. J. Clin. Endocrinol. Metab. 2007, 92, 3712–3718. [Google Scholar] [CrossRef]
- Fytili, P.; Giannatou, E.; Papanikolaou, V.; Stripeli, F.; Karachalios, T.; Malizos, K.; Tsezou, A. Association of Repeat Polymorphisms in the Estrogen Receptors Alpha, Beta, and Androgen Receptor Genes with Knee Osteoarthritis. Clin. Genet. 2005, 68, 268–277. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Hinney, A.; Ziegler, A.; Hermann, H.; Fichter, M.; Mayer, H.; Siegfried, W.; Young, J.K.; Remschmidt, H.; Hebebrand, J. Systematic Mutation Screening of the Estrogen Receptor Beta Gene in Probands of Different Weight Extremes: Identification of Several Genetic Variants. J. Clin. Endocrinol. Metab. 1998, 83, 4524–4527. [Google Scholar] [CrossRef] [PubMed]
- Lang-Muritano, M.; Sproll, P.; Wyss, S.; Kolly, A.; Hürlimann, R.; Konrad, D.; Biason-Lauber, A. Early-Onset Complete Ovarian Failure and Lack of Puberty in a Woman With Mutated Estrogen Receptor β (ESR2). J. Clin. Endocrinol. Metab. 2018, 103, 3748–3756. [Google Scholar] [CrossRef] [PubMed]
- Baetens, D.; Güran, T.; Mendonca, B.B.; Gomes, N.L.; De Cauwer, L.; Peelman, F.; Verdin, H.; Vuylsteke, M.; Van Der Linden, M.; Atay, Z.; et al. Biallelic and Monoallelic ESR2 Variants Associated with 46,XY Disorders of Sex Development. Genet. Med. 2018, 20, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Biason-Lauber, A.; Lang-Muritano, M. Estrogens: Two Nuclear Receptors, Multiple Possibilities. Mol. Cell. Endocrinol. 2022, 554, 111710. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, B.; Ou-Yang, L. Role of Estrogen Receptors in Health and Disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Pike, A.C.W.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engström, O.; Öhman, L.; Greene, G.L.; Gustafsson, J.Å.; Carlquist, M. Molecular Basis of Agonism and Antagonism in the Oestrogen Receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef]
- Paech, K.; Webb, P.; Kuiper, G.G.J.M.; Nilsson, S.; Gustafsson, J.Å.; Kushner, P.J.; Scanlan, T.S. Differential Ligand Activation of Estrogen Receptors ERalpha and ERbeta at AP1 Sites. Science 1997, 277, 1508–1510. [Google Scholar] [CrossRef]
- Fixemer, T.; Remberger, K.; Bonkhoff, H. Differential Expression of the Estrogen Receptor Beta (ERβ) in Human Prostate Tissue, Premalignant Changes, and in Primary, Metastatic, and Recurrent Prostatic Adenocarcinoma. Prostate 2003, 54, 79–87. [Google Scholar] [CrossRef]
- Bonkhoff, H. Estrogen Receptor Signaling in Prostate Cancer: Implications for Carcinogenesis and Tumor Progression. Prostate 2018, 78, 2–10. [Google Scholar] [CrossRef]
- Qu, L.G.; Wardan, H.; Davis, I.D.; Iddawela, M.; Sluka, P.; Pezaro, C.J. Circulating Oestrogen Receptor Mutations and Splice Variants in Advanced Prostate Cancer. BJU Int. 2019, 124 (Suppl. S1), 50–56. [Google Scholar] [CrossRef] [PubMed]
- Thellenberg-Karlsson, C.; Lindström, S.; Malmer, B.; Wiklund, F.; Augustsson-Bälter, K.; Adami, H.O.; Stattin, P.; Nilsson, M.; Dahlman-Wright, K.; Gustafsson, J.Å.; et al. Estrogen Receptor Beta Polymorphism Is Associated with Prostate Cancer Risk. Clin. Cancer Res. 2006, 12, 1936–1941. [Google Scholar] [CrossRef]
- Latil, A.; Biè, I.; Vidaud, D.; Lidereau, R.; Berthon, P.; Cussenot, O.; Vidaud, M. Evaluation of Androgen, Estrogen (ER and ER), and Progesterone Receptor Expression in Human Prostate Cancer by Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction Assays. Cancer Res. 2001, 61, 1919–1926. [Google Scholar] [PubMed]
- Sehgal, P.D.; Bauman, T.M.; Nicholson, T.M.; Vellky, J.E.; Ricke, E.A.; Tang, W.; Xu, W.; Huang, W.; Ricke, W.A. Tissue-Specific Quantification and Localization of Androgen and Estrogen Receptors in Prostate Cancer. Hum. Pathol. 2019, 89, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Sboner, A.; Nair, S.S.; Giannopoulou, E.; Li, R.; Hennig, S.; Mosquera, J.M.; Pauwels, J.; Park, K.; Kossai, M.; et al. The Oestrogen Receptor Alpha-Regulated LncRNA NEAT1 Is a Critical Modulator of Prostate Cancer. Nat. Commun. 2014, 5, 5383. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Shiina, H.; Deguchi, M.; Zhao, H.; Okino, S.T.; Kane, C.J.; Carroll, P.R.; Igawa, M.; Dahiya, R. Age-Dependent Methylation of ESR1 Gene in Prostate Cancer. Biochem. Biophys. Res. Commun. 2004, 321, 455–461. [Google Scholar] [CrossRef]
- Li, L.C.; Chui, R.; Nakajima, K.; Oh, B.R.; Au, H.C.; Dahiya, R. Frequent Methylation of Estrogen Receptor in Prostate Cancer: Correlation with Tumor Progression. Cancer Res. 2000, 60, 702–706. [Google Scholar]
- Hu, C.; Liu, Y.; Jiang, S.; Chen, H.; Xu, H.; Hu, J.; Li, C.; Xia, H. The Variable Association between Expression and Methylation of Estrogen Receptors and the Survival of Patients with Different Tumors. Clin. Transl. Med. 2020, 10, e49. [Google Scholar] [CrossRef]
- Clemons, J.; Michael Glodé, L.; Gao, D.; Flaig, T.W. Low-Dose Diethylstilbestrol for the Treatment of Advanced Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 198–204. [Google Scholar] [CrossRef][Green Version]
- Shamash, J.; Powles, T.; Sarker, S.J.; Protheroe, A.; Mithal, N.; Mills, R.; Beard, R.; Wilson, P.; Tranter, N.; O’Brien, N.; et al. A Multi-Centre Randomised Phase III Trial of Dexamethasone vs Dexamethasone and Diethylstilbestrol in Castration-Resistant Prostate Cancer: Immediate vs Deferred Diethylstilbestrol. Br. J. Cancer 2011, 104, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Grenader, T.; Plotkin, Y.; Gips, M.; Cherny, N.; Gabizon, A. Diethylstilbestrol for the Treatment of Patients with Castration-Resistant Prostate Cancer: Retrospective Analysis of a Single Institution Experience. Oncol. Rep. 2014, 31, 428–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stein, M.; Goodin, S.; Doyle-Lindrud, S.; Silberberg, J.; Kane, M.; Metzger, D.; Eddy, S.; Shih, W.; DiPaola, R.S. Transdermal Estradiol in Castrate and Chemotherapy Resistant Prostate Cancer. Med. Sci. Monit. 2012, 18, CR260–CR264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Konishi, N.; Nakaoka, S.; Hiasa, Y.; Kitahori, Y.; Ohshima, M.; Samma, S.; Okajima, E. Immunohistochemical Evaluation of Estrogen Receptor Status in Benign Prostatic Hypertrophy and in Prostate Carcinoma and the Relationship to Efficacy of Endocrine Therapy. Oncology 1993, 50, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Olczak, M.; Orzechowska, M.J.; Bednarek, A.K.; Lipiński, M. The Transcriptomic Profiles of ESR1 and MMP3 Stratify the Risk of Biochemical Recurrence in Primary Prostate Cancer beyond Clinical Features. Int. J. Mol. Sci. 2023, 24, 8399. [Google Scholar] [CrossRef]
- Yeh, C.R.; Slavin, S.; Da, J.; Hsu, I.; Luo, J.; Xiao, G.Q.; Ding, J.; Chou, F.J.; Yeh, S. Estrogen Receptor α in Cancer Associated Fibroblasts Suppresses Prostate Cancer Invasion via Reducing CCL5, IL6 and Macrophage Infiltration in the Tumor Microenvironment. Mol. Cancer 2016, 15, 7. [Google Scholar] [CrossRef]
- Slavin, S.; Yeh, C.-R.; Da, J.; Yu, S.; Miyamoto, H.; Messing, E.M.; Guancial, E.; Yeh, S. Estrogen Receptor α in Cancer-Associated Fibroblasts Suppresses Prostate Cancer Invasion via Modulation of Thrombospondin 2 and Matrix Metalloproteinase 3. Carcinogenesis 2014, 35, 1301–1309. [Google Scholar] [CrossRef]
- Grindstad, T.; Skjefstad, K.; Andersen, S.; Ness, N.; Nordby, Y.; Al-Saad, S.; Fismen, S.; Donnem, T.; Khanehkenari, M.R.; Busund, L.T.; et al. Estrogen Receptors α and β and Aromatase as Independent Predictors for Prostate Cancer Outcome. Sci. Rep. 2016, 6, 33114. [Google Scholar] [CrossRef]
- Nojima, D.; Li, L.-C.; Dharia, A.; Perinchery, G.; Ribeiro-Filho, L.; Yen, T.-S.B.; Dahiya, R. CpG Hypermethylation of the Promoter Region Inactivates the Estrogen Receptor-Gene in Patients with Prostate Carcinoma. Cancer 2001, 92, 2076–2083. [Google Scholar] [CrossRef]
- Božović, A.; Mandušić, V.; Todorović, L.; Krajnović, M. Estrogen Receptor Beta: The Promising Biomarker and Potential Target in Metastases. Int. J. Mol. Sci. 2021, 22, 1656. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Bilancio, A.; Perillo, B.; Sinisi, A.A.; Migliaccio, A.; Castoria, G. Estrogen Receptors in Epithelial-Mesenchymal Transition of Prostate Cancer. Cancers 2019, 11, 1418. [Google Scholar] [CrossRef]
- Andersson, S.; Sundberg, M.; Pristovsek, N.; Ibrahim, A.; Jonsson, P.; Katona, B.; Clausson, C.M.; Zieba, A.; Ramström, M.; Söderberg, O.; et al. Insufficient Antibody Validation Challenges Oestrogen Receptor Beta Research. Nat. Commun. 2017, 8, 15840. [Google Scholar] [CrossRef]
- Gustafsson, J.A.; Strom, A.; Warner, M. Update on ERbeta. J. Steroid Biochem. Mol. Biol. 2019, 191, 105312. [Google Scholar] [CrossRef]
- Belluti, S.; Rigillo, G.; Imbriano, C. Transcription Factors in Cancer: When Alternative Splicing Determines Opposite Cell Fates. Cells 2020, 9, 760. [Google Scholar] [CrossRef]
- Belluti, S.; Semeghini, V.; Rigillo, G.; Ronzio, M.; Benati, D.; Torricelli, F.; Reggiani Bonetti, L.; Carnevale, G.; Grisendi, G.; Ciarrocchi, A.; et al. Alternative Splicing of NF-YA Promotes Prostate Cancer Aggressiveness and Represents a New Molecular Marker for Clinical Stratification of Patients. J. Exp. Clin. Cancer Res. 2021, 40, 362. [Google Scholar] [CrossRef]
- Munkley, J.; Livermore, K.; Rajan, P.; Elliott, D.J. RNA Splicing and Splicing Regulator Changes in Prostate Cancer Pathology. Hum. Genet. 2017, 136, 1143. [Google Scholar] [CrossRef]
- Del Giudice, M.; Foster, J.G.; Peirone, S.; Rissone, A.; Caizzi, L.; Gaudino, F.; Parlato, C.; Anselmi, F.; Arkell, R.; Guarrera, S.; et al. FOXA1 Regulates Alternative Splicing in Prostate Cancer. Cell Rep. 2022, 40, 111404. [Google Scholar] [CrossRef]
- Flouriot, G.; Brand, H.; Denger, S.; Metivier, R.; Kos, M.; Reid, G.; Sonntag-Buck, V.; Gannon, F. Identification of a New Isoform of the Human Estrogen Receptor-Alpha (HER-α) That Is Encoded by Distinct Transcripts and That Is Able to Repress HER-α Activation Function 1. EMBO J. 2000, 19, 4688–4700. [Google Scholar] [CrossRef]
- Denger, S.; Reid, G.; Koš, M.; Flouriot, G.; Parsch, D.; Brand, H.; Korach, K.S.; Sonntag-Buck, V.; Gannon, F. ERα Gene Expression in Human Primary Osteoblasts: Evidence for the Expression of Two Receptor Proteins. Mol. Endocrinol. 2001, 15, 2064–2077. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, X.; Shen, P.; Loggie, B.W.; Chang, Y.; Deuel, T.F. Identification, Cloning, and Expression of Human Estrogen Receptor-A36, a Novel Variant of Human Estrogen Receptor-A66. Biochem. Biophys. Res. Commun. 2005, 336, 1023–1027. [Google Scholar] [CrossRef]
- Lee, L.M.J.; Cao, J.; Deng, H.; Chen, P.; Gatalica, Z.; Wang, Z.Y. ER-A36, a Novel Variant of ER-α, Is Expressed in ER-Positive and -Negative Human Breast Carcinomas. Anticancer Res. 2008, 28, 479–484. [Google Scholar]
- Wang, Z.Y.; Zhang, X.T.; Shen, P.; Loggie, B.W.; Chang, Y.C.; Deuel, T.F. A Variant of Estrogen Receptor-{alpha}, HER-{alpha}36: Transduction of Estrogen- and Antiestrogen-Dependent Membrane-Initiated Mitogenic Signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 9063–9068. [Google Scholar] [CrossRef]
- Kim, C.K.; Torcaso, A.; Asimes, A.; Chung, W.C.J.; Pak, T.R. Structural and Functional Characteristics of Estrogen Receptor Beta (ERβ) Splice Variants: Implications for the Aging Brain. J. Neuroendocrinol. 2018, 30, e12488. [Google Scholar] [CrossRef]
- Moore, J.T.; McKee, D.D.; Slentz-Kesler, K.; Moore, L.B.; Jones, S.A.; Horne, E.L.; Su, J.L.; Kliewer, S.A.; Lehmann, J.M.; Willson, T.M. Cloning and Characterization of Human Estrogen Receptor β Isoforms. Biochem. Biophys. Res. Commun. 1998, 247, 75–78. [Google Scholar] [CrossRef]
- Leung, Y.K.; Mak, P.; Hassan, S.; Ho, S.M. Estrogen Receptor (ER)-Beta Isoforms: A Key to Understanding ER-Beta Signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 13162–13167. [Google Scholar] [CrossRef]
- Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen Receptor Beta: An Overview and Update. Nucl. Recept. Signal. 2008, 6, e003. [Google Scholar] [CrossRef]
- Faria, M.; Shepherd, P.; Pan, Y.; Chatterjee, S.S.; Navone, N.; Gustafsson, J.-Å.; Strom, A.; Faria, M.; Shepherd, P.; Pan, Y.; et al. The Estrogen Receptor Variants Β2 and Β5 Induce Stem Cell Characteristics and Chemotherapy Resistance in Prostate Cancer through Activation of Hypoxic Signaling. Oncotarget 2018, 9, 36273–36288. [Google Scholar] [CrossRef]
- Formaggio, N.; Rubin, M.A.; Theurillat, J.P. Loss and Revival of Androgen Receptor Signaling in Advanced Prostate Cancer. Oncogene 2021, 40, 1205–1216. [Google Scholar] [CrossRef]
- Leach, D.A.; Fernandes, R.C.; Bevan, C.L. Cellular Specificity of Androgen Receptor, Coregulators, and Pioneer Factors in Prostate Cancer. Endocr. Oncol. 2022, 2, R112–R131. [Google Scholar] [CrossRef]
- Özturan, D.; Morova, T.; Lack, N.A. Androgen Receptor-Mediated Transcription in Prostate Cancer. Cells 2022, 11, 898. [Google Scholar] [CrossRef]
- Lau, K.M.; To, K.F. Importance of Estrogenic Signaling and Its Mediated Receptors in Prostate Cancer. Int. J. Mol. Sci. 2016, 17, 1434. [Google Scholar] [CrossRef]
- Ricke, W.A.; McPherson, S.J.; Bianco, J.J.; Cunha, G.R.; Wang, Y.; Risbridger, G.P. Prostatic Hormonal Carcinogenesis Is Mediated by in Situ Estrogen Production and Estrogen Receptor Alpha Signaling. FASEB J. 2008, 22, 1512–1520. [Google Scholar] [CrossRef]
- Leung, Y.K.; Lam, H.M.; Wu, S.; Song, D.; Levin, L.; Cheng, L.; Wu, C.L.; Ho, S.M. Estrogen Receptor Β2 and Β5 Are Associated with Poor Prognosis in Prostate Cancer, and Promote Cancer Cell Migration and Invasion. Endocr. Relat. Cancer 2010, 17, 675. [Google Scholar] [CrossRef] [PubMed]
- Omoto, Y.; Iwase, H. Clinical Significance of Estrogen Receptor β in Breast and Prostate Cancer from Biological Aspects. Cancer Sci. 2015, 106, 337. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Jonsson, P.; Hartman, J.; Williams, C.; Ström, A.; Gustafsson, J.Å. Estrogen Receptors Β1 and Β2 Have Opposing Roles in Regulating Proliferation and Bone Metastasis Genes in the Prostate Cancer Cell Line PC3. Mol. Endocrinol. 2012, 26, 1991–2003. [Google Scholar] [CrossRef]
- Lombardi, A.P.G.; Vicente, C.M.; Porto, C.S. Estrogen Receptors Promote Migration, Invasion and Colony Formation of the Androgen-Independent Prostate Cancer Cells PC-3 Through β-Catenin Pathway. Front. Endocrinol. 2020, 11, 527553. [Google Scholar] [CrossRef]
- Stevens, J.H.; Bano, A.; Bensaoula, L.; Strom, A.M.; Gustafsson, J.-Å. Estrogen Receptor β Isoforms Regulate Chemotherapy Resistance and the Cancer Stem Cell Population in Prostate Cancer Cells. Receptors 2023, 2, 176–190. [Google Scholar] [CrossRef]
- Leav, I.; Lau, K.M.; Adams, J.Y.; McNeal, J.E.; Taplin, M.E.; Wang, J.; Singh, H.; Ho, S.M. Comparative Studies of the Estrogen Receptors β and α and the Androgen Receptor in Normal Human Prostate Glands, Dysplasia, and in Primary and Metastatic Carcinoma. Am. J. Pathol. 2001, 159, 79–92. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, E.J.; Madison, L.D.; Lazennec, G. Expression of Estrogen Receptor β in Prostate Carcinoma Cells Inhibits Invasion and Proliferation and Triggers Apoptosis. FEBS Lett. 2004, 566, 169–172. [Google Scholar] [CrossRef]
- Corey, E.; Quinn, J.E.; Emond, M.J.; Buhler, K.R.; Brown, L.G.; Vessella, R.L. Inhibition of Androgen-Independent Growth of Prostate Cancer Xenografts by 17beta-Estradiol. Clin. Cancer Res. 2002, 8, 1003–1007. [Google Scholar]
- Weihua, Z.; Mäkelä, S.; Andersson, L.C.; Salmi, S.; Saji, S.; Webster, J.I.; Jensen, E.V.; Nilsson, S.; Warner, M.; Gustafsson, J.Å. A Role for Estrogen Receptor β in the Regulation of Growth of the Ventral Prostate. Proc. Natl. Acad. Sci. USA 2001, 98, 6330–6335. [Google Scholar] [CrossRef]
- Imamov, O.; Morani, A.; Shim, G.J.; Omoto, Y.; Thulin-Andersson, C.; Warner, M.; Gustafsson, J.Å. Estrogen Receptor β Regulates Epithelial Cellular Differentiation in the Mouse Ventral Prostate. Proc. Natl. Acad. Sci. USA 2004, 101, 9375–9380. [Google Scholar] [CrossRef]
- Kumar, V.; Chambon, P. The Estrogen Receptor Binds Tightly to Its Responsive Element as a Ligand-Induced Homodimer. Cell 1988, 55, 145–156. [Google Scholar] [CrossRef]
- Cowley, S.M.; Hoare, S.; Mosselman, S.; Parker, M.G. Estrogen Receptors a and b Form Heterodimers on DNA. J. Biol. Chem. 1997, 272, 19858–19862. [Google Scholar] [CrossRef] [PubMed]
- Jakacka, M.; Ito, M.; Weiss, J.; Chien, P.-Y.; Gehm, B.D.; Jameson, J.L. Estrogen Receptor Binding to DNA Is Not Required for Its Activity through the Nonclassical AP1 Pathway. Pediatrics 2001, 276, 13615–13621. [Google Scholar] [CrossRef] [PubMed]
- McKenna, N.J.; Lanz, R.B.; O’Malley, B.W. Nuclear Receptor Coregulators: Cellular and Molecular Biology. Endocr. Rev. 1999, 20, 321–344. [Google Scholar] [CrossRef] [PubMed]
- O’Lone, R.; Frith, M.C.; Karlsson, E.K.; Hansen, U. Genomic Targets of Nuclear Estrogen Receptors. Mol. Endocrinol. 2004, 18, 1859–1875. [Google Scholar] [CrossRef]
- Frasor, J.; Danes, J.M.; Komm, B.; Chang, K.C.N.; Richard Lyttle, C.; Katzenellenbogen, B.S. Profiling of Estrogen Up- and down-Regulated Gene Expression in Human Breast Cancer Cells: Insights into Gene Networks and Pathways Underlying Estrogenic Control of Proliferation and Cell Phenotype. Endocrinology 2003, 144, 4562–4574. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Shao, Z.-M.; Chen, J.-C.; Li, X.-S.; Hussain, A.; Fontana, J.A. Expression of Estrogen Receptors in Estrogen Receptor–Negative Human Breast Carcinoma Cells: Modulation of Epidermal Growth Factor-receptor (EGF-R) and Transforming Growth Factor α (TGFα) Gene Expression. J. Cell. Biochem. 1994, 54, 289–298. [Google Scholar] [CrossRef]
- Ostano, P.; Mello-grand, M.; Sesia, D.; Gregnanin, I.; Peraldo-neia, C.; Guana, F.; Jachetti, E.; Farsetti, A.; Chiorino, G. Gene Expression Signature Predictive of Neuroendocrine Transformation in Prostate Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 1078. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Uzair, M.; Nazli, A.; Chen, J.Z. An Overview on Estrogen Receptors Signaling and Its Ligands in Breast Cancer. Eur. J. Med. Chem. 2022, 241, 114658. [Google Scholar] [CrossRef] [PubMed]
- Rej, R.K.; Thomas, J.E.; Acharyya, R.K.; Rae, J.M.; Wang, S. Targeting the Estrogen Receptor for the Treatment of Breast Cancer: Recent Advances and Challenges. J. Med. Chem. 2023, 66, 8339–8381. [Google Scholar] [CrossRef] [PubMed]
- Tecalco-Cruz, A.C.; Macías-Silva, M.; Ramírez-Jarquín, J.O.; Ramírez-Jarquín, U.N. Decoding the Therapeutic Implications of the ERα Stability and Subcellular Distribution in Breast Cancer. Front. Endocrinol. 2022, 13, 867448. [Google Scholar] [CrossRef]
- Dey, P.; Ström, A.; Gustafsson, J.A. Estrogen Receptor β Upregulates FOXO3a and Causes Induction of Apoptosis through PUMA in Prostate Cancer. Oncogene 2013, 33, 4213–4225. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xiao, M.; Zou, M.; Xu, W. Estrogen Receptor β Inhibits Prostate Cancer Cell Proliferation through Downregulating TGF-Β1/IGF-1 Signaling. Int. J. Clin. Exp. Pathol. 2017, 10, 8569. [Google Scholar] [PubMed]
- Chaurasiya, S.; Widmann, S.; Botero, C.; Lin, C.Y.; Gustafsson, J.Å.; Strom, A.M. Estrogen Receptor β Exerts Tumor Suppressive Effects in Prostate Cancer through Repression of Androgen Receptor Activity. PLoS ONE 2020, 15, e0226057. [Google Scholar] [CrossRef]
- Wu, W.F.; Maneix, L.; Insunza, J.; Nalvarte, I.; Antonson, P.; Kere, J.; Yu, N.Y.L.; Tohonen, V.; Katayama, S.; Einarsdottir, E.; et al. Estrogen Receptor β, a Regulator of Androgen Receptor Signaling in the Mouse Ventral Prostate. Proc. Natl. Acad. Sci. USA 2017, 114, E3816–E3822. [Google Scholar] [CrossRef]
- Gehrig, J.; Kaulfuß, S.; Jarry, H.; Bremmer, F.; Stettner, M.; Burfeind, P.; Thelen, P.; Gehrig, J.; Kaulfuß, S.; Jarry, H.; et al. Prospects of Estrogen Receptor β Activation in the Treatment of Castration-Resistant Prostate Cancer. Oncotarget 2017, 8, 34971–34979. [Google Scholar] [CrossRef]
- McPherson, S.J.; Hussain, S.; Balanathan, P.; Hedwards, S.L.; Niranjan, B.; Grant, M.; Chandrasiri, U.P.; Toivanen, R.; Wang, Y.; Taylor, R.A.; et al. Estrogen Receptor–β Activated Apoptosis in Benign Hyperplasia and Cancer of the Prostate Is Androgen Independent and TNFα Mediated. Proc. Natl. Acad. Sci. USA 2010, 107, 3123–3128. [Google Scholar] [CrossRef]
- Mak, P.; Leav, I.; Pursell, B.; Bae, D.; Yang, X.; Taglienti, C.A.; Gouvin, L.M.; Sharma, V.M.; Mercurio, A.M. ERβ Impedes Prostate Cancer EMT by Destabilizing HIF-1α and Inhibiting VEGF-Mediated Snail Nuclear Localization: Implications for Gleason Grading. Cancer Cell 2010, 17, 319–332. [Google Scholar] [CrossRef]
- Guerini, V.; Sau, D.; Scaccianoce, E.; Rusmini, P.; Ciana, P.; Maggi, A.; Martini, P.G.V.; Katzenellenbogen, B.S.; Martini, L.; Motta, M.; et al. The Androgen Derivative 5alpha-Androstane-3beta,17beta-Diol Inhibits Prostate Cancer Cell Migration through Activation of the Estrogen Receptor Beta Subtype. Cancer Res. 2005, 65, 5445–5453. [Google Scholar] [CrossRef]
- Dey, P.; Velazquez-Villegas, L.A.; Faria, M.; Turner, A.; Jonsson, P.; Webb, P.; Williams, C.; Gustafsson, J.-Å.; Ström, A.M. Estrogen Receptor Β2 Induces Hypoxia Signature of Gene Expression by Stabilizing HIF-1α in Prostate Cancer. PLoS ONE 2015, 10, e0128239. [Google Scholar] [CrossRef]
- Nelson, A.W.; Tilley, W.D.; Neal, D.E.; Carroll, J.S. Estrogen Receptor Beta in Prostate Cancer: Friend or Foe? Endocr. Relat. Cancer 2014, 21, T219–T234. [Google Scholar] [CrossRef] [PubMed]
- Grubisha, M.J.; Defranco, D.B. Local Endocrine, Paracrine and Redox Signaling Networks Impact Estrogen and Androgen Crosstalk in the Prostate Cancer Microenvironment. Steroids 2013, 78, 538–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grubisha, M.J.; Cifuentes, M.E.; Hammes, S.R.; DeFranco, D.B. A Local Paracrine and Endocrine Network Involving TGFβ, Cox-2, ROS, and Estrogen Receptor β Influences Reactive Stromal Cell Regulation of Prostate Cancer Cell Motility. Mol. Endocrinol. 2012, 26, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, C.; Vlaeminck-Guillem, V.; Trédan, O.; Poulard, C.; Le Romancer, M. Non-Genomic Signaling of Steroid Receptors in Cancer. Mol. Cell. Endocrinol. 2021, 538, 111453. [Google Scholar] [CrossRef]
- Souza, D.S.; Macheroni, C.; Pereira, G.J.S.; Vicente, C.M.; Porto, C.S. Molecular Regulation of Prostate Cancer by Galectin-3 and Estrogen Receptor. Front. Endocrinol. 2023, 14, 1124111. [Google Scholar] [CrossRef]
- Hurtado, A.; Pinós, T.; Barbosa-Desongles, A.; López-Avilés, S.; Barquinero, J.; Petriz, J.; Santamaria-Martínez, A.; Morote, J.; De Torres, I.; Bellmunt, J.; et al. Estrogen Receptor Beta Displays Cell Cycle-Dependent Expression and Regulates the G1 Phase through a Non-Genomic Mechanism in Prostate Carcinoma Cells. Anal. Cell. Pathol. 2008, 30, 349–365. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, H.; Kong, Q.; Liu, C.; Tian, Y.; Zeng, X.; Li, D. Effect of ERβ-Regulated ERK1/2 Signaling on Biological Behaviors of Prostate Cancer Cells. Am. J. Transl. Res. 2017, 9, 2775. [Google Scholar]
- Silva, R.D.S.; Lombardi, A.P.G.; de Souza, D.S.; Vicente, C.M.; Porto, C.S. Activation of Estrogen Receptor Beta (ERβ) Regulates the Expression of N-Cadherin, E-Cadherin and β-Catenin in Androgen-Independent Prostate Cancer Cells. Int. J. Biochem. Cell Biol. 2018, 96, 40–50. [Google Scholar] [CrossRef]
- Augusto, T.V.; Georgina, C.D.S.; Rodrigues, C.M.P.; Teixeira, N.; Amaral, C. Acquired Resistance to Aromatase Inhibitors: Where We Stand! Endocr. Relat. Cancer 2018, 25, R283–R301. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.X.; Reinert, T.; Chmielewska, I.; Ellis, M.J. Mechanisms of Aromatase Inhibitor Resistance. Nat. Rev. Cancer 2015, 15, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. Tamoxifen: A Most Unlikely Pioneering Medicine. Nat. Rev. Drug Discov. 2003, 2, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zwart, W.; Griekspoor, A.; Berno, V.; Lakeman, K.; Jalink, K.; Mancini, M.; Neefjes, J.; Michalides, R. PKA-Induced Resistance to Tamoxifen Is Associated with an Altered Orientation of ERalpha towards Co-Activator SRC-1. EMBO J. 2007, 26, 3534–3544. [Google Scholar] [CrossRef]
- Ali, S.; Buluwela, L.; Coombes, R.C. Antiestrogens and Their Therapeutic Applications in Breast Cancer and Other Diseases. Annu. Rev. Med. 2011, 62, 217–232. [Google Scholar] [CrossRef]
- Shiota, M.; Fujimoto, N.; Kashiwagi, E.; Eto, M. The Role of Nuclear Receptors in Prostate Cancer. Cells 2019, 8, 602. [Google Scholar] [CrossRef]
- Wakeling, A.E.; Dukes, M.; Bowler, J. A Potent Specific Pure Antiestrogen with Clinical Potential. Cancer Res. 1991, 51, 3867–3873. [Google Scholar]
- Movérare-Skrtic, S.; Börjesson, A.E.; Farman, H.H.; Sjögren, K.; Windahl, S.H.; Lagerquist, M.K.; Andersson, A.; Stubelius, A.; Carlsten, H.; Gustafsson, J.-Å.; et al. The Estrogen Receptor Antagonist ICI 182,780 Can Act Both as an Agonist and an Inverse Agonist When Estrogen Receptor α AF-2 Is Modified. Proc. Natl. Acad. Sci. USA 2014, 111, 1180–1185. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, S.; Gustafsson, J.Å. Nuclear Receptors: Recent Drug Discovery for Cancer Therapies. Endocr. Rev. 2019, 40, 1207–1249. [Google Scholar] [CrossRef]
- Burris, T.P.; Solt, L.A.; Wang, Y.; Crumbley, C.; Banerjee, S.; Griffett, K.; Lundasen, T.; Hughes, T.; Kojetin, D.J. Nuclear Receptors and Their Selective Pharmacologic Modulators. Pharmacol. Rev. 2013, 65, 710–778. [Google Scholar] [CrossRef]
- Raza, S.; Meyer, M.; Goodyear, C.; Hammer, K.D.P.; Guo, B.; Ghribi, O. The Cholesterol Metabolite 27-Hydroxycholesterol Stimulates Cell Proliferation via ERβ in Prostate Cancer Cells. Cancer Cell Int. 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.-M.; Laspina, M.; Long, J.; Ho, S.-M. Expression of Estrogen Receptor (ER)-and ER-in Normal and Malignant Prostatic Epithelial Cells: Regulation by Methylation and Involvement in Growth Regulation 1. Cancer Res. 2000, 60, 3175–3182. [Google Scholar] [PubMed]
- Bhattacharyya, R.S.; Krishnan, A.V.; Swami, S.; Feldman, D. Fulvestrant (ICI 182,780) down-Regulates Androgen Receptor Expression and Diminishes Androgenic Responses in LNCaP Human Prostate Cancer Cells. Mol. Cancer Ther. 2006, 5, 1539–1549. [Google Scholar] [CrossRef]
- Gasent Blesa, J.M.; Alberola Candel, V.; Giner Marco, V.; Giner-Bosch, V.; Provencio Pulla, M.; Laforga Canales, J.B. Experience with Fulvestrant Acetate in Castration-Resistant Prostate Cancer Patients. Ann. Oncol. 2010, 21, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Chadha, M.K.; Ashraf, U.; Lawrence, D.; Tian, L.; Levine, E.; Silliman, C.; Escott, P.; Payne, V.; Trump, D.L. Phase II Study of Fulvestrant (Faslodex) in Castration Resistant Prostate Cancer. Prostate 2008, 68, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. The G Protein-Coupled Oestrogen Receptor GPER in Health and Disease: An Update. Nat. Rev. Endocrinol. 2023, 19, 407–424. [Google Scholar] [CrossRef]
- Rekha, P.; Gupta, A.; Goud, K.S.; Biswas, B.; Bhattar, S.; Vijayakumar, G.; Selvaraju, S. GPER Induces Mitochondrial Fission through P44/42 MAPK—Drp1 Pathway in Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2023, 643, 16–23. [Google Scholar] [CrossRef]
- Kampa, M.; Lappano, R.; Grande, F.; Rizzuti, B.; Maggiolini, M.; Castanas, E.; Jacquot, Y. Promising Perspectives of the Antiproliferative GPER Inverse Agonist ERα17p in Breast Cancer. Cells 2023, 12, 653. [Google Scholar] [CrossRef]
- Pal, U.; Manjegowda, M.C.; Singh, N.; Saikia, S.; Philip, B.S.; Jyoti Kalita, D.; Kumar Rai, A.; Sarma, A.; Raphael, V.; Modi, D.; et al. The G-Protein-Coupled Estrogen Receptor, a Gene Co-Expressed with ERα in Breast Tumors, Is Regulated by Estrogen-ERα Signalling in ERα Positive Breast Cancer Cells. Gene 2023, 877, 147548. [Google Scholar] [CrossRef]
- Czogalla, B.; Partenheimer, A.; Jeschke, U.; von Schönfeldt, V.; Mayr, D.; Mahner, S.; Burges, A.; Simoni, M.; Melli, B.; Benevelli, R.; et al. β-Arrestin 2 Is a Prognostic Factor for Survival of Ovarian Cancer Patients Upregulating Cell Proliferation. Front. Endocrinol. 2020, 11, 554733. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a Second-Generation Antiandrogen for Treatment of Advanced Prostate Cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- Ricci, F.; Buzzatti, G.; Rubagotti, A.; Boccardo, F. Safety of Antiandrogen Therapy for Treating Prostate Cancer. Expert. Opin. Drug Saf. 2014, 13, 1483–1499. [Google Scholar] [CrossRef]
- Fizazi, K.; Massard, C.; Bono, P.; Jones, R.; Kataja, V.; James, N.; Garcia, J.A.; Protheroe, A.; Tammela, T.L.; Elliott, T.; et al. Activity and Safety of ODM-201 in Patients with Progressive Metastatic Castration-Resistant Prostate Cancer (ARADES): An Open-Label Phase 1 Dose-Escalation and Randomised Phase 2 Dose Expansion Trial. Lancet Oncol. 2014, 15, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.N.S.; Ferraldeschi, R.; Attard, G.; De Bono, J. Evolution of Androgen Receptor Targeted Therapy for Advanced Prostate Cancer. Nat. Rev. Clin. Oncol. 2014, 11, 365–376. [Google Scholar] [CrossRef]
- Culig, Z. Targeting the Androgen Receptor in Prostate Cancer. Expert. Opin. Pharmacother. 2014, 15, 1427–1437. [Google Scholar] [CrossRef]
- Dalton, J.T.; Mukherjee, A.; Zhu, Z.; Kirkovsky, L.; Miller, D.D. Discovery of Nonsteroidal Androgens. Biochem. Biophys. Res. Commun. 1998, 244, 1–4. [Google Scholar] [CrossRef]
- Schmidt, A.; Meissner, R.S.; Gentile, M.A.; Chisamore, M.J.; Opas, E.E.; Scafonas, A.; Cusick, T.E.; Gambone, C.; Pennypacker, B.; Hodor, P.; et al. Identification of an Anabolic Selective Androgen Receptor Modulator That Actively Induces Death of Androgen-Independent Prostate Cancer Cells. J. Steroid Biochem. Mol. Biol. 2014, 143, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Loddick, S.A.; Ross, S.J.; Thomason, A.G.; Robinson, D.M.; Walker, G.E.; Dunkley, T.P.J.; Brave, S.R.; Broadbent, N.; Stratton, N.C.; Trueman, D.; et al. AZD3514: A Small Molecule That Modulates Androgen Receptor Signaling and Function in Vitro and in Vivo. Mol. Cancer Ther. 2013, 12, 1715–1727. [Google Scholar] [CrossRef]
- Yu, Z.; Cai, C.; Gao, S.; Simon, N.I.; Shen, H.C.; Balk, S.P. Galeterone Prevents Androgen Receptor Binding to Chromatin and Enhances Degradation of Mutant Androgen Receptor. Clin. Cancer Res. 2014, 20, 4075–4085. [Google Scholar] [CrossRef] [PubMed]
- Kach, J.; Long, T.M.; Selman, P.; Tonsing-Carter, E.Y.; Bacalao, M.A.; Lastra, R.R.; De Wet, L.; Comiskey, S.; Gillard, M.; VanOpstall, C.; et al. Selective Glucocorticoid Receptor Modulators (SGRMs) Delay Castrate-Resistant Prostate Cancer Growth. Mol. Cancer Ther. 2017, 16, 1680–1692. [Google Scholar] [CrossRef]
- Taplin, M.E.; Manola, J.; Oh, W.K.; Kantoff, P.W.; Bubley, G.J.; Smith, M.; Barb, D.; Mantzoros, C.; Gelmann, E.P.; Balk, S.P. A Phase II Study of Mifepristone (RU-486) in Castration-Resistant Prostate Cancer, with a Correlative Assessment of Androgen-Related Hormones. BJU Int. 2008, 101, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Bélanger, A.; Luu-The, V.; Labrie, C.; Simard, J.; Cusan, L.; Gomez, J.; Candas, B. Gonadotropin-Releasing Hormone Agonists in the Treatment of Prostate Cancer. Endocr. Rev. 2005, 26, 361–379. [Google Scholar] [CrossRef]
- Thakur, A.; Roy, A.; Ghosh, A.; Chhabra, M.; Banerjee, S. Abiraterone Acetate in the Treatment of Prostate Cancer. Biomed. Pharmacother. 2018, 101, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.D.; Ellison, S.J.; Baker, J.G.; Stagg, D.B.; Wardell, S.E.; Park, S.; Alley, H.M.; Baldi, R.M.; Yllanes, A.; Andreano, K.J.; et al. Androgen Receptor Antagonism Drives Cytochrome P450 17A1 Inhibitor Efficacy in Prostate Cancer. J. Clin. Investig. 2017, 127, 2326–2338. [Google Scholar] [CrossRef]
- Horton, J.; Rosenbaum, C.; Cummings, F.J. Tamoxifen in Advanced Prostate Cancer: An ECOG Pilot Study. Prostate 1988, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- El-Arini, M.O. Response to Tamoxifen in Drug-Resistant Prostatic Carcinoma. Lancet 1979, 2, 588. [Google Scholar] [CrossRef]
- Tong, D. Selective Estrogen Receptor Modulators Contribute to Prostate Cancer Treatment by Regulating the Tumor Immune Microenvironment. J. Immunother. Cancer 2022, 10, e002944. [Google Scholar] [CrossRef]
- Semenas, J.; Wang, T.; Sajid Syed Khaja, A.; Firoj Mahmud, A.K.M.; Simoulis, A.; Grundström, T.; Fällman, M.; Persson, J.L. Targeted Inhibition of ERα Signaling and PIP5K1α/Akt Pathways in Castration-Resistant Prostate Cancer. Mol. Oncol. 2021, 15, 968–986. [Google Scholar] [CrossRef]
- Ho, T.H.; Nunez-Nateras, R.; Hou, Y.X.; Bryce, A.H.; Northfelt, D.W.; Dueck, A.C.; Wong, B.; Stanton, M.L.; Joseph, R.W.; Castle, E.P. A Study of Combination Bicalutamide and Raloxifene for Patients With Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, 196–202.e1. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.C.; Crews, C.M. Induced Protein Degradation: An Emerging Drug Discovery Paradigm. Nat. Rev. Drug Discov. 2017, 16, 101–114. [Google Scholar] [CrossRef]
- Barton, M. Position Paper: The Membrane Estrogen Receptor GPER--Clues and Questions. Steroids 2012, 77, 935–942. [Google Scholar] [CrossRef]
- Gasent Blesa, J.M.; Alberola Candel, V. PSA Decrease with Fulvestrant Acetate in a Hormone-Resistant Metastatic Prostate Cancer Patient. Onkologie 2010, 33, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Féchon, A.; Droz, J.P. Do We Really Need New Trials on Fulvestrant in Prostate Cancer? Onkologie 2010, 33, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus Palbociclib versus Fulvestrant plus Placebo for Treatment of Hormone-Receptor-Positive, HER2-Negative Metastatic Breast Cancer That Progressed on Previous Endocrine Therapy (PALOMA-3): Final Analysis of the Multicentre, Double-Blind, Phase 3 Randomised Controlled Trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Garbán, D.C.; Deng, G.; Comin-Anduix, B.; Garcia, A.J.; Xing, Y.; Chen, H.W.; Cheung-Lau, G.; Hamilton, N.; Jung, M.E.; Pietras, R.J. Antiestrogens in Combination with Immune Checkpoint Inhibitors in Breast Cancer Immunotherapy. J. Steroid Biochem. Mol. Biol. 2019, 193, 105415. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Val Bianchi, G.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Giessrigl, B.; Schmidt, W.M.; Kalipciyan, M.; Jeitler, M.; Bilban, M.; Gollinger, M.; Krieger, S.; Jäger, W.; Mader, R.M.; Krupitza, G. Fulvestrant Induces Resistance by Modulating GPER and CDK6 Expression: Implication of Methyltransferases, Deacetylases and the HSWI/SNF Chromatin Remodelling Complex. Br. J. Cancer 2013, 109, 2751–2762. [Google Scholar] [CrossRef]
- Jung, J. Role of G Protein-Coupled Estrogen Receptor in Cancer Progression. Toxicol. Res. 2019, 35, 209–214. [Google Scholar] [CrossRef]
- Burslem, G.M.; Crews, C.M. Small-Molecule Modulation of Protein Homeostasis. Chem. Rev. 2017, 117, 11269–11301. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K.; Voisin-Chiret, A.S. Estrogen Receptor-α Targeting: PROTACs, SNIPERs, Peptide-PROTACs, Antibody Conjugated PROTACs and SNIPERs. Pharmaceutics 2022, 14, 2523. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xiang, H.; Luo, G. Targeting Estrogen Receptor α for Degradation with PROTACs: A Promising Approach to Overcome Endocrine Resistance. Eur. J. Med. Chem. 2020, 206, 112689. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Verma, R.; Ransick, A.; Stein, B.; Crews, C.M.; Deshaies, R.J. Development of Protacs to Target Cancer-Promoting Proteins for Ubiquitination and Degradation. Mol. Cell. Proteom. 2003, 2, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, T.L.; Hancock, M.; Sun, S.; Gersch, C.L.; Larios, J.M.; David, W.; Hu, J.; Hayes, D.F.; Wang, S.; Rae, J.M. Targeted Degradation of Activating Estrogen Receptor α Ligand-Binding Domain Mutations in Human Breast Cancer. Breast Cancer Res. Treat. 2020, 180, 611–622. [Google Scholar] [CrossRef]
- Savarese, D.M.; Halabi, S.; Hars, V.; Akerley, W.L.; Taplin, M.E.; Godley, P.A.; Hussain, A.; Small, E.J.; Vogelzang, N.J. Phase II Study of Docetaxel, Estramustine, and Low-Dose Hydrocortisone in Men with Hormone-Refractory Prostate Cancer: A Final Report of CALGB 9780. Cancer and Leukemia Group B. J. Clin. Oncol. 2001, 19, 2509–2516. [Google Scholar] [CrossRef]
- Puyang, X.; Furman, C.; Zheng, G.Z.; Wu, Z.J.; Banka, D.; Aithal, K.; Agoulnik, S.; Bolduc, D.M.; Buonamici, S.; Caleb, B.; et al. Discovery of Selective Estrogen Receptor Covalent Antagonists for the Treatment of ERαWT and ERαMUT Breast Cancer. Cancer Discov. 2018, 8, 1176–1193. [Google Scholar] [CrossRef] [PubMed]
- Furman, C.; Puyang, X.; Zhang, Z.; Wu, Z.J.; Banka, D.; Aithal, K.B.; Albacker, L.A.; Hao, M.H.; Irwin, S.; Kim, A.; et al. Covalent ERα Antagonist H3B-6545 Demonstrates Encouraging Preclinical Activity in Therapy-Resistant Breast Cancer. Mol. Cancer Ther. 2022, 21, 890–902. [Google Scholar] [CrossRef]
- Furman, C.; Hao, M.H.; Prajapati, S.; Reynolds, D.; Rimkunas, V.; Zheng, G.Z.; Zhu, P.; Korpal, M. Estrogen Receptor Covalent Antagonists: The Best Is Yet to Come. Cancer Res. 2019, 79, 1740–1745. [Google Scholar] [CrossRef]
- Berry, M.; Metzger, D.; Chambon, P. Role of the Two Activating Domains of the Oestrogen Receptor in the Cell-Type and Promoter-Context Dependent Agonistic Activity of the Anti-Oestrogen 4-Hydroxytamoxifen. EMBO J. 1990, 9, 2811–2818. [Google Scholar] [CrossRef]
- Pagliuca, M.; Donato, M.; D’Amato, A.L.; Rosanova, M.; Russo, A.O.M.; Scafetta, R.; De Angelis, C.; Trivedi, M.V.; André, F.; Arpino, G.; et al. New Steps on an Old Path: Novel Estrogen Receptor Inhibitors in Breast Cancer. Crit. Rev. Oncol. Hematol. 2022, 180, 103861. [Google Scholar] [CrossRef]
- Min, J.; Nwachukwu, J.C.; Min, C.K.; Njeri, J.W.; Srinivasan, S.; Rangarajan, E.S.; Nettles, C.C.; Guillen, V.S.; Ziegler, Y.; Yan, S.; et al. Dual-Mechanism Estrogen Receptor Inhibitors. Proc. Natl. Acad. Sci. USA 2021, 118, e2101657118. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Nair, B.C.; Gonugunta, V.K.; Zhang, Q.G.; Brenner, A.; Brann, D.W.; Tekmal, R.R.; Vadlamudi, R.K. Therapeutic Significance of Estrogen Receptor β Agonists in Gliomas. Mol. Cancer Ther. 2012, 11, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Schüler-Toprak, S.; Häring, J.; Inwald, E.C.; Moehle, C.; Ortmann, O.; Treeck, O. Agonists and Knockdown of Estrogen Receptor β Differentially Affect Invasion of Triple-Negative Breast Cancer Cells in Vitro. BMC Cancer 2016, 16, 951. [Google Scholar] [CrossRef]
- Ruddy, S.C.; Lau, R.; Cabrita, M.A.; McGregor, C.; McKay, B.C.; Murphy, L.C.; Wright, J.S.; Durst, T.; Pratt, M.A.C. Preferential Estrogen Receptor β Ligands Reduce Bcl-2 Expression in Hormone-Resistant Breast Cancer Cells to Increase Autophagy. Mol. Cancer Ther. 2014, 13, 1882–1893. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Moehle, C.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Effect of Estrogen Receptor β Agonists on Proliferation and Gene Expression of Ovarian Cancer Cells. BMC Cancer 2017, 17, 319. [Google Scholar] [CrossRef]
- Warner, M.; Huang, B.; Gustafsson, J.A. Estrogen Receptor β as a Pharmaceutical Target. Trends Pharmacol. Sci. 2017, 38, 92–99. [Google Scholar] [CrossRef]
- Honma, N.; Horii, R.; Iwase, T.; Saji, S.; Younes, M.; Takubo, K.; Matsuura, M.; Ito, Y.; Akiyama, F.; Sakamoto, G. Clinical Importance of Estrogen Receptor-Beta Evaluation in Breast Cancer Patients Treated with Adjuvant Tamoxifen Therapy. J. Clin. Oncol. 2008, 26, 3727–3734. [Google Scholar] [CrossRef]
- Sareddy, G.R.; Li, X.; Liu, J.; Viswanadhapalli, S.; Garcia, L.; Gruslova, A.; Cavazos, D.; Garcia, M.; Strom, A.M.; Gustafsson, J.A.; et al. Selective Estrogen Receptor β Agonist LY500307 as a Novel Therapeutic Agent for Glioblastoma. Sci. Rep. 2016, 6, 24185. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.F.; Wang, L.; Spetsieris, N.; Boukovala, M.; Efstathiou, E.; Brössner, C.; Warner, M.; Gustafsson, J.A. Estrogen Receptor β and Treatment with a Phytoestrogen Are Associated with Inhibition of Nuclear Translocation of EGFR in the Prostate. Proc. Natl. Acad. Sci. USA 2021, 118, e2011269118. [Google Scholar] [CrossRef] [PubMed]
| Isoform | KDa | Expression in Prostate | Function in Prostate Cancer | Refs. |
|---|---|---|---|---|
| ESR1 gene: Estrogen Receptor-α | ||||
| ERα-66 (full-length, often referred to as ERα) | 66–67 | Highly expressed in tumor stroma; ↑ expression in epithelium in PCa; ↑ hg-PIN; ↑ high Gleason score (GS) tumors; ↑↓ (?) CRPC. | Tumor-promoting. | [100,101,104,105,106,116,117,118] |
| ERα-46 | 46 | Expressed in normal and malignant prostate tissue. | Not described in PCa. | [128,129] |
| ERα-36 | 36 | Expressed in normal and malignant prostate tissue. | Not described in PCa. | [102,130,131,132] |
| ESR2 gene: Estrogen Receptor-β | ||||
| ERβ1 (full-length, often referred to as ERβ) | 59–60 | Mostly expressed in prostate epithelial cells; ↓ hg-PIN; ↓ localized tumors; ↓ CRPC; ↓ high Gleason score (GS) tumors. | Tumor-suppressive. | [74,101,137,143,144,145] |
| ERβ2 | 55–56 | Predominantly in the cytoplasm of prostate epithelial cells; ↑ in PCa, especially in the nucleus; ↑ metastatic PCa. | Tumor-promoting; promotes stem cell properties and the development of chemoresistance. | [137,143,144,145,146] |
| ERβ3 | 56 | Not expressed in normal and malignant prostate cells. | Not applicable. | [134,143] |
| ERβ4 | 54 | Expressed in normal and malignant prostate cells. | Not described in PCa; heterodimerizes with ERβ1 and enhances its transcriptional activity (from yeast two-hybrid and promoter luciferase assays). | [135,147] |
| ERβ5 | 53 | Expressed in basal epithelial cells in benign prostate glands; ↑ metastatic PCa. | Tumor-promoting; promotes stem cell properties and the development of chemoresistance. | [135,137,143,145,146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belluti, S.; Imbriano, C.; Casarini, L. Nuclear Estrogen Receptors in Prostate Cancer: From Genes to Function. Cancers 2023, 15, 4653. https://doi.org/10.3390/cancers15184653
Belluti S, Imbriano C, Casarini L. Nuclear Estrogen Receptors in Prostate Cancer: From Genes to Function. Cancers. 2023; 15(18):4653. https://doi.org/10.3390/cancers15184653
Chicago/Turabian StyleBelluti, Silvia, Carol Imbriano, and Livio Casarini. 2023. "Nuclear Estrogen Receptors in Prostate Cancer: From Genes to Function" Cancers 15, no. 18: 4653. https://doi.org/10.3390/cancers15184653
APA StyleBelluti, S., Imbriano, C., & Casarini, L. (2023). Nuclear Estrogen Receptors in Prostate Cancer: From Genes to Function. Cancers, 15(18), 4653. https://doi.org/10.3390/cancers15184653







