Association between Contrast-Enhanced Computed Tomography Radiomic Features, Genomic Alterations and Prognosis in Advanced Lung Adenocarcinoma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Molecular Testing and Radiomic Data
2.3. Statistical Analysis
2.3.1. Radiomic Features Reproducibility
2.3.2. Models for Actionable Gene Status Prediction
2.3.3. Models for Overall Survival Prediction
3. Results
3.1. Patient and Imaging Characteristics
3.2. Models for Actionable Gene Status Prediction
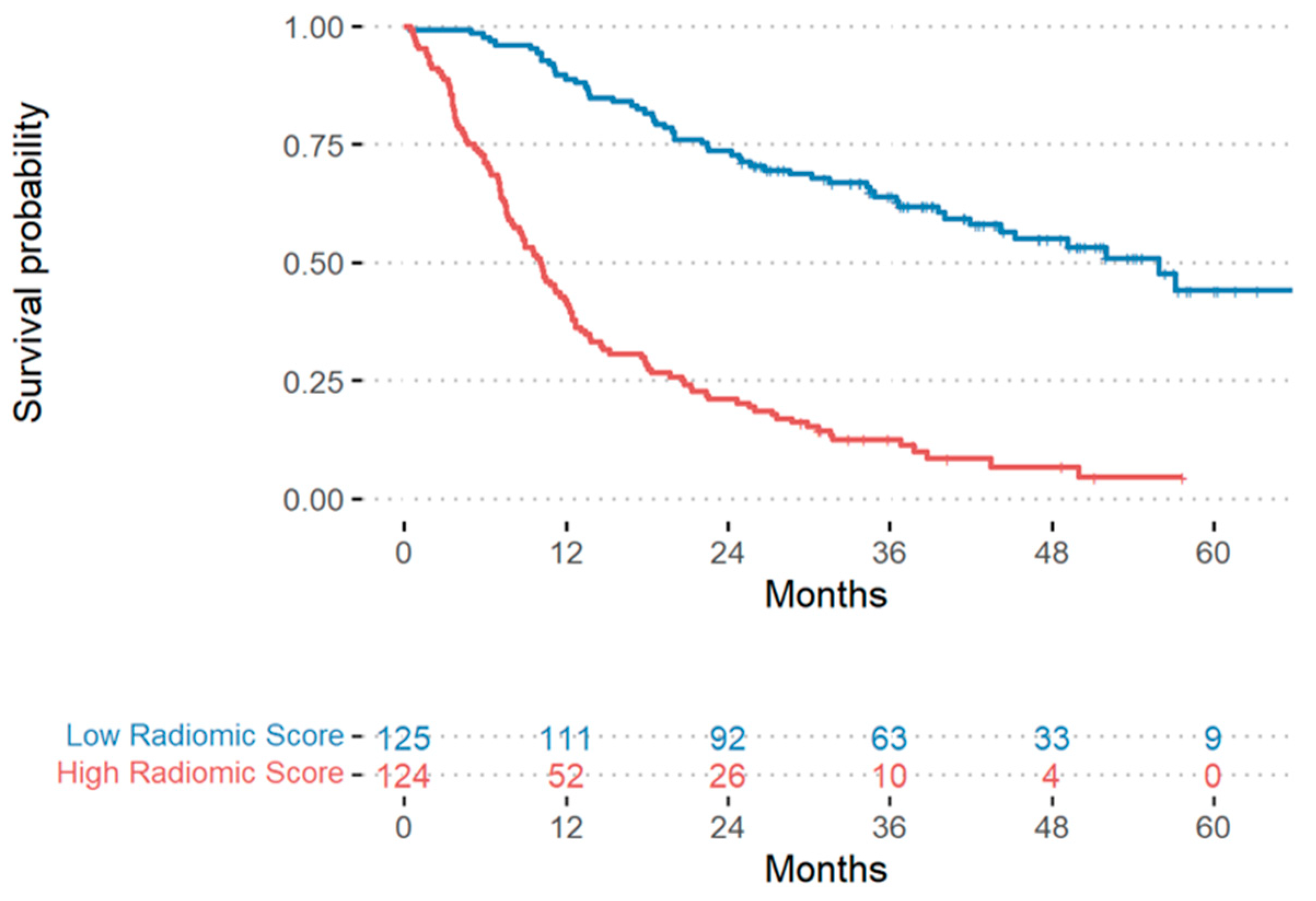
3.3. Models for Overall Survival Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cetin, K.; Ettinger, D.S.; Hei, Y.; O’Malley, C. Survival by Histologic Subtype in Stage IV Nonsmall Cell Lung Cancer Based on Data from the Surveillance, Epidemiology and End Results Program. Clin. Epidemiol. 2011, 3, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, A.; Al-Hashami, Z.; Moore, S.; Pender, A.; Wong, S.K.; Wang, Y.; Leung, B.; Wu, J.; Ho, C. Effect of Targeted Therapy and Immunotherapy on Advanced Nonsmall-Cell Lung Cancer Outcomes in the Real World. Cancer Med. 2022, 11, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Punekar, S.R.; Shum, E.; Grello, C.M.; Lau, S.C.; Velcheti, V. Immunotherapy in Non-Small Cell Lung Cancer: Past, Present, and Future Directions. Front. Oncol. 2022, 12, 877594. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef]
- Ibrahim, A.; Primakov, S.; Beuque, M.; Woodruff, H.C.; Halilaj, I.; Wu, G.; Refaee, T.; Granzier, R.; Widaatalla, Y.; Hustinx, R.; et al. Radiomics for Precision Medicine: Current Challenges, Future Prospects, and the Proposal of a New Framework. Methods 2021, 188, 20–29. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, T.; Han, M. The Application of Radiomics in Predicting Gene Mutations in Cancer. Eur. Radiol. 2022, 32, 4014–4024. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chang, C.-K.; Tu, C.-Y.; Liao, W.-C.; Wu, B.-R.; Chou, K.-T.; Chiou, Y.-R.; Yang, S.-N.; Zhang, G.; Huang, T.-C. Radiomic Features Analysis in Computed Tomography Images of Lung Nodule Classification. PLoS ONE 2018, 13, e0192002. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.; Wang, J.; Li, J.; Wang, X.; Li, B.; Xue, F.; Shao, G.; Xue, H. A Wavelet Features Derived Radiomics Nomogram for Prediction of Malignant and Benign Early-Stage Lung Nodules. Sci. Rep. 2021, 11, 22330. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shi, J.; Dong, D.; Fang, M.; Zhong, W.; Wang, K.; Wu, N.; Huang, Y.; Liu, Z.; Cheng, Y.; et al. A New Approach to Predict Progression-Free Survival in Stage IV EGFR-Mutant NSCLC Patients with EGFR-TKI Therapy. Clin. Cancer Res. 2018, 24, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Goo, J.M.; Lee, K.H.; Kim, Y.T.; Park, C.M. Preoperative CT-Based Deep Learning Model for Predicting Disease-Free Survival in Patients with Lung Adenocarcinomas. Radiology 2020, 296, 216–224. [Google Scholar] [CrossRef]
- Li, H.; Zhang, R.; Wang, S.; Fang, M.; Zhu, Y.; Hu, Z.; Dong, D.; Shi, J.; Tian, J. CT-Based Radiomic Signature as a Prognostic Factor in Stage IV ALK-Positive Non-Small-Cell Lung Cancer Treated with TKI Crizotinib: A Proof-of-Concept Study. Front. Oncol. 2020, 10, 57. [Google Scholar] [CrossRef]
- Dercle, L.; Fronheiser, M.; Lu, L.; Du, S.; Hayes, W.; Leung, D.K.; Roy, A.; Wilkerson, J.; Guo, P.; Fojo, A.T.; et al. Identification of Non–Small Cell Lung Cancer Sensitive to Systemic Cancer Therapies Using Radiomics. Clin. Cancer Res. 2020, 26, 2151–2162. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Yan, W.; Qian, W.; Pang, T.; Gong, Y.; Yang, Z. Machine Learning-Based CT Radiomics Analysis for Prognostic Prediction in Metastatic Non-Small Cell Lung Cancer Patients With EGFR-T790M Mutation Receiving Third-Generation EGFR-TKI Osimertinib Treatment. Front. Oncol. 2021, 11, 719919. [Google Scholar] [CrossRef]
- Tunali, I.; Tan, Y.; Gray, J.E.; Katsoulakis, E.; Eschrich, S.A.; Saller, J.; Aerts, H.J.W.L.; Boyle, T.; Qi, J.; Guvenis, A.; et al. Hypoxia-Related Radiomics and Immunotherapy Response: A Multicohort Study of Non-Small Cell Lung Cancer. JNCI Cancer Spectr. 2021, 5, pkab048. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Zhou, L.; Xia, W.; Zhang, R.; Wei, H.; Feng, J.; Zhao, X.; Jian, J.; Gao, X.; et al. CT-Based Radiomics Signatures Can Predict the Tumor Response of Non-Small Cell Lung Cancer Patients Treated with First-Line Chemotherapy and Targeted Therapy. Eur. Radiol. 2022, 32, 1538–1547. [Google Scholar] [CrossRef]
- Wu, W.; Parmar, C.; Grossmann, P.; Quackenbush, J.; Lambin, P.; Bussink, J.; Mak, R.; Aerts, H.J.W.L. Exploratory Study to Identify Radiomics Classifiers for Lung Cancer Histology. Front. Oncol. 2016, 6, 71. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, D.; Chen, Z.; Fang, M.; Zhang, L.; Song, J.; Yu, D.; Zang, Y.; Liu, Z.; Shi, J.; et al. Radiomic Signature as a Diagnostic Factor for Histologic Subtype Classification of Non-Small Cell Lung Cancer. Eur. Radiol. 2018, 28, 2772–2778. [Google Scholar] [CrossRef]
- Marentakis, P.; Karaiskos, P.; Kouloulias, V.; Kelekis, N.; Argentos, S.; Oikonomopoulos, N.; Loukas, C. Lung Cancer Histology Classification from CT Images Based on Radiomics and Deep Learning Models. Med. Biol. Eng. Comput. 2021, 59, 215–226. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, J.; Balagurunathan, Y.; Li, Q.; Garcia, A.L.; Stringfield, O.; Ye, Z.; Gillies, R.J. Radiomic Features Are Associated with EGFR Mutation Status in Lung Adenocarcinomas. Clin. Lung Cancer 2016, 17, 441–448.e6. [Google Scholar] [CrossRef] [PubMed]
- Rios Velazquez, E.; Parmar, C.; Liu, Y.; Coroller, T.P.; Cruz, G.; Stringfield, O.; Ye, Z.; Makrigiorgos, M.; Fennessy, F.; Mak, R.H.; et al. Somatic Mutations Drive Distinct Imaging Phenotypes in Lung Cancer. Cancer Res. 2017, 77, 3922–3930. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Luo, Y.; Wang, Y.; Gong, J. CT Texture Analysis of Lung Adenocarcinoma: Can Radiomic Features Be Surrogate Biomarkers for EGFR Mutation Statuses. Cancer Imaging 2018, 18, 52. [Google Scholar] [CrossRef]
- Yang, X.; Dong, X.; Wang, J.; Li, W.; Gu, Z.; Gao, D.; Zhong, N.; Guan, Y. Computed Tomography-Based Radiomics Signature: A Potential Indicator of Epidermal Growth Factor Receptor Mutation in Pulmonary Adenocarcinoma Appearing as a Subsolid Nodule. Oncologist 2019, 24, e1156–e1164. [Google Scholar] [CrossRef]
- Tu, W.; Sun, G.; Fan, L.; Wang, Y.; Xia, Y.; Guan, Y.; Li, Q.; Zhang, D.; Liu, S.; Li, Z. Radiomics Signature: A Potential and Incremental Predictor for EGFR Mutation Status in NSCLC Patients, Comparison with CT Morphology. Lung Cancer 2019, 132, 28–35. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, Y.; Xu, Y.; Sun, Y.; Gao, P.; Tan, M.; Ma, W.; Li, C.; Jin, L.; Hua, Y.; et al. The Potential of Radiomics Nomogram in Non-Invasively Prediction of Epidermal Growth Factor Receptor Mutation Status and Subtypes in Lung Adenocarcinoma. Front. Oncol. 2020, 9, 1485. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhu, Z.; Mao, L.; Li, X.; Han, W.; Du, H.; Wu, H.; Song, W.; Jin, Z. Clinical, Conventional CT and Radiomic Feature-Based Machine Learning Models for Predicting ALK Rearrangement Status in Lung Adenocarcinoma Patients. Front. Oncol. 2020, 10, 369. [Google Scholar] [CrossRef]
- Choe, J.; Lee, S.M.; Kim, W.; Do, K.-H.; Kim, S.; Choi, S.; Seo, J.B. CT Radiomics-Based Prediction of Anaplastic Lymphoma Kinase and Epidermal Growth Factor Receptor Mutations in Lung Adenocarcinoma. Eur. J. Radiol. 2021, 139, 109710. [Google Scholar] [CrossRef]
- Le, N.Q.K.; Kha, Q.H.; Nguyen, V.H.; Chen, Y.-C.; Cheng, S.-J.; Chen, C.-Y. Machine Learning-Based Radiomics Signatures for EGFR and KRAS Mutations Prediction in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 9254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cao, Y.; Zhang, J.; Ren, J.; Zhao, Z.; Zhang, X.; Li, S.; Deng, L.; Zhou, J. Predicting EGFR Mutation Status in Lung Adenocarcinoma: Development and Validation of a Computed Tomography-Based Radiomics Signature. Am. J. Cancer Res. 2021, 11, 546. [Google Scholar] [PubMed]
- Rossi, G.; Barabino, E.; Fedeli, A.; Ficarra, G.; Coco, S.; Russo, A.; Adamo, V.; Buemi, F.; Zullo, L.; Dono, M.; et al. Radiomic Detection of EGFR Mutations in NSCLC. Cancer Res. 2021, 81, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yang, P.; Li, J.; Peng, W.; Pu, X.; Chen, B.; Li, J.; Wang, J.; Wu, L. Computed Tomography-Based Radiomics Quantification Predicts Epidermal Growth Factor Receptor Mutation Status and Efficacy of First-Line Targeted Therapy in Lung Adenocarcinoma. Front. Oncol. 2022, 12, 985284. [Google Scholar] [CrossRef]
- El Ayachy, R.; Giraud, N.; Giraud, P.; Durdux, C.; Giraud, P.; Burgun, A.; Bibault, J.E. The Role of Radiomics in Lung Cancer: From Screening to Treatment and Follow-Up. Front. Oncol. 2021, 11, 603595. [Google Scholar] [CrossRef]
- Rinaldi, L.; De Angelis, S.P.; Raimondi, S.; Rizzo, S.; Fanciullo, C.; Rampinelli, C.; Mariani, M.; Lascialfari, A.; Cremonesi, M.; Orecchia, R.; et al. Reproducibility of Radiomic Features in CT Images of NSCLC Patients: An Integrative Analysis on the Impact of Acquisition and Reconstruction Parameters. Eur. Radiol. Exp. 2022, 6, 2. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The Facts and the Challenges of Image Analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Fumagalli, C.; Vacirca, D.; Rappa, A.; Passaro, A.; Guarize, J.; Raviele, P.R.; de Marinis, F.; Spaggiari, L.; Casadio, C.; Viale, G.; et al. The Long Tail of Molecular Alterations in Non-Small Cell Lung Cancer: A Single-Institution Experience of next-Generation Sequencing in Clinical Molecular Diagnostics. J. Clin. Pathol. 2018, 71, 767–773. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Rinaldi, L.; Pezzotta, F.; Santaniello, T.; De Marco, P.; Bianchini, L.; Origgi, D.; Cremonesi, M.; Milani, P.; Mariani, M.; Botta, F. HeLLePhant: A Phantom Mimicking Non-Small Cell Lung Cancer for Texture Analysis in CT Images. Phys. Med. 2022, 97, 13–24. [Google Scholar] [CrossRef]
- Tsao, A.S.; Tang, X.M.; Sabloff, B.; Xiao, L.; Shigematsu, H.; Roth, J.; Spitz, M.; Hong, W.K.; Gazdar, A.; Wistuba, I. Clinicopathologic Characteristics of the EGFR Gene Mutation in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2006, 1, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical Features and Outcome of Patients with Non-Small-Cell Lung Cancer Who Harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Dacic, S.; Shuai, Y.; Yousem, S.; Ohori, P.; Nikiforova, M. Clinicopathological Predictors of EGFR/KRAS Mutational Status in Primary Lung Adenocarcinomas. Mod. Pathol. 2010, 23, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, T.; Lee, S.-H.; Choi, Y.-L.; Han, J. Clinicopathologic Characteristics of EGFR, KRAS, and ALK Alterations in 6,595 Lung Cancers. Oncotarget 2016, 7, 23874–23884. [Google Scholar] [CrossRef] [PubMed]
- Ninatti, G.; Kirienko, M.; Neri, E.; Sollini, M.; Chiti, A. Imaging-Based Prediction of Molecular Therapy Targets in NSCLC by Radiogenomics and AI Approaches: A Systematic Review. Diagnostics 2020, 10, 359. [Google Scholar] [CrossRef]
- Bosc, C.; Ferretti, G.R.; Cadranel, J.; Audigier-Valette, C.; Besse, B.; Barlesi, F.; Decroisette, C.; Lantuejoul, S.; Arbib, F.; Moro-Sibilot, D. Rebiopsy during Disease Progression in Patients Treated by TKI for Oncogene-Addicted NSCLC. Targ. Oncol. 2015, 10, 247–253. [Google Scholar] [CrossRef]
- Murray, S.; Karavasilis, V.; Bobos, M.; Razis, E.; Papadopoulos, S.; Christodoulou, C.; Kosmidis, P.; Fountzilas, G. Molecular Predictors of Response to Tyrosine Kinase Inhibitors in Patients with Non-Small-Cell Lung Cancer. J. Exp. Clin. Cancer Res. 2012, 31, 77. [Google Scholar] [CrossRef]
- Dietel, M.; Bubendorf, L.; Dingemans, A.-M.C.; Dooms, C.; Elmberger, G.; García, R.C.; Kerr, K.M.; Lim, E.; López-Ríos, F.; Thunnissen, E.; et al. Diagnostic Procedures for Non-Small-Cell Lung Cancer (NSCLC): Recommendations of the European Expert Group. Thorax 2016, 71, 177–184. [Google Scholar] [CrossRef]
- Trédan, O.; Wang, Q.; Pissaloux, D.; Cassier, P.; de la Fouchardière, A.; Fayette, J.; Desseigne, F.; Ray-Coquard, I.; de la Fouchardière, C.; Frappaz, D.; et al. Molecular Screening Program to Select Molecular-Based Recommended Therapies for Metastatic Cancer Patients: Analysis from the ProfiLER Trial. Ann. Oncol. 2019, 30, 757–765. [Google Scholar] [CrossRef]
- Malapelle, U.; Tiseo, M.; Vivancos, A.; Kapp, J.; Serrano, M.J.; Tiemann, M. Liquid Biopsy for Biomarker Testing in Non-Small Cell Lung Cancer: A European Perspective. J. Mol. Pathol. 2021, 2, 255–273. [Google Scholar] [CrossRef]
- Cucchiara, F.; Petrini, I.; Romei, C.; Crucitta, S.; Lucchesi, M.; Valleggi, S.; Scavone, C.; Capuano, A.; De Liperi, A.; Chella, A.; et al. Combining Liquid Biopsy and Radiomics for Personalized Treatment of Lung Cancer Patients. State of the Art and New Perspectives. Pharmacol. Res. 2021, 169, 105643. [Google Scholar] [CrossRef]
- Moreno, S.; Bonfante, M.; Zurek, E.; Cherezov, D.; Goldgof, D.; Hall, L.; Schabath, M. A Radiogenomics Ensemble to Predict EGFR and KRAS Mutations in NSCLC. Tomography 2021, 7, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Shiri, I.; Amini, M.; Nazari, M.; Hajianfar, G.; Haddadi Avval, A.; Abdollahi, H.; Oveisi, M.; Arabi, H.; Rahmim, A.; Zaidi, H. Impact of Feature Harmonization on Radiogenomics Analysis: Prediction of EGFR and KRAS Mutations from Non-Small Cell Lung Cancer PET/CT Images. Comput. Biol. Med. 2022, 142, 105230. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, G.; Pereira, T.; Dias, C.; Freitas, C.; Hespanhol, V.; Costa, J.L.; Cunha, A.; Oliveira, H.P. Identifying Relationships between Imaging Phenotypes and Lung Cancer-Related Mutation Status: EGFR and KRAS. Sci. Rep. 2020, 10, 3625. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Peng, H.; Xue, T.; Li, J.; Ge, Y.; Wang, G.; Feng, F. Prediction and Verification of Survival in Patients with Non-Small-Cell Lung Cancer Based on an Integrated Radiomics Nomogram. Clin. Radiol. 2022, 77, e222–e230. [Google Scholar] [CrossRef]
- Luna, J.M.; Barsky, A.R.; Shinohara, R.T.; Roshkovan, L.; Hershman, M.; Dreyfuss, A.D.; Horng, H.; Lou, C.; Noël, P.B.; Cengel, K.A.; et al. Radiomic Phenotypes for Improving Early Prediction of Survival in Stage III Non-Small Cell Lung Cancer Adenocarcinoma after Chemoradiation. Cancers 2022, 14, 700. [Google Scholar] [CrossRef]
- Hou, K.-Y.; Chen, J.-R.; Wang, Y.-C.; Chiu, M.-H.; Lin, S.-P.; Mo, Y.-H.; Peng, S.-C.; Lu, C.-F. Radiomics-Based Deep Learning Prediction of Overall Survival in Non-Small-Cell Lung Cancer Using Contrast-Enhanced Computed Tomography. Cancers 2022, 14, 3798. [Google Scholar] [CrossRef]
- Ferrante, M.; Rinaldi, L.; Botta, F.; Hu, X.; Dolp, A.; Minotti, M.; De Piano, F.; Funicelli, G.; Volpe, S.; Bellerba, F.; et al. Application of NnU-Net for Automatic Segmentation of Lung Lesions on CT Images and Its Implication for Radiomic Models. J. Clin. Med. 2022, 11, 7334. [Google Scholar] [CrossRef]
- Mendoza, D.P.; Stowell, J.; Muzikansky, A.; Shepard, J.-A.O.; Shaw, A.T.; Digumarthy, S.R. Computed tomography imaging characteristics of non-small-cell-lung cancer with anaplastic lymphoma kinase rearrangements: A systematic review and meta-analysis. Clin. Lung Cancer 2019, 20, 339. [Google Scholar] [CrossRef]
- Rizzo, S.; Petrella, F.; Buscarino, V.; De Maria, F.; Raimondi, S.; Barberis, M.; Fumagalli, C.; Spitaleri, G.; Rampinelli, C.; De Marinis, F.; et al. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in Non-Small cell Lung Cancer. Eur. Radiol. 2016, 26, 32. [Google Scholar] [CrossRef]
- Mak, R.H.; Digumarthy, S.R.; Muzikansky, A.; Engelman, J.A.; Shepard, J.-A.O.; Choi, N.C.; Sequist, L.V. Role of 18F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor in non-small-cell lung cancer. Oncologist 2011, 16, 319. [Google Scholar] [CrossRef]
- Passaro, A.; Attili, I.; Rappa, A.; Vacirca, D.; Ranghiero, A.; Fumagalli, C.; Guarize, J.; Spaggiari, L.; de Marinis, F.; Barberis, M.; et al. Genomic Characterization of Concurrent Alterations in Non-Small Cell Lung Cancer (NSCLC) Harboring Actionable Mutations. Cancers 2021, 13, 2172. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
| Dataset 1 | Dataset 2 | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | p-Value 1 | |
| Tumor volume (cm3) | 55.4 (± 99.5) | 62.4 (±116.0) | 0.90 |
| Age (years) | 65.9 (±10.4) | 65.1 (±12.0) | 0.59 |
| N/Total (%) | N/Total (%) | p-value 1 | |
| Sex | |||
| Female | 97/261 (37.2%) | 20/48 (41.7%) | 0.55 |
| Male | 164/261 (62.8%) | 28/48 (58.3%) | |
| Smoking history 2 | 0.55 | ||
| current smoker | 58/252 (23.0%) | 9/47 (19.2%) | |
| ex-smoker | 137/252 (54.4%) | 24/47 (51.1%) | |
| never-smoker | 57/252 (22.6%) | 14/47 (29.8%) | |
| Initial lesion site and side 3 | 0.04 | ||
| Lower-right | 41/256 (16.0%) | 8/48 (16.7%) | |
| Lower-left | 30/256 (11.7%) | 6/48 (12.5%) | |
| Medium-right | 8/256 (3.1%) | 2/48 (4.2%) | |
| Upper-right | 87/256 (33.9%) | 18/48 (37.5%) | |
| Upper-left | 80/256 (31.1%) | 8/48 (16.7%) | |
| Mixed | 10/256 (3.9%) | 6/48 (12.5%) | |
| Stage | 0.001 | ||
| not-IV | 102/261 (39.0%) | 7/48 (14.6%) | |
| IV | 159/261 (60.9%) | 41/48 (85.4%) | |
| Gene alteration status | |||
| EGFR mutation-positive | 52/261 (19.9%) | 14/48 (29.2%) | 0.15 |
| ALK rearrangement-positive | 22/261 (8.4%) | 3/48 (6.25%) | 0.78 |
| KRAS mutation-positive | 106/261 (40.6%) | 16/48 (33.3%) | 0.34 |
| Others (=no alterations in the three investigated driver genes) | 81/261 (31.0%) | 15/48 (31.2%) | 0.98 |
| Prediction | Model | AUC (95% CI) Training | AUC (95% CI) Validation |
|---|---|---|---|
| EGFR+ | Radiomic | 0.97 (0.95, 0.99) | 0.78 (0.65, 0.91) |
| Clinical | 0.82 (0.76, 0.87) | 0.85 (0.74, 0.95) | |
| Clinical-Radiomic | 0.98 (0.96, 0.99) | 0.86 (0.75, 0.96) | |
| KRAS+ | Radiomic | 0.98 (0.97, 0.99) | 0.64 (0.48, 0.80) |
| Clinical | 0.70 (0.64, 0.76) | 0.62 (0.45, 0.79) | |
| Clinical-Radiomic | 0.98 (0.97, 0.99) | 0.61 (0.46, 0.77) | |
| ALK+ | Radiomic | 0.95 (0.92, 0.97) | 0.59 (0.29, 0.90) |
| Clinical | 0.89 (0.80, 0.95) | 0.60 (0.23, 0.97) | |
| Clinical-Radiomic | 0.98 (0.96, 0.99) | 0.65 (0.31, 1.00) |
| Model | C-Index | C-Index Cross-Validation Median (IQR) |
|---|---|---|
| Radiomic | 0.78 | 0.79 (0.71–0.87) |
| Clinical | 0.64 | 0.63 (0.52–0.72) |
| Clinical-Radiomic | 0.80 | 0.80 (0.73–0.87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, L.; Guerini Rocco, E.; Spitaleri, G.; Raimondi, S.; Attili, I.; Ranghiero, A.; Cammarata, G.; Minotti, M.; Lo Presti, G.; De Piano, F.; et al. Association between Contrast-Enhanced Computed Tomography Radiomic Features, Genomic Alterations and Prognosis in Advanced Lung Adenocarcinoma Patients. Cancers 2023, 15, 4553. https://doi.org/10.3390/cancers15184553
Rinaldi L, Guerini Rocco E, Spitaleri G, Raimondi S, Attili I, Ranghiero A, Cammarata G, Minotti M, Lo Presti G, De Piano F, et al. Association between Contrast-Enhanced Computed Tomography Radiomic Features, Genomic Alterations and Prognosis in Advanced Lung Adenocarcinoma Patients. Cancers. 2023; 15(18):4553. https://doi.org/10.3390/cancers15184553
Chicago/Turabian StyleRinaldi, Lisa, Elena Guerini Rocco, Gianluca Spitaleri, Sara Raimondi, Ilaria Attili, Alberto Ranghiero, Giulio Cammarata, Marta Minotti, Giuliana Lo Presti, Francesca De Piano, and et al. 2023. "Association between Contrast-Enhanced Computed Tomography Radiomic Features, Genomic Alterations and Prognosis in Advanced Lung Adenocarcinoma Patients" Cancers 15, no. 18: 4553. https://doi.org/10.3390/cancers15184553
APA StyleRinaldi, L., Guerini Rocco, E., Spitaleri, G., Raimondi, S., Attili, I., Ranghiero, A., Cammarata, G., Minotti, M., Lo Presti, G., De Piano, F., Bellerba, F., Funicelli, G., Volpe, S., Mora, S., Fodor, C., Rampinelli, C., Barberis, M., De Marinis, F., Jereczek-Fossa, B. A., ... Botta, F. (2023). Association between Contrast-Enhanced Computed Tomography Radiomic Features, Genomic Alterations and Prognosis in Advanced Lung Adenocarcinoma Patients. Cancers, 15(18), 4553. https://doi.org/10.3390/cancers15184553









