Reducing the Invasiveness of Low- and High-Grade Endometrial Cancers in Both Primary Human Cancer Biopsies and Cell Lines by the Inhibition of Aquaporin-1 Channels
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pharmacological Agents
2.2. Human EC Biopsy Collection and Primary Cell Culture
2.3. EC Cell Line Cultures and Steroid Hormone Treatments
2.4. Immunohistochemistry for Human EC Biopsy Tissues
2.5. Immunofluorescence for Cell Lines
2.6. Quantitative PCR Analyses for EC Cell Lines
2.7. Analyses of Viability in EC Cell Lines
2.8. Transwell Invasion in EC Biopsy Cells and Cell Lines
2.9. Western Blot
2.10. The Kaplan–Meier Survival Analysis
2.11. Statistical Analyses
3. Results
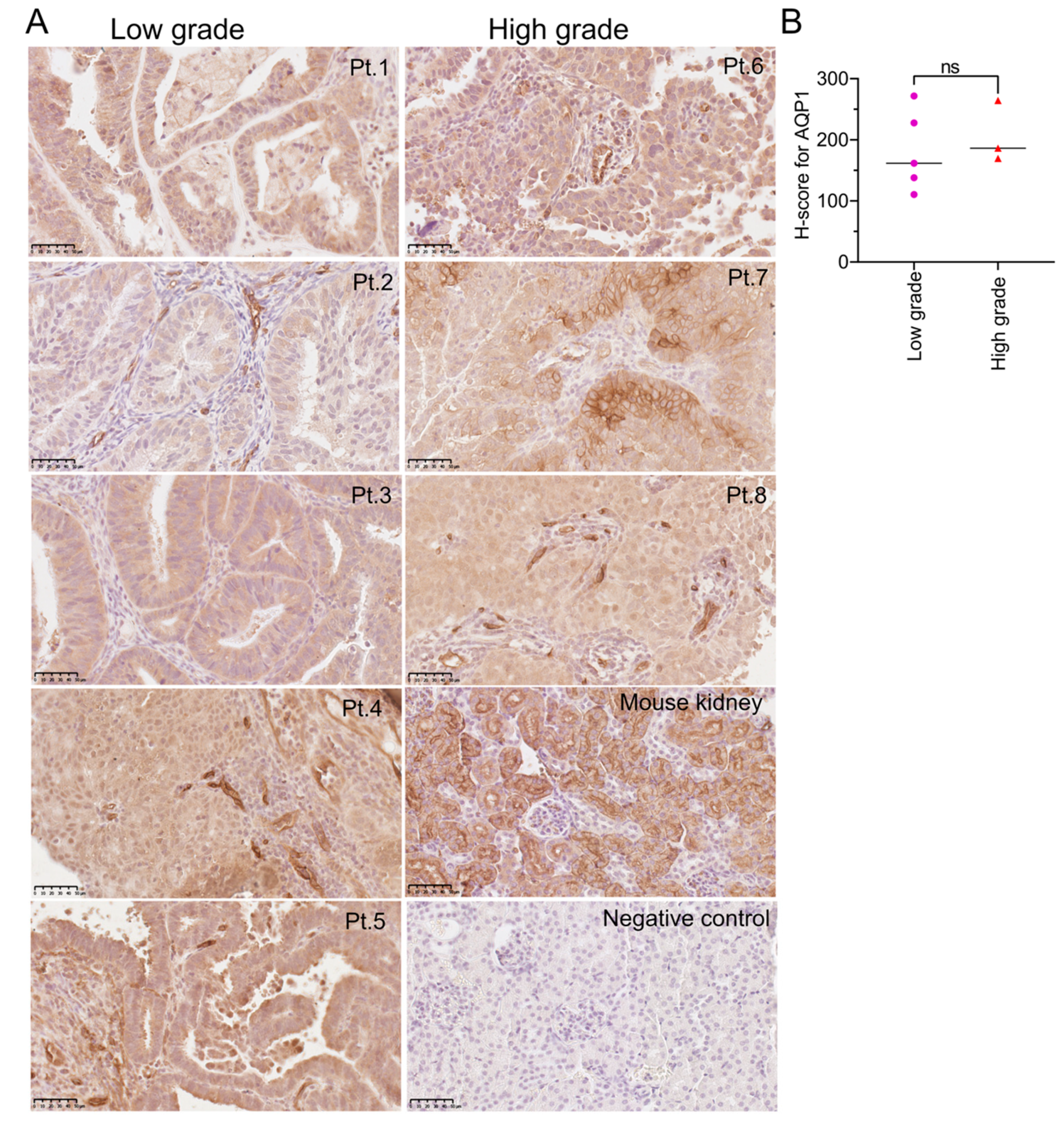
3.1. AQP1 Is Highly Expressed in Both Low- and High-Grade EC Tissues
3.2. High AQP 1, 4 and 11 Transcript Levels Are Related to Poor Prognoses in Grade 3 EC
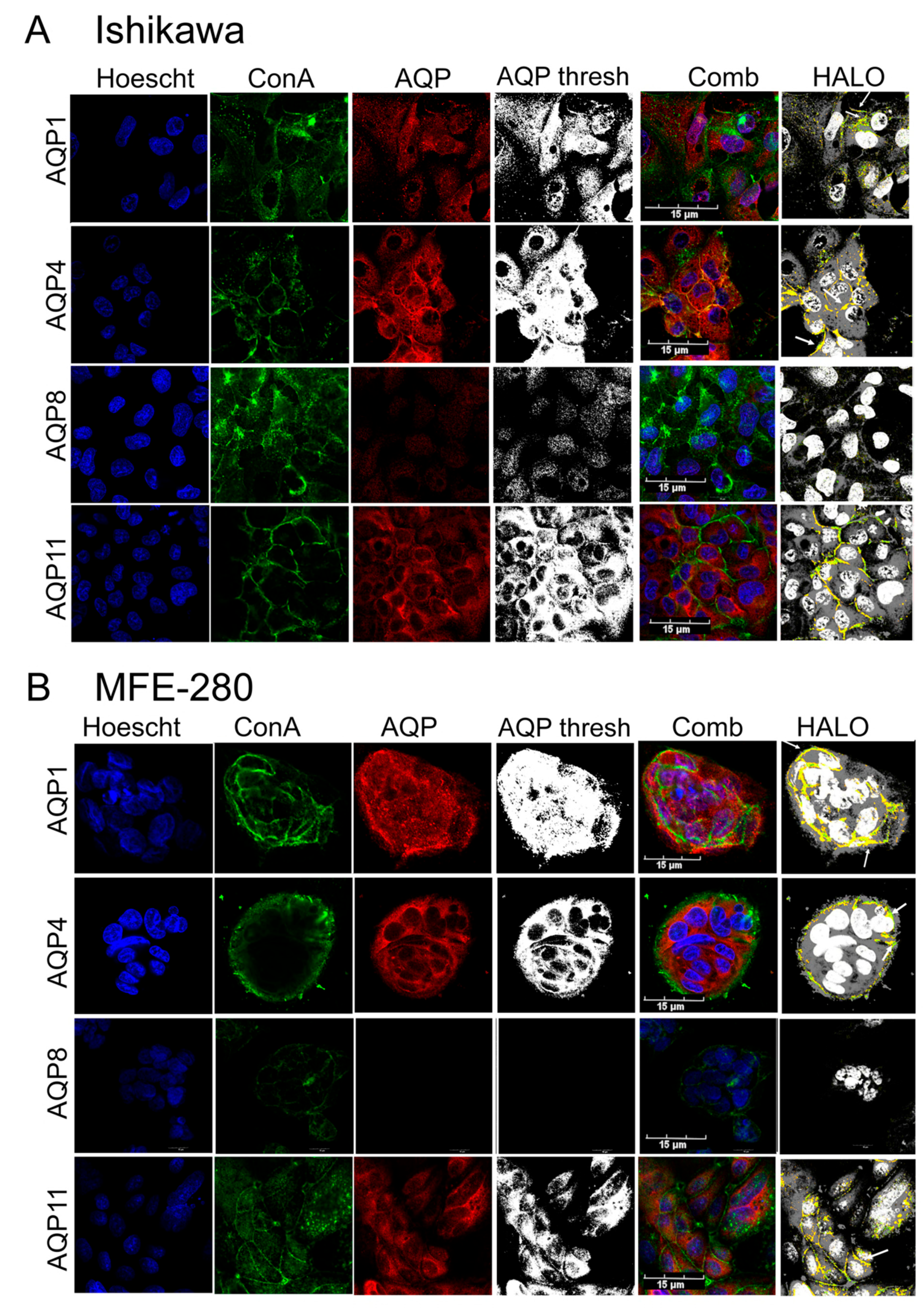
3.3. Plasma Membrane Expression of AQPs 1, 4, and 11 in Ishikawa and MFE-280 Cells
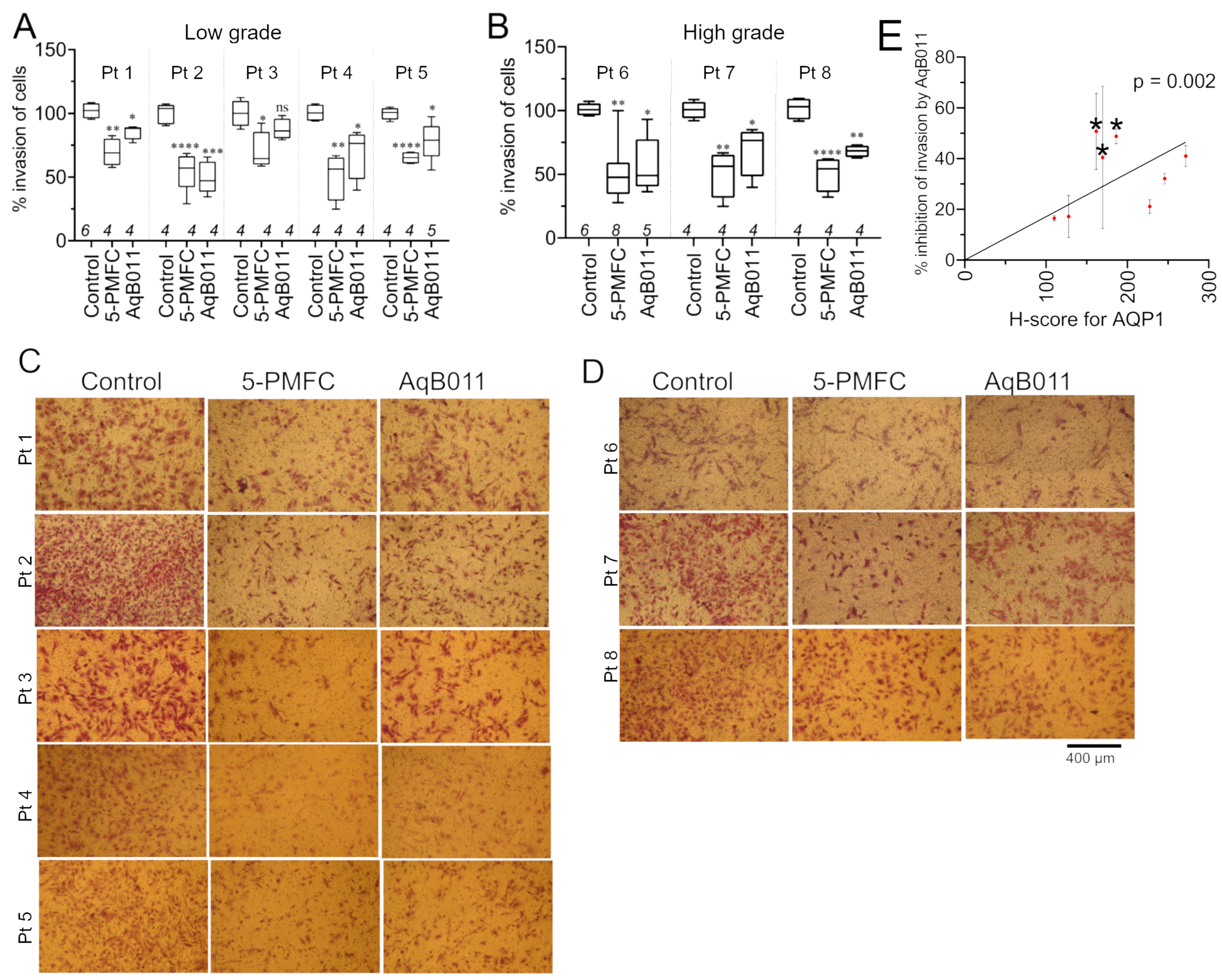
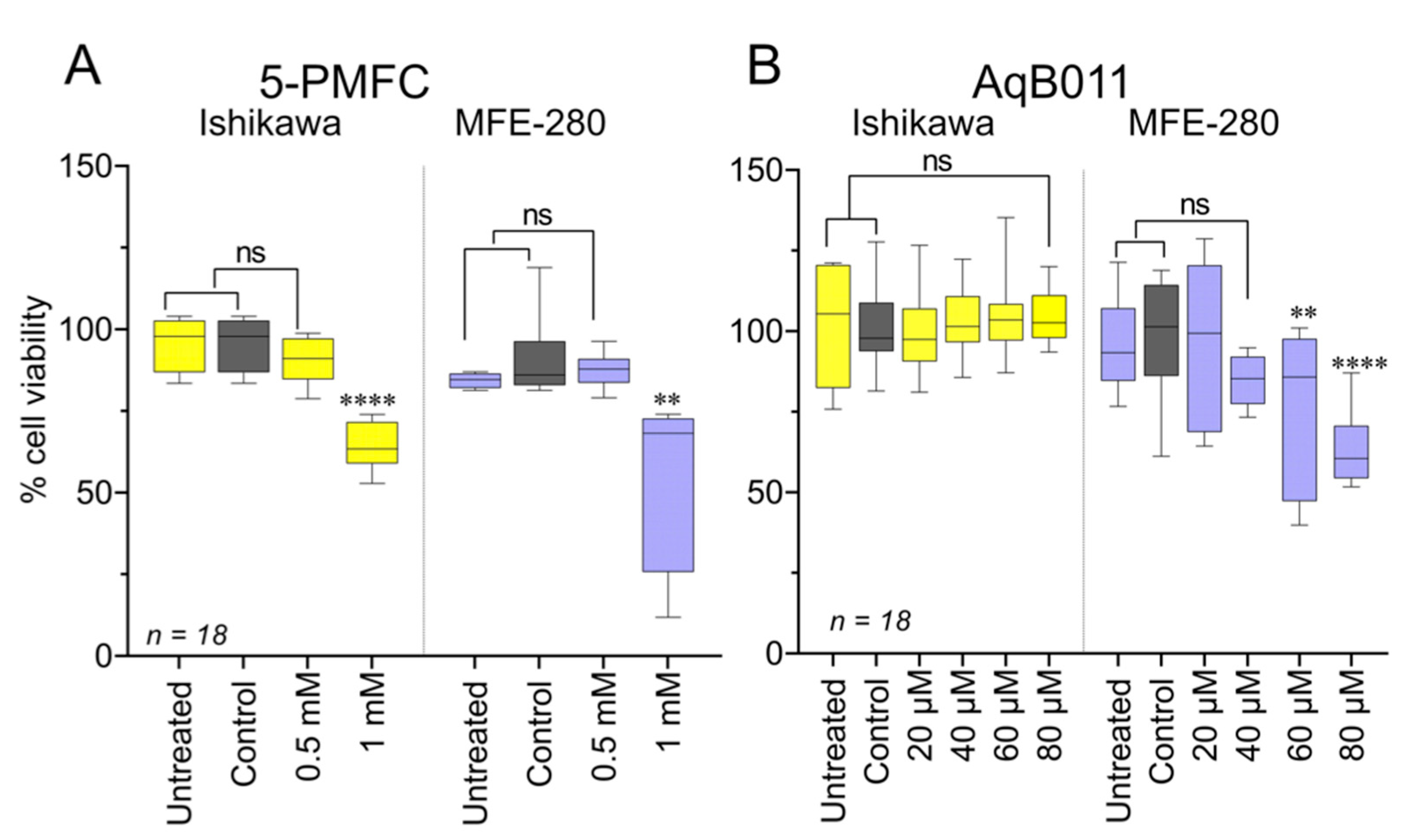
3.4. Comparison of Pharmacological Modulator Effects in Reducing Invasiveness of EC Cell Lines
3.5. AQP1 Ion Channel Inhibitors Restrained Invasiveness of Primary EC Cells
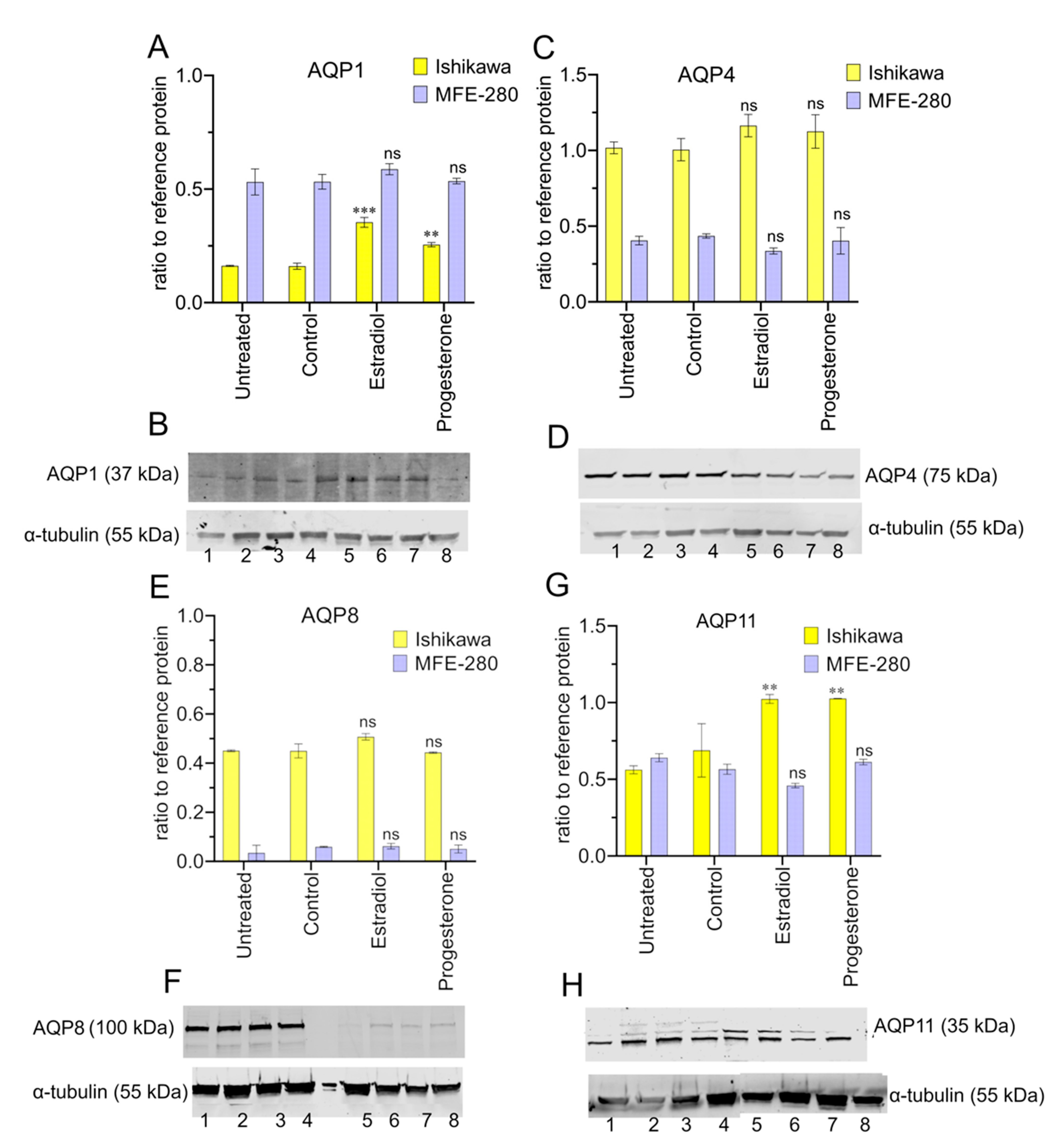
3.6. Aquaporin Expression (RNA and Protein) in Ishikawa and MFE-280 Cells Is Regulated by Estradiol and Progesterone
3.7. Effects of Pharmacological Treatments on EC Cell Viability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International patterns and trends in endometrial cancer incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Abdol Manap, N.; Ng, B.K.; Phon, S.E.; Abdul Karim, A.K.; Lim, P.S.; Fadhil, M. Endometrial Cancer in Pre-Menopausal Women and Younger: Risk Factors and Outcome. Int. J. Environ. Res. Public Health 2022, 19, 9059. [Google Scholar] [CrossRef] [PubMed]
- Pennant, M.; Mehta, R.; Moody, P.; Hackett, G.; Prentice, A.; Sharp, S.; Lakshman, R. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ricciardelli, C.; Yool, A.J. Targeting aquaporins in novel therapies for male and female breast and reproductive cancers. Cells 2021, 10, 215. [Google Scholar] [CrossRef]
- De Ieso, M.L.; Yool, A.J. Mechanisms of aquaporin-facilitated cancer invasion and metastasis. Front. Chem. 2018, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Kitchen, P.; Yool, A.J.; Bill, R.M. Recent breakthroughs and future directions in drugging aquaporins. Trends Pharmacol. Sci. 2022, 43, 30–42. [Google Scholar] [CrossRef]
- Pan, X.-Y.; Guo, H.; Han, J.; Hao, F.; An, Y.; Xu, Y.; Xiaokaiti, Y.; Pan, Y.; Li, X.-J. Ginsenoside Rg3 attenuates cell migration via inhibition of aquaporin 1 expression in PC-3M prostate cancer cells. Eur. J. Pharmacol. 2012, 683, 27–34. [Google Scholar] [CrossRef]
- Jiang, X.X.; Xu, K.H.; Ma, J.Y.; Tian, Y.H.; Guo, X.Y.; Lin, J.; Wu, R.J. Reduced migration of Ishikawa cells associated with downregulation of aquaporin-5. Oncol. Lett. 2012, 4, 257–261. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Yool, A.J.; Pei, J.V.; Townsend, A.R.; Hardingham, J.E. Stereoselective anti-cancer activities of ginsenoside Rg3 on triple negative breast cancer cell models. Pharmaceuticals 2019, 12, 117. [Google Scholar] [CrossRef]
- Kourghi, M.; Pei, J.V.; De Ieso, M.L.; Flynn, G.; Yool, A.J. Bumetanide Derivatives AqB007 and AqB011 Selectively Block the Aquaporin-1 Ion Channel Conductance and Slow Cancer Cell Migration. Mol. Pharmacol. 2016, 89, 133–140. [Google Scholar] [CrossRef] [PubMed]
- De Ieso, M.L.; Pei, J.V.; Nourmohammadi, S.; Smith, E.; Chow, P.H.; Kourghi, M.; Hardingham, J.E.; Yool, A.J. Combined pharmacological administration of AQP1 ion channel blocker AqB011 and water channel blocker Bacopaside II amplifies inhibition of colon cancer cell migration. Sci. Rep. 2019, 9, 12635. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.; Unger, L.; Salman, M.M.; Kitchen, P.; Bill, R.M.; Yool, A.J. Signaling mechanisms and pharmacological modulators governing diverse aquaporin functions in human health and disease. Int. J. Mol. Sci. 2022, 23, 1388. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Raihan, T.; Ahmed, J.; Hakim, A.; Emon, T.H.; Chowdhury, P.A. Human aquaporins: Functional diversity and potential roles in infectious and non-infectious diseases. Front. Genet. 2021, 12, 654865. [Google Scholar] [CrossRef] [PubMed]
- Tyerman, S.D.; McGaughey, S.A.; Qiu, J.; Yool, A.J.; Byrt, C.S. Adaptable and multifunctional ion-conducting aquaporins. Annu. Rev. Plant Biol. 2021, 72, 703–736. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Sun, C.-C.; Zhou, C.-Y.; Huang, H.-F. Expression of aquaporin-1 in normal, hyperplasic, and carcinomatous endometria. Int. J. Gynecol. Obstet. 2008, 101, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.-B.; Zhang, R.-J.; Tan, Y.-J.; Ding, G.-L.; Shi, S.; Zhang, D.; He, R.-H.; Liu, A.-X.; Wang, T.-T.; Leung, P.C. Identification of estrogen response element in the aquaporin-2 gene that mediates estrogen-induced cell migration and invasion in human endometrial carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, E1399–E1408. [Google Scholar] [CrossRef]
- Watanabe, T.; Sato, K.; Kono, T.; Yamagishi, Y.; Kumazawa, F.; Miyamoto, M.; Takano, M.; Tsuda, H. Aquaporin 3 Expression in Endometrioid Carcinoma of the Uterine Body Correlated With Early Stage and Lower Grade. Pathol. Oncol. Res. 2020, 26, 2247–2253. [Google Scholar] [CrossRef]
- Kourghi, M.; Pei, J.V.; De Ieso, M.L.; Nourmohammadi, S.; Chow, P.H.; Yool, A.J. Fundamental structural and functional properties of Aquaporin ion channels found across the kingdoms of life. Clin. Exp. Pharmacol. Physiol. 2018, 45, 401–409. [Google Scholar] [CrossRef]
- Yu, J.; Yool, A.J.; Schulten, K.; Tajkhorshid, E. Mechanism of gating and ion conductivity of a possible tetrameric pore in aquaporin-1. Structure 2006, 14, 1411–1423. [Google Scholar] [CrossRef]
- Suarez, A.A.; Felix, A.S.; Cohn, D.E. Bokhman Redux: Endometrial cancer “types” in the 21st century. Gynecol. Oncol. 2017, 144, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B.; Brewer, M.; Boruta, D.; Herzog, T.J.; Shahin, F.A. Endometrial cancer: A review and current management strategies: Part II. Gynecol. Oncol. 2014, 134, 393–402. [Google Scholar] [CrossRef]
- Khan, S.; Wardill, H.R.; Bowen, J. Role of toll-like receptor 4 (TLR4)-mediated interleukin-6 (IL-6) production in chemotherapy-induced mucositis. Cancer Chemother. Pharmacol. 2018, 82, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K.; Yahata, H.; Yoshikawa, H.; Konishi, I.; Yasugi, T.; Saito, T.; Nakanishi, T.; Sasaki, H.; Saji, F.; Iwasaka, T. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J. Clin. Oncol. 2007, 25, 2798–2803. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xiang, Y.; Mu, S.-M.; Li, T.; Yu, H.-M.; Li, X.-J. Effects of acetazolamide and anordiol on osmotic water permeability in AQP1-cRNA injected Xenopus oocyte. Acta Pharmacol. Sin. 2004, 25, 90–97. [Google Scholar] [PubMed]
- Huber, V.J.; Tsujita, M.; Yamazaki, M.; Sakimura, K.; Nakada, T. Identification of arylsulfonamides as Aquaporin 4 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1270–1273. [Google Scholar] [CrossRef]
- Chow, P.H.; Yool, A.J. Dose-Dependent Inhibition of Colon Cancer Cell Migration by a Natural Medical Plant Extract, KeenMind, Targeting Human AQP1 Water Channel Activity. Ph.D. Thesis, The University of Adelaide, Adelaide, SA, Australia, 2020; p. 146. [Google Scholar]
- Kourghi, M.; De Ieso, M.L.; Nourmohammadi, S.; Pei, J.V.; Yool, A.J. Identification of loop D domain amino acids in the human Aquaporin-1 channel involved in activation of the ionic conductance and inhibition by AqB011. Front. Chem. 2018, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.H.; Cox, C.D.; Pei, J.V.; Anabaraonye, N.; Nourmohammadi, S.; Henderson, S.W.; Martinac, B.; Abdulmalik, O.; Yool, A.J. Inhibition of the aquaporin-1 cation conductance by selected furan compounds reduces red blood cell sickling. Front. Pharmacol. 2022, 12, 794791. [Google Scholar] [CrossRef]
- Chow, P.H.; Kourghi, M.; Pei, J.V.; Nourmohammadi, S.; Yool, A.J. 5-hydroxymethyl-furfural and structurally related compounds block the ion conductance in human aquaporin-1 channels and slow cancer cell migration and invasion. Mol. Pharmacol. 2020, 98, 38–48. [Google Scholar] [CrossRef]
- Guo, C.; Wu, T.; Zhu, H.; Gao, L. Aquaporin 4 blockade attenuates acute lung injury through inhibition of Th17 cell proliferation in mice. Inflammation 2019, 42, 1401–1412. [Google Scholar] [CrossRef]
- Li, J.; Jia, Z.; Xu, W.; Guo, W.; Zhang, M.; Bi, J.; Cao, Y.; Fan, Z.; Li, G. TGN-020 alleviates edema and inhibits astrocyte activation and glial scar formation after spinal cord compression injury in rats. Life Sci. 2019, 222, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, Q.; Chen, K.; Wang, Y.; Chen, L.; Li, X. Curcumin suppresses migration and invasion of human endometrial carcinoma cells. Oncol. Lett. 2015, 10, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Cao, C.; Lu, S.; Kivlin, R.; Amaral, A.; Kouttab, N.; Yang, H.; Chu, W.; Bi, Z.; Di, W. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother. Pharmacol. 2008, 62, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tan, C.; Liu, Y.; Liu, X.; Wang, X.; Gui, Y.; Qin, L.; Deng, F.; Yu, Z.; Hu, C. Resveratrol ameliorates oxidative stress and inhibits aquaporin 4 expression following rat cerebral ischemia-reperfusion injury. Mol. Med. Rep. 2015, 12, 7756–7762. [Google Scholar] [CrossRef] [PubMed]
- Sueblinvong, T.; Ghebre, R.; Iizuka, Y.; Pambuccian, S.E.; Isaksson Vogel, R.; Skubitz, A.P.; Bazzaro, M. Establishment, characterization and downstream application of primary ovarian cancer cells derived from solid tumors. PLoS ONE 2012, 7, e50519. [Google Scholar] [CrossRef] [PubMed]
- Menz, A.; Gorbokon, N.; Viehweger, F.; Lennartz, M.; Hube-Magg, C.; Hornsteiner, L.; Kluth, M.; Völkel, C.; Luebke, A.M.; Fraune, C.; et al. Pan-keratin Immunostaining in Human Tumors: A Tissue Microarray Study of 15,940 Tumors. Int. J. Surg. Pathol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Dembinski, T.C.; Leung, C.K.; Shiu, R.P. Evidence for a novel pituitary factor that potentiates the mitogenic effect of estrogen in human breast cancer cells. Cancer Res. 1985, 45, 3083–3089. [Google Scholar]
- Nishida, M.; Kasahara, K.; Kaneko, M.; Iwasaki, H.; Hayashi, K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 1103–1111. [Google Scholar]
- Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Hackenberg, R.; Hawighorst, T.; Hild, F.; Schulz, K.-D. Establishment of new epithelial carcinoma cell lines by blocking monolayer formation. J. Cancer Res. Clin. Oncol. 1997, 123, 669–673. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Lokman, N.A.; Pyragius, C.E.; Ween, M.P.; Macpherson, A.M.; Ruszkiewicz, A.; Hoffmann, P.; Oehler, M.K. Keratin 5 overexpression is associated with serous ovarian cancer recurrence and chemotherapy resistance. Oncotarget 2017, 8, 17819. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Pellavio, G.; Martinotti, S.; Patrone, M.; Ranzato, E.; Laforenza, U. Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells. Cells 2022, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Glynn, M.W.; McAllister, A.K. Immunocytochemistry and quantification of protein colocalization in cultured neurons. Nat. Protoc. 2006, 1, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Willets, J.M.; Brown, L.; Lambert, D.G.; McDonald, J.; Davies, Q.; Moss, E.L.; Konje, J.C. Validation of endogenous control reference genes for normalizing gene expression studies in endometrial carcinoma. Mol. Hum. Reprod. 2015, 21, 723–735. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Selection of Endogenous Control Reference Genes for Studies on Type 1 or Type 2 Endometrial Cancer. Sci. Rep. 2020, 10, 8468. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. Methods Mol. Biol. 2015, 1250, 333–348. [Google Scholar] [CrossRef]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res./Rev. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef]
- Henderson, S.W.; Nakayama, Y.; Whitelaw, M.L.; Bruning, J.B.; Anderson, P.A.; Tyerman, S.D.; Ramesh, S.A.; Martinac, B.; Yool, A.J. Proteoliposomes reconstituted with human aquaporin-1 reveal novel single-ion-channel properties. Biophys. Rep. 2023, 3, 100100. [Google Scholar] [CrossRef]
- Hnasko, T.S.; Hnasko, R.M. The Western Blot. In ELISA: Methods and Protocols; Hnasko, R., Ed.; Springer: New York, NY, USA, 2015; pp. 87–96. [Google Scholar]
- Faoro, V.; Stanta, G. Membrane Stripping. In Guidelines for Molecular Analysis in Archive Tissues; Springer: Berlin/Heidelberg, Germany, 2011; pp. 277–278. [Google Scholar]
- Miller, L. Analyzing gels and western blots with ImageJ. Lukemiller. org Miscellaneous Topics Vaguely Related to Science. 2010. Available online: https://lukemiller.org/index.php/2010/11/analyzing-gels-and-western-blots-with-image-j/ (accessed on 4 September 2023).
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet. Res. 2021, 23, e27633. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.W.; Mayne, C.G.; Dharmarajan, V.; Carlson, K.E.; Martin, T.A.; Novick, S.J.; Toy, W.; Green, B.; Panchamukhi, S.; Katzenellenbogen, B.S. Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. Elife 2016, 5, e12792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Q.; Wang, Y.; Peng, W.; Cai, H. Effects of curcumin on ion channels and transporters. Front. Physiol. 2014, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Varricchio, A.; Ricciardelli, C.; Yool, A.J. Invasiveness of endometrial cancer cell lines is potentiated by estradiol and blocked by a traditional medicine Guizhi Fuling at clinically relevant doses. Front. Oncol. 2022, 12, 1015708. [Google Scholar] [CrossRef] [PubMed]
- García, F.; Kierbel, A.; Larocca, M.C.; Gradilone, S.A.; Splinter, P.; LaRusso, N.F.; Marinelli, R.A. The water channel aquaporin-8 is mainly intracellular in rat hepatocytes, and its plasma membrane insertion is stimulated by cyclic AMP. J. Biol. Chem. 2001, 276, 12147–12152. [Google Scholar] [CrossRef]
- Mader, S.; Brimberg, L.; Vo, A.; Strohl, J.J.; Crawford, J.M.; Bonnin, A.; Carrión, J.; Campbell, D.; Huerta, T.S.; La Bella, A. In utero exposure to maternal anti–aquaporin-4 antibodies alters brain vasculature and neural dynamics in male mouse offspring. Sci. Transl. Med. 2022, 14, eabe9726. [Google Scholar] [CrossRef]
- Hemley, S.J.; Bilston, L.E.; Cheng, S.; Chan, J.N.; Stoodley, M.A. Aquaporin-4 expression in post-traumatic syringomyelia. J. Neurotrauma 2013, 30, 1457–1467. [Google Scholar] [CrossRef]
- Stael, S.; Miller, L.P.; Fernández-Fernández, Á.D.; Breusegem, F.V. Detection of Damage-Activated Metacaspase Activity by Western Blot in Plants. In Plant Proteases and Plant Cell Death; Springer: Berlin/Heidelberg, Germany, 2022; pp. 127–137. [Google Scholar]
- Kourghi, M.; Nourmohammadi, S.; Pei, J.V.; Qiu, J.; McGaughey, S.; Tyerman, S.D.; Byrt, C.S.; Yool, A.J. Divalent cations regulate the ion conductance properties of diverse classes of aquaporins. Int. J. Mol. Sci. 2017, 18, 2323. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2576–2583. [Google Scholar] [CrossRef]
- Yool, A.J.; Weinstein, A.M. New roles for old holes: Ion channel function in aquaporin-1. Physiology 2002, 17, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.D.; Kwon, I.-K.; Wang, R. cGMP-dependent protein kinases as potential targets for colon cancer prevention and treatment. Future Med. Chem. 2010, 2, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, X.; Li, X.; Xu, G.; Bai, Y.; Wu, J.; Piao, Y.; Shi, Y.; Xiang, R.; Wang, L. Nucleotide de novo synthesis increases breast cancer stemness and metastasis via cGMP-PKG-MAPK signaling pathway. PLoS Biol. 2020, 18, e3000872. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, J.S.; Yang, H. Upregulation of lncRNA DARS-AS1 accelerates tumor malignancy in cervical cancer by activating cGMP-PKG pathway. J. Biochem. Mol. Toxicol 2021, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Campbell, E.M. Structure, function and translational relevance of aquaporin dual water and ion channels. Mol. Asp. Med. 2012, 33, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.F.B.; Ko, Y.-A.; Ghosh, A.; Syed, S.M.; Ius, Y.; O’Sullivan, R.; Netherton, J.K.; Baker, M.A.; Nahar, P.; Jaaback, K. Proteomic and functional characterization of intra-tumor heterogeneity in human endometrial cancer. Cell Rep. Med. 2022, 3, 100738. [Google Scholar] [CrossRef] [PubMed]
- Supernat, A.; Łapińska-Szumczyk, S.; Majewska, H.; Gulczyński, J.; Biernat, W.; Wydra, D.; Żaczek, A.J. Tumor heterogeneity at protein level as an independent prognostic factor in endometrial cancer. Transl. Oncol. 2014, 7, 613–619. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Fukao, Y. Discordance between protein and transcript levels detected by selected reaction monitoring. Plant Signal. Behav. 2015, 10, e1017697. [Google Scholar] [CrossRef]
- Xiang, Y.; Ma, B.; Li, T.; Gao, J.-W.; Yu, H.-M.; Li, X.-J. Acetazolamide inhibits aquaporin-1 protein expression and angiogenesis. Acta Pharmacol. Sin. 2004, 25, 812–816. [Google Scholar] [PubMed]
- Sirohi, V.K.; Popli, P.; Sankhwar, P.; Kaushal, J.B.; Gupta, K.; Manohar, M.; Dwivedi, A. Curcumin exhibits anti-tumor effect and attenuates cellular migration via Slit-2 mediated down-regulation of SDF-1 and CXCR4 in endometrial adenocarcinoma cells. J. Nutr. Biochem. 2017, 44, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PLoS ONE 2014, 9, e102535. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shang, X.; Wu, H.; Gautam, S.C.; Al-Holou, S.; Li, C.; Kuo, J.; Zhang, L.; Chopp, M. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J. Exp. Ther. Oncol. 2009, 8, 25. [Google Scholar] [PubMed]
- Kaneuchi, M.; Sasaki, M.; Tanaka, Y.; Yamamoto, R.; Sakuragi, N.; Dahiya, R. Resveratrol suppresses growth of Ishikawa cells through down-regulation of EGF. Int. J. Oncol. 2003, 23, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen signaling in endometrial cancer: A key oncogenic pathway with several open questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Zhou, J.; Shi, S.; Xu, H.-Y.; Qu, F.; Zhang, D.; Chen, Y.-D.; Yang, J.; Huang, H.-F.; Sheng, J.-Z. Identification of estrogen response element in aquaporin-3 gene that mediates estrogen-induced cell migration and invasion in estrogen receptor-positive breast cancer. Sci. Rep. 2015, 5, 12484. [Google Scholar] [CrossRef]
- Zou, L.-B.; Shi, S.; Zhang, R.-J.; Wang, T.-T.; Tan, Y.-J.; Zhang, D.; Fei, X.-Y.; Ding, G.-L.; Gao, Q.; Chen, C. Aquaporin-1 plays a crucial role in estrogen-induced tubulogenesis of vascular endothelial cells. J. Clin. Endocrinol. Metab. 2013, 98, E672–E682. [Google Scholar] [CrossRef]
- Krakhmal, N.V.; Zavyalova, M.; Denisov, E.; Vtorushin, S.; Perelmuter, V. Cancer invasion: Patterns and mechanisms. Acta Naturae 2015, 7, 17–28. [Google Scholar] [CrossRef]
- Berens, E.B.; Holy, J.M.; Riegel, A.T.; Wellstein, A. A cancer cell spheroid assay to assess invasion in a 3D setting. J. Vis. Exp. 2015, 105, e53409. [Google Scholar]
- Frede, J.; Fraser, S.P.; Oskay-Özcelik, G.; Hong, Y.; Braicu, E.I.; Sehouli, J.; Gabra, H.; Djamgoz, M.B. Ovarian cancer: Ion channel and aquaporin expression as novel targets of clinical potential. Eur. J. Cancer 2013, 49, 2331–2344. [Google Scholar] [CrossRef] [PubMed]
- Otterbach, F.; Callies, R.; Adamzik, M.; Kimmig, R.; Siffert, W.; Schmid, K.W.; Bankfalvi, A. Aquaporin 1 (AQP1) expression is a novel characteristic feature of a particularly aggressive subgroup of basal-like breast carcinomas. Breast Cancer Res. Treat. 2010, 120, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Q.; Yang, Z.F.; Wang, K.J.; Feng, X.Y.; Lv, Z.J.; Li, Y.; Jian, Z.X. AQP8 inhibits colorectal cancer growth and metastasis by down-regulating PI3K/AKT signaling and PCDH7 expression. Am. J. Cancer Res. 2018, 8, 266. [Google Scholar] [PubMed]
- Jablonski, E.M.; Mattocks, M.A.; Sokolov, E.; Koniaris, L.G.; Hughes, F.M., Jr.; Fausto, N.; Pierce, R.H.; McKillop, I.H. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007, 250, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-H.; Tuokan, T.; Lin, C.; Chang, H. Aquaporin 8 involvement in human cervical cancer SiHa migration via the EGFR-Erk1/2 pathway. Asian Pac. J. Cancer Prev. 2014, 15, 6391–6395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, J.; Zhou, C.; Yang, J.; Ding, X.; Zhu, Y.; Chen, X. Expression of AQP6 and AQP8 in epithelial ovarian tumor. J. Mol. Histol. 2016, 47, 129–134. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.J.; Kalbfleisch, T.; Srivastava, S.; Pan, J.; Rai, S.; Petras, R.E.; Ronquillo, N.; Polk, H.C., Jr.; Galandiuk, S. Decreased tumoral expression of colon-specific water channel aquaporin 8 is associated with reduced overall survival in colon adenocarcinoma. Dis. Colon Rectum 2021, 64, 1083–1095. [Google Scholar] [CrossRef]
- Sharpnack, M.; Carbone, D.P.; Dikov, M.M.; Tchekneva, E.E. Aquaporin 11 as a new predictive biomarker of overall survival and platinum-based chemotherapy response in lung adenocarcinoma patients. Cancer Res. 2018, 78, 2620. [Google Scholar] [CrossRef]
- Neri, M.; Peiretti, M.; Melis, G.B.; Piras, B.; Vallerino, V.; Paoletti, A.M.; Madeddu, C.; Scartozzi, M.; Mais, V. Systemic therapy for the treatment of endometrial cancer. Expert Opin. Pharmacother. 2019, 20, 2019–2032. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Zhang, X.D.; Hondermarck, H.; Tanwar, P.S. The Emerging Role of the Microenvironment in Endometrial Cancer. Cancers 2018, 10, 408. [Google Scholar] [CrossRef]
- Jaroch, K.; Jaroch, A.; Bojko, B. Cell cultures in drug discovery and development: The need of reliable in vitro-in vivo extrapolation for pharmacodynamics and pharmacokinetics assessment. J. Pharm. Biomed. Anal. 2018, 147, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From donor to the lab: A fascinating journey of primary cell lines. Front. Cell Dev. Biol. 2021, 9, 711381. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.-Y.; Leem, S.-H.; Lee, J.H.; Kim, H.S. Dual relationship between stromal cells and immune cells in the tumor microenvironment. Front. Immunol. 2022, 13, 864739. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Cang, H.; Guo, B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019, 8, 4709–4721. [Google Scholar] [CrossRef]
- Montiel, V.; Bella, R.; Michel, L.Y.M.; Esfahani, H.; De Mulder, D.; Robinson, E.L.; Deglasse, J.P.; Tiburcy, M.; Chow, P.H.; Jonas, J.C.; et al. Inhibition of aquaporin-1 prevents myocardial remodeling by blocking the transmembrane transport of hydrogen peroxide. Sci. Transl. Med. 2020, 12, eaay2176. [Google Scholar] [CrossRef]
- Varricchio, A.; Khan, S.; Price, Z.K.; Davis, R.A.; Ramesh, S.A.; Yool, A.J. Pharmacological Inhibition of Membrane Signaling Mechanisms Reduces the Invasiveness of U87-MG and U251-MG Glioblastoma Cells In Vitro. Cancers 2023, 15, 1027. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Choi, S.-H.; Hwang, S.-H.; Kim, H.-J.; Lee, J.-H.; Nah, S.-Y. Resveratrol inhibits GABACρ receptor-mediated ion currents expressed in xenopus oocytes. Korean J. Physiol. Pharmacol. 2013, 17, 175–180. [Google Scholar] [CrossRef]
- Vancauwenberghe, E.; Noyer, L.; Derouiche, S.; Lemonnier, L.; Gosset, P.; Sadofsky, L.R.; Mariot, P.; Warnier, M.; Bokhobza, A.; Slomianny, C. Activation of mutated TRPA1 ion channel by resveratrol in human prostate cancer associated fibroblasts (CAF). Mol. Carcinog. 2017, 56, 1851–1867. [Google Scholar] [CrossRef]
- Wei, S.; Liu, T.T.; Hu, W.P.; Qiu, C.Y. Resveratrol inhibits the activity of acid-sensing ion channels in male rat dorsal root ganglion neurons. J. Neurosci. Res. 2022, 100, 1755–1764. [Google Scholar] [CrossRef]




| Compound | Structure | Dose |
|---|---|---|
| Acetazolamide | 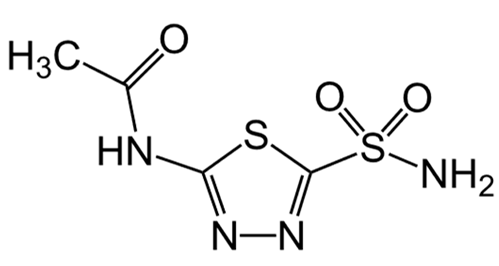 | 1–100 µM [25,26] |
| Ginsenoside (Rg3) | 100 µM [8,10] | |
| Bacopaside II (KeenMind) | 44 µM [27] | |
| AqB011 | 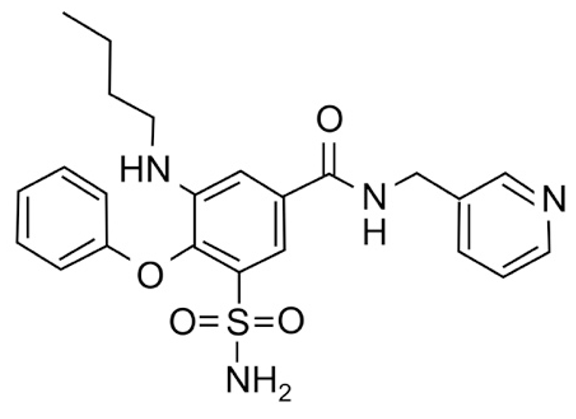 | 80 µM [11,28] |
| 5-HMF | 1 mM [29,30] | |
| 5-PMFC | 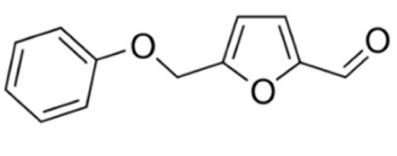 | 0.5 mM [29,30] |
| IMD-0354 |  | 0.2 µM [13] |
| TGN-020 | 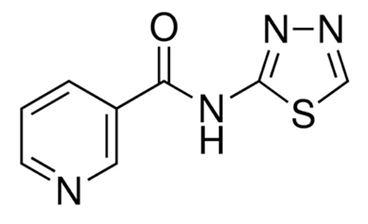 | 3 µM [32] |
| Curcumin | 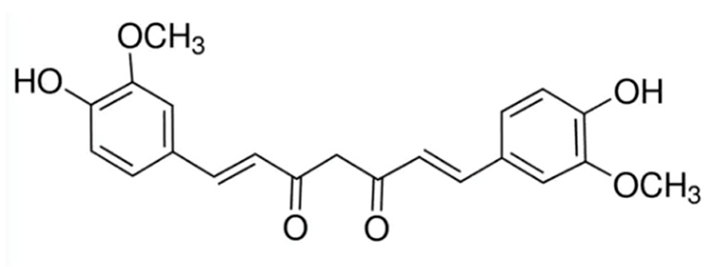 | 20 µM [33,34] |
| Resveratrol | 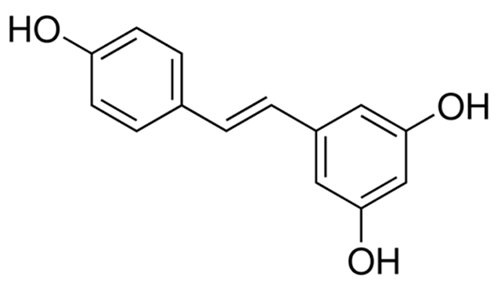 | 40 µM [35] |
| Gene | Primer Sequence | Product Size * | TM °C | Gene ID Number |
|---|---|---|---|---|
| AQP0 | F = CTAGCACTCAACACGTTGCAC | 210 | 60.1 | NM_012064.4 |
| R = AGGATTCATGCCTGCACCAG | 60.4 | |||
| AQP1 | F = CCTTGGACACCTCCTGGCTATTG | 199 | 56.7 | NM_198098.4 |
| R = CTTCACGCGGTCTGTGAGGT | 60.0 | |||
| AQP2 | F = ATGGCGTTTGGCTTGGGTAT | 200 | 60.3 | NM_000486.6 |
| R = GATGTCTGCTGGCGTGATCT | 60.2 | |||
| AQP3 | F = ACCAGCTTTTTGTTTCGGGC | 111 | 59.9 | NM_004925.5 |
| R = AGGCTGTGCCTATGAACTGGT | 61.5 | |||
| AQP4 | F = CCTCGCTGGTGGCCTTTATGA | 207 | 62.5 | NM_001650.7 |
| R = GTCTTTCCCCTTCTTCTCCTCTCC | 61.9 | |||
| AQP5 | F = CCACCTTGTCGGAATCTACTT | 205 | 57.4 | NM_001651.4 |
| R = TTTGATGATGGCCACACGC | 59.1 | |||
| AQP6 | F = CCATCATCATTGGGAAGTTCACAG | 251 | 59.8 | NM_001652.4 |
| R = GCGTAGGCTGTTTCACACACTCTC | 63.9 | |||
| AQP7 | F = CACAGGCGGTCCACCC | 109 | 59.6 | NM_001170.3 |
| R = TCATGAACTCGGCCAGGAAC | 60.0 | |||
| AQP8 | F = ATGTCTGGTCGAACTGCTGG | 231 | 60.0 | NM_001169.3 |
| R = CAGTACGGGAGGAGCATCAC | 60.0 | |||
| AQP9 | F = ATCGTGGGAGAAAATGCAAC | 196 | 58.0 | NM_020980.5 |
| R = CAATAATCAGGAGGCCGATG | 58.0 | |||
| AQP10 | F = TGCAGTGACAGTGTGCCTAT | 178 | 59.3 | NM_080429.3 |
| R = TGGGTGAGGAGCATGAGTACA | 60.3 | |||
| AQP11 | F = TCCGAGTCGACTTGCTCAAA | 165 | 59.3 | NM_173039.3 |
| R = CAGCTCCTGTTAGACTTCCTCC | 59.8 | |||
| AQP12a | F = CCTGCTCTTCCTGCTCTTCC | 102 | 60.1 | NM_198998.3 |
| R = AGAGACTGCTCGGCCATGA | 60.7 | |||
| AQP12b | F = TCTTTGCCACCTTCACCCTC | 136 | 59.9 | NM_001102467.2 |
| R = GTCCTCATCTCCAGGAAGCA | 58.8 | |||
| IPO8 | F = GGTGGGGTGTGAGGTAATCC | 201 | 59.7 | NM_006390.4 |
| R = ACTGGTTGAGCTCGTTCTCG | 60.0 | |||
| PSMC4 | F = TGGAGGTGCAGGAGGAATACA | 162 | 60.6 | NM_006503.4 |
| R = CTGTGGTAGAGCCCACGATG | 60.2 |
| Gene | Grade 1 HR (95% Confidence Interval) | Grade 3 HR (95% Confidence Interval) |
|---|---|---|
| AQP1 | HR = 0 (0−Inf) | HR = 1.81 (1.08−3.05) |
| logrank P = 0.4 NS | logrank P = 0.024 * | |
| AQP2 | HR = not determined (>108) | HR = 0.55 (0.3–1.01) |
| logrank P = 0.35 NS | logrank P = 0.05 NS | |
| AQP3 | HR = not determined (>108) | HR = 0.44 (0.41–1.08) |
| logrank P = 0.048 NS | logrank P = 0.094 NS | |
| AQP4 | HR = 0 (0−Inf) | HR = 2.16 (1.33−3.51) |
| logrank P = 0.021 NS | logrank P = 0.0014 * | |
| AQP5 | HR = 0 (0−Inf) | HR = 0.68 (0.4–1.17) |
| logrank P = 0.2 NS | logrank P = 0.16 NS | |
| AQP8 | HR = 0.23 (0.01−3.7) | HR = 1.5 (0.94−2.41) |
| logrank P = 0.26 NS | logrank P = 0.09 NS | |
| AQP11 | HR = not determined (>108) | HR = 1.93 (1.19−3.12) |
| logrank P = 0.1 NS | logrank P = 0.0066 * | |
| AQP12A | HR = 3.31 (0.21–53.07) | HR = 1.84 (1.13–2.9) |
| logrank P = 0.37 NS | logrank P = 0.012 NS | |
| AQP12B | HR = 2.8 (0.17–44.86) | HR = 0.75 (0.46–1.23) |
| logrank P = 0.45 NS | logrank P = 0.26 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Lokman, N.A.; Oehler, M.K.; Ricciardelli, C.; Yool, A.J. Reducing the Invasiveness of Low- and High-Grade Endometrial Cancers in Both Primary Human Cancer Biopsies and Cell Lines by the Inhibition of Aquaporin-1 Channels. Cancers 2023, 15, 4507. https://doi.org/10.3390/cancers15184507
Khan S, Lokman NA, Oehler MK, Ricciardelli C, Yool AJ. Reducing the Invasiveness of Low- and High-Grade Endometrial Cancers in Both Primary Human Cancer Biopsies and Cell Lines by the Inhibition of Aquaporin-1 Channels. Cancers. 2023; 15(18):4507. https://doi.org/10.3390/cancers15184507
Chicago/Turabian StyleKhan, Sidra, Noor A. Lokman, Martin K. Oehler, Carmela Ricciardelli, and Andrea J. Yool. 2023. "Reducing the Invasiveness of Low- and High-Grade Endometrial Cancers in Both Primary Human Cancer Biopsies and Cell Lines by the Inhibition of Aquaporin-1 Channels" Cancers 15, no. 18: 4507. https://doi.org/10.3390/cancers15184507
APA StyleKhan, S., Lokman, N. A., Oehler, M. K., Ricciardelli, C., & Yool, A. J. (2023). Reducing the Invasiveness of Low- and High-Grade Endometrial Cancers in Both Primary Human Cancer Biopsies and Cell Lines by the Inhibition of Aquaporin-1 Channels. Cancers, 15(18), 4507. https://doi.org/10.3390/cancers15184507






