Role of Prehabilitation and Rehabilitation on Functional Recovery and Quality of Life in Thyroid Cancer Patients: A Comprehensive Review
Abstract
Simple Summary
Abstract
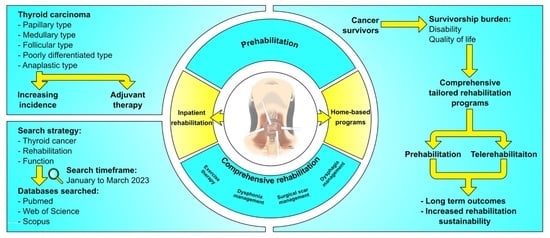
1. Introduction
2. Research Methodology
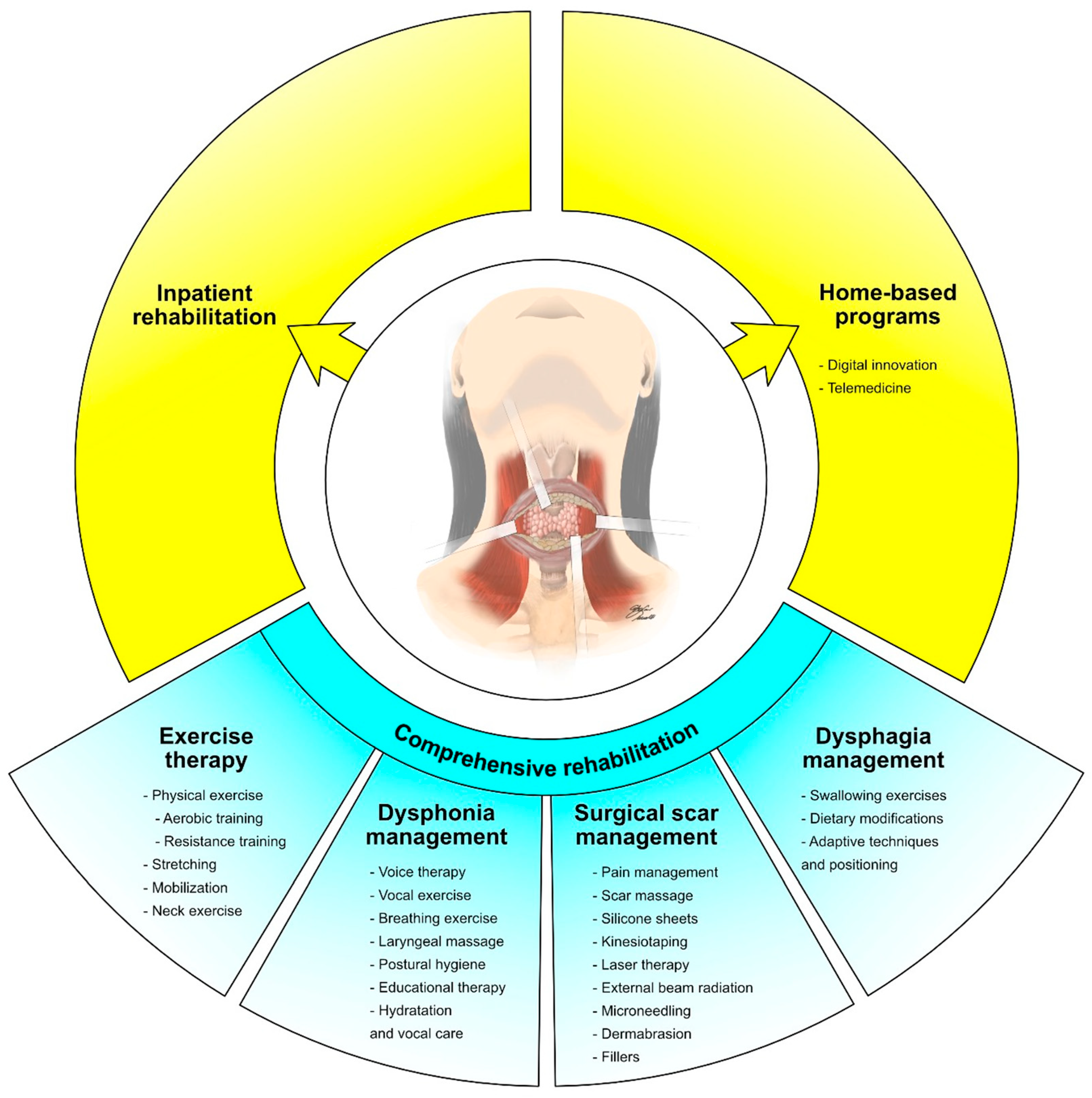
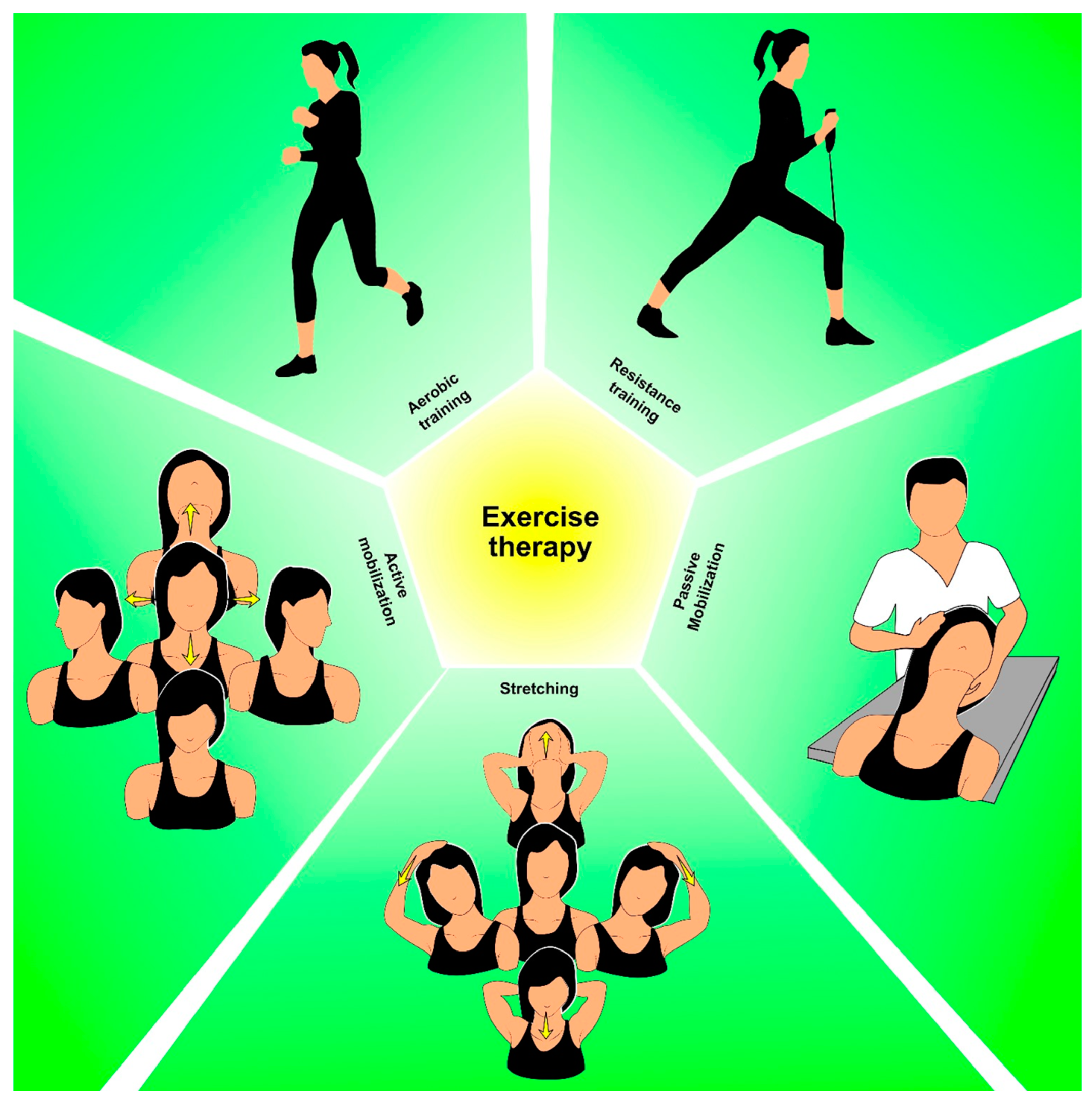
3. Physical Exercise and Rehabilitation
3.1. Role of Physical Exercise in the Rehabilitation of Thyroid Cancer Survivors
3.2. Considerations for Designing Exercise Programs
4. Neck Disorders and Rehabilitation
Rehabilitation Strategies for Neck Disorders
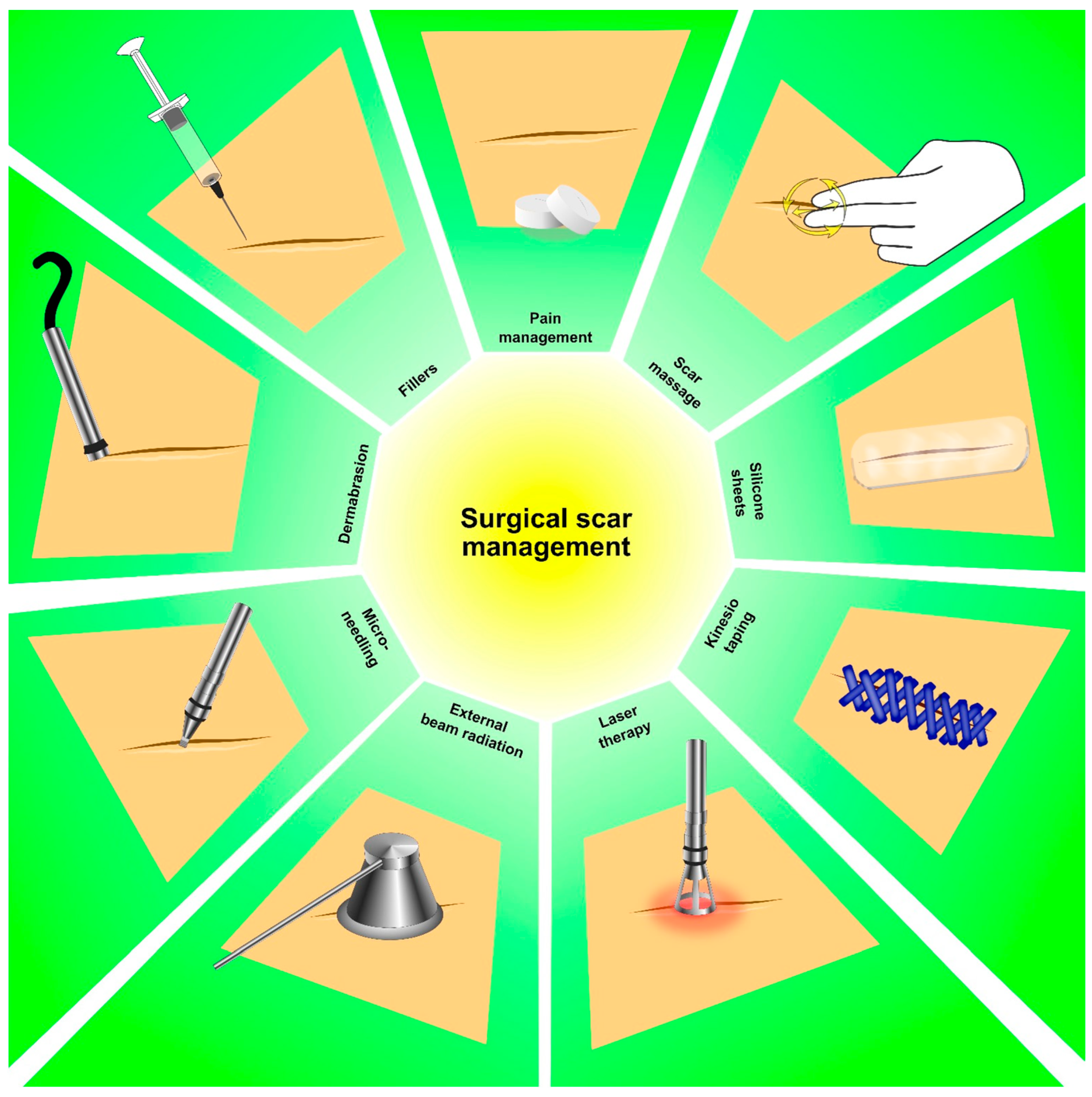
5. Surgical Scar Rehabilitation in Thyroid Cancer Survivors
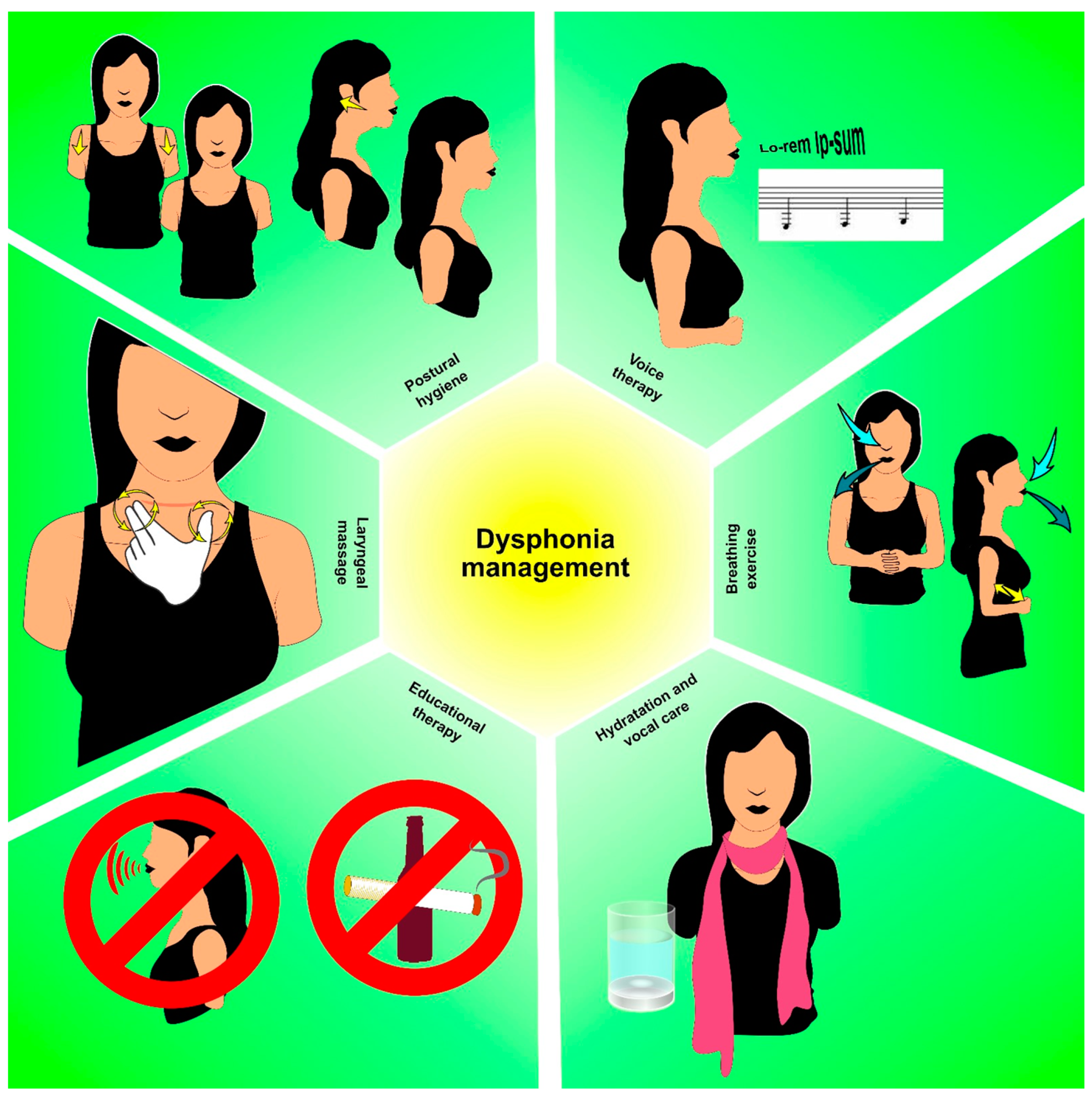
6. Dysphonia in Thyroid Cancer Survivors
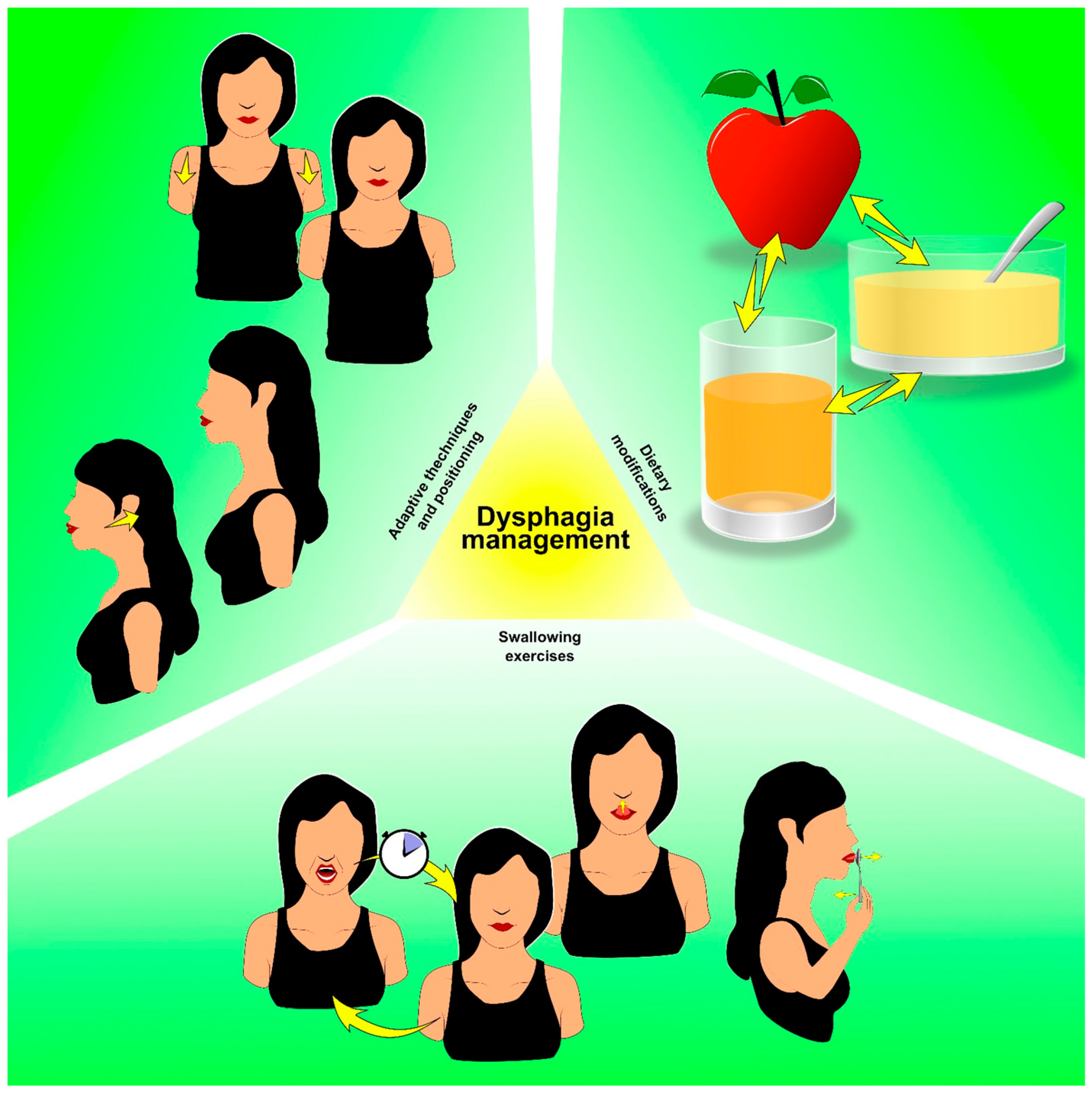
7. Dysphagia in Thyroid Cancer Survivors
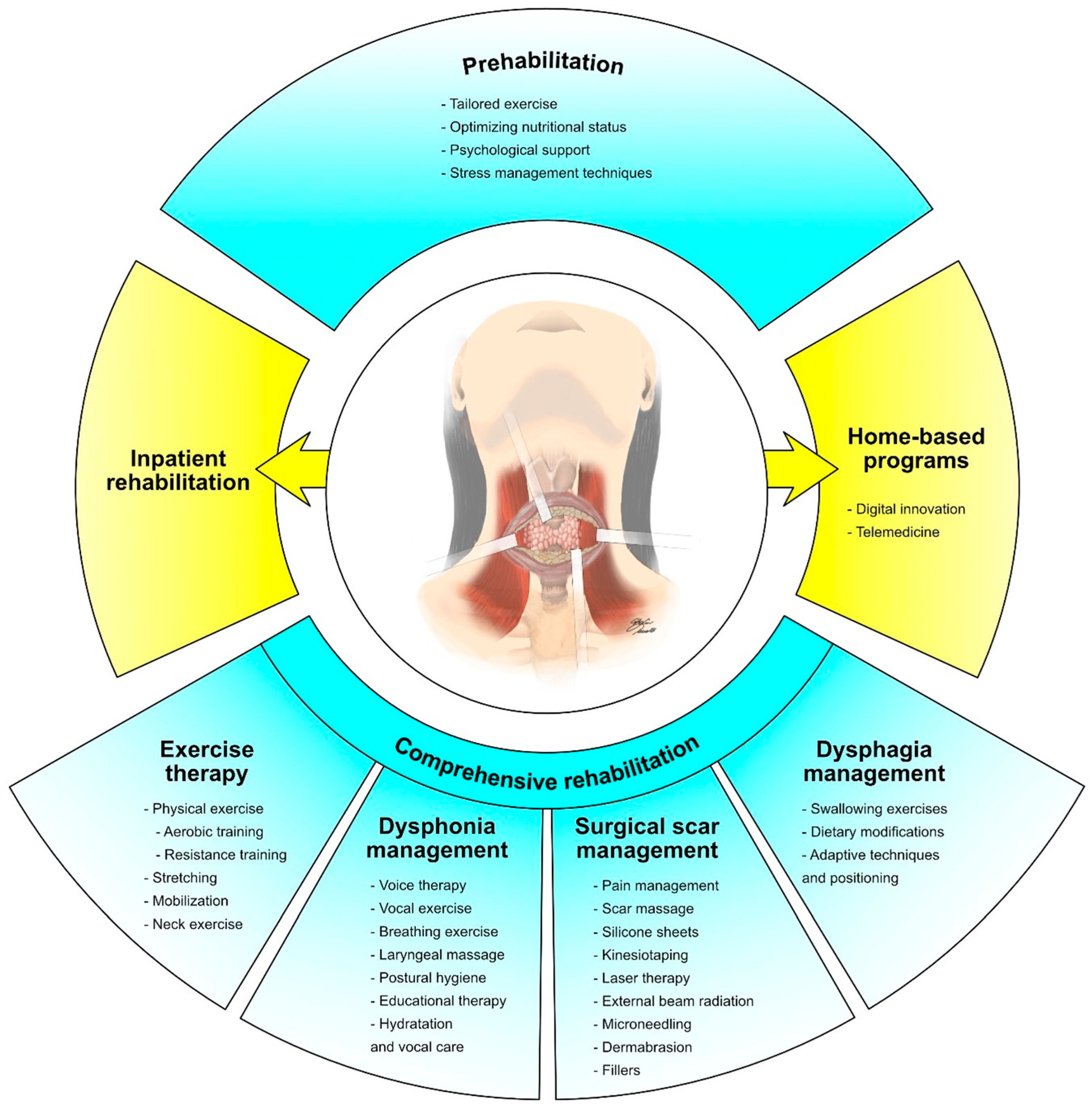
8. Prehabilitation in Thyroid Cancer Patients
9. Comprehensive Rehabilitation Programs: Barriers to and Strategies for Improving Patients’ Engagement
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olson, E.; Wintheiser, G.; Wolfe, K.M.; Droessler, J.; Silberstein, P.T. Epidemiology of Thyroid Cancer: A Review of the National Cancer Database, 2000–2013. Cureus 2019, 11, e4127. [Google Scholar] [CrossRef]
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 23–35. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Leboulleux, S. Current practice in patients with differentiated thyroid cancer. Nat. Rev. Endocrinol. 2021, 17, 176–188. [Google Scholar] [CrossRef]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyroid J. 2019, 8, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Nervo, A.; Retta, F.; Ragni, A.; Piovesan, A.; Gallo, M.; Arvat, E. Management of Progressive Radioiodine-Refractory Thyroid Carcinoma: Current Perspective. Cancer Manag. Res. 2022, 14, 3047–3062. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, H.; Wang, M.; Li, N.; Tian, T.; Wu, Y.; Xu, P.; Yang, S.; Zhai, Z.; Zhou, L. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw. Open 2020, 3, e208759. [Google Scholar] [CrossRef]
- Walshaw, E.G.; Smith, M.; Kim, D.; Wadsley, J.; Kanatas, A.; Rogers, S.N. Systematic review of health-related quality of life following thyroid cancer. Tumori 2022, 108, 291–314. [Google Scholar] [CrossRef]
- Marotta, N.; Lippi, L.; Ammendolia, V.; Calafiore, D.; Inzitari, M.T.; Pinto, M.; Invernizzi, M.; de Sire, A. Efficacy of kinesio taping on upper limb volume reduction in patients with breast cancer-related lymphedema: A systematic review of randomized controlled trials. Eur. J. Phys. Rehabil. Med. 2023, 59, 237–247. [Google Scholar] [CrossRef]
- Invernizzi, M.; Lippi, L.; Folli, A.; Turco, A.; Zattoni, L.; Maconi, A.; de Sire, A.; Fusco, N. Integrating molecular biomarkers in breast cancer rehabilitation. What is the current evidence? A systematic review of randomized controlled trials. Front. Mol. Biosci. 2022, 9, 930361. [Google Scholar] [CrossRef]
- de Sire, A.; Losco, L.; Lippi, L.; Spadoni, D.; Kaciulyte, J.; Sert, G.; Ciamarra, P.; Marcasciano, M.; Cuomo, R.; Bolletta, A.; et al. Surgical Treatment and Rehabilitation Strategies for Upper and Lower Extremity Lymphedema: A Comprehensive Review. Medicina. 2022, 58, 954. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Saco-Ledo, G.; Santos-Lozano, A.; Morales, J.S.; Castillo-García, A.; Simpson, R.J.; Lucia, A.; Fiuza-Luces, C. Exercise Training and Natural Killer Cells in Cancer Survivors: Current Evidence and Research Gaps Based on a Systematic Review and Meta-analysis. Sports Med. Open 2022, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Pérez, I.M.M.; Pérez, S.E.M.; García, R.P.; Lupgens, D.Z.; Martínez, G.B.; González, C.R.; Yán, N.K.; Hernández, F.R. Exercise-based rehabilitation on functionality and quality of life in head and neck cancer survivors. A systematic review and meta-analysis. Sci. Rep. 2023, 13, 8523. [Google Scholar] [CrossRef]
- De Nys, L.; Anderson, K.; Ofosu, E.F.; Ryde, G.C.; Connelly, J.; Whittaker, A.C. The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 143, 105843. [Google Scholar] [CrossRef] [PubMed]
- Himbert, C.; Thompson, H.; Ulrich, C.M. Effects of Intentional Weight Loss on Markers of Oxidative Stress, DNA Repair and Telomere Length—A Systematic Review. Obes. Facts 2017, 10, 648–665. [Google Scholar] [CrossRef]
- Wu, T.; Yan, F.; Wei, Y.; Yuan, C.; Jiao, Y.; Pan, Y.; Zhang, Y.; Zhang, H.; Ma, Y.; Han, L. Effect of exercise therapy on cancer-related fatigue in patients with breast cancer: A systematic review and network meta-analysis. Am. J. Phys. Med. Rehabil. 2023, 10, 1097. [Google Scholar] [CrossRef]
- Voorn, M.J.J.; Driessen, E.J.M.; Reinders, R.; van Kampen-van den Boogaart, V.E.M.; Bongers, B.C.; Janssen-Heijnen, M.L.G. Effects of exercise prehabilitation and/or rehabilitation on health-related quality of life and fatigue in patients with non-small cell lung cancer undergoing surgery: A systematic review. Eur. J. Surg. Oncol. 2023, 23, 106909. [Google Scholar] [CrossRef]
- Dionisi-Vici, M.; Fantoni, M.; Botto, R.; Nervo, A.; Felicetti, F.; Rossetto, R.; Gallo, M.; Arvat, E.; Torta, R.; Leombruni, P. Distress, anxiety, depression and unmet needs in thyroid cancer survivors: A longitudinal study. Endocrine 2021, 74, 603–610. [Google Scholar] [CrossRef]
- Vigário Pdos, S.; Chachamovitz, D.S.; Cordeiro, M.F.; Teixeira Pde, F.; de Castro, C.L.; de Oliveira, F.P.; Vaisman, M. Effects of physical activity on body composition and fatigue perception in patients on thyrotropin-suppressive therapy for differentiated thyroid carcinoma. Thyroid 2011, 21, 695–700. [Google Scholar] [CrossRef]
- Vigário Pdos, S.; Chachamovitz, D.S.; Teixeira Pde, F.; Rocque Mde, L.; Santos, M.L.; Vaisman, M. Exercise is associated with better quality of life in patients on TSH-suppressive therapy with levothyroxine for differentiated thyroid carcinoma. Arq. Bras Endocrinol. Metab. 2014, 58, 274–281. [Google Scholar] [CrossRef][Green Version]
- Doubleday, A.; Sippel, R.S. Surgical options for thyroid cancer and post-surgical management. Expert Rev. Endocrinol. Metab. 2018, 13, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.; Smith, D.; Booth, A. Beyond PICO: The SPIDER tool for qualitative evidence synthesis. Qual. Health Res. 2012, 22, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Chachamovitz, D.S.; dos Santos Vigário, P.; Nogueira Cordeiro, M.F.; de Castro, C.L.; Vaisman, M.; dos Santos Teixeira Pde, F. Quality of life, muscle strength, and fatigue perception in patients on suppressive therapy with levothyroxine for differentiated thyroid carcinoma. Am. J. Clin. Oncol. 2013, 36, 354–361. [Google Scholar] [CrossRef]
- Ayhan, H.; Tastan, S.; Iyigün, E.; Oztürk, E.; Yildiz, R.; Görgülü, S. The Effectiveness of Neck Stretching Exercises Following Total Thyroidectomy on Reducing Neck Pain and Disability: A Randomized Controlled Trial. Worldviews Evid. Based Nurs. 2016, 13, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Han, D.W.; Koo, B.N.; Chung, W.Y.; Park, C.S.; Kim, S.Y.; Palmer, P.P.; Kim, K.J. Preoperative greater occipital nerve block in total thyroidectomy patients can reduce postoperative occipital headache and posterior neck pain. Thyroid 2006, 16, 599–603. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Y.; Yu, J.; Wang, C. Clinical Treatment Efficacy of Total Thyroidectomy Combined with Radioactive Iodine on Treatment of Thyroid Cancer and Its Effect on the Quality of Life of Patients. Iran J. Public Health 2019, 48, 1461–1468. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, W.; Xiao, S.; Xu, L.; Wen, Q.; Bai, L.; Ma, Q.; Ji, B. Mindfulness-based stress reduction in patients with differentiated thyroid cancer receiving radioactive iodine therapy: A randomized controlled trial. Cancer Manag. Res. 2019, 11, 467–474. [Google Scholar] [CrossRef]
- Alhashemi, A.; Jones, J.M.; Goldstein, D.P.; Mina, D.S.; Thabane, L.; Sabiston, C.M.; Chang, E.K.; Brierley, J.D.; Sawka, A.M. An exploratory study of fatigue and physical activity in Canadian thyroid cancer patients. Thyroid 2017, 27, 1156–1163. [Google Scholar] [CrossRef]
- Kim, K.; Gu, M.O.; Jung, J.H.; Hahm, J.R.; Kim, S.K.; Kim, J.H.; Woo, S.H. Efficacy of a home-based exercise program after thyroidectomy for thyroid cancer patients. Thyroid 2018, 28, 236–245. [Google Scholar] [CrossRef]
- Hilfiker, R.; Meichtry, A.; Eicher, M.; Nilsson Balfe, L.; Knols, R.H.; Verra, M.L.; Taeymans, J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect-comparisons meta-analysis. Br. J. Sports Med. 2018, 52, 651–658. [Google Scholar] [CrossRef]
- Patel, H.; Alkhawam, H.; Madanieh, R.; Shah, N.; Kosmas, C.E.; Vittorio, T.J. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J. Cardiol. 2017, 9, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.; Sandmael, J.A.; Stene, G.B.; Thorsen, L.; Balstad, T.R.; Solheim, T.S.; Pripp, A.H.; Oldervoll, L.M. Exercise and Nutrition Interventions in Patients with Head and Neck Cancer during Curative Treatment: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 3233. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.K.; Park, C.; Tussing-Humphreys, L.; Fernhall, B.; Phillips, S.; Doorenbos, A.Z. The Effectiveness of Sarcopenia Interventions for Cancer Patients Receiving Chemotherapy: A Systematic Review and Meta-analysis. Cancer Nurs. 2023, 46, e81–e90. [Google Scholar] [CrossRef]
- de Sire, A.; Lippi, L.; Marotta, N.; Folli, A.; Calafiore, D.; Moalli, S.; Turco, A.; Ammendolia, A.; Fusco, N.; Invernizzi, M. Impact of Physical Rehabilitation on Bone Biomarkers in Non-Metastatic Breast Cancer Women: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 921. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, L.C.; Boldt, K.R.; Lau, H.; Shirt, L.; Bultz, B.; Culos-Reed, S.N. A clinic-supported group exercise program for head and neck cancer survivors: Managing cancer and treatment side effects to improve quality of life. Support. Care Cancer 2015, 23, 1001–1007. [Google Scholar] [CrossRef]
- Lavigne, C.; Twomey, R.; Lau, H.; Francis, G.; Culos-Reed, S.N.; Millet, G.Y. Feasibility of eccentric overloading and neuromuscular electrical stimulation to improve muscle strength and muscle mass after treatment for head and neck cancer. J. Cancer Surviv. 2020, 14, 790–805. [Google Scholar] [CrossRef]
- Rodríguez-Torres, J.; López-López, L.; Cabrera-Martos, I.; Torres-Sánchez, I.; Ortíz-Rubio, A.; Valenza, M.C. Musculoskeletal neck disorders in thyroid cancer patients after thyroidectomy. Eur. J. Cancer Care 2019, 28, e13053. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Klose, P.; Lange, S.; Langhorst, J.; Dobos, G.J. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst. Rev. 2017, 1, Cd010802. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.M.; Lee, M.S.; Novakowski, J.; Osypiuk, K.; Ligibel, J.; Carlson, L.E.; Song, R. Tai Chi and Qigong for cancer-related symptoms and quality of life: A systematic review and meta-analysis. J. Cancer Surviv. 2018, 12, 256–267. [Google Scholar] [CrossRef]
- Ferrante, M.; Distefano, G.; Distefano, C.; Copat, C.; Grasso, A.; Oliveri Conti, G.; Cristaldi, A.; Fiore, M. Benefits of Physical Activity during and after Thyroid Cancer Treatment on Fatigue and Quality of Life: A Systematic Review. Cancers 2022, 14, 3657. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Spei, M.E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef]
- Alfano, C.M.; Zucker, D.S.; Pergolotti, M.; Ness, K.K.; Jones, L.W.; Price, N.D.; Schmitz, K.H.; Ligibel, J.A. A precision medicine approach to improve cancer rehabilitation’s impact and integration with cancer care and optimize patient wellness. Curr. Phys. Med. Rehabil. Rep. 2017, 5, 64–73. [Google Scholar] [CrossRef]
- Kudre, D.; Chen, Z.; Richard, A.; Cabaset, S.; Dehler, A.; Schmid, M.; Rohrmann, S. Multidisciplinary outpatient Cancer rehabilitation can improve Cancer patients’ physical and psychosocial status—A systematic review. Curr. Oncol. Rep. 2020, 22, 122. [Google Scholar] [CrossRef]
- Midtgaard, J.; Hammer, N.M.; Andersen, C.; Larsen, A.; Bruun, D.-M.; Jarden, M. Cancer survivors’ experience of exercise-based cancer rehabilitation—A meta-synthesis of qualitative research. Acta Oncol. 2015, 54, 609–617. [Google Scholar] [CrossRef]
- Miyauchi, A.; Ito, Y.; Miya, A. Stretching Exercise for the Prevention of Postoperative Neck Symptoms Following Thyroid Surgery. VideoEndocrinology 2021, 8, 1. [Google Scholar] [CrossRef]
- Takamura, Y.; Miyauchi, A.; Tomoda, C.; Uruno, T.; Ito, Y.; Miya, A.; Kobayashi, K.; Matsuzuka, F.; Amino, N.; Kuma, K. Stretching exercises to reduce symptoms of postoperative neck discomfort after thyroid surgery: Prospective randomized study. World J. Surg. 2005, 29, 775–779. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.P.; Ryu, J.S.; Woo, S.H. Effect of wound massage on neck discomfort and voice changes after thyroidectomy. Surgery 2018, 164, 965–971. [Google Scholar] [CrossRef]
- Liu, K.; Bhatia, K.; Chu, W.; He, L.; Leung, S.; Ahuja, A. Shear wave elastography–a new quantitative assessment of post-irradiation neck fibrosis. Ultraschall Der Med. Eur. J. Ultrasound 2014, 36, 348–354. [Google Scholar] [CrossRef]
- Kazi, F.; Patil, S.; Pathan, H. Physiotherapy Combined With Voice Exercises in a Patient With Unilateral Vocal Cord Palsy Following a Total Thyroidectomy Surgery: A Case Report. Cureus 2023, 15, e35217. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Melissa, S.; Francesco, G.; Francesco, F.; Rocco, B.; Patrizia, L.; Antonella, P.; Ettore, C.; Zhang, D.; Gianlorenzo, D. Posture and dysphonia associations in patients undergoing total thyroidectomy: Stabilometric analysis. Updates Surg. 2020, 72, 1143–1149. [Google Scholar] [CrossRef]
- Goswami, S.; Peipert, B.J.; Mongelli, M.N.; Kurumety, S.K.; Helenowski, I.B.; Yount, S.E.; Sturgeon, C. Clinical factors associated with worse quality-of-life scores in United States thyroid cancer survivors. Surgery 2019, 166, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bãrbuş, E.; Peştean, C.; Larg, M.I.; Piciu, D. Quality of life in thyroid cancer patients: A literature review. Clujul. Med. 2017, 90, 147. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.L.; Kerolus, J.L. Management of Surgical Scars. Facial Plast. Surg. Clin. North Am. 2019, 27, 513–517. [Google Scholar] [CrossRef]
- Lubczyńska, A.; Garncarczyk, A.; Wcisło-Dziadecka, D. Effectiveness of various methods of manual scar therapy. Ski. Res. Technol. 2023, 29, e13272. [Google Scholar] [CrossRef] [PubMed]
- Shokri, T.; Smith, J.; Ducic, Y. Paradigms in complex facial scar management. In Proceedings of the Seminars in Plastic Surgery, New York, NY, USA, 24 December 2020; pp. 305–313. [Google Scholar]
- Chung, J.H.; Kim, D.S.; Cheon, J.H.; Yoon, J.M.; Baek, S.K.; Jung, K.Y.; Yoon, E.S.; Park, S.H. Current protocol for aesthetic scar management in thyroid surgery. Laryngoscope 2021, 131, E2188–E2195. [Google Scholar] [CrossRef]
- Betarbet, U.; Blalock, T.W. Keloids: A Review of Etiology, Prevention, and Treatment. J. Clin. Aesthetic Dermatol. 2020, 13, 33–43. [Google Scholar]
- O’Reilly, S.; Crofton, E.; Brown, J.; Strong, J.; Ziviani, J. Use of tape for the management of hypertrophic scar development: A comprehensive review. Scars Burn Health 2021, 7, 20595131211029206. [Google Scholar] [CrossRef]
- Krajczy, M.; Krajczy, E.; Bogacz, K.; Łuniewski, J.; Lietz-Kijak, D.; Szczegielniak, J. The possibility of the use of Kinesio Taping in internal, oncologic, and neurologic diseases: A systematic review and meta-analysis. Explore 2020, 16, 44–49. [Google Scholar] [CrossRef]
- Augustsson, S.R.; Reinodt, S.; Sunesson, E.; Haglund, E. Short-term effects of postural taping on pain and forward head posture: A randomized controlled trial. BMC Musculoskelet Disord 2022, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Hadedeya, D.; Shalaby, M.; Akkera, M.; Lee, G.; Harris, K.; Kholmatov, R.; Anwar, M.; Murad, F.; Alawaad, S.; Kandil, E. Prophylactic external beam radiation therapy for keloid prevention in thyroid surgery patients. Gland Surg. 2021, 10, 65. [Google Scholar] [CrossRef]
- Riedemann, H.I.; Schmidt, M.F.; Baron, J.M. Therapy of pathological scars. J. Dtsch Dermatol. Ges. 2023, 11, 1139–1157. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.H.; Hwang, Y.J.; Yoon, E.S.; Jung, K.Y.; Park, S.H.; Chung, J.H. Effectiveness of a combination therapy using non-ablative fractional laser and intralesional triamcinolone injection for thyroidectomy scar treatment: A prospective, randomized, blinded pilot study. J. Cosmet. Dermatol. 2022, 21, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Lin, C.; He, Q.; Gao, H.; Jin, L.; Zheng, X. Ultrasound-guided bilateral superficial cervical plexus blocks enhance the quality of recovery in patients undergoing thyroid cancer surgery: A randomized controlled trial. J. Clin. Anesth. 2020, 61, 109651. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Ghiani, M.; Argenone, A.; Depenni, R. Is pain part of a systemic syndrome in head and neck cancer? Support. Care Cancer 2020, 28, 451–459. [Google Scholar] [CrossRef]
- Scott, H.C.; Stockdale, C.; Robinson, A.; Robinson, L.S.; Brown, T. Is massage an effective intervention in the management of post-operative scarring? A scoping review. J. Hand Ther. 2022, 35, 186–199. [Google Scholar] [CrossRef]
- de Pauli Paglioni, M.; Alves, C.G.B.; Fontes, E.K.; Lopes, M.A.; Ribeiro, A.C.P.; Brandão, T.B.; Migliorati, C.A.; Santos-Silva, A.R. Is photobiomodulation therapy effective in reducing pain caused by toxicities related to head and neck cancer treatment? A systematic review. Support. Care Cancer 2019, 27, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.S.; Koo, D.H.; Kim, J.E.; Cho, J.-m.; Park, J.-O. Effect of botulinum toxin A on scar healing after thyroidectomy: A prospective double-blind randomized controlled trial. J. Clin. Med. 2020, 9, 868. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, H.J.; Cho, S.H.; Lee, J.D.; Kim, H.S. Early postoperative treatment of thyroidectomy scars using botulinum toxin: A split-scar, double-blind randomized controlled trial. Wound Repair Regen. 2014, 22, 605–612. [Google Scholar] [CrossRef]
- Lippi, L.; de Sire, A.; Folli, A.; D’Abrosca, F.; Grana, E.; Baricich, A.; Carda, S.; Invernizzi, M. Multidimensional Effectiveness of Botulinum Toxin in Neuropathic Pain: A Systematic Review of Randomized Clinical Trials. Toxins 2022, 14, 308. [Google Scholar] [CrossRef]
- Reyes-Long, S.; Alfaro-Rodríguez, A.; Cortes-Altamirano, J.L.; Lara-Padilla, E.; Herrera-Maria, E.; Romero-Morelos, P.; Salcedo, M.; Bandala, C. The Mechanisms of Action of Botulinum Toxin Type A in Nociceptive and Neuropathic Pathways in Cancer Pain. Curr. Med. Chem. 2021, 28, 2996–3009. [Google Scholar] [CrossRef]
- Maeda, T.; Saito, M.; Otsuki, N.; Morimoto, K.; Takahashi, M.; Iwaki, S.; Inoue, H.; Tomoda, C.; Miyauchi, A.; Nibu, K.-I. Voice quality after surgical treatment for thyroid cancer. Thyroid 2013, 23, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Kovatch, K.J.; Reyes-Gastelum, D.; Hughes, D.T.; Hamilton, A.S.; Ward, K.C.; Haymart, M.R. Assessment of voice outcomes following surgery for thyroid cancer. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.H.; Lee, S.J.; Cho, J.G.; Choi, I.J.; Choi, Y.S.; Hong, Y.T.; Jung, S.Y.; Kim, J.W.; Lee, D.Y.; Lee, D.K.; et al. Care and Management of Voice Change in Thyroid Surgery: Korean Society of Laryngology, Phoniatrics and Logopedics Clinical Practice Guideline. Clin. Exp. Otorhinolaryngol. 2022, 15, 24–48. [Google Scholar] [CrossRef]
- Bonetti, A.; Šimić, I.; Živković-Ivanović, T. Voice Outcomes as a Results of Voice Therapy after Lobectomy and Thyroidectomy. Acta Clin. Croat. 2020, 59, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.V.; Wu, C.W. Speech therapy after thyroidectomy. Gland Surg. 2017, 6, 501–509. [Google Scholar] [CrossRef]
- Hasan, H.Q.; Al Dabbagh, A. A Prospective study for effect of wound or scar tissue massage after thyroidectomy on neck discomfort and voice change. Iraq Med. J. 2021, 5, 4. [Google Scholar]
- Hashemian, M.; Khorasani, B.; Tarameshlu, M.; Haghani, H.; Ghelichi, L.; Nakhostin Ansari, N. Effects of Dysphagia Therapy on Swallowing Dysfunction after Total Thyroidectomy. Iran J. Otorhinolaryngol. 2019, 31, 329–334. [Google Scholar] [CrossRef]
- Galluzzi, F.; Garavello, W. Dysphagia following uncomplicated thyroidectomy: A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 2661–2671. [Google Scholar] [CrossRef]
- Scerrino, G.; Tudisca, C.; Bonventre, S.; Raspanti, C.; Picone, D.; Porrello, C.; Paladino, N.C.; Vernuccio, F.; Cupido, F.; Cocorullo, G. Swallowing disorders after thyroidectomy: What we know and where we are. A systematic review. Int. J. Surg. 2017, 41, S94–S102. [Google Scholar] [CrossRef]
- Han, T.R.; Kim, H.R.; Kim, S.J. Dysphagia development after surgery unrelated to laryngeal and pharyngeal structures. Dysphagia 2009, 24, 167–171. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Ferrillo, M.; Gennari, A.; Cisari, C.; Pasqua, S.; Bonda, P.F.; Invernizzi, M.; Migliario, M. Bone health, vitamin D status and oral hygiene screening in breast cancer women before starting osteoporosis treatment: A cross-sectional study. J. Biol. Regul. Homeost. Agents 2021, 35, 287–292. [Google Scholar]
- Palanca, A.; Ampudia-Blasco, F.J.; Real, J.T. The controversial role of vitamin d in thyroid cancer prevention. Nutrients 2022, 14, 2593. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Sakuma, K. Pathophysiology of cachexia and characteristics of dysphagia in chronic diseases. Asia-Pac. J. Oncol. Nurs. 2022, 9, 100120. [Google Scholar] [CrossRef]
- Krekeler, B.N.; Wendt, E.; Macdonald, C.; Orne, J.; Francis, D.O.; Sippel, R.; Connor, N.P. Patient-reported dysphagia after thyroidectomy: A qualitative study. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 342–348. [Google Scholar] [CrossRef]
- Di Pede, C.; Mantovani, M.E.; Del Felice, A.; Masiero, S. Dysphagia in the elderly: Focus on rehabilitation strategies. Aging Clin. Exp. Res. 2016, 28, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Damanti, S.; Bertagnoli, L.; Lucchi, T.; Cesari, M. Sarcopenia and swallowing disorders in older people. Aging Clin. Exp. Res. 2019, 31, 799–805. [Google Scholar] [CrossRef]
- Hadde, E.K.; Chen, J. Texture and texture assessment of thickened fluids and texture-modified food for dysphagia management. J. Texture Stud. 2021, 52, 4–15. [Google Scholar] [CrossRef]
- Denk-Linnert, D.-M.; Farneti, D.; Nawka, T.; am Zehnhoff-Dinnesen, A.; Moerman, M.; Zorowka, P.; Farahat, M.; Schindler, A.; Geneid, A. Position Statement of the Union of European Phoniatricians (UEP): Fees and Phoniatricians’ Role in Multidisciplinary and Multiprofessional Dysphagia Management Team. Dysphagia 2023, 38, 711–718. [Google Scholar] [CrossRef]
- Martinovic, D.; Tokic, D.; Puizina Mladinic, E.; Usljebrka, M.; Kadic, S.; Lesin, A.; Vilovic, M.; Lupi-Ferandin, S.; Ercegovic, S.; Kumric, M. Nutritional Management of Patients with Head and Neck Cancer—A Comprehensive Review. Nutrients 2023, 15, 1864. [Google Scholar] [CrossRef]
- Carli, F.; Gillis, C.; Scheede-Bergdahl, C. Promoting a culture of prehabilitation for the surgical cancer patient. Acta Oncol. 2017, 56, 128–133. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hong, N.; Park, S.H.; Shin, D.Y.; Lee, C.R.; Kang, S.W.; Lee, J.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; et al. The relationship of comorbidities to mortality and cause of death in patients with differentiated thyroid carcinoma. Sci. Rep. 2019, 9, 11435. [Google Scholar] [CrossRef] [PubMed]
- PANEL, E. American college of sports medicine roundtable on exercise guidelines for cancer survivors. J. ACSM 2010, 42, 1409–1426. [Google Scholar]
- Silver, J.K.; Baima, J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am. J. Phys. Med. Rehabil. 2013, 92, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Baima, J.; Reynolds, S.-G.; Edmiston, K.; Larkin, A.; Ward, B.M.; O’Connor, A. Teaching of independent exercises for prehabilitation in breast cancer. J. Cancer Educ. 2017, 32, 252–256. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, S.; Ross, D.C.; Doherty, T.J.; Doherty, C.D.; Miller, T.A. Spinal accessory nerve injury: A potentially missed cause of a painful, droopy shoulder. J. Back Musculoskelet. Rehabil. 2016, 29, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Patekar, S.; Ishwarya, M.; Padmakshan, S.; Bradoo, R. Shoulder Dysfunction Post Spinal Accessory Nerve Preserving Neck Dissections: Our Experience. Indian J. Otolaryngol Head Neck Surg. 2023, 75, 675–679. [Google Scholar] [CrossRef]
- Kim, J.; Jeon, J.Y.; Choi, Y.J.; Choi, J.K.; Kim, S.-B.; Jung, K.H.; Ahn, J.-H.; Kim, J.E.; Seo, S. Characteristics of metastatic brachial plexopathy in patients with breast cancer. Support. Care Cancer 2020, 28, 1913–1918. [Google Scholar] [CrossRef]
- Gane, E.M.; O’Leary, S.P.; Hatton, A.L.; Panizza, B.J.; McPhail, S.M. Neck and Upper Limb Dysfunction in Patients following Neck Dissection: Looking beyond the Shoulder. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2017, 157, 631–640. [Google Scholar] [CrossRef]
- Baldoman, D.; Vandenbrink, R. Physical Therapy Challenges in Head and Neck Cancer. Cancer Treat Res. 2018, 174, 209–223. [Google Scholar] [CrossRef]
- Palma, S.; Hasenoehrl, T.; Jordakieva, G.; Ramazanova, D.; Crevenna, R. High-intensity interval training in the prehabilitation of cancer patients—A systematic review and meta-analysis. Support. Care Cancer 2021, 29, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Beeram, M.; Kennedy, A.; Hales, N. Barriers to Comprehensive Multidisciplinary Head and Neck Care in a Community Oncology Practice. Am. Soc. Clin. Oncol. Educ Book 2021, 41, e236–e245. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, I.A.; Tolstanov, O.K. Improving clinical management of patients with thyroid cancer. Wiadomości Lek. 2022, 75, 1090. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; De Leo, S.; Trevisan, M.; Giancola, N.; Scaltrito, A.; Fugazzola, L. Daily Management of Patients on Multikinase Inhibitors’ Treatment. Front. Oncol. 2022, 12, 903532. [Google Scholar] [CrossRef]
- APd, O.; Joaquin, C.; Vazquez, F.; Martinez, E.; Etxanitz, O.; Barez, M.; Fuentes, S.; Puig-Domingo, M.; Reverter, J. Multimodal Prehabilitation in Radioactive Iodine-refractory Differentiated Thyroid Cancer Treated with Lenvatinib. Res. Sq. 2021. [Google Scholar]
- Gkaintatzi, E.; Nikolaou, C.K.; Rampal, T.; Laza-Cagigas, R.; Zand, N.; McCrone, P. Cost Analysis of a Digital Multimodal Cancer Prehabilitation. Curr. Oncol. 2022, 29, 9305–9313. [Google Scholar] [CrossRef]
- Chang, P.; Asher, A. Cancer telerehabilitation. Phys. Med. Rehabil. Clin. 2021, 32, 277–289. [Google Scholar] [CrossRef]
- Lippi, L.; D’Abrosca, F.; Folli, A.; Dal Molin, A.; Moalli, S.; Maconi, A.; Ammendolia, A.; de Sire, A.; Invernizzi, M. Closing the gap between inpatient and outpatient settings: Integrating pulmonary rehabilitation and technological advances in the comprehensive management of frail patients. Int. J. Environ. Res. Public Health 2022, 19, 9150. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.M.; Chang, H.; Kim, B.W.; Lee, Y.S.; Kwon, S.S.; Shin, H.; Chang, H.S.; Park, C.S. Cost for treatment and follow-up of thyroid cancer increases according to the severity of disease. Head Neck 2019, 41, 2376–2379. [Google Scholar] [CrossRef]
- Lubitz, C.C.; Kong, C.Y.; McMahon, P.M.; Daniels, G.H.; Chen, Y.; Economopoulos, K.P.; Gazelle, G.S.; Weinstein, M.C. Annual financial impact of well-differentiated thyroid cancer care in the United States. Cancer 2014, 120, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, M.; Lim, W.; Kim, B.H.; Park, S.K. The Concept of Economic Evaluation and Its Application in Thyroid Cancer Research. Endocrinol. Metab. 2021, 36, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.K. Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. Semin. Oncol. Nurs. 2015, 31, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.K.; Baima, J.; Mayer, R.S. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA A Cancer J. Clin. 2013, 63, 295–317. [Google Scholar] [CrossRef]
| S | PI | D | E | R |
|---|---|---|---|---|
| Sample | Phenomenon of Interest | Design | Evaluation | Research Type |
| Patients with thyroid cancer | Rehabilitation Strategies | Any | Functional Outcomes | Qualitative |
| “Thyroid Cancer” “Neck Cancer” | “Exercise”“Prehabilitation”“Rehabilitation”“Physical Activity” “Voice Therapy” | “Function”“Performance”“Disability”, “Quality of Life” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippi, L.; Turco, A.; Moalli, S.; Gallo, M.; Curci, C.; Maconi, A.; de Sire, A.; Invernizzi, M. Role of Prehabilitation and Rehabilitation on Functional Recovery and Quality of Life in Thyroid Cancer Patients: A Comprehensive Review. Cancers 2023, 15, 4502. https://doi.org/10.3390/cancers15184502
Lippi L, Turco A, Moalli S, Gallo M, Curci C, Maconi A, de Sire A, Invernizzi M. Role of Prehabilitation and Rehabilitation on Functional Recovery and Quality of Life in Thyroid Cancer Patients: A Comprehensive Review. Cancers. 2023; 15(18):4502. https://doi.org/10.3390/cancers15184502
Chicago/Turabian StyleLippi, Lorenzo, Alessio Turco, Stefano Moalli, Marco Gallo, Claudio Curci, Antonio Maconi, Alessandro de Sire, and Marco Invernizzi. 2023. "Role of Prehabilitation and Rehabilitation on Functional Recovery and Quality of Life in Thyroid Cancer Patients: A Comprehensive Review" Cancers 15, no. 18: 4502. https://doi.org/10.3390/cancers15184502
APA StyleLippi, L., Turco, A., Moalli, S., Gallo, M., Curci, C., Maconi, A., de Sire, A., & Invernizzi, M. (2023). Role of Prehabilitation and Rehabilitation on Functional Recovery and Quality of Life in Thyroid Cancer Patients: A Comprehensive Review. Cancers, 15(18), 4502. https://doi.org/10.3390/cancers15184502