Simple Summary
Many previous studies have focused on the prevalence of high-risk HPV16 and HPV18 strains, highly associated with cervical and oral cancers covered by the original HPV vaccine. However, little research is currently available regarding the oral prevalence of other high-risk HPV strains, such as HPV31 and HPV33, which are part of the recently revised nine-strain HPV vaccine. This study conducted one of the first oral prevalence studies of these high-risk HPV strains among a multi-ethnic patient population at a public dental school in Nevada. The results of this investigation revealed a significant percentage of children and adults in this study harbored one or more of these high-risk strains, which were mostly found among patients within the recommended vaccination or catch-up age range (9–45 years).
Abstract
Many human papillomavirus (HPV) strains induce cancer in the cervix and the oral cavity. Although high-risk strains including HPV16 and HPV18 are commonly known, additional high-risk strains including HPV31, HPV33, and HPV35 may also induce carcinogenesis, and much less is known about their prevalence. Using an approved protocol, samples from a salivary biorepository were screened to find pediatric and adult samples from a multi-ethnic, university-based patient clinic population. A total of N = 86 samples from the saliva biorepository met the quality and concentration standards and were screened for high-risk HPV. qPCR screening of adult samples revealed n = 10/45 or 22% were HPV31- or HPV33-positive. In addition, a total of n = 9/41 or 21.9% of pediatric samples were either HPV31- or HPV33-positive (or both). No samples harbored HPV35. Most samples were derived from patients within the recommended vaccination or catch-up age range (age 9–45 years). These results demonstrated that a significant percentage of patients harbor additional high-risk HPV strains within the oral cavity, including HPV31 and HPV33. These data support oral healthcare provider recommendations for the newer nine-valent vaccine, which includes both HPV31 and HPV33.
1. Introduction
Human papillomavirus (HPV) is a non-enveloped, double-stranded DNA virus that is epitheliotropic in nature and is known to cause disease in a variety of tissues [1,2]. There are between 150–200 different strains of HPV, some of which are known to cause human disease, including cancer [3,4]. Approximately 40 types of HPV are known to cause cutaneous or mucosal infections within human hosts [5,6].
HPV infections can spread through skin-to-skin contact through both sexual and non-sexual transmission pathways [7,8]. Epidemiologic prevalence estimates suggest that more than 40 million people in the United States are currently infected with some form of HPV, with an incidence of more than 10 million new cases per year [9,10,11]. Although most HPV infections are cleared by the immune system (90–95% by some estimates), some infections result in long-term infections that cause precancerous abnormalities and malignancies if left untreated [12,13,14].
The main clinical distinction between the many human papillomavirus (HPV) strains lies in their ability to mediate cellular transformation into cancer within various tissues, which are broadly categorized into low-risk (LR) or high-risk (HR) depending upon their most frequently associated clinical outcomes [15,16]. The low-risk HPV strains 6 and 11 are responsible for >90% of anogenital warts and lesions, but also include additional commonly identified strains such as 40, 42, 43, and 44 [17,18]. In addition, high-risk strains HPV16 and 18 are responsible for the vast majority of cervical as well as the majority of HPV-associated oropharyngeal cancers, although there are many additional high-risk HPV strains commonly identified including HPV31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 [19,20].
Although the high-risk strains HPV16 and HPV18 are the most frequently identified, additional high-risk strains found in the cervix and oral cavity may induce carcinogenesis, including HPV31, HPV33, and HPV35, although much less is known about their prevalence [21,22,23]. Based upon this understanding of additional clinically-relevant strains of HPV, the new nine-valent HPV vaccine incorporates not only the most common high-risk (16 and 18) and low-risk (6 and 11) HPV strains, but also includes additional high-risk strains such as HPV31, 33, 45, 52, and 58 [24,25,26]. Although several studies from this institution have evaluated the prevalence of high-risk oral HPV strains 16 and 18 among both adult and pediatric patient populations, no study to date has evaluated the additional high-risk strains of HPV31, 33, and 35 [27,28,29,30].
Based upon lack of evidence regarding these high-risk strains, the primary objective of this project is to evaluate the prevalence of these strains among pediatric and adult patients through screening of clinical saliva samples. The working hypothesis for this project was that additional high-risk strains of HPV, such as HPV31, 33, and 35, would be identified if the appropriate screening for these additional HPV strains was conducted.
2. Materials and Methods
2.1. Study Approval
The review of the protocol for this study was completed by the Office for the Protection of Research Subjects (OPRS) and the Institutional Review Board at the University of Nevada, Las Vegas (UNLV). Protocol #1717625-1, titled “Retrospective analysis of microbial prevalence from DNA isolated from saliva samples originally obtained from the University of Nevada, Las Vegas (UNLV) School of Dental Medicine (SDM) pediatric and clinical population”, was approved by the UNLV-IRB and OPRS on 3 March 2021.
2.2. Human Subjects and Informed Consent
The original collection protocol was approved by the UNLV-IRB under protocol OPRS#1305-4466M, “The Prevalence of Oral Microbes in Saliva from the UNLV School of Dental Medicine Pediatric and Adult Clinical Population”. Inclusion criteria included patients between the ages of 5 to 45 years of age that agreed to provide informed consent (adult over 18 years of age) or pediatric assent with Informed consent (children under 18 years of age with guardian or parental permission and consent). Exclusion criteria included any patients (or parents/guardians that refused to provide informed consent or pediatric assent) and any samples from patients outside the UNLV School of Dental Medicine.
2.3. Original Sample Collection Protocol
Original sample collection from UNLV–SDM clinic patients involved only voluntary study participants. Following voluntary agreement to study participation and the provision of informed consent and/or pediatric assent, clinic patients were provided a sterile 50 mL saliva collection tube. Up to 5.0 mL of unstimulated saliva was collected from each study participant. To prevent the collection of any personal or patient information, all samples were assigned a non-duplicated, randomly-generated number for labeling purposes. Only basic demographic characteristics, such as patient age at the time of collection, self-reported ethnicity or race, and sex were noted at the time of sample collection. All samples and other materials were stored in a biomedical laboratory for subsequent analysis and processing.
2.4. DNA Isolation and Analysis
A total of N = 253 samples from the biomedical sample repository were identified for potential inclusion in the current retrospective analysis. DNA was isolated from each sample using the phenol:chloroform extraction method. In brief, samples were thawed and vortexed, then 500 µL was transferred to a sterile microcentrifuge tube and mixed with 500 µL of TRIzol DNA isolation reagent from Invitrogen (Waltham, MA, USA). To each sample, 200 µL of molecular-grade chloroform from Invitrogen (Waltham, MA, USA) was added prior to incubation on ice for 15 min. Each sample was subsequently centrifuged using an Eppendorf Model 5425 (Hamburg, Germany) refrigerated microcentrifuge for fifteen minutes at 12,000 × relative centrifugal force (RCF) at 4 °C.
The upper aqueous phase (approximately 400–500 µL) was transferred to a new, sterile microcentrifuge tube and mixed with molecular-grade isopropanol from Invitrogen (Waltham, MA, USA) to precipitate the DNA. Each sample was then centrifuged using the settings described above. Following removal of the isopropanol, the pellet containing sample DNA was washed using molecular-grade ethanol from Invitrogen (Waltham, MA, USA) prior to centrifugation for ten additional minutes. After removal of the ethanol supernatant, DNA from each sample was resuspended in 100 µL of nuclease-free water obtained from Thermo Fisher Scientific (Waltham, MA, USA). Analysis of quality and quantity of DNA was facilitated with a NanoDrop 2000 spectrophotometer obtained from Thermo Fisher Scientific (Waltham, MA, USA) using absorbances of A260 and A280 nm, as specified by the manufacturer protocol. All samples that met minimum quantity standards (>10 ng/µL) as well as quality standards (A260:A280 ratio > 1.65) were then utilized in the current study for molecular HPV screening using qPCR.
2.5. qPCR Screening
The samples that met the minimum criteria for DNA quantity and DNA purity (N = 86) were screened for high-risk HPV strains 31, 33, and 35 using quantitative polymerase chain reaction (qPCR). Each reaction consisted of 15 µL Fast SYBR Green Master Mix from Applied Biosystems (Waltham, MA, USA), 1.5 µL of forward primer, 1.5 µL of reverse primer, 2.0 µL of sample DNA, and 5.0 µL of nuclease-free water. Reactions were performed using the QuantStudio 3 from Thermo Fisher Scientific (Waltham, MA, USA) and the following validated primers:
- HPV31 forward: ATTCCACAACATAGGAGGAAGGTG;
- HPV31 reverse: CACTTGGGTTTCAGTACGAGGTCT;
- HPV33 forward: ATATTTCGGGGTCGTTGGGCA;
- HPV33 reverse: ACGTCACAGTGCAGTTTCTCTACGT;
- HPV35 forward: TCGGTGTATGTCTGTTGGAAAC;
- HPV35 reverse: CATAGTCTTGCAATGTAGTTATTTCTCCA.
2.6. Statistical Analysis
Demographic variables for the study sample were compiled and presented as simple, descriptive statistics. Analysis of differences between the study sample and the overall clinic population with respect to categorical variables, such as sex and race or ethnicity, were done using chi-square statistics which is appropriate for non-parametric data analysis. Analysis of qPCR screening results was also presented as simple descriptive statistics, such as percentages, and the differences between HPV-positive and HPV-negative samples were also analyzed using chi-square statistics and the GraphPad Prism software, Version 8 (San Diego, CA, USA). Comparisons for parametric data, such as age, were completed using two-tailed Student’s t-tests, using an alpha level of 0.05 for statistical significance.
3. Results
A total of N = 86 samples from an existing biorepository met the minimum DNA quality and quantity standards for inclusion in this retrospective study, which were nearly equally divided between adults (52.3% or n = 45/86, Table 1) and pediatric (47.7% or n = 41/86) patients (Table 2). Analysis of the adults in the study sample revealed approximately half were derived from females (55.6% or n = 25/45), which closely approximates the percentage of females in the overall clinic population (49.1%), p = 0.1614 (Table 1). In addition, analysis of the demographic characteristics demonstrated that the majority of the adult study samples were derived from racial or ethnic minorities (55.6% or n = 25/45), which was slightly lower but not significantly different from the percentages observed within the overall main clinic population (65.4%), p = 0.0592. Finally, the average age of the adult samples was found to be 41.5 years (range: 18 to 73 years), which closely approximates the average age of the main patient clinic population of 42.3 years (range: 18 to 89 years), p = 0.7738.

Table 1.
Demographic analysis of adult study samples.

Table 2.
Demographic analysis of pediatric study samples.
Analysis of the pediatric patients in the study sample revealed approximately half were derived from females (56.1% or n = 23/41), which was similar to the proportion of females observed from the pediatric clinic patient population (52.8%), p = 0.5478 (Table 2). Demographic analysis of the ethnic and racial characteristics of the study sample demonstrated that most patient samples from the pediatric clinic had self-identified as an ethnic or racial (non-White) minority (82.96% or n = 34/41), which was not deemed to be statistically significant from the observed proportion of minority (non-White) patients from the overall the pediatric clinic population (75.34%), p = 0.0647. In addition, the average age of samples within this study was 12.7 years, ranging between 5 and 17 years, which was slightly higher than the average age of the overall clinical population of pediatric patients of 10.4 years, ranging between 0 and 17 years, p = 0.2531.
Screening of the adult patients revealed that n = 10/45 or 22.2% different samples harbored one or more of the three high-risk strains of HPV analyzed, including 31, 33, and 35 (Figure 1). More specifically, n = 4/10 or 40% of the HPV-positive samples harbored HPV31 while n = 7/10 or 70% harbored HPV33, including one that was also positive for HPV31. However, none of the samples evaluated harbored HPV35.
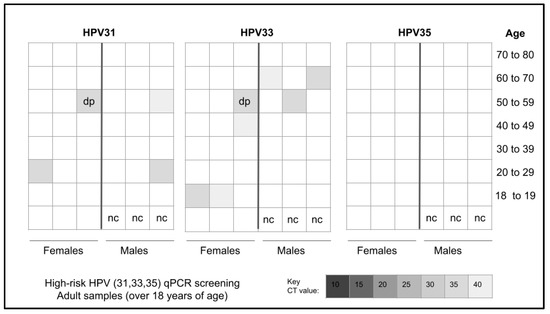
Figure 1.
Heatmap analysis for qPCR screening of adult samples for high-risk HPV. A total of n = 10 samples tested positive for HPV with n = 4/10 or 40% testing positive for HPV31, n = 6/10 testing positive for HPV33, and one sample testing positive for both HPV31 and HPV33. No samples tested positive for HPV35. nc = negative control, dp = double positive.
More detailed analysis of the adult samples revealed an equal distribution of HPV-positive samples between males (n = 5/10 or 50%) and females (n = 5/10 or 50%), which closely matched the distribution of HPV-negative samples from males and females, p = 0.5478 (Table 3). In addition, analysis of race and ethnicity revealed the majority of HPV-positive samples were derived from minority patients (60%), which closely matched the proportion of HPV-negative samples from minority patients (54.3%), p = 0.2286. Finally, the proportion of HPV-positive samples and HPV-negative samples from patients within the catch-up range (under 45 years) was nearly equal and not significantly different (40%, 42.9%, respectively), p = 0.5445.

Table 3.
Demographic analysis of adult HPV-positive and HPV-negative samples.
Screening of the pediatric patient samples revealed that n = 9/41 or 21.9% samples harbored one or more of the three high-risk strains of HPV, such as 31, 33, and 35 (Figure 2). More specifically, n = 6/9 or 66.7% of the HPV-positive samples harbored HPV31 while n = 7/9 or 77.8% harbored HPV33—including four that were double positive for both HPV31 and HPV33. However, none of the samples evaluated harbored HPV35.
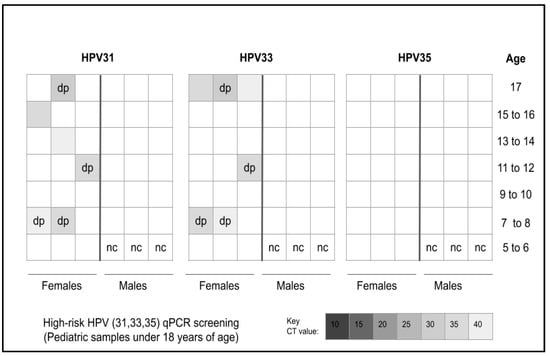
Figure 2.
Analysis of heatmap for qPCR screening of pediatric samples for high-risk HPV. A total of n = 9/41 or 21.9% of samples tested positive for HPV with n = 6/9 or 66.7% testing positive for HPV31, n = 7/9 or 77.8% testing positive for HPV33 and four samples testing positive for both HPV31 and HPV33. No samples tested positive for HPV35. nc = negative control, dp = double positive.
More detailed analysis of the pediatric samples revealed an unequal distribution of HPV-positive samples between males (n = 3/9 or 33.3%) and females (n = 6/9 or 66.7%), which was significantly different from the distribution of HPV-negative samples from males and females, p = 0.005 (Table 4). In addition, analysis of race and ethnicity revealed the majority of HPV-positive samples were derived from minority patients (n = 7/9 or 77.8%), which did not differ significantly from the proportion of HPV-negative samples from minority patients (n = 27/32 or 84.4%), p = 0.1017. Finally, the proportion of HPV-positive samples and HPV-negative samples from patients within the HPV vaccination age range (11 to 17 years) was significantly different (77.8%, 83.7%, respectively), p = 0.0001.

Table 4.
Demographic analysis of pediatric HPV-positive and HPV-negative samples.
4. Discussion
The primary goal of this study was to assess the prevalence of high-risk HPV strains 31, 33, and 35 using an existing biorepository including both pediatric and adult clinical saliva samples. The results of this study successfully demonstrated that HPV31 and HPV33 were found among both pediatric and adult samples in similar proportions (21.9% and 22.2%, respectively), although no samples tested positive for HPV35. These data represent the first clinical descriptions of non-HPV16 and non-HPV18 high-risk HPV prevalence within this patient population [27,28,29,30].
However, some notable differences were found regarding the prevalence of these high-risk HPV strains compared with previous studies of HPV strains HPV16 and HPV18. For example, this study found nearly one-quarter of adults (22.2%) harbored either HPV31, HPV33, or both. This is somewhat lower than the most recent study of HPV16 and HPV18 prevalence among adults within this patient population, which found an overall prevalence of 30.2% [27]. However, it is also much higher than the first description of HPV16 and HPV18 prevalence among adults from nearly a decade earlier that found an overall prevalence of only 2.6% within the same clinical patient population [30]. Moreover, these data also confirm other recent observations of HPV31 and HPV33 oral prevalence within other patient populations, which ranged between 5.7% and 14.3% [31,32,33]. These data may therefore support the overwhelming evidence that HPV16 and HPV18 remain the dominant oral strains of concern, but that other high-risk HPV strains may also be present if the appropriate screening is performed.
In addition, these data also demonstrated that HPV31 and HPV35 were found in approximately one-fifth of pediatric patient samples (21.9%), which corresponds to similar prevalence levels of HPV16 and HPV18 found within these patients as recently as last year (19.5%) [27]. Although this represents the first non-HPV16, non-HPV18 screening within this patient population, the more troubling aspect is the rise in pediatric oral HPV prevalence observed with HPV16 and HPV18, which was 2.5% in 2012, 9.2% in 2016, and 19.5% in 2022 [27,28,29]. As more studies confirm oral prevalence levels of high-risk HPV among pediatric populations at similar levels, the case for screening and evaluating these additional HPV strains becomes more critical [34,35].
Despite the significance of epidemiological data regarding high-risk HPV prevalence, as more studies are now screening for HPV strains other than HPV16 and HPV18, there are some limitations associated with this type of study that should also be considered [36,37]. Specifically, this was a retrospective study of previously collected saliva samples from an existing biorepository and may not reflect the most current oral prevalence, which may have shifted due to behavioral and vaccination practice changes following the onset of the SARS-CoV-2 (COVID-19) pandemic [38,39]. In addition, due to the retrospective nature of this study, no other health information (oral or systemic) was available to determine if other factors, such as smoking, vaping, or oral microbiota, might have influenced the outcomes of this study [40,41,42]. Finally, due to the parameters of the original protocol, these samples were part of cross-sectional studies involving one-time saliva collections, and therefore have no information regarding the temporal nature of the HPV detected and whether each was a short- or long-term infection.
However, due to the fact that high-risk oral HPV strains may have important functions in both the development and progression of oral cancers, any information regarding the prevalence of these non-HPV16 and non-HPV18 strains becomes critical for oral health researchers, clinicians, and epidemiologists to evaluate and consider [43,44]. Moreover, the findings from this study that most of the HPV-positive samples were from vaccination age-appropriate patients also suggest that more emphasis on the public health benefits and prevention of HPV-related diseases through vaccination may have the potential to yield significant results through more widespread awareness of the risks and prevalence of high-risk HPV and acceptance of these effective and low-cost prevention methods [45,46].
5. Conclusions
The importance of these findings, including the increased prevalence of high-risk strains HPV31 and HPV33 detected in this study combined with previous data about the increasing prevalence of HPV16 and HPV18 within this patient population, may suggest a more robust and focused effort on both HPV vaccination and awareness of oral HPV infection [27,28,29,30]. The results further highlight this need since the oral prevalence of HPV31 and HPV33 previously published may be underreported with current screenings [31,32,33]. However, recent evidence regarding increasing levels of vaccine hesitancy also suggest that more evidence may be needed to demonstrate the relevance of HPV prevention, particularly among this patient population [47,48]. This study is among the first to provide this type of evidence through the assessment and evaluation of oral HPV infection outside of the conventional HPV strains of HPV16 and HPV18.
Author Contributions
H.H., S.C., J.R.S. and K.K. were responsible for methodology, investigation, formal analysis, and writing—original draft preparation. H.H. and K.K. were responsible for conceptualization, methodology, resources, data curation, formal analysis, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Office of Research at the University of Nevada, Las Vegas—School of Dental Medicine and the Department of Advanced Education—Orthodontic Dental Residency Program.
Institutional Review Board Statement
This study involved an analysis of collected saliva biomedical repositories. The protocol and procedures for this study were reviewed and subsequently approved by the Institutional Review Board (IRB) of the University of Nevada, Las Vegas (UNLV) as Exempt under Protocol #171612-1 “Retrospective analysis of microbial prevalence from DNA isolated from saliva samples originally obtained from the University of Nevada, Las Vegas (UNLV) School of Dental Medicine (SDM) pediatric and clinical population” on 3 March 2021.
Informed Consent Statement
The original protocol for the collection of saliva samples from the UNLV–SDM clinic was approved under OPRS#1305-4466M titled “The Prevalence of Oral Microbes in Saliva from the UNLV School of Dental Medicine Pediatric and Adult Clinical Population”. Under this protocol, saliva samples were collected from volunteer patients at the beginning of their clinic appointment. Informed consent was collected from adult patients who chose to participate, while pediatric patients above the age of seven were also required to provide pediatric assent in addition to the informed consent and approval of the accompanying guardian or parent.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the presentation of preliminary data from this manuscript by S.C. and J.R.S. at the American Association for Dental Oral and Craniofacial Research (AADOCR) conference in 2023. The authors would like to acknowledge the Office of Research at the University of Nevada, Las Vegas—School of Dental Medicine and the Department of Advanced Education—Orthodontic Dental Residency Program for support of this project. K.K. is a co-investigator on the National Institute of Health (NIH) grant R15DE028431.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spurgeon, M.E. Small DNA tumor viruses and human cancer: Preclinical models of virus infection and disease. Tumour Virus Res. 2022, 14, 200239. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Wanjari, U.R.; Gopalakrishnan, A.V.; Kannampuzha, S.; Murali, R.; Namachivayam, A.; Ganesan, R.; Renu, K.; Dey, A.; Vellingiri, B.; et al. Exploring the Molecular Pathogenesis, Pathogen Association, and Therapeutic Strategies against HPV Infection. Pathogens 2022, 12, 25. [Google Scholar] [CrossRef]
- Oyouni, A.A.A. Human papillomavirus in cancer: Infection, disease transmission, and progress in vaccines. J. Infect. Public Health 2023, 16, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Huang, Y.; Li, Z. Prevention and treatment of human papillomavirus in men benefits both men and women. Front. Cell. Infect. Microbiol. 2022, 12, 1077651. [Google Scholar] [CrossRef]
- La Rosa, G.; Fratini, M.; Accardi, L.; D’Oro, G.; Della Libera, S.; Muscillo, M.; Di Bonito, P. Mucosal and cutaneous human papillomaviruses detected in raw sewages. PLoS ONE 2013, 8, e52391. [Google Scholar] [CrossRef]
- Stanley, M. Host defence and persistent human papillomavirus infection. Curr. Opin. Virol. 2021, 51, 106–110. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.-C.; Sandru, F.; Dumitrascu, M.C. Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar] [CrossRef]
- Liu, Z.; Rashid, T.; Nyitray, A.G. Penises not required: A systematic review of the potential for human papillomavirus horizontal transmission that is non-sexual or does not include penile penetration. Sex. Health 2016, 13, 10–21. [Google Scholar] [CrossRef]
- Rintala, S.; Dahlstrom, K.R.; Franco, E.L.; Louvanto, K. A synthesis of evidence for cancer-specific screening interventions: A Preventive Medicine Golden Jubilee Review. Prev. Med. 2023, 167, 107395. [Google Scholar] [CrossRef] [PubMed]
- Seay, J.; Matsuno, R.; Buechel, J.; Tannenbaum, K.; Wells, N. HPV-Related Cancers: A Growing Threat to U.S. Military Health and Readiness. Mil. Med. 2022, 187, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Stenger, M.R.; Baral, S.; Stahlman, S.; Wohlfeiler, D.; Barton, J.E.; Peterman, T. As through a glass, darkly: The future of sexually transmissible infections among gay, bisexual and other men who have sex with men. Sex. Health 2017, 14, 18–27. [Google Scholar] [CrossRef]
- Wijstma, E.S.; Jongen, V.W.; Alberts, C.J.; de Melker, H.E.; Hoes, J.; van der Loeff, M.F.S. Approaches to Estimating Clearance Rates for Human Papillomavirus Groupings: A Systematic Review and Real Data Examples. Epidemiology 2022, 34, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Andrei, E.C.; Baniță, I.M.; Munteanu, M.C.; Busuioc, C.J.; Mateescu, G.O.; Mălin, R.D.; Pisoschi, C.G. Oral Papillomatosis: Its Relation with Human Papilloma Virus Infection and Local Immunity—An Update. Medicina 2022, 58, 1103. [Google Scholar] [CrossRef] [PubMed]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection. Front. Cell. Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef]
- Plotzker, R.E.; Vaidya, A.; Pokharel, U.; Stier, E.A. Sexually Transmitted Human Papillomavirus: Update in Epidemiology, Prevention, and Management. Infect. Dis. Clin. North Am. 2023, 37, 289–310. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Tocut, S.M.; Popa, M.I.; Tampa, M. New Insights in the Pathogenesis of HPV Infection and the Associated Carcinogenic Processes: The Role of Chronic Inflammation and Oxidative Stress. J. Immunol. Res. 2018, 2018, 5315816. [Google Scholar] [CrossRef] [PubMed]
- Boda, D.; Neagu, M.; Constantin, C.; Voinescu, R.N.; Caruntu, C.; Zurac, S.; Spandidos, D.A.; Drakoulis, N.; Tsoukalas, D.; Tsatsakis, A.M. HPV strain distribution in patients with genital warts in a female population sample. Oncol. Lett. 2016, 12, 1779–1782. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Lai, Y.; Liu, Q.; Yan, J.; Tang, Y. Characteristics of human papillomaviruses infection in men with genital warts in Shanghai. Oncotarget 2016, 7, 53903–53910. [Google Scholar] [CrossRef][Green Version]
- Balmagambetova, S.; Tinelli, A.; Mynbaev, O.A.; Koyshybaev, A.; Urazayev, O.; Kereyeva, N.; Ismagulova, E. Human Papillomavirus Selected Properties and Related Cervical Cancer Prevention Issues. Curr. Pharm. Des. 2020, 26, 2073–2086. [Google Scholar] [CrossRef]
- Gravitt, P.E. The known unknowns of HPV natural history. J. Clin. Investig. 2011, 121, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Arndt, O.; Johannes, A.; Zeise, K.; Brock, J. HPV-Typen vom “high risk type” in oralen und laryngealen Papillomen und Leukoplakien [High-risk HPV types in oral and laryngeal papilloma and leukoplakia]. Laryngorhinootologie 1997, 76, 142–149. [Google Scholar] [CrossRef]
- Wood, Z.C.; Bain, C.J.; Smith, D.D.; Whiteman, D.C.; Antonsson, A. Oral human papillomavirus infection incidence and clearance: A systematic review of the literature. J. Gen. Virol. 2017, 98, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Bhatia, R.K.; Messeguer, A.L.; González, P.; Herrero, R.; Giuliano, A.R. Oral Human Papillomavirus in Healthy Individuals: A Systematic Review of the Literature. Sex. Transm. Dis. 2010, 37, 386–391. [Google Scholar] [CrossRef]
- Zhu, K.; Tian, Y.; Dong, X.; Akinwunmi, B.O.; Zhang, C.J.P.; Huang, J.; Ming, W.-K. The cost-effectiveness of bivalent, quadrivalent, and nine-valent HPV vaccination in Asia: A systematic review. Arch. Gynecol. Obstet. 2022, 306, 173–187. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H. Could the human papillomavirus vaccine prevent recurrence of ano-genital warts?: A systematic review and meta-analysis. Int. J. STD AIDS 2020, 31, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; Odone, A.; Ciorba, V.; Cella, P.; Audisio, R.A.; Lombardi, A.; Mariani, L.; Mennini, F.S.; Pecorelli, S.; Rezza, G.; et al. Human papillomavirus 9-valent vaccine for cancer prevention: A systematic review of the available evidence. Epidemiology Infect. 2017, 145, 1962–1982. [Google Scholar] [CrossRef]
- Kornhaber, M.S.; Florence, T.; Davis, T.; Kingsley, K. Assessment of Oral Human Papillomavirus Prevalence in Pediatric and Adult Patients within a Multi-Ethnic Clinic Population. Dent. J. 2022, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Tiku, V.; Todd, C.J.; Kingsley, K. Assessment of Oral Human Papillomavirus Prevalence in a Multi-ethnic Pediatric Clinic Population. Compend. Contin. Educ. Dent. 2016, 37, e1–e4. [Google Scholar]
- Flake, C.; Arafa, J.; Hall, A.; Ence, E.; Howard, K.; Kingsley, K. Screening and detection of human papillomavirus (HPV) high-risk strains HPV16 and HPV18 in saliva samples from subjects under 18 years old in Nevada: A pilot study. BMC Oral Health 2012, 12, 43. [Google Scholar] [CrossRef]
- Turner, D.O.; Williams-Cocks, S.J.; Bullen, R.; Catmull, J.; Falk, J.; Martin, D.; Mauer, J.; Barber, A.E.; Wang, R.C.; Gerstenberger, S.L.; et al. High-risk human papillomavirus (HPV) screening and detection in healthy patient saliva samples: A pilot study. BMC Oral Health 2011, 11, 28. [Google Scholar] [CrossRef]
- Cho, H.; Kishikawa, T.; Tokita, Y.; Suzuki, M.; Takemoto, N.; Hanamoto, A.; Fukusumi, T.; Yamamoto, M.; Fujii, M.; Ohno, Y.; et al. Prevalence of human papillomavirus in oral gargles and tonsillar washings. Oral Oncol. 2020, 105, 104669. [Google Scholar] [CrossRef] [PubMed]
- Saghravanian, N.; Ghazvini, K.; Babakoohi, S.; Firooz, A.; Mohtasham, N. Low prevalence of high risk genotypes of human papilloma virus in normal oral mucosa, oral leukoplakia and verrucous carcinoma. Acta Odontol. Scand. 2011, 69, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Kansky, A.A.; Seme, K.; Maver, P.J.; Luzar, B.; Gale, N.; Poljak, M. Human papillomaviruses (HPV) in tissue specimens of oral squamous cell papillomas and normal oral mucosa. Anticancer Res. 2006, 26, 3197–3201. [Google Scholar]
- Di Spirito, F.; Pantaleo, G.; Di Palo, M.P.; Amato, A.; Raimondo, A.; Amato, M. Oral Human Papillomavirus Benign Lesions and HPV-Related Cancer in Healthy Children: A Systematic Review. Cancers 2023, 15, 1096. [Google Scholar] [CrossRef]
- Pinheiro, R.d.S.; de França, T.R.T.; Ferreira, D.d.C.; Ribeiro, C.M.B.; Leão, J.C.; Castro, G.F. Human papillomavirus in the oral cavity of children. J. Oral Pathol. Med. 2011, 40, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nemesio, I.; Cury, F.; Longatto-Filho, A.; Fregnani, J.H.; Musselwhite, L.; Vazquez, F.; Peters, A.C.; Oliveira, C. Identification of human papillomavirus in oral rinse specimens from women with and without cervical intraepithelial lesions. Sex. Transm. Infect. 2020, 96, 408–410. [Google Scholar] [CrossRef]
- Walline, H.M.; Komarck, C.; McHugh, J.B.; Byrd, S.A.; Spector, M.E.; Hauff, S.J.; Graham, M.P.; Bellile, E.; Moyer, J.S.; Prince, M.E.; et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and oral cavity cancers: Comparison of multiple methods. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1320–1327. [Google Scholar] [CrossRef]
- Grigolato, R.; Accorona, R.; Lombardo, G.; Corrocher, G.; Garagiola, U.; Massari, F.; Nicoli, S.; Rossi, S.; Calabrese, L. Oral cancer in non-smoker non-drinker patients. Could comparative pet oncology help to understand risk factors and pathogenesis? Crit. Rev. Oncol. Hematol. 2021, 166, 103458. [Google Scholar] [CrossRef]
- Koskinen, A.I.; Hemminki, O.; Försti, A.; Hemminki, K. Incidence and survival in oral and pharyngeal cancers in Finland and Sweden through half century. BMC Cancer 2022, 22, 227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; D’souza, G.; Fakhry, C.; Bigelow, E.O.; Usyk, M.; Burk, R.D.; Zhao, N. Oral Human Papillomavirus Associated With Differences in Oral Microbiota Beta Diversity and Microbiota Abundance. J. Infect. Dis. 2022, 226, 1098–1108. [Google Scholar] [CrossRef]
- Muzio, L.L.; Ballini, A.; Cantore, S.; Bottalico, L.; Charitos, I.A.; Ambrosino, M.; Nocini, R.; Malcangi, A.; Dioguardi, M.; Cazzolla, A.P.; et al. Overview of Candida albicans and Human Papillomavirus (HPV) Infection Agents and their Biomolecular Mechanisms in Promoting Oral Cancer in Pediatric Patients. BioMed Res. Int. 2021, 2021, 7312611. [Google Scholar] [CrossRef] [PubMed]
- Klawinski, D.; Hanna, I.; Breslin, N.K.; Katzenstein, H.M.; Indelicato, D.J. Vaping the Venom: Oral Cavity Cancer in a Young Adult With Extensive Electronic Cigarette Use. Pediatrics 2021, 147, e2020022301. [Google Scholar] [CrossRef]
- Kansy, K.; Thiele, O.; Freier, K. The role of human papillomavirus in oral squamous cell carcinoma: Myth and reality. Oral Maxillofac. Surg. 2014, 18, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Pinkiewicz, M.; Dorobisz, K.; Zatoński, T. Human Papillomavirus-Associated Head and Neck Cancers. Where are We Now? A Systematic Review. Cancer Manag. Res. 2022, 14, 3313–3324. [Google Scholar] [CrossRef]
- Jiang, S.; Dong, Y. Human papillomavirus and oral squamous cell carcinoma: A review of HPV-positive oral squamous cell carcinoma and possible strategies for future. Curr. Probl. Cancer 2017, 41, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.J.; Jakobsen, K.K.; Jensen, J.S.; Grønhøj, C.; Von Buchwald, C. The Effect of Prophylactic HPV Vaccines on Oral and Oropharyngeal HPV Infection—A Systematic Review. Viruses 2021, 13, 1339. [Google Scholar] [CrossRef] [PubMed]
- Maginot, R.; Esteves, C.; Kingsley, K. Changing Perspectives on Pediatric Human Papillomavirus (HPV) Vaccination among Dental Students and Residents Reveals Recent Increase in Vaccine Hesitancy. Vaccines 2022, 10, 570. [Google Scholar] [CrossRef]
- Mann, S.K.; Kingsley, K. Human Papillomavirus (HPV) Vaccine Knowledge, Awareness and Acceptance among Dental Students and Post-Graduate Dental Residents. Dent. J. 2020, 8, 45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).