Acceptability and Usability of the Family Gene Toolkit for Swiss and Korean Families Harboring BRCA1/BRAC2 Pathogenic Variants: A Web-Based Platform for Cascade Genetic Testing
Abstract
:Simple Summary
Abstract
1. Introduction
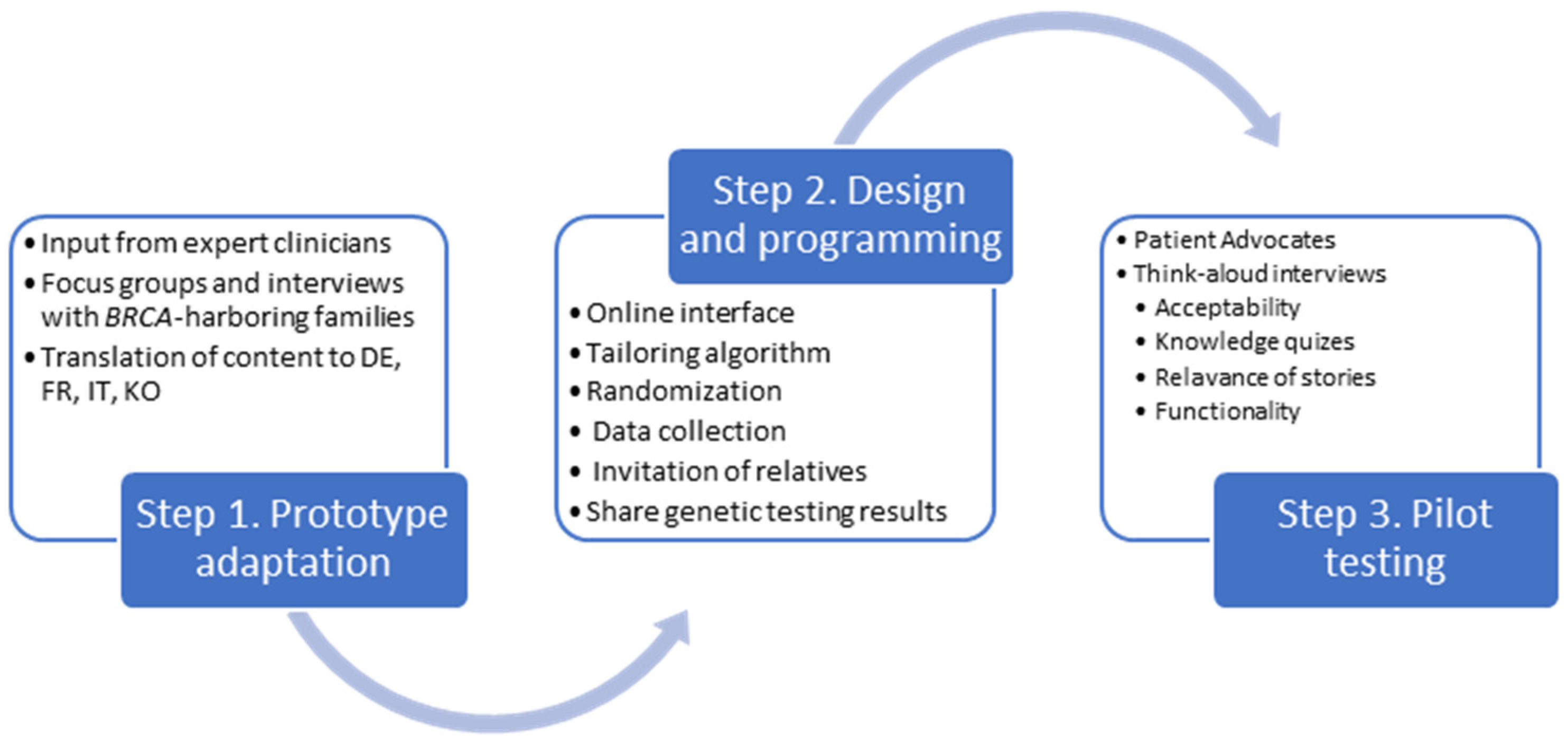
2. Materials and Methods
2.1. Step 1. Adaptation of the Prototype
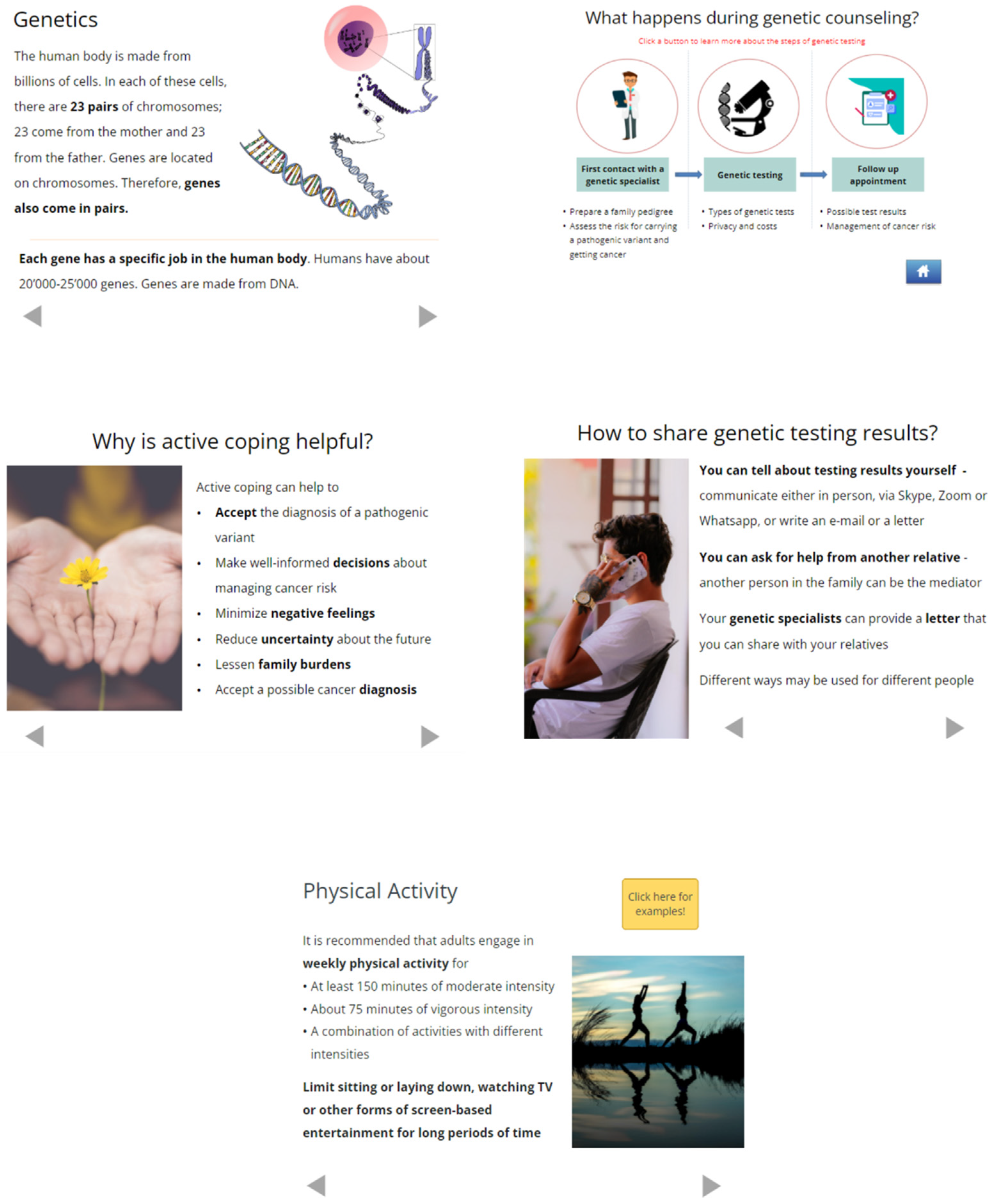
2.2. Design and Programming
2.3. Acceptability and Usability Testing
3. Results
3.1. Adaptation of the Prototype
3.2. Design and Programming
- Enable secure, password-protected log-in for potential participants, assess eligibility, and provide a web-based consent form;
- Deliver a baseline questionnaire to collect information used for message tailoring and for evaluating outcomes;
- Facilitate the invitation of at-risk relatives to the web application via email and SMS messaging;
- Randomize participants either to the Family Gene Toolkit or a comparator website. At-risk relatives will be automatically assigned to the same group as the person who invited them to the study;
- Deliver the Family Gene Toolkit or the comparator, a non-interactive generic website that provides basic information related to HBOC;
- Deliver an evaluation questionnaire to assess satisfaction with the content of the Family Gene Toolkit and the comparator and with the technical aspects of navigating the web application;
- Deliver a follow-up questionnaire that will be used for evaluating primary and secondary outcomes related to family communication of testing results and cascade testing of relatives.
3.3. Acceptability and Usability Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samadder, N.J.; Giridhar, K.V.; Baffy, N.; Riegert-Johnson, D.; Couch, F.J. Hereditary Cancer Syndromes-A Primer on Diagnosis and Management: Part 1: Breast-Ovarian Cancer Syndromes. Mayo Clin. Proc. 2019, 94, 1084–1098. [Google Scholar] [CrossRef]
- Prevention Centers for Disease Control and Cascade Testing for Hereditary Breast and Ovarian Cancer. Available online: https://www.cdc.gov/genomics/disease/cascade_testing/cascade_hboc.htm (accessed on 19 April 2023).
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021, 384, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 3.2023: Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2023, 18, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, N.B.; Wagner, J.K.; Hampel, H.; Devore, C.; Shridhar, N.; Williams, J.L.; Donohue, K.E.; Kullo, I.; Prince, A.E.R. What guidance does HIPAA offer to providers considering familial risk notification and cascade genetic testing? J. Law Biosci. 2020, 7, lsaa071. [Google Scholar] [CrossRef]
- Healey, E.; Taylor, N.; Greening, S.; Wakefield, C.E.; Warwick, L.; Williams, R.; Tucker, K. Quantifying family dissemination and identifying barriers to communication of risk information in Australian BRCA families. Genet. Med. 2017, 19, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Marleen Van Den Heuvel, L.; Stemkens, D.; Van Zelst-Stams, W.A.G.; Willeboordse, F.; Christiaans, I. How to inform at-risk relatives? Attitudes of 1379 Dutch patients, relatives, and members of the general population. J. Genet. Couns. 2020, 29, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzani, C.; Aceti, M.; Schweighoffer, R.; Kaiser-Grolimund, A.; Bürki, N.; Chappuis, P.O.; Graffeo, R.; Monnerat, C.; Pagani, O.; Rabaglio, M.; et al. The Communication Chain of Genetic Risk: Analyses of Narrative Data Exploring Proband-Provider and Proband-Family Communication in Hereditary Breast and Ovarian Cancer. J. Pers. Med. 2022, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.; Kim, S.; Katapodi, M.C. Informational needs of individuals from families harboring BRCA pathogenic variants: A systematic review and content analysis. Genet. Med. 2023, 25, 100001. [Google Scholar] [CrossRef]
- Taber, J.M.; Chang, C.Q.; Lam, T.K.; Gillanders, E.M.; Hamilton, J.G.; Schully, S.D. Prevalence and correlates of receiving and sharing high-penetrance cancer genetic test results: Findings from the Health Information National Trends Survey. Public Health Genom. 2015, 18, 67–77. [Google Scholar] [CrossRef]
- Srinivasan, S.; Won, N.Y.; Dotson, W.D.; Wright, S.T.; Roberts, M.C. Barriers and facilitators for cascade testing in genetic conditions: A systematic review. Eur. J. Hum. Genet. 2020, 28, 1631–1644. [Google Scholar] [CrossRef]
- Kehm, R.D.; Llanos, A.A.M.; Mcdonald, J.A.; Tehranifar, P.; Terry, M.B. Evidence-Based Interventions for Reducing Breast Cancer Disparities: What Works and Where the Gaps Are? Cancers 2022, 14, 4122. [Google Scholar] [CrossRef] [PubMed]
- Law, W.K.; Yaremych, H.E.; Ferrer, R.A.; Richardson, E.; Wu, Y.P.; Turbitt, E. Decision-making about genetic health information among family dyads: A systematic literature review. Health Psychol. Rev. 2022, 16, 412–429. [Google Scholar] [CrossRef]
- Wiens, M.E.; Wilson, B.J.; Honeywell, C.; Etchegary, H. A family genetic risk communication framework: Guiding tool development in genetics health services. J. Community Genet. 2013, 4, 233–242. [Google Scholar] [CrossRef]
- Witt Magdalena, M.; Jankowska Katarzyna, A. Breaking bad news in genetic counseling—Problems and communication tools. J. Appl. Genet. 2018, 59, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Dheensa, S.; Lucassen, A.; Fenwick, A. Limitations and Pitfalls of Using Family Letters to Communicate Genetic Risk: A Qualitative Study with Patients and Healthcare Professionals. J. Genet. Couns. 2018, 27, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.V.; Frederiksen, B.L.; Lautrup, C.K.; Lindberg, L.J.; Ladelund, S.; Nilbert, M. Unsolicited information letters to increase awareness of Lynch syndrome and familial colorectal cancer: Reactions and attitudes. Fam. Cancer 2019, 18, 43–51. [Google Scholar] [CrossRef]
- Rosenlund, M.; Kinnunen, U.M.; Saranto, K. The Use of Digital Health Services Among Patients and Citizens Living at Home: Scoping Review. J. Med. Internet Res. 2023, 25, e44711. [Google Scholar] [CrossRef] [PubMed]
- Biesecker, B.B.; Lewis, K.L.; Umstead, K.L.; Johnston, J.J.; Turbitt, E.; Fishler, K.P.; Patton, J.H.; Miller, I.M.; Heidlebaugh, A.R.; Biesecker, L.G. Web Platform vs In-Person Genetic Counselor for Return of Carrier Results From Exome Sequencing: A Randomized Clinical Trial. JAMA Intern Med. 2018, 178, 338–346. [Google Scholar] [CrossRef]
- Gaieski, J.B.; Patrick-Miller, L.; Egleston, B.L.; Maxwell, K.N.; Walser, S.; DiGiovanni, L.; Brower, J.; Fetzer, D.; Ganzak, A.; McKenna, D.; et al. Research participants’ experiences with return of genetic research results and preferences for web-based alternatives. Mol. Genet. Genomic Med. 2019, 7, e898. [Google Scholar] [CrossRef] [PubMed]
- Krassuski, L.; Vennedey, V.; Stock, S.; Kautz-Freimuth, S. Effectiveness of decision aids for female BRCA1 and BRCA2 mutation carriers: A systematic review. BMC Med. Inform. Decis. Mak. 2019, 19, 154. [Google Scholar] [CrossRef]
- Leighton, S.; Forrest, L.E.; Young, M.A.; Delatycki, M.B.; Lynch, E. Social media usage in family communication about genetic information: ‘I no longer speak with my sister but she needed to know’. J. Genet. Couns. 2021, 30, 180–190. [Google Scholar] [CrossRef]
- Kautz-Freimuth, S.; Redaèlli, M.; Rhiem, K.; Vodermaier, A.; Krassuski, L.; Nicolai, K.; Schnepper, M.; Kuboth, V.; Dick, J.; Vennedey, V.; et al. Development of decision aids for female BRCA1 and BRCA2 mutation carriers in Germany to support preference-sensitive decision-making. BMC Med. Inform. Decis. Mak. 2021, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Oshima, S.M.; Tait, S.D.; Thomas, S.M.; Fayanju, O.M.; Ingraham, K.; Barrett, N.J.; Hwang, E.S. Association of Smartphone Ownership and Internet Use With Markers of Health Literacy and Access: Cross-sectional Survey Study of Perspectives From Project PLACE (Population Level Approaches to Cancer Elimination). J. Med. Internet Res. 2021, 23, e24947. [Google Scholar] [CrossRef] [PubMed]
- Paradis, S.; Roussel, J.; Bosson, J.L.; Kern, J.B. Use of Smartphone Health Apps Among Patients Aged 18 to 69 Years in Primary Care: Population-Based Cross-sectional Survey. JMIR Form. Res. 2022, 6, e34882. [Google Scholar] [CrossRef] [PubMed]
- Baroutsou, V.; Underhill-Blazey, M.L.; Appenzeller-Herzog, C.; Katapodi, M.C. Interventions Facilitating Family Communication of Genetic Testing Results and Cascade Screening in Hereditary Breast/Ovarian Cancer or Lynch Syndrome: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 925. [Google Scholar] [CrossRef]
- Campbell-Salome, G.; Jones, L.K.; Walters, N.L.; Morgan, K.M.; Brangan, A.; Ladd, I.G.; McGowan, M.P.; Wilemon, K.; Schmidlen, T.J.; Simmons, E.; et al. Optimizing communication strategies and designing a comprehensive program to facilitate cascade testing for familial hypercholesterolemia. BMC Health Serv. Res. 2023, 23, 340. [Google Scholar] [CrossRef]
- Caswell-Jin, J.L.; Zimmer, A.D.; Stedden, W.; Kingham, K.E.; Zhou, A.Y.; Kurian, A.W. Cascade Genetic Testing of Relatives for Hereditary Cancer Risk: Results of an Online Initiative. J. Natl. Cancer Inst. 2019, 111, 95–98. [Google Scholar] [CrossRef]
- Goodman, S.; Skirton, H.; Jackson, L.; Jones, R.B. Development of a Secure Website to Facilitate Information Sharing in Families at High Risk of Bowel Cancer-The Familyweb Study. Cancers 2021, 13, 2404. [Google Scholar] [CrossRef]
- Haas, C.B.; Ralston, J.; Fullerton, S.M.; Scrol, A.; Henrikson, N.B. Environmental scan of family chart linking for genetic cascade screening in a U.S. integrated health system. Front. Genet. 2022, 13, 886650. [Google Scholar] [CrossRef]
- Peshkin, B.N.; Ladd, M.K.; Isaacs, C.; Segal, H.; Jacobs, A.; Taylor, K.L.; Graves, K.D.; O’neill, S.C.; Schwartz, M.D. The Genetic Education for Men (GEM) Trial: Development of Web-Based Education for Untested Men in BRCA1/2-Positive Families. J. Cancer Educ. 2021, 36, 72–84. [Google Scholar] [CrossRef]
- Pollard, S.; Weymann, D.; Loewen, R.; Nuk, J.; Sun, S.; Schrader, K.A.; Hessels, C.; Regier, D.A. Development and early-stage evaluation of a patient portal to enhance familial communication about hereditary cancer susceptibility testing: A patient-driven approach. Health Expect. 2023, 26, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Schmidlen, T.; Jones, C.L.; Campbell-Salome, G.; Mccormick, C.Z.; Vanenkevort, E.; Sturm, A.C. Use of a chatbot to increase uptake of cascade genetic testing. J. Genet. Couns. 2022, 31, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Katapodi, M.C.; Jung, M.; Schafenacker, A.M.; Milliron, K.J.; Mendelsohn-Victor, K.E.; Merajver, S.D.; Northouse, L.L. Development of a Web-based Family Intervention for BRCA Carriers and Their Biological Relatives: Acceptability, Feasibility, and Usability Study. JMIR Cancer 2018, 4, e7. [Google Scholar] [CrossRef]
- Folkman, S.; Lazarus, R.S.; Dunkel-Schetter, C.; Delongis, A.; Gruen, R.J. Dynamics of a stressful encounter: Cognitive appraisal, coping, and encounter outcomes. J. Pers. Soc. Psychol. 1986, 50, 992–1003. [Google Scholar] [CrossRef]
- Rolland, J.S.; Williams, J.K. Toward a biopsychosocial model for 21st-century genetics. Fam. Process 2005, 44, 3–24. [Google Scholar] [CrossRef]
- Gooding, H.C.; Organista, K.; Burack, J.; Biesecker, B.B. Genetic susceptibility testing from a stress and coping perspective. Soc. Sci. Med. 2006, 62, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Janis, I.L.; Mann, L. Decision Making: A Psychological Analysis of Conflict, Choice, and Commitment; Free Press: New York, NY, USA, 1977. [Google Scholar]
- Elwyn, G.; O’connor, A.; Stacey, D.; Volk, R.; Edwards, A.; Coulter, A.; Thomson, R.; Barratt, A.; Barry, M.; Bernstein, S.; et al. Developing a quality criteria framework for patient decision aids: Online international Delphi consensus process. BMJ 2006, 333, 417. [Google Scholar] [CrossRef] [PubMed]
- Han, S.A.; Kim, S.W.; Kang, E.; Park, S.K.; Ahn, S.H.; Lee, M.H.; Nam, S.J.; Han, W.; Bae, Y.T.; Kim, H.A.; et al. The prevalence of BRCA mutations among familial breast cancer patients in Korea: Results of the Korean Hereditary Breast Cancer study. Fam. Cancer 2013, 12, 75–81. [Google Scholar] [CrossRef]
- Kang, E.; Kim, S.W. The korean hereditary breast cancer study: Review and future perspectives. J. Breast Cancer 2013, 16, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, D.; Azzarello-Burri, S.; Steindl, K.; Boonsawat, P.; Zweier, M.; Dedes, K.J.; Joset, P.; Fink, D.; Rauch, A. Prevalence of genetic susceptibility for breast and ovarian cancer in a non-cancer related study population: Secondary germline findings from a Swiss single centre cohort. Swiss Med. Wkly. 2019, 149, w20092. [Google Scholar] [CrossRef] [PubMed]
- Prevention Centers for Disease Control and Simply Put: A Guide for Creating Easy-to-Understand Materials. Available online: https://www.cdc.gov/healthliteracy/pdf/simply_put.pdf (accessed on 19 April 2023).
- Weber, W.P.; Morrow, M.; Boniface, J.; Pusic, A.; Montagna, G.; Kappos, E.A.; Ritter, M.; Haug, M.; Kurzeder, C.; Saccilotto, R.; et al. Knowledge gaps in oncoplastic breast surgery. Lancet Oncol. 2020, 21, e375–e385. [Google Scholar] [CrossRef] [PubMed]
- Katapodi, M.C.; Viassolo, V.; Caiata-Zufferey, M.; Nikolaidis, C.; Bührer-Landolt, R.; Buerki, N.; Graffeo, R.; Horváth, H.C.; Kurzeder, C.; Rabaglio, M.; et al. Cancer Predisposition Cascade Screening for Hereditary Breast/Ovarian Cancer and Lynch Syndromes in Switzerland: Study Protocol. JMIR Res. Protoc. 2017, 6, e184. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Aceti, M.; Baroutsou, V.; Bürki, N.; Caiata-Zufferey, M.; Cattaneo, M.; Chappuis, P.O.; Ciorba, F.M.; Graffeo-Galbiati, R.; Heinzelmann-Schwarz, V.; et al. Using a Tailored Digital Health Intervention for Family Communication and Cascade Genetic Testing in Swiss and Korean Families With Hereditary Breast and Ovarian Cancer: Protocol for the DIALOGUE Study. JMIR Res. Protoc. 2021, 10, e26264. [Google Scholar] [CrossRef]
- Darejeh, A.; Singh, D. A review on user interface design principles to increase software usability for users with less computer literacy. J. Comput. Sci. 2013, 9, 1443. [Google Scholar] [CrossRef]
- Kalbach, J. Designing Web Navigation: Optimizing the User Experience; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2007. [Google Scholar]
- Horton, S.; Quesenbery, W. A Web for Everyone: Designing Accessible User Experiences; Rosenfeld Media: New York, NY, USA, 2014. [Google Scholar]
- Kushniruk, A.W.; Patel, V.L. Cognitive and usability engineering methods for the evaluation of clinical information systems. J. Biomed. Inform. 2004, 37, 56–76. [Google Scholar] [CrossRef]
- Bastien, J.M. Usability testing: A review of some methodological and technical aspects of the method. Int. J. Med. Inform. 2010, 79, e18–e23. [Google Scholar] [CrossRef] [PubMed]
- Hartson, H.R.; Andre, T.S.; Williges, R.C. Criteria for evaluating usability evaluation methods. Int. J. Hum. Comput. Interact. 2001, 13, 373–410. [Google Scholar] [CrossRef]
- Team R Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/index.html (accessed on 13 February 2023).
- Fonteyn, M.E.; Kuipers, B.; Grobe, S.J. A description of think aloud method and protocol analysis. Qual. Health Res. 1993, 3, 430–441. [Google Scholar] [CrossRef]
- Statistics Federal Office of Languages. Available online: https://www.bfs.admin.ch/bfs/en/home/statistics/population/languages-religions/languages.html (accessed on 19 April 2023).
- Wikipedia. Korean Diaspora. Available online: https://en.wikipedia.org/wiki/Korean_diaspora (accessed on 7 July 2023).
- Gaff, C.L.; Bylund, C.L. Chapter 15: Facilitating family communication about genetics in practice. In Family Communication about Genetics: Theory and Practice; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Katapodi, M.C.; Ming, C.; Northouse, L.L.; Duffy, S.A.; Duquette, D.; Mendelsohn-Victor, K.E.; Milliron, K.J.; Merajver, S.D.; Dinov, I.D.; Janz, N.K. Genetic Testing and Surveillance of Young Breast Cancer Survivors and Blood Relatives: A Cluster Randomized Trial. Cancers 2020, 12, 2526. [Google Scholar] [CrossRef]
- Underhill, M.L.; Jones, T.; Habin, K. Disparities in Cancer Genetic Risk Assessment and Testing. Oncol. Nurs. Forum 2016, 43, 519–523. [Google Scholar] [CrossRef]
- Kim, S.H.; Choe, Y.H.; Kim, D.H. Patient Empowerment in Cancer Care: A Scoping Review. Cancer Nurs 2023. [Google Scholar] [CrossRef] [PubMed]
- Seiler, A.; Jenewein, J. Resilience in Cancer Patients. Front. Psychiatry 2019, 10, 208. [Google Scholar] [CrossRef]
- Mackenzie, C.; Stoljar, N. Relational Autonomy: Feminist Perspectives on Autonomy, Agency, and the Social Self; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Weller, S.; Lyle, K.; Lucassen, A. Re-imagining ‘the patient’: Linked lives and lessons from genomic medicine. Soc. Sci. Med. 2022, 297, 114806. [Google Scholar] [CrossRef]
- Korngiebel, D.M.; Mooney, S.D. Considering the possibilities and pitfalls of Generative Pre-trained Transformer 3 (GPT-3) in healthcare delivery. npj Digit. Med. 2021, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Mozilla Web Docs Mdn. Using the Web Speech API. Available online: https://developer.mozilla.org/en-US/docs/Web/API/Web_Speech_API/Using_the_Web_Speech_API (accessed on 26 April 2023).
- National Academies of Sciences, Engineering, and Medicine. Understanding Disparities in Access to Genomic Medicine: Proceedings of a Workshop; National Academies Press: Washington, CA, USA, 2018. [Google Scholar]
- Grimmett, C.; Pickett, K.; Shepherd, J.; Welch, K.; Recio-Saucedo, A.; Streit, E.; Seers, H.; Armstrong, A.; Cutress, R.I.; Evans, D.G.; et al. Systematic review of the empirical investigation of resources to support decision-making regarding BRCA1 and BRCA2 genetic testing in women with breast cancer. Patient Educ. Couns. 2018, 101, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Ayme, A.; Viassolo, V.; Rapiti, E.; Fioretta, G.; Schubert, H.; Bouchardy, C.; Chappuis, P.O.; Benhamou, S. Determinants of genetic counseling uptake and its impact on breast cancer outcome: A population-based study. Breast Cancer Res. Treat. 2014, 144, 379–389. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Swiss Sample N = 46 | Korean Sample N = 22 |
|---|---|---|
| Age (mean, range) | 50 (32–72) | 42 (27–68) |
| Linguistic region | (n, %) | (n, %) |
| French-speaking | 25 (54%) | Not applicable |
| German-speaking | 14 (31%) | |
| Italian-speaking | 7 (15%) | |
| Education | ||
| Compulsory/High school/Technical school | 28 (61%) | 7 (32%) |
| University/Post-graduate degree | 18 (39%) | 15 (68%) |
| Employment | ||
| Yes | 36 (78%) | 8 (36%) |
| No | 10 (22%) | 14 (64%) |
| Marital status | ||
| Married/Partnered | 35 (76%) | 15 (68%) |
| Divorced/Separated/Widowed | 7 (15%) | 1 (5%) |
| Single | 4 (9%) | 6 (27%) |
| Previous cancer diagnosis | ||
| Yes (breast, ovarian, other) | 29 (63%) | 17 (77%) |
| No | 17 (37%) | 5 (23%) |
| Characteristic | N = 18 |
|---|---|
| Age (mean, range) | 51 (28–70) |
| Linguistic region | |
| French-speaking | 7 (39%) |
| German-speaking | 5 (28%) |
| Italian-speaking | 1 (6%) |
| Korean-speaking | 5 (28%) |
| Education | |
| Compulsory/High school/Technical school | 10 (56%) |
| University/Post-graduate degree | 8 (44%) |
| Employment | |
| Yes | 12 (67%) |
| No | 6 (33%) |
| Marital status | |
| Married/Partnered | 13 (72%) |
| Divorced/Separated/Widowed | 2 (12%) |
| Single | 3 (16%) |
| Previous cancer diagnosis | |
| Yes (breast, ovarian, other) | 12 (67%) |
| No | 6 (33%) |
| Question | Median (IQR) * |
|---|---|
| The Family Gene Toolkit had helpful information for… | |
| risk factors for hereditary breast and ovarian cancer syndrome | 7 (1) |
| the genetic counseling and genetic testing process | 7 (1) |
| how to find genetic services | 7 (1) |
| cancer screening for people at higher risk | 7 (1) |
| tips for family communication of genetic testing results | 7 (0) |
| tips for family support in genetic cancer syndromes | 7 (0) |
| The Family Gene Toolkit… | |
| was easy to understand | 7 (1) |
| took too much time to review | 3 (4) |
| made me nervous | 1 (1) |
| was important to me | 7 (1) |
| made me think about ways to help my family | 6 (2) |
| was not useful to me | 1 (1) |
| I would suggest this study to other people | 7 (1) |
| The study was important | 7 (1) |
| Topic | Question | Quotes from “Think Aloud” Interviews |
|---|---|---|
| Content | How did you like the content of the Family Gene Toolkit? | “I’d like to show it to my son…there is a lot of information about men.” |
| “I had no idea that there are medications that could reduce cancer risk.” | ||
| “I found the quiz really helpful; it helps the information to stick in my mind.” | ||
| Missing information | Is there any information that you needed but it was not addressed? | “I would like to find more information about my personal cancer risk. And a specific risk estimate.…That would be more helpful for me.“ |
| Timing of intervention | When do you think is the best time to deliver this information? | “I wish I had this intervention before I even started thinking about genetic testing and dealing with my cancer risk.” |
| “I think this intervention would be more helpful when someone is just being diagnosed with the mutation.” | ||
| Navigation | How easy or difficult was it to navigate the web application? | “I expected that clicking on the arrow would take me back to the main menu, but it didn’t. It was not clear to me what this ‘home’ button was.” |
| “The quizzes are very nice, but I would also like to have a detailed explanation when I selected the correct answer.” (This comment was addressed in subsequent interviews.) | ||
| “It was not clear that I could find more links and see more stories when I clicked on words that were blue and bold.” | ||
| Overall satisfaction | Overall, what do you think about the information covered in the Family Gene Toolkit? | “The intervention is very well-done, with clear and comprehensive information, and made me feel that I want to read more.” |
| “It contains everything and exhausted all the information.” | ||
| “Overall, I think the intervention is nice, has beautiful pictures, and is user-friendly. I had no trouble navigating through and finding what I needed.” | ||
| Overall, was there something that you did not like? | “The intervention was very informative and well-structured, but I feel that this is very long.” | |
| “I think it would be stressful for some people to get this information. Maybe the intervention needs some more positive content.” | ||
| “I felt burdoned to tell my relatives. To me, it was hard to share results with my family members.” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroutsou, V.; Duong, V.; Signorini, A.; Saccilotto, R.; Ciorba, F.M.; Bürki, N.; Caiata-Zufferey, M.; Ryu, J.M.; Kim, S.-W.; Lim, M.C.; et al. Acceptability and Usability of the Family Gene Toolkit for Swiss and Korean Families Harboring BRCA1/BRAC2 Pathogenic Variants: A Web-Based Platform for Cascade Genetic Testing. Cancers 2023, 15, 4485. https://doi.org/10.3390/cancers15184485
Baroutsou V, Duong V, Signorini A, Saccilotto R, Ciorba FM, Bürki N, Caiata-Zufferey M, Ryu JM, Kim S-W, Lim MC, et al. Acceptability and Usability of the Family Gene Toolkit for Swiss and Korean Families Harboring BRCA1/BRAC2 Pathogenic Variants: A Web-Based Platform for Cascade Genetic Testing. Cancers. 2023; 15(18):4485. https://doi.org/10.3390/cancers15184485
Chicago/Turabian StyleBaroutsou, Vasiliki, Vu Duong, Alice Signorini, Ramon Saccilotto, Florina M. Ciorba, Nicole Bürki, Maria Caiata-Zufferey, Jai Min Ryu, Sung-Won Kim, Myong Cheol Lim, and et al. 2023. "Acceptability and Usability of the Family Gene Toolkit for Swiss and Korean Families Harboring BRCA1/BRAC2 Pathogenic Variants: A Web-Based Platform for Cascade Genetic Testing" Cancers 15, no. 18: 4485. https://doi.org/10.3390/cancers15184485






