Neuroimaging and Neurocognitive Outcomes in Older Patients with Multiple Myeloma Treated with Chemotherapy and Autologous Stem Cell Transplantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Measures
3. Statistical, Imaging, and Cytokine Analyses
Cytokine Panel Analysis
4. Results
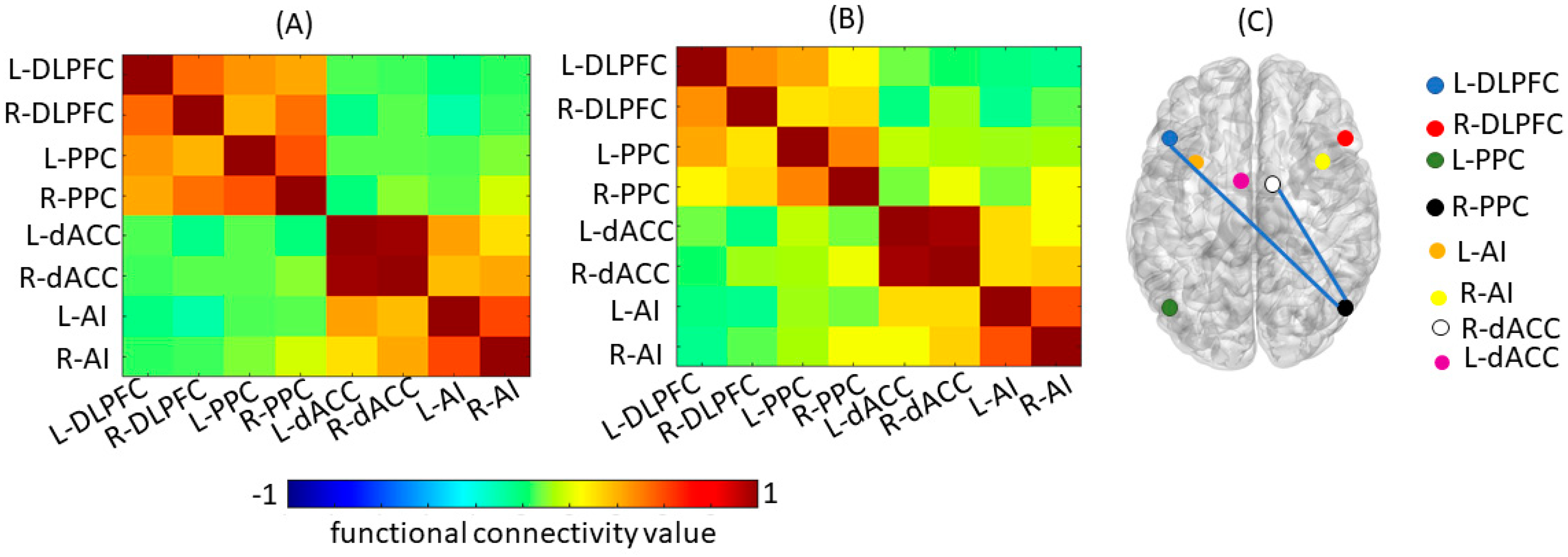
4.1. Structural and Functional Imaging
4.2. Neurocognitive Function and Self-Report Scales
4.3. Multiplex Cytokine Panel
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dietrich, J.; Monje, M.; Wefel, J.; Meyers, C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 2008, 13, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Monje, M. Microglia in Cancer Therapy-Related Cognitive Impairment. Trends Neurosci. 2021, 44, 441–451. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Smith, D.J.; West, J.D.; Saykin, A.J. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: A replication and extension study. Brain Behav. Immun. 2012, 30, S117–S125. [Google Scholar] [CrossRef]
- Kesler, S.R. Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiol. Aging 2014, 35 (Suppl. S2), S11–S19. [Google Scholar] [CrossRef]
- Correa, D.D.; Root, J.C.; Baser, R.; Moore, D.; Peck, K.K.; Lis, E.; Shore, T.B.; Thaler, H.T.; Jakubowski, A.; Relkin, N. A prospective evaluation of changes in brain structure and cognitive functions in adult stem cell transplant recipients. Brain Imaging Behav. 2013, 7, 478–490. [Google Scholar] [CrossRef]
- Correa, D.D.; Wang, Y.; West, J.D.; Peck, K.K.; Root, J.C.; Baser, R.E.; Thaler, H.T.; Shore, T.B.; Jakubowski, A.; Saykin, A.J.; et al. Prospective assessment of white matter integrity in adult stem cell transplant recipients. Brain Imaging Behav. 2016, 10, 486–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buchbinder, D.; Kelly, D.L.; Duarte, R.F.; Auletta, J.J.; Bhatt, N.; Byrne, M.; DeFilipp, Z.; Gabriel, M.; Mahindra, A.; Norkin, M.; et al. Neurocognitive dysfunction in hematopoietic cell transplant recipients: Expert review from the late effects and Quality of Life Working Committee of the CIBMTR and complications and Quality of Life Working Party of the EBMT. Bone Marrow Transpl. 2018, 53, 535–555. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Vichaya, E.G.; Wang, X.S.; Sailors, M.H.; Cleeland, C.S.; Wefel, J.S. Acute cognitive impairment in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplant. Cancer 2013, 119, 4188–4195. [Google Scholar] [CrossRef]
- Bury-Kamińska, M.; Szudy-Szczyrek, A.; Nowaczyńska, A.; Jankowska-Łęcka, O.; Hus, M.; Kot, K. Chemotherapy-Related Differences in Cognitive Functioning and Their Biological Predictors in Patients with Multiple Myeloma. Brain Sci. 2021, 11, 1166. [Google Scholar] [CrossRef]
- Jacobs, S.R.; Small, B.J.; Booth-Jones, M.; Jacobsen, P.B.; Fields, K.K. Changes in cognitive functioning in the year after hematopoietic stem cell transplantation. Cancer 2007, 110, 1560–1567. [Google Scholar] [CrossRef]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediat. Inflamm. 2017, 2017, 1852517. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Mandelblatt, J.S.; Jacobsen, P.B.; Ahles, T. Cognitive Effects of Cancer Systemic Therapy: Implications for the Care of Older Patients and Survivors. J. Clin. Oncol. 2014, 32, 2617–2626. [Google Scholar] [CrossRef]
- Vachha, B.A.; Gohel, S.; Root, J.C.; Kryza-Lacombe, M.; Hensley, M.L.; Correa, D.D. Altered regional homogeneity in patients with ovarian cancer treated with chemotherapy: A resting state fMRI study. Brain Imaging Behav. 2022, 16, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Williams, S.; Howard, R.; Frackowiak, R.S.J.; Turner, R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Lezak, M.D. Neuropsychological Assessment, 5th ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2012; Volume XXV, 1161p. [Google Scholar]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Pscyhol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Cella, D. FACIT Manual: Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System; Research and Education Core, Evanston Northwestern Healthcare: Evanston, IL, USA, 1997. [Google Scholar]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Supekar, K.S.; Ryali, S.; Menon, V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011, 31, 18578–18589. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gohel, S.; Zhang, Z.; Hatzoglou, V.; Holodny, A.I.; Vachha, B.A. Glioma-Induced Disruption of Resting-State Functional Connectivity and Amplitude of Low-Frequency Fluctuations in the Salience Network. AJNR Am. J. Neuroradiol. 2021, 42, 551–558. [Google Scholar] [CrossRef]
- Yarkoni, T.; Poldrack, R.A.; Nichols, T.E.; van Essen, D.C.; Wager, T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 2011, 8, 665–670. [Google Scholar] [CrossRef]
- Kurzrock, R.; Voorhees, P.M.; Casper, C.; Furman, R.R.; Fayad, L.; Lonial, S.; Borghaei, H.; Jagannath, S.; Sokol, L.; Usmani, S.Z.; et al. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin. Cancer Res. 2013, 19, 3659–3670. [Google Scholar] [CrossRef]
- Chen, F.; Teachey, D.T.; Pequignot, E.; Frey, N.; Porter, D.; Maude, S.L.; Grupp, S.A.; June, C.H.; Melenhorst, J.J.; Lacey, S.F. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J. Immunol. Methods 2016, 434, 1–8. [Google Scholar] [CrossRef]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Nee, D.E.; D’Esposito, M. Causal evidence for lateral prefrontal cortex dynamics supporting cognitive control. Elife 2017, 6, e28040. [Google Scholar] [CrossRef]
- Vincent, J.L.; Kahn, I.; Snyder, A.Z.; Raichle, M.E.; Buckner, R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008, 100, 3328–3342. [Google Scholar] [CrossRef]
- Shenhav, A.; Botvinick, M.M.; Cohen, J.D. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 2013, 79, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.F.; Zheng, L.J.; Shi, Z.; Huang, W.; Zhang, L.J. Network-level functional connectivity alterations in chemotherapy treated breast cancer patients: A longitudinal resting state functional MRI study. Cancer Imaging 2020, 20, 73. [Google Scholar] [CrossRef]
- Correa, D.D.; Root, J.C.; Kryza-Lacombe, M.; Mehta, M.; Karimi, S.; Hensley, M.L.; Relkin, N. Brain structure and function in patients with ovarian cancer treated with first-line chemotherapy: A pilot study. Brain Imaging Behav. 2017, 11, 1652–1663. [Google Scholar] [CrossRef]
- McDonald, B.C. Structural Neuroimaging Findings Related to Adult Non-CNS Cancer and Treatment: Review, Integration, and Implications for Treatment of Cognitive Dysfunction. Neurotherapeutics 2021, 18, 792–810. [Google Scholar] [CrossRef]
- Harrison, R.A.; Sharafeldin, N.; Rexer, J.L.; Streck, B.; Petersen, M.; Henneghan, A.M.; Kesler, S.R. Neurocognitive Impairment After Hematopoietic Stem Cell Transplant for Hematologic Malignancies: Phenotype and Mechanisms. Oncologist 2021, 26, e2021–e2033. [Google Scholar] [CrossRef] [PubMed]
- Syrjala, K.L.; Dikmen, S.; Langer, S.L.; Roth-Roemer, S.; Abrams, J.R. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood 2004, 104, 3386–3392. [Google Scholar] [CrossRef] [PubMed]
- Rollin-Sillaire, A.; Delbeuck, X.; Pollet, M.; Mackowiak, M.-A.; Lenfant, P.; Noel, M.-P.; Facon, T.; Leleu, X.; Pasquier, F.; Le Rhun, E. Memory loss during lenalidomide treatment: A report on two cases. BMC Pharmacol. Toxicol. 2013, 14, 41. [Google Scholar] [CrossRef]
- Prado, C.E.; Crowe, S.F. Corticosteroids and Cognition: A Meta-Analysis. Neuropsychol. Rev. 2019, 29, 288–312. [Google Scholar] [CrossRef]
- Calvi, E.; Marchetti, M.; Santagata, F.; Luppi, C.; Coppo, E.; Massaia, M.; Isaia, G.C. Similar neurocognitive patterns in patients treated with lenalidomide: Chemobrain effect? Neurocase 2019, 25, 259–262. [Google Scholar] [CrossRef]
- Phillips, K.M.; McGinty, H.L.; Cessna, J.; Asvat, Y.; Gonzalez, B.; Cases, M.G.; Small, B.J.; Jacobsen, P.B.; Pidala, J.; Jim, H.S. A systematic review and meta-analysis of changes in cognitive functioning in adults undergoing hematopoietic cell transplantation. Bone Marrow Transpl. 2013, 48, 1350–1357. [Google Scholar] [CrossRef]
- Wardill, H.R.; Mander, K.A.; Van Sebille, Y.Z.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Sonis, S.T. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int. J. Cancer 2016, 139, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Ng, T.; Shwe, M.; Ho, H.K.; Foo, K.M.; Cham, M.T.; Lee, J.A.; Fan, G.; Tan, Y.P.; Yong, W.S.; et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: A multi-centered, prospective, cohort study. Ann. Oncol. 2015, 26, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.; Janelsins, M.; Koovakkattu, D.; Palesh, O.; Mustian, K.; Morrow, G.; Dhabhar, F.S. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav. Immun. 2013, 30, S109–S116. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, A.I.; Nelson, A.M.; Gonzalez, B.D.; Small, B.J.; Breen, E.C.; Sutton, S.K.; Syrjala, K.L.; Bower, J.E.; Pidala, J.; Booth-Jones, M.; et al. Worsening cognitive performance is associated with increases in systemic inflammation following hematopoietic cell transplantation. Brain Behav. Immun. 2019, 80, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Mustian, K.M.; Palesh, O.G.; Mohile, S.G.; Peppone, L.J.; Sprod, L.K.; Heckler, C.E.; Roscoe, J.A.; Katz, A.W.; Williams, J.P.; et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: Implications for cognitive impairment research. Support Care Cancer 2012, 20, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Condomines, M.; Veyrune, J.-L.; Larroque, M.; Quittet, P.; Latry, P.; Lugagne, C.; Hertogh, C.; Kanouni, T.; Rossi, J.-F.; Klein, B. Increased plasma-immune cytokines throughout the high-dose melphalan-induced lymphodepletion in patients with multiple myeloma: A window for adoptive immunotherapy. J. Immunol. 2010, 184, 1079–1084. [Google Scholar] [CrossRef]
- Speake, C.; Habib, T.; Lambert, K.; Hundhausen, C.; Lord, S.; Dufort, M.J.; Skinner, S.O.; Hu, A.; Kinsman, M.; Jones, B.E.; et al. IL-6-targeted therapies to block the cytokine or its receptor drive distinct alterations in T cell function. JCI Insight 2022, 7, e159436. [Google Scholar] [CrossRef]
- Kostek, M.C.; Nagaraju, K.; Pistilli, E.; Sali, A.; Lai, S.-H.; Gordon, B.; Chen, Y.-W. IL-6 signaling blockade increases inflammation but does not affect muscle function in the mdx mouse. BMC Musculoskelet. Disord. 2012, 13, 106. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Alterations in brain activation during working memory processing associated with breast cancer and treatment: A prospective functional magnetic resonance imaging study. J. Clin. Oncol. 2012, 30, 2500–2508. [Google Scholar] [CrossRef]
- Hoogland, A.I.; Barata, A.; Logue, J.; Kommalapati, A.; Hyland, K.A.; Nelson, A.M.; Eisel, S.L.; Small, B.J.; James, B.W.; Christy, S.M.; et al. Change in Neurocognitive Performance Among Patients with Non-Hodgkin Lymphoma in the First Year after Chimeric Antigen Receptor T Cell Therapy. Transpl. Cell. Ther. 2022, 28, 305.e1–305.e9. [Google Scholar] [CrossRef]
| Network Name | Region Name | X | Y | Z |
|---|---|---|---|---|
| Default Mode Network | ||||
| Posterior Cingulate Cortex | −2 | −54 | 26 | |
| Medial Prefrontal Cortex | 2 | 50 | −6 | |
| L-Angular Gyrus | −50 | −62 | 32 | |
| R-Angular Gyrus | 46 | −70 | 32 | |
| Salience Network | ||||
| L-Dorsal Anterior Cingulate Cortex | −5 | 26 | 31 | |
| R-Dorsal Anterior Cingulate Cortex | 5 | 26 | 31 | |
| L-Anterior Insula | −34 | 15 | −4 | |
| R-Anterior Insula | 37 | 20 | −6 | |
| Central Executive Network | ||||
| L-Dorsolateral Prefrontal cortex | −46 | 20 | 44 | |
| R-Dorsolateral Prefrontal cortex | 46 | 20 | 44 | |
| L-Posterior Parietal Cortex | −40 | −56 | 44 | |
| R-Posterior Parietal Cortex | 52 | −52 | 50 |
| Demographics | |
|---|---|
| Sex (M/F) | 10/8 |
| Handedness (R/L) | 17/1 |
| Education (years) | |
| Mean (SD) | 14.33 (3.58) |
| Median (Range) | 14.5 (13–18) |
| Age at study entry (Years) | |
| Mean (SD) | 66.11 (3.60) |
| Treatment regimen pre-ASCT | |
| RVd | 5 (28%) |
| KRd | 5 (28%) |
| Lenalidomide (alone) | 4 (22%) |
| KRd + RVd | 1 (5.5%) |
| CyBorD ± KRd | 2 (11%) |
| CyBordD + KRd + RVd | 1 (5.5%) |
| Response to pre-ASCT treatment | |
| CR | 7 (39%) |
| VGPR | 9 (50%) |
| PR | 2 (11%) |
| Time since pre-ASCT treatment | |
| 0–1 months | 11 (61%) |
| 2 months | 5 (28%) |
| >2 months | 2 (11%) |
| ASCT Conditioning Regimen | |
| Melphalan—Single Dose | 17 (95%) |
| Melphalan—Two-Dose | 1 (5%) |
| Time from Baseline * to ASCT | |
| ≤1 month | 15 (83%) |
| 1.1–4 months | 3 (17%) |
| Relevant Medications | |
| Pre-ASCT | |
| Dexamethasone | 6 (33%) |
| Lenalidomide | 4 (22%) |
| Post-ASCT | |
| Dexamethasone | 0 (0%) |
| Lenalidomide (maintenance) | 3 (17%) |
| Measures | Pre-ASCT n = 15 Mean (SD) | Post-ASCT n = 15 Mean (SD) |
|---|---|---|
| Attention/Working Memory | ||
| LDSF | 0.16 (1.18) | 0.20 (1.21) |
| LDSB | −0.02 (0.96) | 0.50 (1.09) * |
| LLSS | −0.14 (1.05) | 0.17 (1.14) |
| BTA | −0.07 (1.07) | 0.33 (0.97) |
| ACT-T | −0.24 (1.02) | −0.46 (1.10) |
| ACT-P | −1.60 (1.41) | −1.64 (1.55) |
| Executive Functions | ||
| TMT A | −0.35 (0.80) | −0.05 (1.01) |
| TMT B | −0.71 (1.03) | −0.16 (1.13) |
| COWA | −0.94 (1.17) | −0.51 (1.08) |
| Verbal Memory | ||
| HVLT-R-T | −0.63 (1.09) | −0.49 (0.93) |
| HVLT-R-D | −0.58 (0.98) | −0.55 (1.42) |
| HVLT-R-DI | −0.02 (0.86) | 0.34 (0.69) |
| Self-Report Scales | ||
| CES-D | 9.67 (5.49) | 9.73 (6.37) |
| FACIT-FS | 38.89 (7.55) | 39.40 (6.72) |
| Cytokines | Pre-ASCT n = 13 Median (Range) | Post-ASCT n = 13 Median (Range) |
|---|---|---|
| IL-1β | 0.00 (0.00–0.00) | 0.00 (0.00–0.18) * |
| IL-2 | 0.08 (0.01–0.28) | 0.35 (0.19–1.05) * |
| IL-4 | 0.01 (0.00–0.02) | 0.01 (0.00–2.53) * |
| IL-6 | 0.78 (0.65–0.86) | 2.30 (0.58–1572) |
| IL-8 | 4.53 (3.41–6.62) | 7.70 (6.75–10.96) * |
| IL-10 | 0.19 (0.12–0.42) | 0.28 (0.15–0.48) |
| IL-12 | 0.05 (0.01–0.14) | 0.10 (0.08–3.03) * |
| IL-13 | 0.00 (0.00–0.13) | 0.25 (0.00–0.88) * |
| IFNγ | 2.39 (1.82–6.91) | 4.15 (2.43–7.38) |
| TNFα | 0.56 (0.44–0.75) | 0.94 (0.60–1.04) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa, D.D.; Vachha, B.A.; Baser, R.E.; Koch, A.; Wong, P.; Gohel, S.; Giralt, S.; Root, J.C. Neuroimaging and Neurocognitive Outcomes in Older Patients with Multiple Myeloma Treated with Chemotherapy and Autologous Stem Cell Transplantation. Cancers 2023, 15, 4484. https://doi.org/10.3390/cancers15184484
Correa DD, Vachha BA, Baser RE, Koch A, Wong P, Gohel S, Giralt S, Root JC. Neuroimaging and Neurocognitive Outcomes in Older Patients with Multiple Myeloma Treated with Chemotherapy and Autologous Stem Cell Transplantation. Cancers. 2023; 15(18):4484. https://doi.org/10.3390/cancers15184484
Chicago/Turabian StyleCorrea, Denise D., Behroze A. Vachha, Raymond E. Baser, Adrian Koch, Phillip Wong, Suril Gohel, Sergio Giralt, and James C. Root. 2023. "Neuroimaging and Neurocognitive Outcomes in Older Patients with Multiple Myeloma Treated with Chemotherapy and Autologous Stem Cell Transplantation" Cancers 15, no. 18: 4484. https://doi.org/10.3390/cancers15184484
APA StyleCorrea, D. D., Vachha, B. A., Baser, R. E., Koch, A., Wong, P., Gohel, S., Giralt, S., & Root, J. C. (2023). Neuroimaging and Neurocognitive Outcomes in Older Patients with Multiple Myeloma Treated with Chemotherapy and Autologous Stem Cell Transplantation. Cancers, 15(18), 4484. https://doi.org/10.3390/cancers15184484





