Targeted Therapy in Follicular Lymphoma: Towards a Chemotherapy-Free Approach
Abstract
:Simple Summary
Abstract
1. Introduction
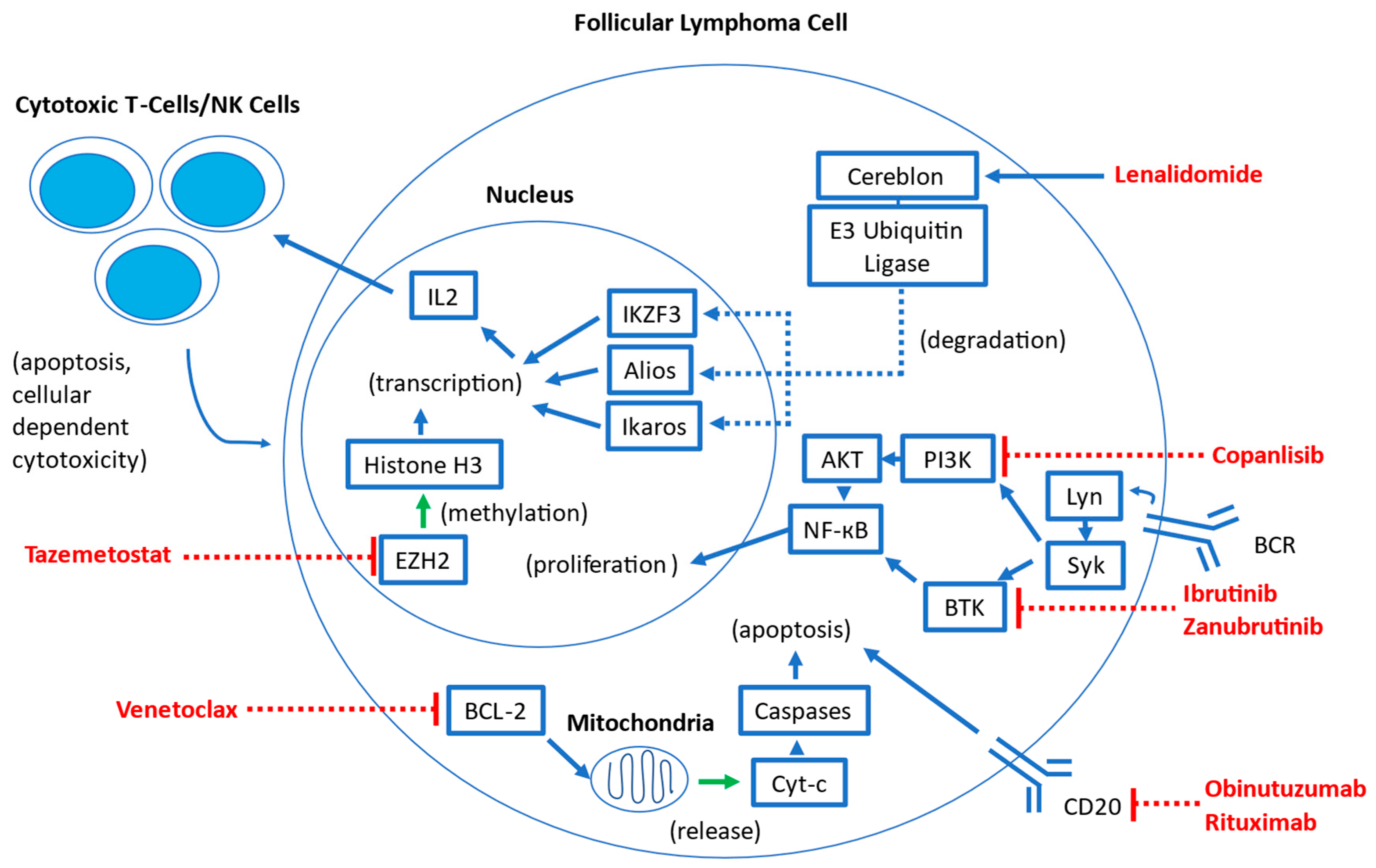
2. Overview of Targeted Therapies
3. Therapies in the Relapsed/Refractory (R/R) Setting
3.1. Rituximab with Lenalidomide (R2) in the Relapsed/Refractory Setting
3.2. Obinutuzumab with Lenalidomide in the Relapsed/Refractory Setting
3.3. Venetoclax and Rituximab with or without Chemotherapy in the Relapsed/Refractory Setting
3.4. Ibrutinib Monotherapy in the Relapsed/Refractory Setting
3.5. Zanubrutinib + Obinutuzumab in the Relapsed Refractory Setting
3.6. Tazemetostat in the Relapsed/Refractory Setting
3.7. Copanlisib in the Relapsed/Refractory Setting
3.8. Bispecific T-Cell Engager Antibody Monotherapy in the Relapsed–Refractory Setting
3.9. Bispecific T-Cell Engager Antibody Combination Therapy in the Relapsed–Refractory Setting
3.10. Chimeric Antigen Receptor T-Cell Therapy in the Relapsed–Refractory Setting
4. Therapies in the Front-Line Setting
4.1. Rituximab with Lenalidomide in the Front-Line Setting
| Referenced Study | Phase | Therapies Studied | FL Grades | FLIPI Score | ORR (CR) | PFS (Median) | OS (Median) | POD24 (%) | Notable AEs |
|---|---|---|---|---|---|---|---|---|---|
| [66,67] (RELEVANCE) | III | Rituximab + Lenalidomide | 1–3a | 0–1: 15% 2: 36% 3–5: 49% | 61% (48%) | 3 yr: 77% 6 yr: 60% (NR) | 3 yr: 94% 6 yr: 89% (NR) | 13% | Cutaneous reactions, diarrhea, rash, neutropenia |
| [69] (GALEN) | Ib/II | Obinutuzumab + Lenalidomide | 1–3a | 0–1: 17% 2: 40% 3–5: 43% | 92% (47%) | 3 yr: 82% (NR) | 3 yr: 94% (NR) | 14% | Asthenia, neutropenia, constipation, diarrhea, cough |
| [13] (PCYC-1125-CA) | II | Ibrutinib + Rituximab | 1–3a | 0–1: 12% 2: 38% 3–5: 50% | 85% (40%) | 30 mo: 67% (41.9 mo) | 30 mo: 97% (NR) | N/A | Fatigue, diarrhea, nausea, bleeding, cardiac events |
| [14] | II | Obinutuzumab + Ibrutinib + Venetoclax | 1–3a | 0–1: 12.5% 2: 25% 3–5: 62.5% | 100% (100%) | 12 mo: 100% (NR) | 12 mo: 100% (NR) | N/A | Fatigue, lymphopenia, diarrhea, neutropenia, rash, thrombocytopenia |
4.2. Obinutuzumab with Lenalidomide in the Front-Line Setting
4.3. Ibrutinib in Combination with Anti-CD20 Therapy and Venetoclax in the Front-Line Setting
5. The Role of Molecular Testing in Selecting Targeted Therapies
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Jacobsen, E. Follicular lymphoma: 2023 update on diagnosis and management. Am. J. Hematol. 2022, 97, 1638–1651. [Google Scholar] [CrossRef]
- Salles, G. How do I sequence therapy for follicular lymphoma? Hematology 2020, 2020, 287–294. [Google Scholar] [CrossRef]
- Gandhi, A.K.; Kang, J.; Havens, C.G.; Conklin, T.; Ning, Y.; Wu, L.; Ito, T.; Ando, H.; Waldman, M.F.; Thakurta, A.; et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br. J. Haematol. 2014, 164, 811–821. [Google Scholar] [CrossRef]
- Lopez-Girona, A.; Mendy, D.; Ito, T.; Miller, K.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef]
- Mössner, E.; Brünker, P.; Moser, S.; Püntener, U.; Schmidt, C.; Herter, S.; Grau, R.; Gerdes, C.; Nopora, A.; van Puijenbroek, E.; et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell–mediated B-cell cytotoxicity. Blood 2010, 115, 4393–4402. [Google Scholar] [CrossRef]
- Bachy, E.; Seymour, J.F.; Feugier, P.; Offner, F.; López-Guillermo, A.; Belada, D.; Xerri, L.; Catalano, J.V.; Brice, P.; Lemonnier, F.; et al. Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients with Follicular Lymphoma: Long-Term Results of the PRIMA Study. J. Clin. Oncol. 2019, 37, 2815–2824. [Google Scholar] [CrossRef]
- Sehn, L.H.; Chua, N.; Mayer, J.; Dueck, G.; Trněný, M.; Bouabdallah, K.; Fowler, N.; Delwail, V.; Press, O.; Salles, G.; et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): A randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016, 17, 1081–1093. [Google Scholar] [CrossRef]
- Marcus, R.; Davies, A.; Ando, K.; Klapper, W.; Opat, S.; Owen, C.; Phillips, E.; Sangha, R.; Schlag, R.; Seymour, J.F.; et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N. Engl. J. Med. 2017, 377, 1331–1344. [Google Scholar] [CrossRef]
- Townsend, W.; Hiddemann, W.; Buske, C.; Cartron, G.; Cunningham, D.; Dyer, M.J.; Gribben, J.; Phillips, E.; Dreyling, M.; Seymour, J.F.; et al. S206: Obinutuzumab Plus Chemotherapy Demonstrates Long-Term Benefit Over Rituximab Plus Chemotherapy in Patients with Previously Untreated Follicular Lymphoma: Final Analysis of the GALLIUM Study. HemaSphere 2022, 6, 107–108. [Google Scholar] [CrossRef]
- Honigberg, L.A.; Smith, A.M.; Sirisawad, M.; Verner, E.; Loury, D.; Chang, B.; Li, S.; Pan, Z.; Thamm, D.H.; Miller, R.A.; et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080. [Google Scholar] [CrossRef]
- Bartlett, N.L.; Costello, B.A.; LaPlant, B.R.; Ansell, S.M.; Kuruvilla, J.G.; Reeder, C.B.; Thye, L.S.; Anderson, D.M.; Krysiak, K.; Ramirez, C.; et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: A phase 2 consortium trial. Blood 2018, 131, 182–190. [Google Scholar] [CrossRef]
- Frustaci, A.M.; Deodato, M.; Zamprogna, G.; Cairoli, R.; Montillo, M.; Tedeschi, A. Next Generation BTK Inhibitors in CLL: Evolving Challenges and New Opportunities. Cancers 2023, 15, 1504. [Google Scholar] [CrossRef]
- Fowler, N.H.; Nastoupil, L.; De Vos, S.; Knapp, M.; Flinn, I.W.; Chen, R.; Advani, R.H.; Bhatia, S.; Martin, P.; Mena, R.; et al. The combination of ibrutinib and rituximab demonstrates activity in first-line follicular lymphoma. Br. J. Haematol. 2020, 189, 650–660. [Google Scholar] [CrossRef]
- Othman, T.; Rosenberg, A.S.; Timmerman, J.; Heyman, B.; Abdulhaq, H.; Tuscano, J.M. A Phase II Trial of the Combination of Obinutuzumab, Venetoclax, and Ibrutinib in Patients with Previously Untreated Follicular Lymphoma. Blood 2022, 140, 11954–11955. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Mayer, J.; Auer, R.; Bijou, F.; Oliveira, A.C.d.; Flowers, C.; Merli, M.; Bouabdallah, K.; Ganly, P.S.; Johnson, R.; et al. Zanubrutinib plus obinutuzumab (ZO) versus obinutuzumab (O) monotherapy in patients (pts) with relapsed or refractory (R/R) follicular lymphoma (FL): Primary analysis of the phase 2 randomized ROSEWOOD trial. J. Clin. Oncol. 2022, 40, 7510. [Google Scholar] [CrossRef]
- Bazinet, A.; Darbaniyan, F.; Jabbour, E.; Montalban-Bravo, G.; Ohanian, M.; Chien, K.; Kadia, T.; Takahashi, K.; Masarova, L.; Short, N.; et al. Azacitidine plus venetoclax in patients with high-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia: Phase 1 results of a single-centre, dose-escalation, dose-expansion, phase 1-2 study. Lancet Haematol. 2022, 9, e756–e765. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Eichhorst, B.; Niemann, C.U.; Kater, A.P.; Fürstenau, M.; von Tresckow, J.; Zhang, C.; Robrecht, S.; Gregor, M.; Juliusson, G.; Thornton, P.; et al. First-Line Venetoclax Combinations in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 1739–1754. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Ennishi, D. Biological and clinical significance of epigenetic alterations in B-cell lymphomas. Int. J. Hematol. 2022, 116, 821–827. [Google Scholar] [CrossRef]
- Morschhauser, F.; Tilly, H.; Chaidos, A.; McKay, P.; Phillips, T.; Assouline, S.; Batlevi, C.L.; Campbell, P.; Ribrag, V.; Damaj, G.L.; et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: An open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 2020, 21, 1433–1442. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769. [Google Scholar] [CrossRef]
- Richardson, N.C.; Kasamon, Y.; Pazdur, R.; Gormley, N. The saga of PI3K inhibitors in haematological malignancies: Survival is the ultimate safety endpoint. Lancet Oncol. 2022, 23, 563–566. [Google Scholar] [CrossRef]
- Incyte Provides Update on Parsaclisib and MCLA-145. Available online: https://investor.incyte.com/news-releases/news-release-details/incyte-provides-update-parsaclisib-and-mcla-145 (accessed on 3 August 2023).
- Lynch, R.C.; Avigdor, A.; McKinney, M.S.; Paneesha, S.; Wahlin, B.; Hrom, J.S.; Cunningham, D.; Morley, N.; Canales, M.; Bastos-Oreiro, M.; et al. Efficacy and Safety of Parsaclisib in Patients with Relapsed or Refractory Follicular Lymphoma: Primary Analysis from a Phase 2 Study (CITADEL-203). Blood 2021, 138, 813. [Google Scholar] [CrossRef]
- Zelenetz, A.D.; Jurczak, W.; Ribrag, V.; Linton, K.M.; Collins, G.P.; Bishton, M.; López Jiménez, J.; Dholaria, B.; Mengarelli, A.; Phillips, T.J.; et al. Efficacy and Safety of Single-Agent Zandelisib Administered by Intermittent Dosing in Patients with Relapsed or Refractory (R/R) Follicular Lymphoma (FL): Final Results of the Tidal Phase 2 Study. Blood 2022, 140, 3595–3597. [Google Scholar] [CrossRef]
- MEI Pharma and Kyowa Kirin Announce Discontinuation of Zandelisib Development Outside of Japan Following Recent FDA Meeting. Available online: https://www.businesswire.com/news/home/20221205005800/en/MEI-Pharma-and-Kyowa-Kirin-Announce-Discontinuation-of-Zandelisib-Development-Outside-of-Japan-Following-Recent-FDA-Meeting (accessed on 3 August 2023).
- Sun, L.L.; Ellerman, D.; Mathieu, M.; Hristopoulos, M.; Chen, X.; Li, Y.; Yan, X.; Clark, R.; Reyes, A.; Stefanich, E.; et al. Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies. Sci. Transl. Med. 2015, 7, 287ra270. [Google Scholar] [CrossRef]
- Salvaris, R.; Ong, J.; Gregory, G.P. Bispecific Antibodies: A Review of Development, Clinical Efficacy and Toxicity in B-Cell Lymphomas. J. Pers. Med. 2021, 11, 355. [Google Scholar] [CrossRef]
- Duell, J.; Lammers, P.E.; Djuretic, I.; Chunyk, A.G.; Alekar, S.; Jacobs, I.; Gill, S. Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin. Pharmacol. Ther. 2019, 106, 781–791. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Mosunetuzumab-Axgb for Relapsed or Refractory Follicular Lymphoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mosunetuzumab-axgb-relapsed-or-refractory-follicular-lymphoma (accessed on 11 May 2023).
- Van der Horst, H.J.; de Jonge, A.V.; Hiemstra, I.H.; Gelderloos, A.T.; Berry, D.; Hijmering, N.J.; van Essen, H.F.; de Jong, D.; Chamuleau, M.E.D.; Zweegman, S.; et al. Epcoritamab induces potent anti-tumor activity against malignant B-cells from patients with DLBCL, FL and MCL, irrespective of prior CD20 monoclonal antibody treatment. Blood Cancer J. 2021, 11, 38. [Google Scholar] [CrossRef]
- Bröske, A.E.; Korfi, K.; Belousov, A.; Wilson, S.; Ooi, C.H.; Bolen, C.R.; Canamero, M.; Alcaide, E.G.; James, I.; Piccione, E.C.; et al. Pharmacodynamics and molecular correlates of response to glofitamab in relapsed/refractory non-Hodgkin lymphoma. Blood Adv. 2022, 6, 1025–1037. [Google Scholar] [CrossRef]
- Bannerji, R.; Arnason, J.E.; Advani, R.H.; Brown, J.R.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; O’Brien, S.M.; Chávez, J.C.; Duell, J.; et al. Odronextamab, a human CD20 × CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): Results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022, 9, e327–e339. [Google Scholar] [CrossRef]
- Bannerji, R.; Allan, J.N.; Arnason, J.E.; Brown, J.R.; Advani, R.; Ansell, S.M.; O’Brien, S.M.; Duell, J.; Martin, P.; Joyce, R.M.; et al. Odronextamab (REGN1979), a Human CD20 x CD3 Bispecific Antibody, Induces Durable, Complete Responses in Patients with Highly Refractory B-Cell Non-Hodgkin Lymphoma, Including Patients Refractory to CAR T Therapy. Blood 2020, 136, 42–43. [Google Scholar] [CrossRef]
- Smith, E.J.; Olson, K.; Haber, L.J.; Varghese, B.; Duramad, P.; Tustian, A.D.; Oyejide, A.; Kirshner, J.R.; Canova, L.; Menon, J.; et al. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci. Rep. 2015, 5, 17943. [Google Scholar] [CrossRef]
- Falchi, L.; Leppä, S.; Wahlin, B.E.; Nijland, M.; Christensen, J.H.; Vos, S.D.; Holte, H.; Linton, K.M.; Abbas, A.; Wang, L.; et al. Subcutaneous epcoritamab with rituximab + lenalidomide (R2) in patients (pts) with relapsed or refractory (R/R) follicular lymphoma (FL): Update from phase 1/2 trial. J. Clin. Oncol. 2022, 40, 7524. [Google Scholar] [CrossRef]
- Falchi, L.; Morschhauser, F.; Gribben, J.G.; Huang, H.; Dinh, M.; Conlon, R.; Chen, X.; Elliot, B.; Seymour, J.F. Phase 3 Trial of Subcutaneous Epcoritamab in Combination with Rituximab and Lenalidomide (R2) Vs R2 Among Patients with Relapsed or Refractory Follicular Lymphoma (EPCORE FL-1). Blood 2022, 140, 9338–9339. [Google Scholar] [CrossRef]
- Locke, F.L.; Go, W.Y.; Neelapu, S.S. Development and Use of the Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy Axicabtagene Ciloleucel in Large B-Cell Lymphoma: A Review. JAMA Oncol. 2020, 6, 281–290. [Google Scholar] [CrossRef]
- Denlinger, N.; Bond, D.; Jaglowski, S. CAR T-cell therapy for B-cell lymphoma. Curr. Probl. Cancer 2022, 46, 100826. [Google Scholar] [CrossRef]
- Maude, S.L.; Pulsipher, M.A.; Boyer, M.W.; Grupp, S.A.; Davies, S.M.; Phillips, C.L.; Verneris, M.R.; August, K.J.; Schlis, K.; Driscoll, T.A.; et al. Efficacy and Safety of CTL019 in the First US Phase II Multicenter Trial in Pediatric Relapsed/Refractory Acute Lymphoblastic Leukemia: Results of an Interim Analysis. Blood 2016, 128, 2801. [Google Scholar] [CrossRef]
- Fowler, N.H.; Dickinson, M.; Dreyling, M.; Martinez-Lopez, J.; Kolstad, A.; Butler, J.; Ghosh, M.; Popplewell, L.; Chavez, J.C.; Bachy, E.; et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: The phase 2 ELARA trial. Nat. Med. 2022, 28, 325–332. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 91–103. [Google Scholar] [CrossRef]
- Morschhauser, F.; Dahiya, S.; Palomba, M.L.; Garcia-Sancho, A.M.; Ortega, J.L.R.; Kuruvilla, J.; Jager, U.; Cartron, G.; Izutsu, K.; Dreyling, M.; et al. TRANSCEND FL: Phase 2 Study Results of Lisocabtagene Maraleucel (LISO-CEL) in Patients (PTS) with Relapsed/Refractory (R/R) Follicular Lymphoma (FL). Hematol. Oncol. 2023, 41, 877–880. [Google Scholar] [CrossRef]
- Jain, M.D.; Smith, M.; Shah, N.N. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood 2023, 141, 2430–2442. [Google Scholar] [CrossRef]
- Gasiorowski, R.; Trotman, J. CAR-T for follicular lymphoma: Are we good to go? Blood 2022, 140, 799–800. [Google Scholar] [CrossRef]
- Rivas-Delgado, A.; Magnano, L.; Moreno-Velázquez, M.; García, O.; Nadeu, F.; Mozas, P.; Dlouhy, I.; Baumann, T.; Rovira, J.; González-Farre, B.; et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br. J. Haematol. 2019, 184, 753–759. [Google Scholar] [CrossRef]
- Leonard, J.P.; Trneny, M.; Izutsu, K.; Fowler, N.H.; Hong, X.; Zhu, J.; Zhang, H.; Offner, F.; Scheliga, A.; Nowakowski, G.S.; et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J. Clin. Oncol. 2019, 37, 1188–1199. [Google Scholar] [CrossRef]
- Leonard, J.P.; Trneny, M.; Offner, F.; Mayer, J.; Zhang, H.; Nowakowski, G.S.; Scheinberg, P.; Gkasiamis, A.; Mikita-Geoffroy, J.; Rowe, E.; et al. Five-Year Results and Overall Survival Update from the Phase 3 Randomized Study Augment: Lenalidomide Plus Rituximab (R2) Vs Rituximab Plus Placebo in Patients with Relapsed/Refractory Indolent Non-Hodgkin Lymphoma. Blood 2022, 140, 561–563. [Google Scholar] [CrossRef]
- Morschhauser, F.; Le Gouill, S.; Feugier, P.; Bailly, S.; Nicolas-Virelizier, E.; Bijou, F.; Salles, G.A.; Tilly, H.; Fruchart, C.; Van Eygen, K.; et al. Obinutuzumab combined with lenalidomide for relapsed or refractory follicular B-cell lymphoma (GALEN): A multicentre, single-arm, phase 2 study. Lancet Haematol. 2019, 6, e429–e437. [Google Scholar] [CrossRef]
- Fowler, N.H.; Nastoupil, L.J.; Chin, C.; Strati, P.; Hagemeister, F.B.; Fayad, L.E.; Westin, J.R.; Forbes, S.G.; Arafat, J.; Feng, L.; et al. A Phase I/II Study of Lenalidomide Plus Obinutuzumab in Relapsed Indolent Lymphoma. Blood 2019, 134, 348. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Flinn, I.W.; Yuen, S.L.S.; Topp, M.S.; Rusconi, C.; Fleury, I.; Le Dû, K.; Arthur, C.; Pro, B.; Gritti, G.; et al. Venetoclax-rituximab with or without bendamustine vs bendamustine-rituximab in relapsed/refractory follicular lymphoma. Blood 2020, 136, 2628–2637. [Google Scholar]
- Dreyling, M.; Santoro, A.; Mollica, L.; Leppä, S.; Follows, G.; Lenz, G.; Kim, W.S.; Nagler, A.; Dimou, M.; Demeter, J.; et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am. J. Hematol. 2020, 95, 362–371. [Google Scholar] [CrossRef]
- Dreyling, M.; Santoro, A.; Mollica, L.; Leppä, S.; Follows, G.A.; Lenz, G.; Kim, W.S.; Nagler, A.; Panayiotidis, P.; Demeter, J.; et al. Phosphatidylinositol 3-Kinase Inhibition by Copanlisib in Relapsed or Refractory Indolent Lymphoma. J. Clin. Oncol. 2017, 35, 3898–3905. [Google Scholar] [CrossRef]
- Budde, L.E.; Sehn, L.H.; Matasar, M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: A single-arm, multicentre, phase 2 study. Lancet Oncol. 2022, 23, 1055–1065. [Google Scholar] [CrossRef]
- Hutchings, M.; Mous, R.; Clausen, M.R.; Johnson, P.; Linton, K.M.; Chamuleau, M.E.D.; Lewis, D.J.; Sureda Balari, A.; Cunningham, D.; Oliveri, R.S.; et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: An open-label, phase 1/2 study. Lancet 2021, 398, 1157–1169. [Google Scholar] [CrossRef]
- Hutchings, M.; Morschhauser, F.; Iacoboni, G.; Carlo-Stella, C.; Offner, F.C.; Sureda, A.; Salles, G.; Martínez-Lopez, J.; Crump, M.; Thomas, D.N.; et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J. Clin. Oncol. 2021, 39, 1959–1970. [Google Scholar] [CrossRef]
- Morschhauser, F.; Carlo-Stella, C.; Dickinson, M.; Phillips, T.; Houot, R.; Offner, F.; Haioun, C.; Corradini, P.; Hutchings, M.; Sureda, A.; et al. Glofitamab As Monotherapy and in Combination with Obinutuzumab Induces High Complete Response Rates in Patients (pts) with Multiple Relapsed or Refractory (R/R) Follicular Lymphoma (FL). Blood 2021, 138, 128. [Google Scholar] [CrossRef]
- Kim, T.M.; Taszner, M.; Cho, S.-G.; Novelli, S.; Le Gouill, S.; Poon, M.L.; Villasboas, J.C.; Champion, R.; Bachy, E.; Guidez, S.; et al. Odronextamab in Patients with Relapsed/Refractory (R/R) Follicular Lymphoma (FL) Grade 1-3a: Results from a Prespecified Analysis of the Pivotal Phase II Study ELM-2. Blood 2022, 140, 2280–2282. [Google Scholar] [CrossRef]
- Belada, D.; Sureda, A.; Leppä, S.; Vermaat, J.S.P.; Holte, H.; Hutchings, M.; Lugtenburg, P.; Vos, S.d.; Abrisqueta, P.; Nijland, M.; et al. Epcoritamab + R2 regimen and responses in high-risk follicular lymphoma, regardless of POD24 status. J. Clin. Oncol. 2023, 41, 7506. [Google Scholar] [CrossRef]
- Morschhauser, F.; Bishton, M.; Eyre, T.A.; Bachy, E.; Cartron, G.; Ysebaert, L.; Bobillo, S.; Gutierrez, N.C.; Budde, L.E.; Fox, C.P.; et al. Mosunetuzumab in Combination with Lenalidomide Has a Manageable Safety Profile and Encouraging Activity in Patients with Relapsed/Refractory Follicular Lymphoma: Initial Results from a Phase Ib Study. Blood 2021, 138, 129. [Google Scholar] [CrossRef]
- Rummel, M.; Kaiser, U.; Balser, C.; Stauch, M.; Brugger, W.; Welslau, M.; Niederle, N.; Losem, C.; Boeck, H.P.; Weidmann, E.; et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: A multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol. 2016, 17, 57–66. [Google Scholar] [CrossRef]
- Tam, C.S.; Quach, H.; Nicol, A.; Badoux, X.; Rose, H.; Prince, H.M.; Leahy, M.F.; Eek, R.; Wickham, N.; Patil, S.S.; et al. Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv. 2020, 4, 4802–4811. [Google Scholar] [CrossRef]
- Matasar, M.J.; Capra, M.; Özcan, M.; Lv, F.; Li, W.; Yañez, E.; Sapunarova, K.; Lin, T.; Jin, J.; Jurczak, W.; et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 678–689. [Google Scholar] [CrossRef]
- Nastoupil, L.J.; Morschhauser, F.; Scholz, C.W.; Bishton, M.; Yoon, S.-S.; Giri, P.; Wei, M.C.; Knapp, A.; Li, C.-C.; Bottos, A.; et al. CELESTIMO: A phase III trial evaluating the efficacy and safety of mosunetuzumab plus lenalidomide versus rituximab plus lenalidomide in patients with relapsed or refractory follicular lymphoma who have received ≥ 1 line of systemic therapy. J. Clin. Oncol. 2022, 40, TPS7588. [Google Scholar] [CrossRef]
- Morschhauser, F.; Nastoupil, L.; Feugier, P.; Schiano de Colella, J.M.; Tilly, H.; Palomba, M.L.; Bachy, E.; Fruchart, C.; Libby, E.N.; Casasnovas, R.O.; et al. Six-Year Results From RELEVANCE: Lenalidomide Plus Rituximab (R(2)) Versus Rituximab-Chemotherapy Followed by Rituximab Maintenance in Untreated Advanced Follicular Lymphoma. J. Clin. Oncol. 2022, 40, 3239–3245. [Google Scholar] [CrossRef]
- Morschhauser, F.; Fowler, N.H.; Feugier, P.; Bouabdallah, R.; Tilly, H.; Palomba, M.L.; Fruchart, C.; Libby, E.N.; Casasnovas, R.O.; Flinn, I.W.; et al. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N. Engl. J. Med. 2018, 379, 934–947. [Google Scholar] [CrossRef]
- Delfau-Larue, M.H.; Boulland, M.L.; Beldi-Ferchiou, A.; Feugier, P.; Maisonneuve, H.; Casasnovas, R.O.; Lemonnier, F.; Pica, G.M.; Houot, R.; Ysebaert, L.; et al. Lenalidomide/rituximab induces high molecular response in untreated follicular lymphoma: LYSA ancillary RELEVANCE study. Blood Adv. 2020, 4, 3217–3223. [Google Scholar] [CrossRef]
- Bachy, E.; Houot, R.; Feugier, P.; Bouabdallah, K.; Bouabdallah, R.; Virelizier, E.N.; Maerevoet, M.; Fruchart, C.; Snauwaert, S.; Le Gouill, S.; et al. Obinutuzumab plus lenalidomide in advanced, previously untreated follicular lymphoma in need of systemic therapy: A LYSA study. Blood 2022, 139, 2338–2346. [Google Scholar] [CrossRef]
- Passerini, V.; Jurinovic, V.; Bolen, C.R.; Knapp, A.; Bottos, A.; Richter, J.; Fitzgibbon, J.; Klapper, W.; Davies, A.; Marcus, R.; et al. An EZH2 Gene Expression Signature Is Predictive of Differential Efficacy of Chemotherapy Irrespective of EZH2 Mutation Status in Patients with Follicular Lymphoma Treated within the Gallium Trial. Blood 2021, 138, 39. [Google Scholar] [CrossRef]
- Proudman, D.G.; Gupta, D.; Nellesen, D.; Yang, J.; Kamp, B.A.; Mamlouk, K.; Cheson, B.D. Tazemetostat in relapsed/refractory follicular lymphoma: A propensity score-matched analysis of E7438-G000-101 trial outcomes. Oncotarget 2022, 13, 677–683. [Google Scholar] [CrossRef]
- Witzig, T.E.; Wiernik, P.H.; Moore, T.; Reeder, C.; Cole, C.; Justice, G.; Kaplan, H.; Voralia, M.; Pietronigro, D.; Takeshita, K.; et al. Lenalidomide Oral Monotherapy Produces Durable Responses in Relapsed or Refractory Indolent Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2009, 27, 5404–5409. [Google Scholar] [CrossRef]
- Strati, P.; Takahashi, K.; Peterson, C.B.; Keating, M.J.; Thompson, P.A.; Daver, N.G.; Jain, N.; Burger, J.A.; Estrov, Z.; O’Brien, S.M.; et al. Efficacy and predictors of response of lenalidomide and rituximab in patients with treatment-naive and relapsed CLL. Blood Adv. 2019, 3, 1533–1539. [Google Scholar] [CrossRef]
- Liu, D.; Tian, Y.; Sun, D.; Sun, H.; Jin, Y.; Dong, M. The FCGR3A polymorphism predicts the response to rituximab-based therapy in patients with non-Hodgkin lymphoma: A meta-analysis. Ann. Hematol. 2016, 95, 1483–1490. [Google Scholar] [CrossRef]
- Huntington, S.F.; Ollila, T.; Pelcovits, A.; McMahon, J.B.; Yakirevich, I.; Sturtevant, A.; Chorzalska, A.; Morgan, J.; Dubielecka, P. A phase 2 trial of mosunetuzumab with lenalidomide augmentation as first-line therapy for follicular (FL) and marginal zone lymphoma (MZL). J. Clin. Oncol. 2023, 41, TPS7588. [Google Scholar] [CrossRef]

| Referenced Study | Phase | Agents Studied | FL Grades | Percentage POD24 pts | FLIPI Score Distribution | Median Prior Therapies | Refractory to Last Therapy | ORR (CR) | PFS (Median) | OS (Median) | Notable AEs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [48,49] (AUGMENT) | III | Lenalidomide + Rituximab | 1–3a | 31% | 0–1: 29% 2: 31% 3–5: 39% | 1 | 17% | 78% (34%) | 2 yr: 58% (39.4 mo) | 2 yr: 93% (NR) | Pulmonary embolism, infection, neutropenia, cutaneous reactions |
| [50] (GALEN) | II | Obinutuzumab + Lenalidomide | 1–3a | 27% | 0–1: 23% 2: 35% 3–5: 42% | 2 | 17% | 79% (38%) | 2 yr: 65% (NR) | 2 yr: 87% (NR) | Asthenia, cytopenias, rash, bronchitis, diarrhea |
| [51] | I/II | Obinutuzumab + Lenalidomide | 1–3a | N/A | N/A | 2 | N/A | 98% (72%) | 2 yr: 73% | N/A | Neutropenia, thrombocytopenia, fatigue, rash, cough |
| [52] (CONTRALTO) | II | Venetoclax + Rituximab + Bendamustine | 1–3a | N/A | N/A | 3 | 37.3% | 84% (75%) | 18 mo: 61.7% | N/A | Cytopenias, nausea, vomiting, diarrhea, fatigue |
| Venetoclax + Rituximab | 1–3a | N/A | N/A | 3 | 50.0% | 35% (17%) | 18 mo: 26.9% | N/A | Cytopenias, diarrhea, infusion reactions | ||
| [11] | II | Ibrutinib | 1–3a | N/A | 0–1: 15% 2: 35% 3–5: 50% | 3 | 35% | 38% (13%) | 2 yr: 20.4% (14.0 mo) | 2 yr: 79% (NR) | Neutropenia, anemia, infection, diarrhea |
| [15] (ROSEWOOD) | II | Zanubrutinib + Obinutuzumab | N/A | 28% | 0–1: N/A 2: N/A 3–5: 53% | 3 | 32% | 68.3% (37.2%) | (27.4 mo) | 18 mo: 85.4% (NR) | Thrombocytopenia, neutropenia, diarrhea, fatigue, constipation |
| [21] | II | Tazemetostat | 1–3b | 42% (EZH2mt) | N/A | 2 (EZH2mt) | 49% (EZH2mt) | 69% (13%) (EZH2mt) | (13.8 mo) (EZH2mt) | (NR) (EZH2mt) | Sepsis, anemia, neutropenia, thrombocytopenia |
| 59% (EZH2wt) | N/A | 3 (EZH2wt) | 41% (EZH2wt) | 35% (4%) (EZH2wt) | (11.1 mo) (EZH2wt) | (NR) (EZH2wt) | |||||
| [53,54] (CHRONOS-1) | II | Copanlisib | 1–3a | N/A | N/A | 3 | 61% | 59% (20%) | 2 yr: 34% (12.5 mo) | 2 yr: 69% (42.6 mo) | Serious infections, pneumocystis, hyperglycemia, hypertension, neutropenia |
| [55] (GO29781) | II | Mosunetuzumab | 1–3a | 52% | 0–1: 29% 2: 27% 3–5: 44% | 3 | 69% | 80.0% (60.0%) | 18 mo: 47.0% (17.9 mo) | 18 mo: NR (NR) | CRS, neutropenia, tumor flare reaction, infections, ICANS |
| [56] | I/II | Epcoritamab | N/A | N/A | N/A | 4.5 | 83% | 90% (50%) | N/A | N/A | CRS, pyrexia, fatigue, anemia, dyspnea |
| [57] | I | Glofitamab | 1–3a | N/A | N/A | N/A | N/A | 70.5% (47.7%) | (11.8 mo) | N/A | CRS, neutropenia, pyrexia, thrombocytopenia, anemia, fatigue |
| [58] | I | Glofitamab | 1–3a | 36% | 0–1: N/A 2: N/A 3–5: 53% | 3 | 53% | 81% (70%) | N/A | N/A | CRS, neurologic AE, pyrexia, neutropenia |
| [59] (ELM-2) | II | Odronextamab | 1–3a | 48% | 0–1: N/A 2: N/A 3–5: 58% | 3 | 74% | 81% (75%) | (20.2 mo) | (NR) | CRS, pyrexia, anemia, infusion reactions |
| [60] (EPCORE NHL-2) | Ib/II | Epcoritamab + Lenalidomide + Rituximab | N/A | 38% | 0–1: N/A 2: N/A 3–5: 56% | 1 | 49% | 97% (86%) | 6 mo: 93% | N/A | CRS, neutropenia, fatigue, ICANS |
| [61] | Ib | Mosunetuzumab + Lenalidomide | 1–3a | 11% | N/A | 1 | N/A | 92% (77%) | N/A | N/A | CRS, neutropenia |
| [58] | I | Glofitamab + Obinutuzumab | 1–3a | 53% | 0–1: N/A 2: N/A 3–5: 58% | 2 | 42% | 100% (73.7%) | N/A | N/A | CRS, neurologic AE, pyrexia, neutropenia, thrombocytopenia |
| [43] (ZUMA-5) | II | Axicabtagene ciloleucel | 1–3a | 55% | 0–1: 18% 2: 39% 3–5: 44% | 3 | 68% | 94% (79%) | 18 mo: 64.8% (NR) | 18 mo: 87.4% (NR) | CRS, ICANS, hypotension, cytopenias, infections |
| [42] (ELARA) | II | Tisagenlecleucel | 1–3a | 62.9% | 0–1: NA 2: NA 3–5: 59.8% | 4 | 78.4% | 86.2% (69.1%) | 12 mo: 67% (NR) | (NR) | CRS, ICANS, hypotension, cytopenias, infections |
| [44] (TRANSCEND FL) | II | Lisocabtagene Maraleucel | N/A | 43% | 0–1: NA 2: NA 3–5: 57% | 3 | N/A | 97.0% (94.1%) | 12 mo: 80.7% (NR) | N/A | CRS, neurologic AE, cytopenias, infections |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-J.J.; Choi, M.Y.; Heyman, B.M. Targeted Therapy in Follicular Lymphoma: Towards a Chemotherapy-Free Approach. Cancers 2023, 15, 4483. https://doi.org/10.3390/cancers15184483
Chen C-JJ, Choi MY, Heyman BM. Targeted Therapy in Follicular Lymphoma: Towards a Chemotherapy-Free Approach. Cancers. 2023; 15(18):4483. https://doi.org/10.3390/cancers15184483
Chicago/Turabian StyleChen, Chung-Jiah J., Michael Y. Choi, and Benjamin M. Heyman. 2023. "Targeted Therapy in Follicular Lymphoma: Towards a Chemotherapy-Free Approach" Cancers 15, no. 18: 4483. https://doi.org/10.3390/cancers15184483
APA StyleChen, C.-J. J., Choi, M. Y., & Heyman, B. M. (2023). Targeted Therapy in Follicular Lymphoma: Towards a Chemotherapy-Free Approach. Cancers, 15(18), 4483. https://doi.org/10.3390/cancers15184483






