Colorectal Cancer Survival in German–Danish Border Regions—A Registry-Based Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Stage Distribution
3.2. Treatment Patterns
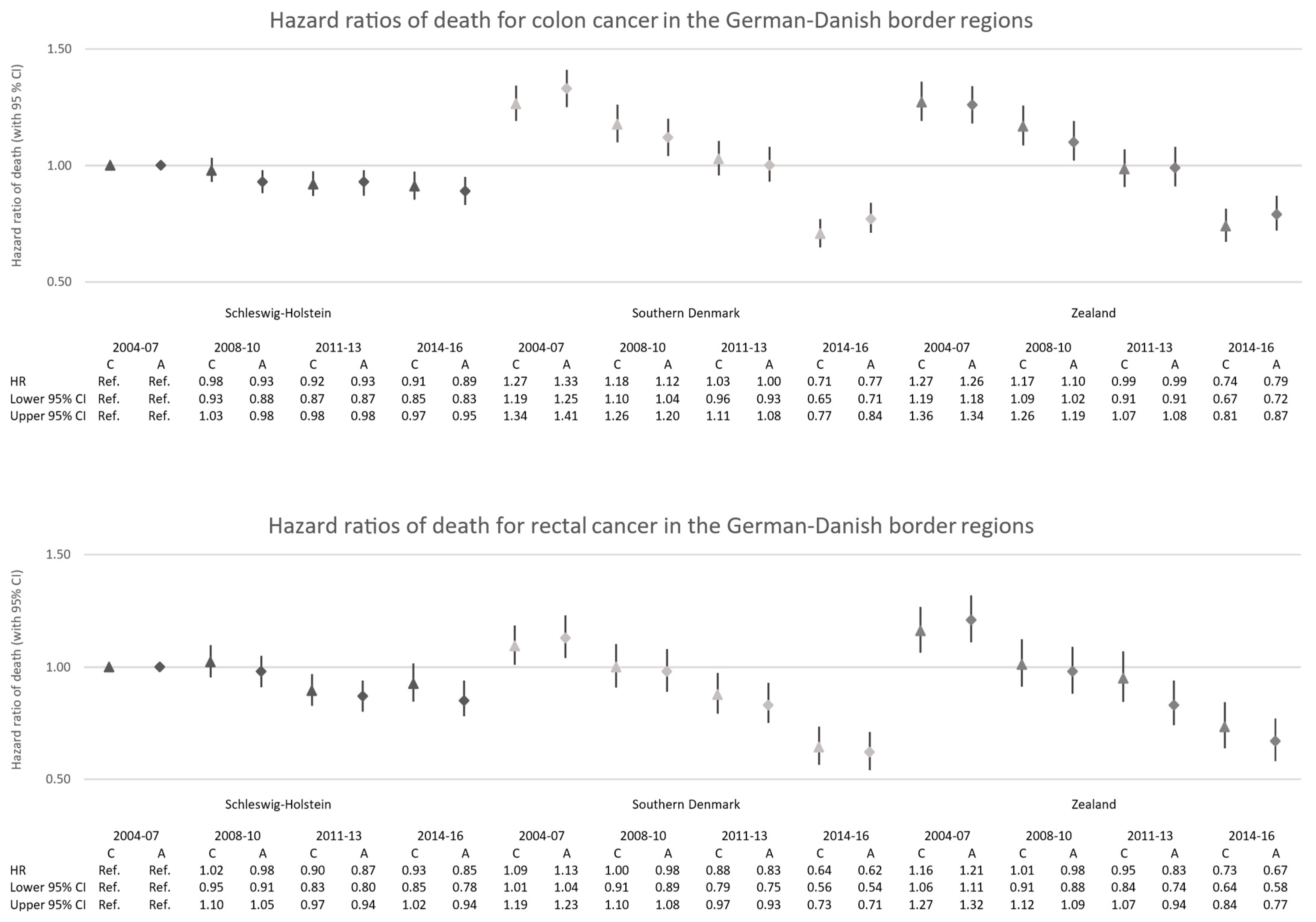
3.3. Survival Differences
4. Discussion
4.1. Secondary Prevention for Early Detection
4.2. Therapeutic Advances
4.3. Health Care System Reforms in Denmark
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, F.E.; Birgisson, H.; Johannesen, T.B.; Engholm, G.; Virtanen, A.; Pettersson, D.; Ólafsdóttir, E.J.; Lambe, M.; Lambert, P.C.; Mørch, L.S.; et al. Survival trends in patients diagnosed with colon and rectal cancer in the Nordic countries 1990–2016: The NORDCAN survival studies. Eur. J. Cancer 2022, 172, 76–84. [Google Scholar] [CrossRef]
- Araghi, M.; Arnold, M.; Rutherford, M.J.; Guren, M.G.; Cabasag, C.J.; Bardot, A.; Ferlay, J.; Tervonen, H.; Shack, L.; Woods, R.R.; et al. Colon and rectal cancer survival in seven high-income countries 2010–2014: Variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut 2021, 70, 114–126. [Google Scholar] [CrossRef]
- Tichanek, F.; Försti, A.; Liska, V.; Hemminki, A.; Hemminki, K. Survival in Colon, Rectal and Small Intestinal Cancers in the Nordic Countries through a Half Century. Cancers 2023, 15, 991. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Baili, P.; Capocaccia, R.; Caldora, M.; Carrani, E.; Minicozzi, P.; Pierannunzio, D.; Santaquilani, M.; Trama, A.; Allemani, C.; et al. The EUROCARE-5 study on cancer survival in Europe 1999–2007: Database, quality checks and statistical analysis methods. Eur. J. Cancer 2015, 51, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Coleman, M.P. Public health surveillance of cancer survival in the United States and worldwide: The contribution of the CONCORD programme. Cancer 2017, 123, 4977–4981. [Google Scholar] [CrossRef]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Holleczek, B.; Rossi, S.; Domenic, A.; Innos, K.; Minicozzi, P.; Francisci, S.; Hackl, M.; Eisemann, N.; Brenner, H.; Zielonke, N.; et al. On-going improvement and persistent differences in the survival for patients with colon and rectum cancer across Europe 1999–2007—Results from the EUROCARE-5 study. Eur. J. Cancer 2015, 51, 2158–2168. [Google Scholar] [CrossRef]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.-S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Rachet, B.; Weir, H.K.; Richardson, L.C.; Lepage, C.; Faivre, J.; Gatta, G.; Capocaccia, R.; Sant, M.; Baili, P.; et al. Colorectal cancer survival in the USA and Europe: A CONCORD high-resolution study. BMJ Open 2013, 3, e003055. [Google Scholar] [CrossRef] [PubMed]
- Storm, H.H.; Engholm, G.; Pritzkuleit, R.; Kejs, A.M.T.; Katalinic, A.; Dunst, J.; Holländer, N.H. Less pitfalls and variation in population based cancer survival comparisons within the European Union: Lessons from colorectal cancer patients in neighbouring regions in Denmark and Germany—The Fehmarn Belt project. Eur. J. Cancer 2015, 51, 1188–1198. [Google Scholar] [CrossRef]
- Rudolph, C.E.; Engholm, G.; Pritzkuleit, R.; Storm, H.H.; Katalinic, A. Survival of breast cancer patients in German-Danish border regions—A registry-based cohort study. Cancer Epidemiol. 2021, 74, 102001. [Google Scholar] [CrossRef]
- The Commonwealth Fund. International Profiles of Health Care Systems, 2015; The Commonwealth Fund: New York, NY, USA; Washington, DC, USA, 2016. [Google Scholar]
- European Commission. Strengthening Cross-Border Cancer Research and Care. Available online: https://ec.europa.eu/regional_policy/en/projects/germany/strengthening-cross-border-cancer-research-and-care (accessed on 7 December 2022).
- Danckert, B.; Ferlay, J.; Engholm, G.; Hansen, H.L.; Johannesen, T.B.; Khan, S.; Køtlum, J.E.; Ólafsdóttir, E.; Schmidt, L.K.H.; Virtanen, A.; et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries; Association of the Nordic Cancer Registries; Danish Cancer Society: Copenhagen, Denmark, 2019; Version 8.2. [Google Scholar]
- Henschel, S.; Katalinic, A. Das Manual der Epidemiologischen Krebsregistrierung; Zuckschwerdt: München, Germany, 2008. [Google Scholar]
- European Network for Cancer Registries; European Commission—Joint Research Centre. ENCR-JRC Call for Data; Version 1.1; European Network for Cancer Registries: Ispra, Italy, 2015. [Google Scholar]
- IARC. International Rules for Multiple Primary Cancers, 3rd ed.; IARC: Lyon, France, 2004. [Google Scholar]
- Zheng, S.; Schrijvers, J.J.A.; Greuter, M.J.W.; Kats-Ugurlu, G.; Lu, W.; de Bock, G.H. Effectiveness of Colorectal Cancer (CRC) Screening on All-Cause and CRC-Specific Mortality Reduction: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1948. [Google Scholar] [CrossRef]
- Veeravalli, R.S.; Vejandla, B.; Gude, S.S.; Venigalla, T.; Chintagumpala, V. Colorectal Cancer Diagnostic Methods: The Present and Future. Cureus 2023, 15, e37622. [Google Scholar] [CrossRef]
- Njor, S.H.; Larsen, M.B.; Søborg, B.; Andersen, B. Colorectal cancer mortality after randomized implementation of FIT-based screening—A nationwide cohort study. J. Med. Screen. 2022, 29, 241–248. [Google Scholar] [CrossRef]
- Hossain, S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Saltz, L.B.; Cox, J.V.; Blanke, C.; Rosen, L.S.; Fehrenbacher, L.; Moore, M.J.; Maroun, J.A.; Ackland, S.P.; Locker, P.K.; Pirotta, N.; et al. Irinotecan plus Flourouracil and Leucovorin for meta-static colorectal cancer. N. Engl. J. Med. 2000, 343, 905–914. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Groot, C.A.U.-D.; Heine, R.; Krol, M.; Verweij, J. Unequal Access to Newly Registered Cancer Drugs Leads to Potential Loss of Life-Years in Europe. Cancers 2020, 12, 2313. [Google Scholar] [CrossRef] [PubMed]
- Hofmarcher, T.; Brådvik, G.; Svedman, C.; Lindgren, P.; Jönsson, B.; Wilking, N. Comparator Report on Cancer in Europe 2019—Disease Burden, Costs and Access to Medicines; IHE Report 2019:7; The Swedish Institute for Health Economics: Lund, Sweden, 2020. [Google Scholar]
- Iversen, L.H.; Ingeholm, P.; Gögenur, I.; Laurberg, S. Major Reduction in 30-Day Mortality After Elective Colorectal Cancer Surgery: A Nationwide Population-Based Study in Denmark 2001–2011. Ann. Surg. Oncol. 2014, 21, 2267–2273. [Google Scholar] [CrossRef]
- Probst, H.B.; Hussain, Z.B.; Andersen, O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians—A national Danish project. Health Policy 2012, 105, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sundhedsstyrelsen. National Kræftplan, Status Og Forslag Til Initiativer I Relation Til Kræftbehandlingen; Sundhedsstyrelsen: Copenhagen, Denmark, 2000. [Google Scholar]
- Sundhedsstyrelsen. National Cancer Plan II—Denmark: National Board of Health Recommendations for Improving Cancer Healthcare Services; Sundhedsstyrelsen: Copenhagen, Denmark, 2005. [Google Scholar]
- Sundhedsstyrelsen. Styrket Indsats På Kræftområdet—Et Sundhedsfagligt Oplæg; Sundhedsstyrelsen: Copenhagen, Denmark, 2010. [Google Scholar]
- Regeringen. Patienternes Kræftplan: Kræftplan IV; Regeringen: Copenhagen, Denmark, 2016. [Google Scholar]
- Ministeriet for Sundhed og Forebyggelse. Bekendtgørelse om Maksimale Ventetider ved Behandling af Kræft og Visse Tilstande ved Iskæmiske Hjertesygdomme (BEK nr 584 af 28/04/2015). Available online: https://www.retsinformation.dk/eli/lta/2015/584 (accessed on 11 April 2023).
- Dyrop, H.B.; Safwat, A.; Vedsted, P.; Maretty-Nielsen, K.; Hansen, B.H.; Jørgensen, P.H.; Baad-Hansen, T.; Bünger, C.; Keller, J. Cancer Patient Pathways shortens waiting times and accelerates the diagnostic process of suspected sarcoma patients in Denmark. Health Policy 2013, 113, 110–117. [Google Scholar] [CrossRef]
- Pukkala, E.; Engholm, G.; Schmidt, L.K.H.; Storm, H.; Khan, S.; Lambe, M.; Pettersson, D.; Ólafsdóttir, E.; Tryggvadóttir, L.; Hakanen, T.; et al. Nordic Cancer Registries—An overview of their procedures and data comparability. Acta Oncol. 2018, 57, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Robert-Koch-Institut. Gesellschaft Der Epidemiologischen Krebsregister in Deutschland, Krebs in Deutschland 2005/2006; Robert-Koch-Institut: Berlin, Germany, 2010. [Google Scholar]
- Robert-Koch-Institut. Gesellschaft Der Epidemiologischen Krebsregister in Deutschland, Krebs in Deutschland 2007/2008; Robert-Koch-Institut: Berlin, Germany, 2012. [Google Scholar]
- Robert-Koch-Institut. Gesellschaft Der Epidemiologischen Krebsregister in Deutschland, Krebs in Deutschland 2009/2010; Robert-Koch-Institut: Berlin, Germany, 2013. [Google Scholar]
- Robert Koch-Institut. Gesellschaft Der Epidemiologischen Krebsregister in Deutschland, Krebs in Deutschland 2011/2012; Robert-Koch-Institut: Berlin, Germany, 2015. [Google Scholar]
- Robert Koch-Institut. Gesellschaft Der Epidemiologischen Krebsregister in Deutschland, Krebs in Deutschland 2013/2014; Robert-Koch-Institut: Berlin, Germany, 2017. [Google Scholar]
- Robert Koch-Institut. Gesellschaft Der Epidemiologischen Krebsregister in Deutschland, Krebs in Deutschland 2015/2016; Robert-Koch-Institut: Berlin, Germany, 2019. [Google Scholar]
- Katalinic, A.; Halber, M.; Meyer, M.; Pflüger, M.; Eberle, A.; Nennecke, A.; Kim-Wanner, S.-Z.; Hartz, T.; Weitmann, K.; Stang, A.; et al. Population-Based Clinical Cancer Registration in Germany. Cancers 2023, 15, 3934. [Google Scholar] [CrossRef]
- Holleczek, B.; Brenner, H. Reduction of population-based cancer survival estimates by trace back of death certificate notifications: An empirical illustration. Eur. J. Cancer 2012, 48, 797–804. [Google Scholar] [CrossRef]
- Andersson, T.M.-L.; Myklebust, T.; Rutherford, M.J.; Møller, B.; Soerjomataram, I.; Arnold, M.; Bray, F.; Parkin, D.M.; Sasieni, P.; Bucher, O.; et al. The impact of excluding or including Death Certificate Initiated (DCI) cases on estimated cancer survival: A simulation study. Cancer Epidemiol. 2021, 71, 101881. [Google Scholar] [CrossRef]
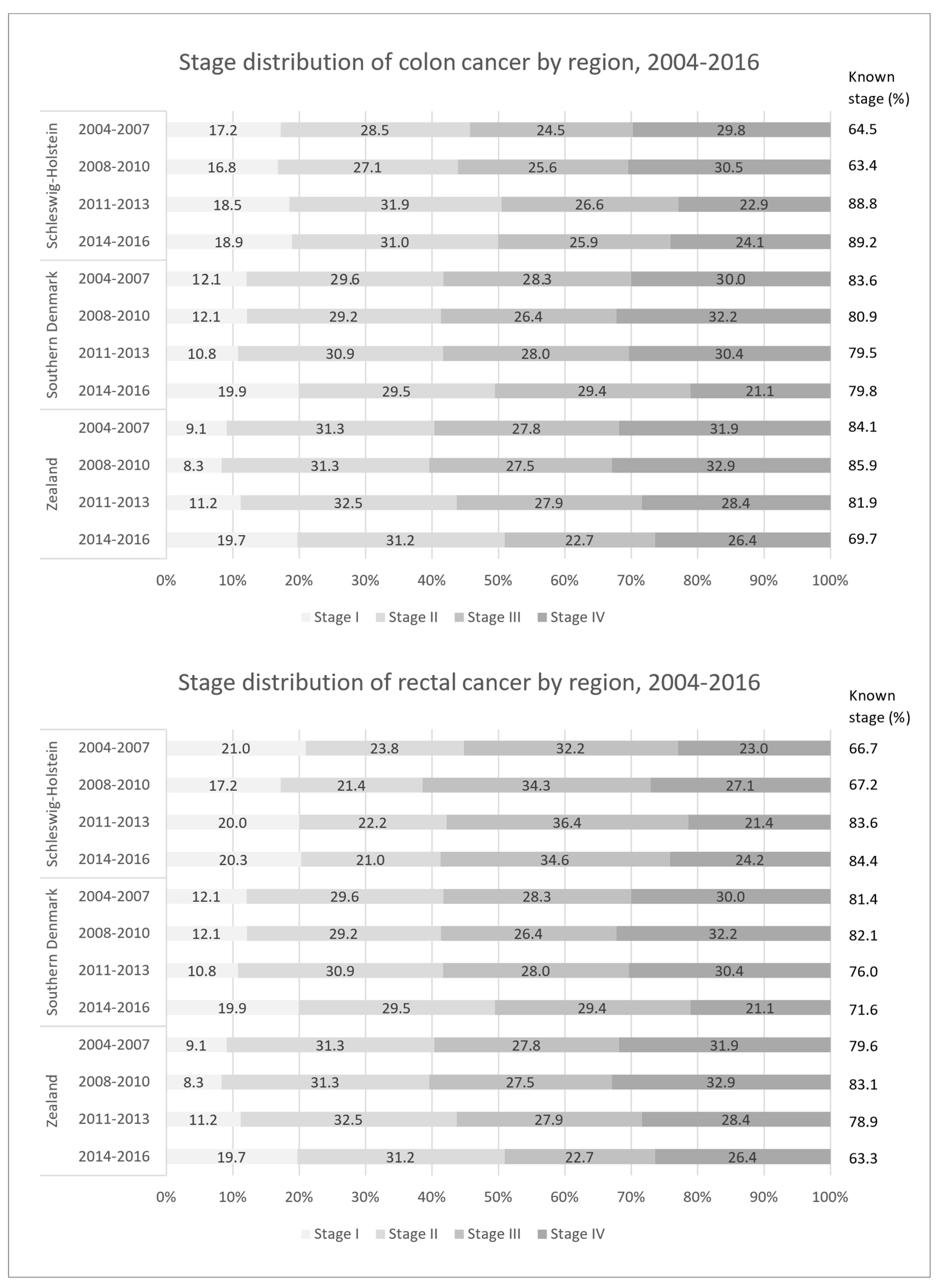
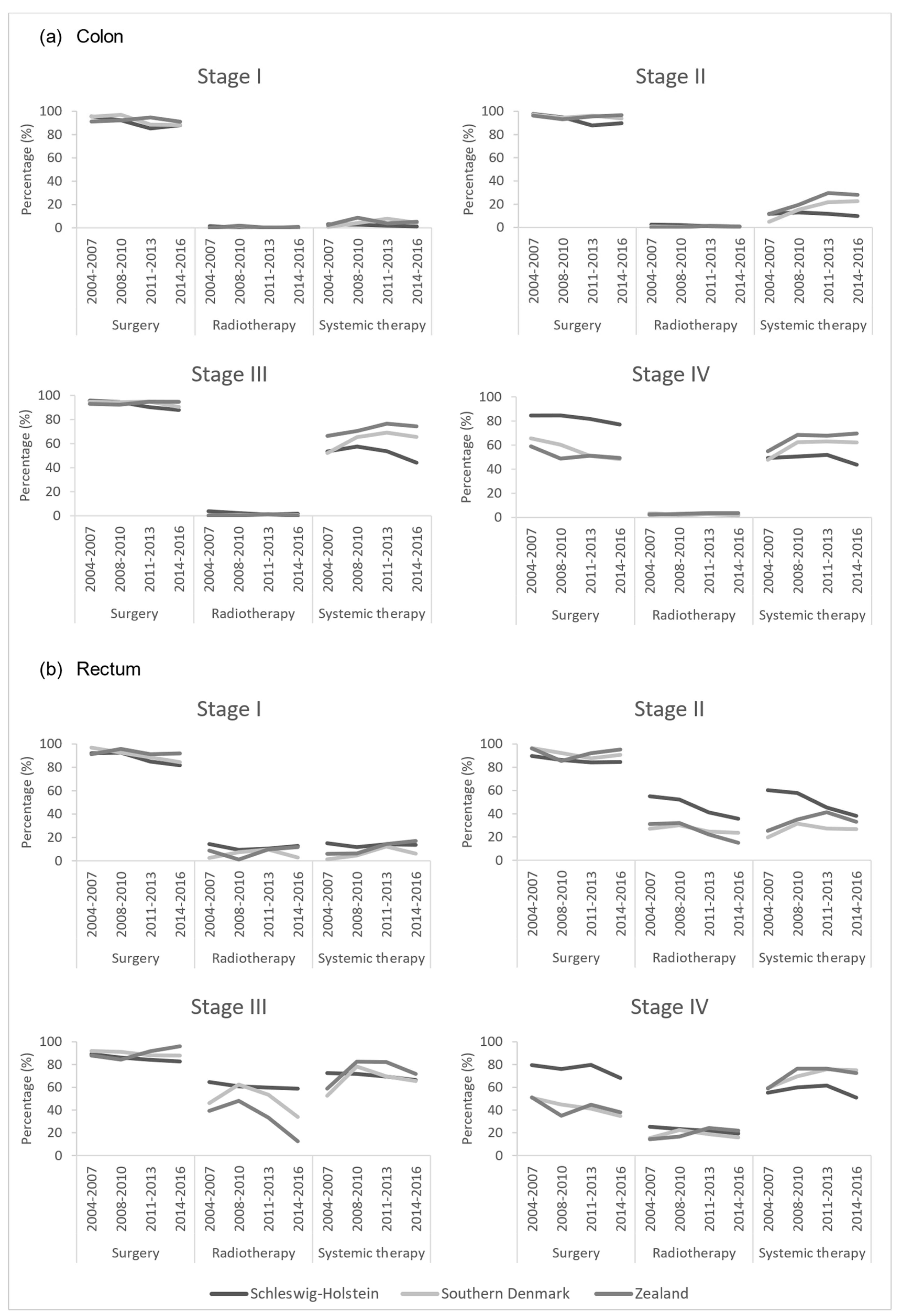
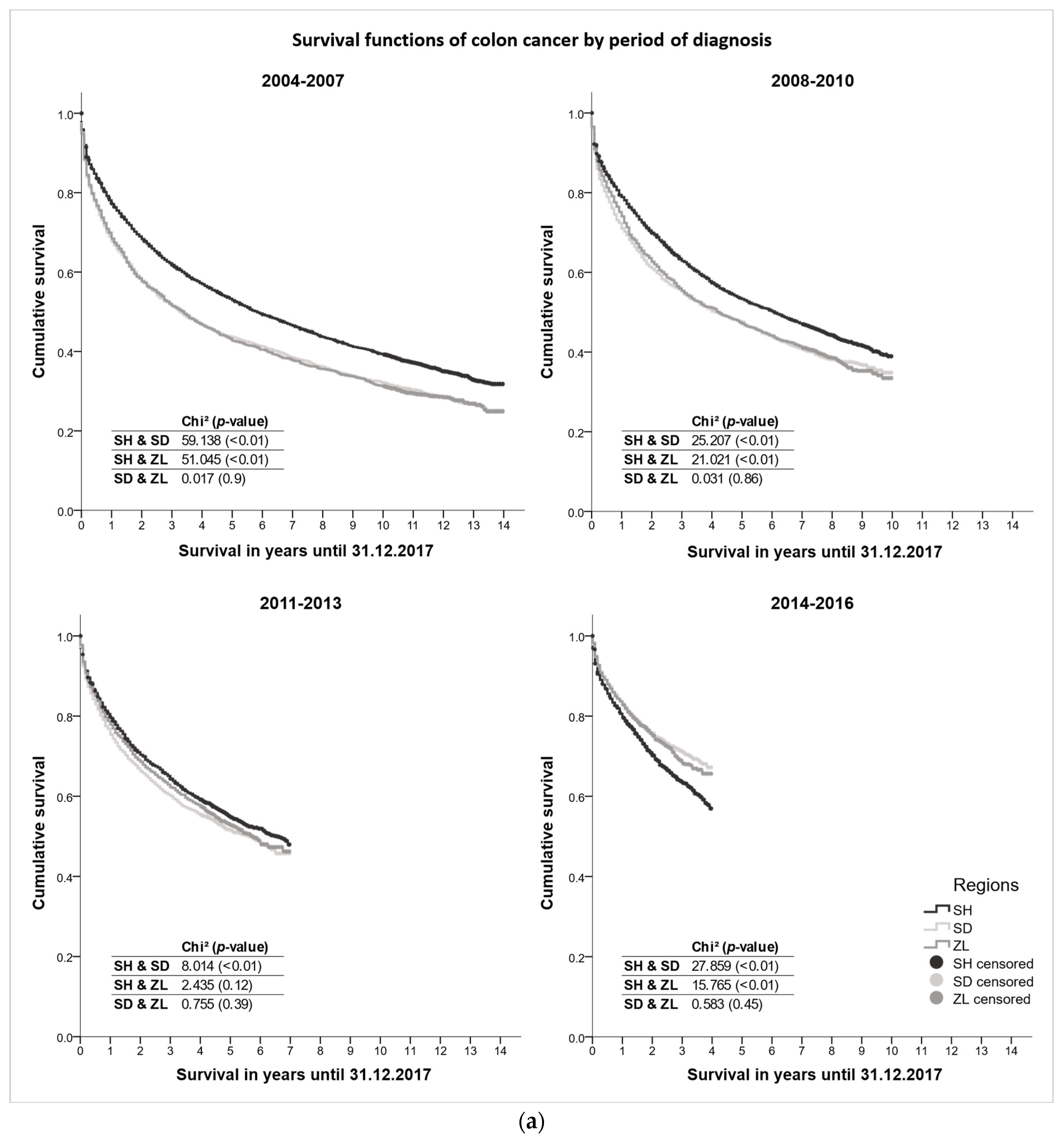
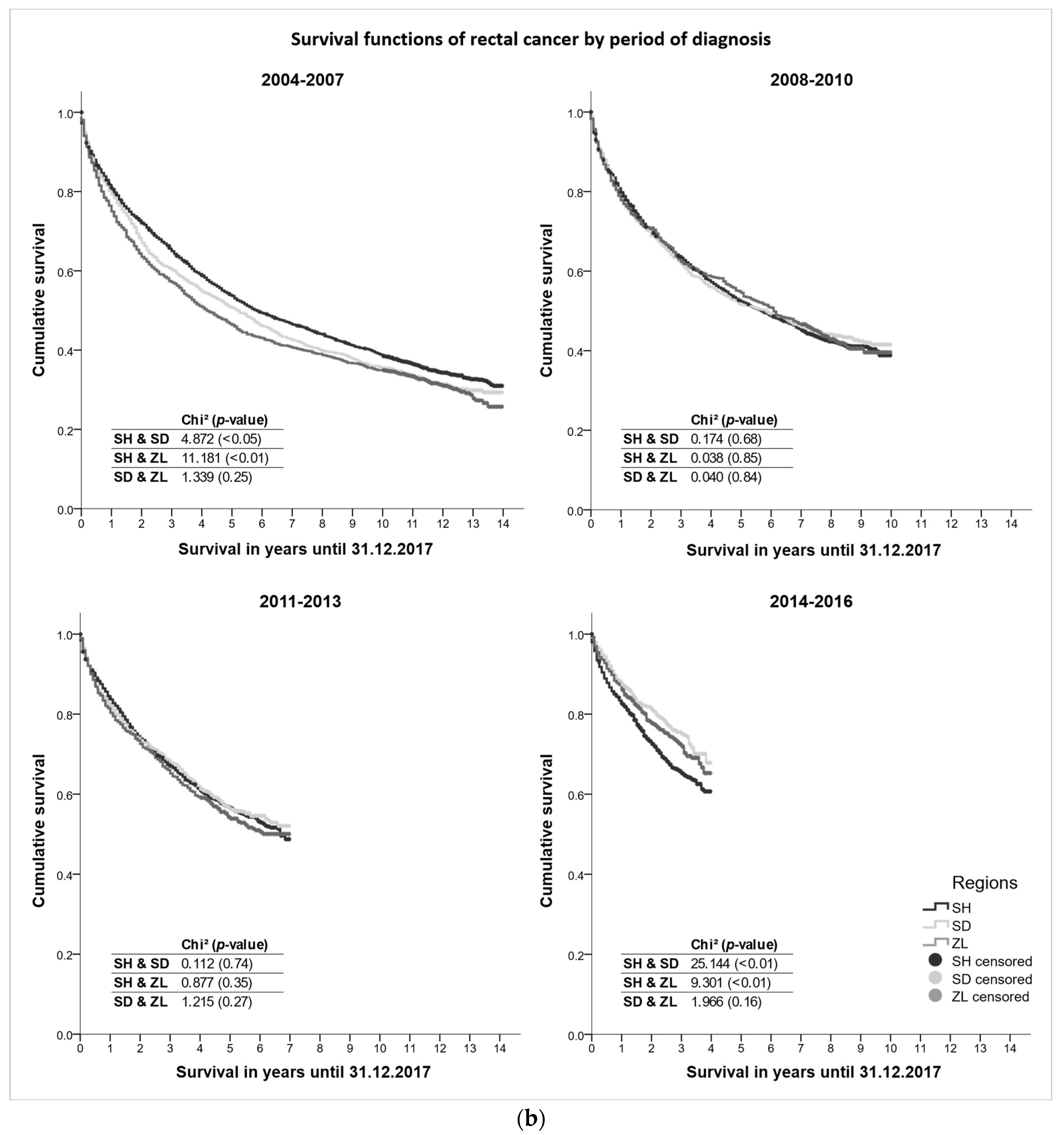
| Schleswig-Holstein | Southern Denmark | Zealand | ||||
|---|---|---|---|---|---|---|
| Colon | Rectum | Colon | Rectum | Colon | Rectum | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| 2004–2007 | ||||||
| Patients in cancer register | 6192 (100.0) | 3386 (100.0) | 2280 (100.0) | 1302 (100.0) | 1698 (100.0) | 987 (100.0) |
| Death certificates only (DCO)/incidental finding at autopsy * | 537 (8.7) | 191 (5.6) | 30 (1.3) | 9 (0.7) | 20 (1.2) | 8 (0.8) |
| Age 90+ at diagnosis * | 305 (4.9) | 125 (3.7) | 75 (3.3) | 38 (2.9) | 40 (2.4) | 19 (1.9) |
| Included in survival analysis | 5475 (88.4) | 3116 (92.0) | 2178 (95.5) | 1255 (96.4) | 1639 (96.5) | 960 (97.3) |
| 2008–2010 | ||||||
| Patients in cancer register | 4781 (100.0) | 2423 (100.0) | 1808 (100.0) | 957 (100.0) | 1494 (100.0) | 751 (100.0) |
| Death certificates only (DCO)/incidental finding at autopsy * | 549 (11.5) | 173 (7.1) | 12 (0.7) | 5 (0.5) | 14 (0.9) | 0 (0.0) |
| Age 90+ at diagnosis * | 208 (4.4) | 59 (2.4) | 67 (3.7) | 25 (2.6) | 34 (2.3) | 7 (0.9) |
| Included in survival analysis | 4134 (86.5) | 2219 (91.6) | 1730 (95.7) | 928 (97.0) | 1449 (97.0) | 744 (99.1) |
| 2011–2013 | ||||||
| Patients in cancer register | 4532 (100.0) | 2335 (100.0) | 2025 (100.0) | 1065 (100.0) | 1486 (100.0) | 706 (100.0) |
| Death certificates only (DCO)/incidental finding at autopsy * | 508 (11.2) | 171 (7.3) | 17 (0.8) | 4 (0.4) | 14 (0.9) | 2 (0.3) |
| Age 90+ at diagnosis * | 234 (5.2) | 91 (3.9) | 57 (2.8) | 28 (2.6) | 43 (2.9) | 12 (1.7) |
| Included in survival analysis | 3909 (86.3) | 2114 (90.5) | 1952 (96.4) | 1034 (97.1) | 1433 (96.4) | 692 (98.0) |
| 2014–2016 | ||||||
| Patients in cancer register | 4581 (100.0) | 2311 (100.0) | 2525 (100.0) | 1156 (100.0) | 1877 (100.0) | 901 (100.0) |
| Death certificates only (DCO)/incidental finding at autopsy * | 494 (10.8) | 161 (7.0) | 23 (0.9) | 1 (0.1) | 23 (1.2) | 2 (0.2) |
| Age 90+ at diagnosis * | 289 (6.3) | 78 (3.4) | 76 (3.0) | 30 (2.6) | 55 (2.9) | 19 (2.1) |
| Included in survival analysis | 3935 (85.9) | 2105 (91.1) | 2430 (96.2) | 1125 (97.3) | 1803 (96.1) | 880 (97.7) |
| 2004–2016 | ||||||
| Patients in cancer register | 20,086 (100.0) | 10,455 (100.0) | 8638 (100.0) | 4480 (100.0) | 6555 (100.0) | 3345 (100.0) |
| Death certificates only (DCO)/incidental finding at autopsy * | 2088 (10.4) | 696 (6.7) | 82 (0.9) | 19 (0.4) | 71 (1.1) | 12 (0.4) |
| Age 90+ at diagnosis * | 1036 (5.2) | 353 (3.4) | 275 (3.2) | 121 (2.7) | 172 (2.6) | 57 (1.7) |
| Included in survival analysis | 17,453 (86.9) | 9554 (91.4) | 8290 (96.0) | 4342 (96.9) | 6324 (96.5) | 3276 (97.9) |
| Schleswig-Holstein | Southern Denmark | Zealand | ||||
|---|---|---|---|---|---|---|
| Colon | Rectum | Colon | Rectum | Colon | Rectum | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| 2004–2007 | ||||||
| Surgery | 4571 (83.5) | 2417 (77.6) | 1835 (84.3) | 995 (79.3) | 1295 (79.0) | 735 (76.6) |
| Radiotherapy | 115 (2.1) | 1051 (33.7) | 27 (1.2) | 340 (27.1) | 15 (0.9) | 246 (25.6) |
| Systemic therapy | 1330 (24.3) | 1294 (41.5) | 593 (27.2) | 446 (35.5) | 571 (34.8) | 370 (38.5) |
| No treatment/Missing | 859 (15.7) | 560 (18.0) | 232 (10.7) | 114 (9.1) | 219 (13.4) | 113 (11.8) |
| 2008–2010 | ||||||
| Surgery | 3437 (83.1) | 1725 (77.7) | 1430 (82.7) | 725 (78.1) | 1099 (75.8) | 535 (71.9) |
| Radiotherapy | 62 (1.5) | 730 (32.9) | 22 (1.3) | 313 (33.7) | 22 (1.5) | 207 (27.8) |
| Systemic therapy | 1073 (26.0) | 983 (44.3) | 636 (36.8) | 464 (50.0) | 657 (45.3) | 391 (52.6) |
| No treatment/Missing | 649 (15.7) | 357 (16.1) | 173 (10.0) | 66 (7.1) | 168 (11.6) | 59 (7.9) |
| 2011–2013 | ||||||
| Surgery | 3166 (81.0) | 1607 (76.0) | 1574 (80.6) | 795 (76.9) | 1142 (79.7) | 517 (74.7) |
| Radiotherapy | 49 (1.3) | 697 (33.0) | 30 (1.5) | 335 (32.4) | 24 (1.7) | 165 (23.8) |
| Systemic therapy | 1081 (27.7) | 952 (45.0) | 791 (40.5) | 512 (49.5) | 679 (47.4) | 392 (56.6) |
| No treatment/Missing | 667 (17.1) | 400 (18.9) | 191 (9.8) | 75 (7.3) | 138 (9.6) | 51 (7.4) |
| 2014–2016 | ||||||
| Surgery | 3137 (79.7) | 1496 (71.1) | 1968 (81.0) | 852 (75.7) | 1488 (82.5) | 671 (76.3) |
| Radiotherapy | 49 (1.2) | 680 (32.3) | 27 (1.1) | 270 (24.0) | 27 (1.5) | 177 (20.1) |
| Systemic therapy | 916 (23.3) | 864 (41.0) | 902 (37.1) | 487 (43.3) | 771 (42.8) | 425 (48.3) |
| No treatment/Missing | 692 (17.6) | 424 (20.1) | 253 (10.4) | 94 (8.4) | 155 (8.6) | 71 (8.1) |
| 2004–2016 | ||||||
| Surgery | 14,311 (82.0) | 7245 (75.8) | 6807 (82.1) | 3367 (77.5) | 5024 (79.4) | 2458 (75.0) |
| Radiotherapy | 275 (1.6) | 3158 (33.1) | 106 (1.3) | 1258 (29.0) | 88 (1.4) | 795 (24.3) |
| Systemic therapy | 4400 (25.2) | 4093 (42.8) | 2922 (35.2) | 1909 (44.0) | 2678 (42.3) | 1578 (48.2) |
| No treatment/Missing | 2867 (16.4) | 1741 (18.2) | 849 (10.2) | 349 (8.0) | 680 (10.8) | 294 (9.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolph, C.; Engholm, G.; Pritzkuleit, R.; Storm, H.H.; Katalinic, A. Colorectal Cancer Survival in German–Danish Border Regions—A Registry-Based Cohort Study. Cancers 2023, 15, 4474. https://doi.org/10.3390/cancers15184474
Rudolph C, Engholm G, Pritzkuleit R, Storm HH, Katalinic A. Colorectal Cancer Survival in German–Danish Border Regions—A Registry-Based Cohort Study. Cancers. 2023; 15(18):4474. https://doi.org/10.3390/cancers15184474
Chicago/Turabian StyleRudolph, Christiane, Gerda Engholm, Ron Pritzkuleit, Hans H. Storm, and Alexander Katalinic. 2023. "Colorectal Cancer Survival in German–Danish Border Regions—A Registry-Based Cohort Study" Cancers 15, no. 18: 4474. https://doi.org/10.3390/cancers15184474
APA StyleRudolph, C., Engholm, G., Pritzkuleit, R., Storm, H. H., & Katalinic, A. (2023). Colorectal Cancer Survival in German–Danish Border Regions—A Registry-Based Cohort Study. Cancers, 15(18), 4474. https://doi.org/10.3390/cancers15184474





