Serum Paraprotein Is Associated with Adverse Prognostic Factors and Outcome, across Different Subtypes of Mature B-Cell Malignancies—A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
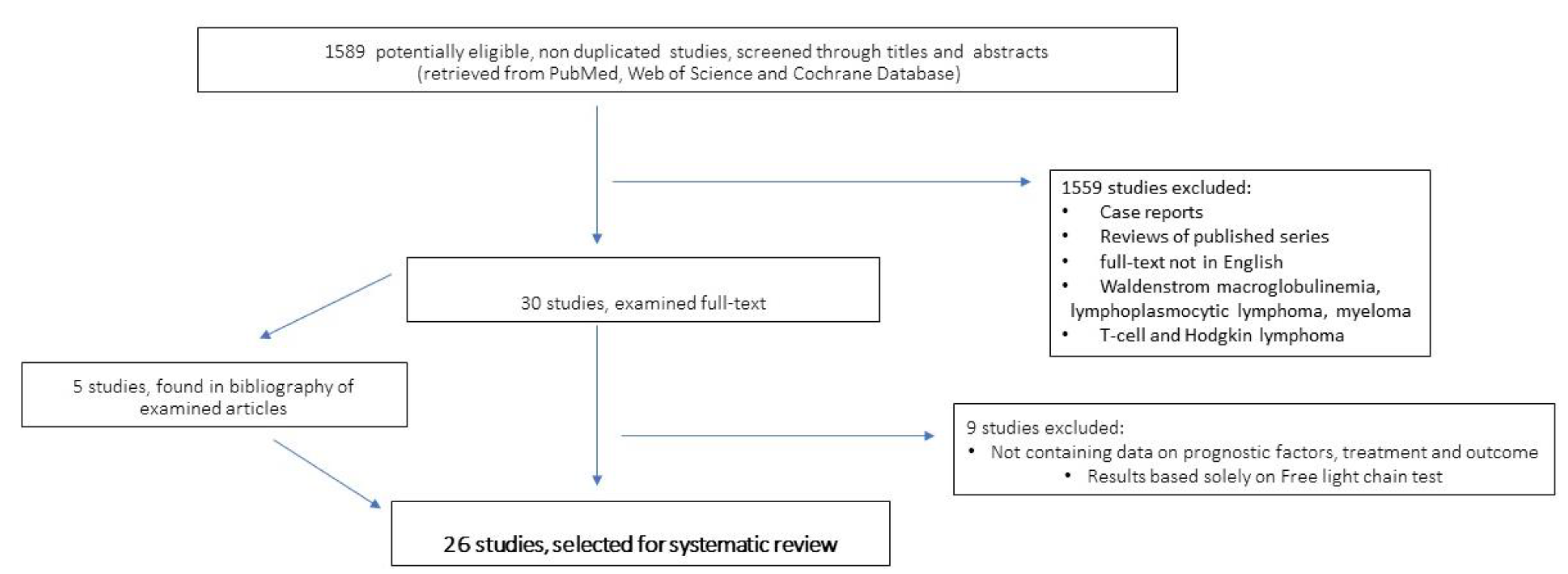
2. Methods and Criteria of Bibliographic Research and Articles Selection
3. Search Results
4. Paraprotein-Associated Mature B-Cell Lymphoproliferative Diseases
4.1. Chronic Lymphocytic Leukaemia
4.2. Diffuse Large B-Cell Lymphoma
4.3. Follicular Lymphoma
4.4. Marginal Zone Lymphoma
4.4.1. Extranodal Marginal Zone Lymphoma
4.4.2. Primary Cutaneous Marginal Zone Lymphoma (PCMZL)
4.4.3. Splenic Marginal Zone Lymphoma and CBL-MZ
4.5. Other B-Cell Lymphoproliferative Disorders
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Liu, J.; Burrows, P.D.; Wang, J.Y. B Cell Development and Maturation. Adv. Exp. Med. Biol. 2020, 1254, 1–22. [Google Scholar] [CrossRef]
- Meng, X.; Min, Q.; Wang, J.Y. B Cell Lymphoma. Adv. Exp. Med. Biol. 2020, 1254, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Garcia-Ibanez, L.; Toellner, K.M. Regulation of germinal center B-cell differentiation. Immunol. Rev. 2016, 270, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Agathangelidis, A.; Chatzidimitriou, A.; Chatzikonstantinou, T.; Tresoldi, C.; Davis, Z.; Giudicelli, V.; Kossida, S.; Belessi, C.; Rosenquist, R.; Ghia, P.; et al. Immunoglobulin gene sequence analysis in chronic lymphocytic leukemia: The 2022 update of the recommendations by ERIC, the European Research Initiative on CLL. Leukemia 2022, 36, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Efremov, D.G.; Laurenti, L. Recent advances in the pathogenesis and treatment of chronic lymphocytic leukemia. Prilozi 2014, 35, 105–120. [Google Scholar] [CrossRef][Green Version]
- Landau, D.A.; Tausch, E.; Taylor-Weiner, A.N.; Stewart, C.; Reiter, J.G.; Bahlo, J.; Kluth, S.; Bozic, I.; Lawrence, M.; Böttcher, S.; et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015, 526, 525–530. [Google Scholar] [CrossRef]
- Fermand, J.P.; Bridoux, F.; Dispenzieri, A.; Jaccard, A.; Kyle, R.A.; Leung, N.; Merlini, G. Monoclonal gammopathy of clinical significance: A novel concept with therapeutic implications. Blood 2018, 132, 1478–1485. [Google Scholar] [CrossRef]
- Girard, L.P.; Soekojo, C.Y.; Ooi, M.; Poon, L.M.; Chng, W.J.; de Mel, S. Immunoglobulin m paraproteinaemias. Cancers 2020, 12, 1688. [Google Scholar] [CrossRef]
- Singh, G. Serum free light chain assay and κ/λ ratio performance in patients without monoclonal gammopathies: High false-positive rate. Am. J. Clin. Pathol. 2016, 146, 207–214. [Google Scholar] [CrossRef]
- Singh, G. Serum and Urine Protein Electrophoresis and Serum-Free Light Chain Assays in the Diagnosis and Monitoring of Monoclonal Gammopathies. J. Appl. Lab. Med. 2020, 5, 1358–1371. [Google Scholar] [CrossRef]
- Maurer, M.J. The potential of serum light chains in diffuse large B-cell lymphoma. Leuk. Lymphoma 2013, 54, 1857–1858. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.J.; Cerhan, J.R.; Katzmann, J.A.; Link, B.K.; Allmer, C.; Zent, C.S.; Call, T.G.; Rabe, K.G.; Hanson, C.A.; Kay, N.E.; et al. Monoclonal and polyclonal serum free light chains and clinical outcome in chronic lymphocytic leukemia. Blood 2011, 118, 2821–2826. [Google Scholar] [CrossRef]
- Maurer, M.J.; Micallef, I.N.M.; Cerhan, J.R.; Katzmann, J.A.; Link, B.K.; Colgan, J.P.; Habermann, T.M.; Inwards, D.J.; Markovic, S.N.; Ansell, S.M.; et al. Elevated serum free light chains are associated with event-free and overall survival in two independent cohorts of patients with diffuse large B-cell lymphoma. J. Clin. Oncol. 2011, 29, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Morabito, F.; De Filippi, R.; Laurenti, L.; Zirlik, K.; Recchia, A.G.; Gentile, M.; Morelli, E.; Vigna, E.; Gigliotti, V.; Calemma, R.; et al. The cumulative amount of serum-free light chain is a strong prognosticator in chronic lymphocytic leukemia. Blood J. Am. Soc. Hematol. 2011, 118, 6353–6361. [Google Scholar] [CrossRef][Green Version]
- Pratt, G.; Harding, S.; Holder, R.; Fegan, C.; Pepper, C.; Oscier, D.; Gardiner, A.; Bradwell, A.R.; Mead, G. Abnormal serum free light chain ratios are associated with poor survival and may reflect biological subgroups in patients with chronic lymphocytic leukaemia. Br. J. Haematol. 2009, 144, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Maurer, M.J.; Habermann, T.M.; Link, B.K.; Micallef, I.N.M.; Nowakowski, G.S.; Ansell, S.M.; Colgan, J.P.; Inwards, D.J.; Porrata, L.F.; et al. Elevated monoclonal and polyclonal serum immunoglobulin free light chain as prognostic factors in B- and T-cell non-Hodgkin lymphoma. Am. J. Hematol. 2014, 89, 1116–1120. [Google Scholar] [CrossRef][Green Version]
- Bosch, F.; Dalla-Favera, R. Chronic lymphocytic leukaemia: From genetics to treatment. Nat. Rev. Clin. Oncol. 2019, 16, 684–701. [Google Scholar] [CrossRef]
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic lymphocytic leukaemia. Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef]
- Hampel, P.J.; Parikh, S.A. Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer J. 2022, 12, 161. [Google Scholar] [CrossRef]
- Lin, P.; Hao, S.; Handy, B.C.; Bueso-Ramos, C.E.; Medeiros, L.J. Lymphoid neoplasms associated with IgM paraprotein: A study of 382 patients. Am. J. Clin. Pathol. 2005, 123, 200–205. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y.H.; Fan, L.; Fang, C.; Zhu, D.X.; Wang, D.M.; Qiao, C.; Wu, Y.J.; Li, J.Y. Prognostic significance of serum immunoglobulin paraprotein in patients with chronic lymphocytic leukemia. Leuk. Res. 2011, 35, 1060–1065. [Google Scholar] [CrossRef]
- Rizzo, D.; Chauzeix, J.; Trimoreau, F.; Woillard, J.B.; Genevieve, F.; Bouvier, A.; Labrousse, J.; Poli, C.; Guerin, E.; Dmytruk, N.; et al. IgM peak independently predicts treatment-free survival in chronic lymphocytic leukemia and correlates with accumulation of adverse oncogenetic events. Leukemia 2015, 29, 337–345. [Google Scholar] [CrossRef]
- Corbingi, A.; Innocenti, I.; Tomasso, A.; Pasquale, R.; Visentin, A.; Varettoni, M.; Flospergher, E.; Autore, F.; Morelli, F.; Trentin, L.; et al. Monoclonal Gammopathy and Serum Immunoglobulin Levels as Prognostic Factors in Chronic Lymphocytic Leukaemia. Br. J. Haematol. 2020, 190, 901–908. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B. de O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G.; Nagel, I.; Siebert, R.; Roschke, A.V.; Sanger, W.; Wright, G.W.; Dave, S.S.; Tan, B.; Zhao, H.; Rosenwald, A.; et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J. Exp. Med. 2007, 204, 633–643. [Google Scholar] [CrossRef]
- Alkodsi, A.; Cervera, A.; Zhang, K.; Louhimo, R.; Meriranta, L.; Pasanen, A.; Leivonen, S.K.; Holte, H.; Leppä, S.; Lehtonen, R.; et al. Distinct Subtypes of Diffuse Large B-Cell Lymphoma Defined by Hypermutated Genes. Leukemia 2019, 33, 2662–2672. [Google Scholar] [CrossRef]
- Heise, N.; de Silva, N.S.; Silva, K.; Carette, A.; Simonetti, G.; Pasparakis, M.; Klein, U. Germinal Center B Cell Maintenance and Differentiation Are Controlled by Distinct NF-ΚB Transcription Factor Subunits. J. Exp. Med. 2014, 211, 2103–2118. [Google Scholar] [CrossRef]
- Havranek, O.; Xu, J.; Köhrer, S.; Wang, Z.; Becker, L.; Comer, J.M.; Henderson, J.; Ma, W.; Ma, J.M.C.; Westin, J.R.; et al. Tonic B-Cell Receptor Signaling in Diffuse Large B-Cell Lymphoma. Blood 2017, 130, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular Subtypes of Diffuse Large B-Cell Lymphoma Are Associated with Distinct Pathogenic Mechanisms and Outcomes. Nat. Med. 2018, 24, 679. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.L.; et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494.e15. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. M. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Miao, Y.; Medeiros, L.J.; Li, Y.; Li, J.; Young, K.H. Genetic Alterations and Their Clinical Implications in DLBCL. Nat. Rev. Clin. Oncol. 2019, 16, 634–652. [Google Scholar] [CrossRef]
- Jardin, F.; Delfau-Larue, M.H.; Molina, T.J.; Copie-Bergman, C.; Brière, J.; Petrella, T.; Canioni, D.; Fabiani, B.; Jais, J.P.; Figeac, M.; et al. Immunoglobulin Heavy Chain/Light Chain Pair Measurement Is Associated with Survival in Diffuse Large B-Cell Lymphoma. Leuk Lymphoma 2013, 54, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Maiolo, E.; Alma, E.; Napodano, C.; Gulli, F.; Bellesi, S.; Cuccaro, A.; Pocino, K.; D’Alo’, F.; Iachini, M.; Martini, M.; et al. The Prognostic Impact of Monoclonal Immune Globulin and Free Light Chain Secretion in Diffuse Large B Cell Lymphoma (DLBCL). Leuk Lymphoma 2020, 61, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.C.; Di Napoli, A.; Scarpino, S.; Salerno, G.; Tatarelli, C.; Talerico, C.; Lombardi, M.; Monarca, B.; Amadori, S.; Ruco, L. Clinicopathologic Characterization of Diffuse-Large-B-Cell Lymphoma with an Associated Serum Monoclonal IgM Component. PLoS ONE 2014, 9, e93903. [Google Scholar] [CrossRef]
- Cox, M.C.; Marcheselli, L.; Scafetta, G.; Visco, C.; Hohaus, S.; Annibali, O.; Musuraca, G.; Fabbri, A.; Cantonetti, M.; Pelliccia, S.; et al. IgM-Secreting Diffuse Large B-Cell Lymphoma: Results of a Multicentre Clinicopathological and Molecular Study. Leukemia 2022, 36, 2719–2723. [Google Scholar] [CrossRef]
- Dührsen, U.; Müller, S.; Hertenstein, B.; Thomssen, H.; Kotzerke, J.; Mesters, R.; Berdel, W.E.; Franzius, C.; Kroschinsky, F.; Weckesser, M.; et al. Positron Emission Tomography-Guided Therapy of Aggressive Non-Hodgkin Lymphomas (PETAL): A Multicenter, Randomized Phase III Trial. J. Clin. Oncol. 2018, 36, 2024–2034. [Google Scholar] [CrossRef]
- Johansson, P.; Alig, S.; Richter, J.; Hanoun, C.; Rekowski, J.; Dürig, J.; Ylstra, B.; de Jong, D.; Klapper, W.; Alizadeh, A.A.; et al. Outcome Prediction by Interim Positron Emission Tomography and IgM Monoclonal Gammopathy in Diffuse Large B-Cell Lymphoma. Ann. Hematol. 2023. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, S.J.; Cheong, J.W.; Kim, Y.; Jang, J.E.; Lee, J.Y.; Min, Y.H.; Song, J.W.; Yang, W.I.; Kim, J.S. Monoclonal and Polyclonal Gammopathy Measured by Serum Free Light Chain and Immunofixation Subdivide the Clinical Outcomes of Diffuse Large B-Cell Lymphoma According to Molecular Classification. Ann. Hematol. 2014, 93, 1867–1877. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhu, H.Y.; Liang, J.H.; Wu, W.; Wu, J.Z.; Xia, Y.; Fan, L.; Li, J.Y.; Xu, W. Prognostic Significance of Serum Immunoglobulin Paraprotein in Patients with Diffuse Large B Cell Lymphoma. Br. J. Haematol. 2018, 182, 131–134. [Google Scholar] [CrossRef]
- Papageorgiou, S.G.; Thomopoulos, T.P.; Spathis, A.; Bouchla, A.; Glezou, I.; Stavroulaki, G.; Gkontopoulos, K.; Bazani, E.; Foukas, P.G.; Pappa, V. Prognostic Significance of Monoclonal Gammopathy in Diffuse Large B-Cell Lymphoma. Hematol. Oncol. 2019, 37, 634–637. [Google Scholar]
- Zhang, Y.; Wei, Z.; Li, J.; Gao, R.; Liu, P. Monoclonal Gammopathies Regardless of Subtypes Are Associated with Poor Prognosis of Diffuse Large B-Cell Lymphoma A STROBE-Compliant Article. Medicine (United States) 2018, 97, e11719. [Google Scholar] [CrossRef]
- Mozas, P.; Rivero, A.; Rivas-Delgado, A.; Fabregat, A.; Piñeyroa, J.A.; Correa, J.G.; Nadeu, F.; Oliver, A.; Bataller, A.; Giné, E.; et al. Baseline Correlations and Prognostic Impact of Serum Monoclonal Proteins in Follicular Lymphoma. Br. J. Haematol. 2021, 193, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Xochelli, A.; Kalpadakis, C.; Gardiner, A.; Baliakas, P.; Vassilakopoulos, T.P.; Mould, S.; Davis, Z.; Stalika, E.; Kanellis, G.; Angelopoulou, M.K.; et al. Clonal B-cell lymphocytosis exhibiting immunophenotypic features consistent with a marginal-zone origin: Is this a distinct entity? Blood 2014, 123, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Zucca, E.; Bertoni, F. The Spectrum of MALT Lymphoma at Different Sites: Biological and Therapeutic Relevance. Blood 2016, 127, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Wöhrer, S.; Streubel, B.; Bartsch, R.; Chott, A.; Raderer, M. Monoclonal Immunoglobulin Production Is a Frequent Event in Patients with Mucosa-Associated Lymphoid Tissue Lymphoma. Clin. Cancer. Res. 2004, 10, 7179–7181. [Google Scholar] [CrossRef] [PubMed]
- Asatiani, E.; Cohen, P.; Ozdemirli, M.; Kessler, C.M.; Mavromatis, B.; Cheson, B.D. Monoclonal Gammopathy in Extranodal Marginal Zone Lymphoma (ENMZL) Correlates with Advanced Disease and Bone Marrow Involvement. Am. J. Hematol. 2004, 77, 144–146. [Google Scholar] [CrossRef]
- Arcaini, L.; Burcheri, S.; Rossi, A.; Passamonti, F.; Paulli, M.; Boveri, E.; Brusamolino, E.; Orlandi, E.; Molteni, A.; Pulsoni, A.; et al. Nongastric Marginal-Zone B-Cell MALT Lymphoma: Prognostic Value of Disease Dissemination. Oncologist 2006, 11, 285–291. [Google Scholar] [CrossRef]
- Alderuccio, J.P.; Zhao, W.; Desai, A.; Ramdial, J.; Gallastegui, N.; Kimble, E.; de la Fuente, M.I.; Husnain, M.; Rosenblatt, J.D.; Alencar, A.J.; et al. Short Survival and Frequent Transformation in Extranodal Marginal Zone Lymphoma with Multiple Mucosal Sites Presentation. Am. J. Hematol. 2019, 94, 585–596. [Google Scholar] [CrossRef]
- Ren, Y.M.; Shang, C.Y.; Liang, J.H.; Yin, H.; Xia, Y.; Wu, J.Z.; Wang, L.; Jian-Yong, L.; Li, Y.; Xu, W. Prognostic Significance of Serum Immunoglobulin Paraprotein in Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Br. J. Haematol. 2022, 196, 1353–1361. [Google Scholar] [CrossRef]
- Thieblemont, C.; Cascione, L.; Conconi, A.; Kiesewetter, B.; Raderer, M.; Gaidano, G.; Martelli, M.; Laszlo, D.; Coiffier, B.; Lopez Guillermo, A.; et al. A MALT Lymphoma Prognostic Index. Blood 2017, 130, 1409–1417. [Google Scholar] [CrossRef]
- Frings, V.; Röding, K.; Strate, A.; Rosenwald, A.; Roth, S.; Kneitz, H.; Goebeler, M.; Geissinger, E.; Wobser, M. Paraproteinaemia in Primary Cutaneous Marginal Zone Lymphoma. Acta. Dermato-Venereologica 2018, 98, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Felman, P.; Berger, F.; Dumontet, C.; Arnaud, P.; Hequet, O.; Arcache, J.; Callet-Bauchu, E.; Salles, G.; Coeffier, B. Treatment of Splenic Marginal Zone B-Cell Lymphoma: An Analysis of 81 Patients. Clin. Lymphoma 2002, 3, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Montalbán, C.; Abraira, V.; Arcaini, L.; Domingo-Domenech, E.; Guisado-Vasco, P.; Iannito, E.; Mollejo, M.; Matutes, E.; Ferreri, A.; Salar, A.; et al. Risk Stratification for Splenic Marginal Zone Lymphoma Based on Haemoglobin Concentration, Platelet Count, High Lactate Dehydrogenase Level and Extrahilar Lymphadenopathy: Development and Validation on 593 Cases. Br. J. Haematol. 2012, 159, 164–171. [Google Scholar] [CrossRef]
- Epperla, N.; Welkie, R.L.; Torka, P.; Shouse, G.; Karmali, R.; Shea, L.; Anampa-Guzmán, A.; Oh, T.S.; Reaves, H.; Tavakkoli, M.; et al. Impact of Early Relapse within 24 Months after First-Line Systemic Therapy (POD24) on Outcomes in Patients with Marginal Zone Lymphoma: A US Multisite Study. J. Hematol. Oncol. 2023, 16, 49. [Google Scholar]
- Epperla, N.; Zhao, Q.; Karmali, R.; Torka, P.; Shea, L.; Oh, T.S.; Anampa-Guzmán, A.; Reves, H.; Tavakkoli, M.; Greenwell, I.B.; et al. Impact of monoclonal protein at diagnosis on outcomes in marginal zone lymphoma: A multicenter cohort study. Blood Adv. 2023, 7, 5038–5046. [Google Scholar] [CrossRef] [PubMed]
- Luminari, S.; Merli, M.; Rattotti, S.; Tarantino, V.; Marcheselli, L.; Cavallo, F.; Varettoni, M.; Bianchi, B.; Merli, F.; Tedeschi, A.; et al. Brief Report Early Progression as a Predictor of Survival in Marginal Zone Lymphomas: An Analysis from the FIL-NF10 Study. Blood 2019, 134, 798–801. [Google Scholar]
- Randen, U.; Trøen, G.; Tierens, A.; Steen, C.; Warsame, A.; Beiske, K.; Tjønnfjord, G.E.; Berentsen, S.; Delabie, J. Primary Cold Agglutinin-Associated Lymphoproliferative Disease: A B-Cell Lymphoma of the Bone Marrow Distinct from Lymphoplasmacytic Lymphoma. Haematologica 2014, 99, 497–504. [Google Scholar] [CrossRef]
- Ollila, T.A.; Kurt, H.; Waroich, J.; Vatkevich, J.; Sturtevant, A.; Patel, N.R.; Dubielecka, P.M.; Treaba, D.O.; Olszewski, A.J. Genomic Subtypes May Predict the Risk of Central Nervous System Recurrence in Diffuse Large B-Cell Lymphoma. Blood 2021, 137, 1120–1124. [Google Scholar] [CrossRef]
| Disease | PP+ve/All (%) | PP Subtypes (%) | Advanced Stage | p | AdversePrognosticFactors | p | Outcome | p | Reference |
|---|---|---|---|---|---|---|---|---|---|
| CLL | 27/133 (20%) | IgM = 44% IgG = 44% | PP+ve vs. ref | <0.001 | 17p-/11q-- PP+ve = 67% ref = 33% | 0.032 | OS MV IgG IgM HR = 0.208 | ns 0.04 | Xu 2011 |
| CLL | 222/1505 (17.3%) | IgM = 33% IgG = 67% | IgM = 39.% IgG = 23% hypo-y = 31% ref = 25% | 0.002 | 17p- IgM = 15% IgG = 3% hypo-y = 5% ref = 6% | 0.022 | TFS MV IgM HR = 2.187 IgG HR = 1.609 Hypo-y HR = 1.699 | <0.0001 0.001 <0.0001 | Corbingi 2020 |
| OS MV | ns | ||||||||
| CLL | 52/150 (34.6%) | IgM = 52% IgG = 48% | IgM = 63% IgG = 37.5% hypo-y = 22% ref = 3.5% | <0.0001 | 17p-/11q-/+12 IgM = 60% IgG = 50% Hypo-y = 19% ref = 23% | <0.001 | TFS MV IgM HR = 2.63 IgG HR = 3.55 Hypo-y HR = 2.34 | 0.0031 0.0002 0.0059 | Rizzo 2015 |
| OS MV | ns | ||||||||
| DLBCL | 158/492 IgM = 102 IgG = 52 IgA = 3 Other = 1 | IgM = 83% O-PP = 68% ref = 65% | 0.002 | IPI = 3–5 IgM = 55% O-PP = 40% ref = 36% CNS-IPI = 4–6 IgM = 36% O-PP = 14% ref = 15% | <0.001 <0.001 | PFS MV IgM, HR = 3.62 O-PP, HR = 1.54 | <0.001 ns | Cox 2022 | |
| OS MV IgM, HR = 3.57 O-PP, HR = 1.53 | <0.001 ns | ||||||||
| DLBCL | 154/409 (37.6%) | IgM = 51.6% IgG = 17% IgA = 8% LC = 24.5% | IgM vs. ref LC vs. ref O-PP n.a. | 0.002 0.001 | IPI-score 3–5 IgM vs. ref LC vs. ref O-PP vs. ref Age-adjusted IPI = 2–3 IgM vs. ref LC vs. ref O-PP n.a. | <0.001 0.008 n.a. 0.002 0.013 | PFS MV IgM HR = 1.53 O-PP | 0.032 ns | Jardin 2013 |
| OS MV IgM O-PP | ns ns | ||||||||
| DLBCL | 19/108 (18%) | n.a. | ref = 51% IgM = 74% | 0.079 | IPI-score = 3–5 IgM vs. ref | 0.025 | PFS MV IgM, HR = 5.44 OS MV IgM, HR = 8.15 | <0.001 <0.001 | Johansson 2023 |
| FL | 82/299 (27%) | IgM = 25.6% IgG = 50% IgA = 5% LC = 11% Biclonal = 9% | PP vs. ref | ns | Extranodal sites Beta2 > N POD24 | 0.05 0.002 0.02 | PFS (10-years) ref vs. PP+ve PFS MV | 0.0076 ns | Mozas 2021 |
| OS (10-years) ref vs. PP OS MV All PP+ve vs. ref >60y PP+ve vs. ref | 0.045 ns 0.02 | ||||||||
| EMZL (non gastric) | 36/208 (17%) | IgM: 66% IgG: 25% IgA: 8% | n.a. | n.a. | OS MV (HR n.a.) | 0.04 | Arcaini 2006 | ||
| EZML | 42/176 (19.3%) | IgM = 64% IgG = 14.3% Biclonal = 12% LC = 2.4% | PP 74% vs. ref 29% | <0.0001 | Extranodal > 2 Beta2 > N Nodal involvement MALT-IPI | <0.0001 <0.0001 <0.0001 0.001 | PFS MV PP+ve, HR = 2.31 OS MV PP+ve, HR = 4.14 | 0.018 0.026 | Ren 2022 |
| EZML | 35/328 (10.7%) | IgM = 43% IgG = 37% IgA = 14% Biclonal = 3% LC = 3% | n.a. | n.a. | PFS (5-years) ref vs. PP+ve PP+ve PFS MV | 0.015 ns | Alderuccio 2019 | ||
| OS (5-years) ref vs. PP OS MV | 0.038 ns | ||||||||
| MZL EMZL NMZL SMZL | 173/547 (32%) 86/319 (50%) 49/121 (28%) 38/107 (22%) | IgM = 56% IgG = 31% IgA = 5% Biclonal = 3% LC = 5% | PP 73% vs. ref 54% | 0.01 | Age (median) BM infiltration PS Ecog HT POD24 | <0.01 0.001 0.01 0.001 <0.0001 | TFS ref vs. PP+ve | ns | Epperla 2023 Blood adv & J Hematology & Oncol |
| PFS MV All PP+ve HR = 1.74 R-Benda PP+ve | 0.004 ns | ||||||||
| OS MV PP+ve | ns | ||||||||
| SMZL | 34/81 (42%) | IgM 43% IgG 37% IgA 3% biclonal 2% | n.a. | n.a. | TTP MV PP+ve | <0.006 | Thieblemont 2002 | ||
| OS MV PP+ve | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, M.C.; Esposito, F.; Postorino, M.; Venditti, A.; Di Napoli, A. Serum Paraprotein Is Associated with Adverse Prognostic Factors and Outcome, across Different Subtypes of Mature B-Cell Malignancies—A Systematic Review. Cancers 2023, 15, 4440. https://doi.org/10.3390/cancers15184440
Cox MC, Esposito F, Postorino M, Venditti A, Di Napoli A. Serum Paraprotein Is Associated with Adverse Prognostic Factors and Outcome, across Different Subtypes of Mature B-Cell Malignancies—A Systematic Review. Cancers. 2023; 15(18):4440. https://doi.org/10.3390/cancers15184440
Chicago/Turabian StyleCox, Maria Christina, Fabiana Esposito, Massimiliano Postorino, Adriano Venditti, and Arianna Di Napoli. 2023. "Serum Paraprotein Is Associated with Adverse Prognostic Factors and Outcome, across Different Subtypes of Mature B-Cell Malignancies—A Systematic Review" Cancers 15, no. 18: 4440. https://doi.org/10.3390/cancers15184440
APA StyleCox, M. C., Esposito, F., Postorino, M., Venditti, A., & Di Napoli, A. (2023). Serum Paraprotein Is Associated with Adverse Prognostic Factors and Outcome, across Different Subtypes of Mature B-Cell Malignancies—A Systematic Review. Cancers, 15(18), 4440. https://doi.org/10.3390/cancers15184440







