Is Computed-Tomography-Based Body Composition a Reliable Predictor of Chemotherapy-Related Toxicity in Pancreatic Cancer Patients?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Clinical Data Recording
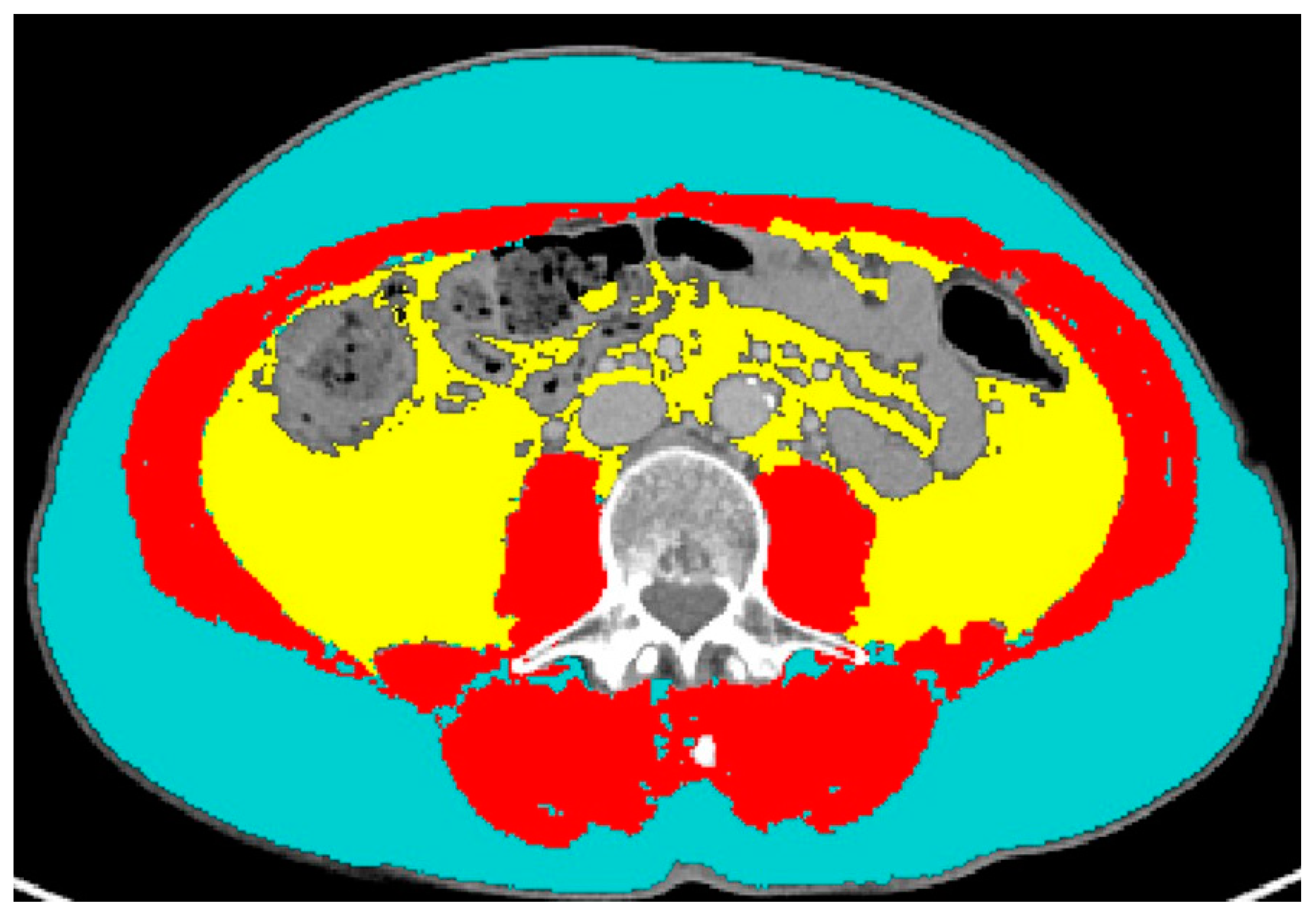
2.3. CT Data Extraction
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer. A Review. J. Am. Med. Assoc. 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Frulloni, L.; Minniti, C.; Bassi, C.; Barugola, G.; D’onofrio, M.; Crippa, S.; Falconi, M. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig. Liver Dis. 2012, 44, 945–951. [Google Scholar] [CrossRef]
- Emori, T.; Itonaga, M.; Ashida, R.; Tamura, T.; Kawaji, Y.; Hatamaru, K.; Yamashita, Y.; Shimokawa, T.; Koike, M.; Sonomura, T.; et al. Impact of sarcopenia on prediction of progression-free survival and overall survival of patients with pancreatic ductal adenocarcinoma receiving first-line gemcitabine and nab-paclitaxel chemotherapy. Pancreatology 2021, 22, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Del Grande, M.; Rizzo, S.; Nicolino, G.M.; Colombo, I.; Rossi, L.; Manganaro, L.; Del Grande, F. Computed Tomography–Based Body Composition in Patients With Ovarian Cancer: Association With Chemotoxicity and Prognosis. Front. Oncol. 2021, 11, 718815. [Google Scholar] [CrossRef]
- Rizzo, S.; Petrella, F.; Bardoni, C.; Bramati, L.; Cara, A.; Mohamed, S.; Radice, D.; Raia, G.; Del Grande, F.; Spaggiari, L. CT-Derived Body Composition Values and Complications After Pneumonectomy in Lung Cancer Patients: Time for a Sex-Related Analysis? Front. Oncol. 2022, 12, 826058. [Google Scholar] [CrossRef]
- Sanchez, A.; Kissel, S.; Coletta, A.; Scott, J.; Furberg, H. Impact of body size and body composition on bladder cancer outcomes: Risk stratification and opportunity for novel interventions. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 713–718. [Google Scholar] [CrossRef]
- Basile, D.; Corvaja, C.; Caccialanza, R.; Aprile, G. Sarcopenia: Looking to muscle mass to better manage pancreatic cancer patients. Curr. Opin. Support. Palliat. Care 2019, 13, 279–285. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yagyu, T.; Uchinaka, E.; Miyatani, K.; Hanaki, T.; Kihara, K.; Matsunaga, T.; Yamamoto, M.; Tokuyasu, N.; Honjo, S.; et al. Sarcopenia as a prognostic factor in patients with recurrent pancreatic cancer: A retrospective study. World J. Surg. Oncol. 2020, 18, 221. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef]
- DiMagno, E.P. Pancreatic cancer: Clinical presentation, pitfalls and early clues. Ann. Oncol. 1999, 10, S140–S142. [Google Scholar] [CrossRef]
- Zalite, I.O.; Zykus, R.; Gonzalez, M.F.; Saygili, F.; Pukitis, A.; Gaujoux, S.; Charnley, R.; Lyadov, V. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: A systematic review. Pancreatology 2015, 15, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Na, J.Y.; Kim, J.; Oh, J.-W.; Kim, Y.J.; Choi, Y.-J. Age-Specific Characteristics of Adult and Pediatric Respiratory Viral Infections: A Retrospective Single-Center Study. J. Clin. Med. 2022, 11, 3197. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chan, M.Y.; Chok, K.S.H. Sarcopenia in pancreatic cancer—Effects on surgical outcomes and chemotherapy. World J. Gastrointest. Oncol. 2019, 11, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Wang, C.-J.; Chao, Y.-J.; Chen, H.-Y.; Wang, H.-C.; Tung, H.-L.; Lin, J.-T.; Shan, Y.-S. Elevated Serum Interleukin-8 Level Correlates with Cancer-Related Cachexia and Sarcopenia: An Indicator for Pancreatic Cancer Outcomes. J. Clin. Med. 2018, 7, 502. [Google Scholar] [CrossRef]
- Bachmann, J.; Heiligensetzer, M.; Krakowski-Roosen, H.; Büchler, M.W.; Friess, H.; Martignoni, M.E. Cachexia Worsens Prognosis in Patients with Resectable Pancreatic Cancer. J. Gastrointest. Surg. 2008, 12, 1193–1201. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Chen, C.-Y.; Huang, C.-J.; Wang, C.-J.; Chao, Y.-J.; Chiang, N.-J.; Wang, H.-C.; Tung, H.-L.; Liu, H.-C.; Shan, Y.-S. The Differential Clinical Impacts of Cachexia and Sarcopenia on the Prognosis of Advanced Pancreatic Cancer. Cancers 2022, 14, 3137. [Google Scholar] [CrossRef]
- Sjøblom, B.; Grønberg, B.H.; Benth, J.Š.; Baracos, V.E.; Fløtten, Ø.; Hjermstad, M.J.; Aass, N.; Jordhøy, M. Low muscle mass is associated with chemotherapy-induced haematological toxicity in advanced non-small cell lung cancer. Lung Cancer 2015, 90, 85–91. [Google Scholar] [CrossRef]
- Tan, B.H.; Birdsell, L.A.; Martin, L.; Baracos, V.E.; Fearon, K.C. Sarcopenia in an Overweight or Obese Patient Is an Adverse Prognostic Factor in Pancreatic Cancer. Clin. Cancer Res. 2009, 15, 6973–6979. [Google Scholar] [CrossRef]
- Hopkins, J.J.; Sawyer, M.B. A review of body composition and pharmacokinetics in oncology. Expert Rev. Clin. Pharmacol. 2017, 10, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Cousin, S.; Hollebecque, A.; Koscielny, S.; Mir, O.; Varga, A.; Baracos, V.E.; Soria, J.C.; Antoun, S. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Investig. New Drugs 2013, 32, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Choi, S.J.; Kim, Y.S.; Ahn, H.K.; Hong, J.; Sym, S.J.; Park, J.; Cho, E.K.; Lee, J.H.; Shin, Y.J.; et al. Prognostic Factors for Risk Stratification of Patients with Recurrent or Metastatic Pancreatic Adenocarcinoma Who Were Treated with Gemcitabine-Based Chemotherapy. Cancer Res. Treat. 2016, 48, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.B.; Slack, R.; Fogelman, D.; Holmes, H.M.; Petzel, M.; Parker, N.; Balachandran, A.; Garg, N.; Ngo-Huang, A.; Varadhachary, G.; et al. Characterization of Anthropometric Changes that Occur During Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2014, 22, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Kurita, Y.; Kobayashi, N.; Tokuhisa, M.; Goto, A.; Kubota, K.; Endo, I.; Nakajima, A.; Ichikawa, Y. Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy. Pancreatology 2018, 19, 127–135. [Google Scholar] [CrossRef]
- Asama, H.; Ueno, M.; Kobayashi, S.; Fukushima, T.; Kawano, K.; Sano, Y.; Tanaka, S.; Nagashima, S.; Morimoto, M.; Ohira, H.; et al. Sarcopenia: Prognostic Value for Unresectable Pancreatic Ductal Adenocarcinoma Patients Treated With Gemcitabine Plus Nab-Paclitaxel. Pancreas 2022, 51, 148–152. [Google Scholar] [CrossRef]
- Thormann, M.; Hinnerichs, M.; Ordonez, F.B.; Saalfeld, S.; Perrakis, A.; Croner, R.; Omari, J.; Pech, M.; Zamsheva, M.; Meyer, H.-J.; et al. Sarcopenia is an Independent Prognostic Factor in Patients With Pancreatic Cancer—A Meta-analysis. Acad. Radiol. 2022, 30, 1552–1561. [Google Scholar] [CrossRef]
- Youn, S.; Chen, A.; Ha, V.; Chambers, C.; Eurich, D.T.; McCall, M.; Sawyer, M.B. An exploratory study of body composition as a predictor of dose-limiting toxicity in metastatic pancreatic cancer treated with gemcitabine plus nab-paclitaxel. Clin. Nutr. 2021, 40, 4888–4892. [Google Scholar] [CrossRef]
- Rizzo, S.; Scala, I.; Robayo, A.R.; Cefalì, M.; De Dosso, S.; Cappio, S.; Xhepa, G.; Del Grande, F. Body composition as a predictor of chemotherapy-related toxicity in pancreatic cancer patients: A systematic review. Front. Oncol. 2022, 12, 974116. [Google Scholar] [CrossRef]
- Rizzo, S.M.R.; Kalra, M.K.; Schmidt, B.; Raupach, R.; Maher, M.M.; Blake, M.A.; Saini, S. CT Images of Abdomen and Pelvis: Effect of Nonlinear Three-dimensional Optimized Reconstruction Algorithm on Image Quality and Lesion Characteristics. Radiology 2005, 237, 309–315. [Google Scholar] [CrossRef]
- Pediconi, F.; Rizzo, V.; Schiaffino, S.; Cozzi, A.; Della Pepa, G.; Galati, F.; Catalano, C.; Sardanelli, F. Visceral adipose tissue area predicts intensive care unit admission in COVID-19 patients. Obes. Res. Clin. Pract. 2020, 15, 89–92. [Google Scholar] [CrossRef]
- Rietjens, M.; Villa, G.; Toesca, A.; Rizzo, S.; Raimondi, S.; Rossetto, F.; Sangalli, C.; De Lorenzi, F.; Manconi, A.; Matthes, A.G.Z.; et al. Appropriate Use of Magnetic Resonance Imaging and Ultrasound to Detect Early Silicone Gel Breast Implant Rupture in Postmastectomy Reconstruction. Plast. Reconstr. Surg. 2014, 134, 13e–20e. [Google Scholar] [CrossRef] [PubMed]
- Cascinu, S.; Falconi, M.; Valentini, V.; Jelic, S. Pancreatic cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v55–v58. [Google Scholar] [CrossRef] [PubMed]
- Bellomi, M.; Rizzo, S.; Travaini, L.L.; Bazzi, L.; Trifirò, G.; Zampino, M.G.; Radice, D.; Paganelli, G. Role of multidetector CT and FDG-PET/CT in the diagnosis of local and distant recurrence of resected rectal cancer. La Radiol. Medica 2007, 112, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Botta, F.; Raimondi, S.; Rinaldi, L.; Bellerba, F.; Corso, F.; Bagnardi, V.; Origgi, D.; Minelli, R.; Pitoni, G.; Petrella, F.; et al. Association of a CT-Based Clinical and Radiomics Score of Non-Small Cell Lung Cancer (NSCLC) with Lymph Node Status and Overall Survival. Cancers 2020, 12, 1432. [Google Scholar] [CrossRef]
- Ionescu, C.M.; Ghita, M.; Copot, D.; Derom, E.; Verellen, D. A Minimal PKPD Interaction Model for Evaluating Synergy Effects of Combined NSCLC Therapies. J. Clin. Med. 2020, 9, 1832. [Google Scholar] [CrossRef]
- Dalal, T.; Kalra, M.K.; Rizzo, S.M.R.; Schmidt, B.; Suess, C.; Flohr, T.; Blake, M.A.; Saini, S. Metallic Prosthesis: Technique to Avoid Increase in CT Radiation Dose with Automatic Tube Current Modulation in a Phantom and Patients. Radiology 2005, 236, 671–675. [Google Scholar] [CrossRef]
- Zaffina, C.; Wyttenbach, R.; Pagnamenta, A.; Grasso, R.F.; Biroli, M.; Del Grande, F.; Rizzo, S. Body composition assessment: Comparison of quantitative values between magnetic resonance imaging and computed tomography. Quant. Imaging Med. Surg. 2022, 12, 1450–1466. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v56–v68. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. nab-Paclitaxel Plus Gemcitabine for Metastatic Pancreatic Cancer: Long-Term Survival From a Phase III Trial. Gynecol. Oncol. 2014, 107, dju413. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Fang, P.; Hu, J.-H.; Cheng, Z.-G.; Liu, Z.-F.; Wang, J.-L.; Jiao, S.-C. Efficacy and Safety of Bevacizumab for the Treatment of Advanced Hepatocellular Carcinoma: A Systematic Review of Phase II Trials. PLoS ONE 2012, 7, e49717. [Google Scholar] [CrossRef]
- Takeda, T.; Sasaki, T.; Suzumori, C.; Mie, T.; Furukawa, T.; Yamada, Y.; Kasuga, A.; Matsuyama, M.; Ozaka, M.; Sasahira, N. The impact of cachexia and sarcopenia in elderly pancreatic cancer patients receiving palliative chemotherapy. Int. J. Clin. Oncol. 2021, 26, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, A.; Monti, D.; Santoro, A. Editorial: Adipose Tissue: Which Role in Aging and Longevity? Front. Endocrinol. 2020, 11, 583. [Google Scholar] [CrossRef]
- Rossi, T.; Bandini, E.; Balzi, W.; Fabbri, F.; Massa, I.; Maltoni, R. Obesity and Dose of Anti-cancer Therapy: Are We Sure to Be on the Right Track in the Precision Medicine Era? Front. Med. 2021, 8, 725346. [Google Scholar] [CrossRef]
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front. Endocrinol. 2020, 10, 861. [Google Scholar] [CrossRef]
- Morrish, G.A.; Pai, M.P.; Green, B. The effects of obesity on drug pharmacokinetics in humans. Expert Opin. Drug Metab. Toxicol. 2011, 7, 697–706. [Google Scholar] [CrossRef]
- Chagnac, A.; Herman, M.; Zingerman, B.; Erman, A.; Rozen-Zvi, B.; Hirsh, J.; Gafter, U. Obesity-induced glomerular hyperfiltration: Its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol. Dial. Transplant. 2008, 23, 3946–3952. [Google Scholar] [CrossRef]
- McGovern, J.; Dolan, R.D.; Horgan, P.G.; Laird, B.J.; McMillan, D.C. Computed tomography-defined low skeletal muscle index and density in cancer patients: Observations from a systematic review. J. Cachex-Sarcopenia Muscle 2021, 12, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Anraku, M.; Kawahara, T.; Karasaki, T.; Konoeda, C.; Kitano, K.; Sato, M.; Nakajima, J. Combination of Skeletal Muscle Mass and Density Predicts Postoperative Complications and Survival of Patients With Non-Small Cell Lung Cancer. Ann. Surg. Oncol. 2022, 29, 1816–1824. [Google Scholar] [CrossRef]
- van der Zanden, V.; van Soolingen, N.J.; Viddeleer, A.R.; Trum, J.W.; Amant, F.; Mourits, M.J.; Portielje, J.E.; Bos, F.V.D.; de Kroon, C.D.; Kagie, M.J.; et al. Low preoperative skeletal muscle density is predictive for negative postoperative outcomes in older women with ovarian cancer. Gynecol. Oncol. 2021, 162, 360–367. [Google Scholar] [CrossRef]
- Kim, I.-H.; Choi, M.H.; Lee, I.S.; Hong, T.H.; Lee, M.A. Clinical significance of skeletal muscle density and sarcopenia in patients with pancreatic cancer undergoing first-line chemotherapy: A retrospective observational study. BMC Cancer 2021, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Yoon, S.B. Sarcopenia in pancreatic cancer: Effect on patient outcomes. World J. Gastrointest. Oncol. 2022, 14, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
| No. of Patients | 131 |
|---|---|
| Age (years) | |
| Mean (SD) | 69.7 (9.0) |
| Range | 42–87 |
| Gender, n (%) | |
| Female | 59 (45.0%) |
| Male | 72 (55.0%) |
| Tumor stage, n (%) | |
| Locally advanced | 24 (18.5%) |
| Metastatic | 106 (81.5%) |
| ECOG pre, n (%) | |
| 0 | 45 (34.6%) |
| 1 | 68 (52.3%) |
| 2 | 16 (12.3%) |
| 3 | 1 (0.8%) |
| Body composition variables, mean (SD) | |
| SAT (cm2) | |
| Mean (SD) | 164.1 (81.9) |
| Range | 24.3–390.1 |
| VAT (cm2) | |
| Mean (SD) | 137.4 (96.2) |
| Range | 96.2–499.4 |
| SMA (cm2) | |
| Mean (SD) | 130.6 (33.8) |
| Range | 36.4–285.2 |
| SMD (HU) | |
| Mean (SD) | 32.6 (14.2) |
| Range | −8.3–60.7 |
| SMI (cm2/m2) | |
| Mean (SD) | 45.9 (9.8) |
| Range | 13.5–85.9 |
| Sarcopenia 1 | 59 (45.0%) |
| BMI, kg/m2 | |
| Mean (SD) | 24.2 (4.2) |
| Range | 15.2–38.9 |
| Chemotoxicity, n (%) | |
| Dose reduction | 63 (49.2%) |
| Cycle delays | 30 (23.4%) |
| Early discontinuation | 19 (14.8%) |
| G3-4 toxicity | 43 (37.1%) |
| Second-line treatment | 65 (50.0%) |
| Follow-up (months), mean (SD) (range) | |
| Mean (SD) | 10.8 (7.8) |
| Range | 1–44 |
| Progression-free survival, n (%) | 10 (7.6%) |
| Death, n (%) | 15 (11.5%) |
| Pre | Post | p-Value | |
|---|---|---|---|
| Hb (g/dL) | |||
| Mean (SD) | 12.5 (1.9) | 11.3 (2.0) | <0.001 |
| Range | 8.4–20.0 | 7.6–26.0 | |
| LDH (U/L) | |||
| Mean (SD) | 373.7 (285.6) | 371.5 (161.5) | 0.099 |
| Range | 101–2636 | 124–1109 | |
| Albumin (g/L) | |||
| Mean (SD) | 37.3 (5.9) | 34.9 (5.5) | 0.001 |
| Range | 23–52 | 17–44 | |
| White blood count (K/µL) | |||
| Mean (SD) | 8.0 (3.8) | 6.9 (4.0) | 0.002 |
| Range | 2.34–23.4 | 1.11–35.8 | |
| Lymphocytes (×103 cells/µL) | |||
| Mean (SD) | 1.6 (1.1) | 1.3 (0.7) | 0.002 |
| Range | 0.15–7.2 | 0.1–4.7 |
| Univariate OR (95% CI) | Multivariate OR (95% CI) | Univariate Adjusted OR (95% CI) | Multivariate Adjusted OR (95% CI) | |
|---|---|---|---|---|
| SAT (cm2) | 1.00 (1.00; 1.01) | 1.00 (0.99; 1.00) | 1.00 (1.00; 1.01) | 1.00 (1.00; 1.01) |
| VAT (cm2) | 1.00 (1.00; 1.01) | 1.00 (1.00; 1.01) | 1.00 (1.00; 1.01) | 1.00 (1.00; 1.01) |
| SMA (cm2) | 1.00 (1.00; 1.01) | 1.00 (0.96; 1.02) | 1.01 (1.00; 1.02) | 1.00 (0.94; 1.04) |
| SMD (HU) | 0.96 *** (0.95; 0.97) | 0.96 *** (0.94; 0.98) | 0.96 *** (0.95; 0.97) | 0.96 *** (0.95; 0.98) |
| SMI (cm2/m2) | 1.03 *** (1.02; 1.04) | 1.04 (0.93; 1.10) | 1.04 *** (1.03; 1.05) | 1.05 (0.96; 1.16) |
| BMI (kg2/m2) | 1.07 ** (1.00; 1.14) | 0.41 (0.07; 2.32) | 1.07 ** (1.01; 1.14) | 1.03 (0.93; 1.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cefalì, M.; Scala, I.; Pavone, G.; Helbling, D.; Hussung, S.; Fritsch, R.; Reiner, C.; Stocker, S.; Koeberle, D.; Kissling, M.; et al. Is Computed-Tomography-Based Body Composition a Reliable Predictor of Chemotherapy-Related Toxicity in Pancreatic Cancer Patients? Cancers 2023, 15, 4398. https://doi.org/10.3390/cancers15174398
Cefalì M, Scala I, Pavone G, Helbling D, Hussung S, Fritsch R, Reiner C, Stocker S, Koeberle D, Kissling M, et al. Is Computed-Tomography-Based Body Composition a Reliable Predictor of Chemotherapy-Related Toxicity in Pancreatic Cancer Patients? Cancers. 2023; 15(17):4398. https://doi.org/10.3390/cancers15174398
Chicago/Turabian StyleCefalì, Marco, Isabel Scala, Giuliana Pavone, Daniel Helbling, Saskia Hussung, Ralph Fritsch, Cäcilia Reiner, Soleen Stocker, Dieter Koeberle, Marc Kissling, and et al. 2023. "Is Computed-Tomography-Based Body Composition a Reliable Predictor of Chemotherapy-Related Toxicity in Pancreatic Cancer Patients?" Cancers 15, no. 17: 4398. https://doi.org/10.3390/cancers15174398
APA StyleCefalì, M., Scala, I., Pavone, G., Helbling, D., Hussung, S., Fritsch, R., Reiner, C., Stocker, S., Koeberle, D., Kissling, M., Chianca, V., Del Grande, F., De Dosso, S., & Rizzo, S. (2023). Is Computed-Tomography-Based Body Composition a Reliable Predictor of Chemotherapy-Related Toxicity in Pancreatic Cancer Patients? Cancers, 15(17), 4398. https://doi.org/10.3390/cancers15174398







