Swallowing after Oral Oncological Treatment: A Five-Year Prospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
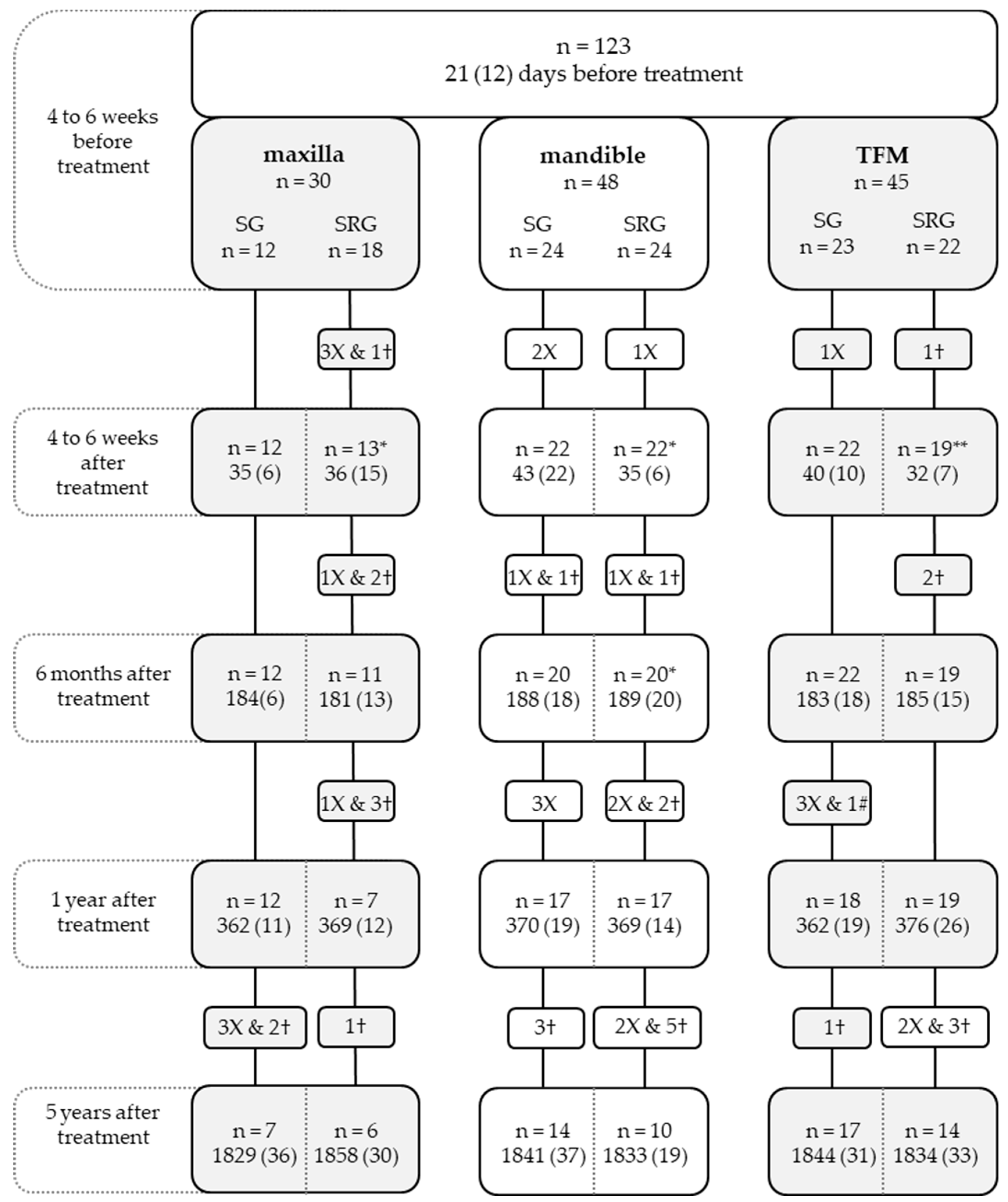
2.1. Patients
2.2. Standardized Assessment Protocol
2.3. Swallowing
2.4. Maximum Tongue Force
2.5. Tongue Mobility
2.6. Sensory Function of the Tongue
2.7. Dental Status
2.8. Obturator Prosthesis
2.9. Statistical Analysis
3. Results
3.1. Patient Population
| Categorical Variables (n, %) | Maxilla (n = 30) | Mandible (n = 48) | TFM (n = 45) | p-Value | |
|---|---|---|---|---|---|
| Gender | Male | 14 (47) | 25 (52) | 30 (67) | 0.179 a |
| Female | 16 (53) | 23 (48) | 15 (33) | ||
| Smoking habit | Daily smokers | 8 (27) | 18 (38) | 16 (36) | 0.599 a |
| Non-smokers and infrequent smokers | 22 (73) | 30 (62) | 29 (64) | ||
| Alcohol consumption | >1 unit alcohol per day | 8 (27) | 15 (31) | 19 (42) | 0.328 a |
| ≤1 unit alcohol per day | 22 (73) | 33 (69) | 26 (58) | ||
| Tumor size by T classification [19] | T1 | 5 (17) | 14 (29) | 23 (51) | 0.000 b*** |
| T2 | 11 (37) | 13 (27) | 14 (31) | ||
| T3 | 1 (3) | 3 (6) | 4 (9) | ||
| T4 | 13 (43) | 18 (38) | 4 (9) | ||
| Treatment | Surgery | 12 (40) | 24 (50) | 23 (51) | 0.600 a |
| Surgery and radiotherapy | 18 (60) | 24 (50) | 22 (49) | ||
| Surgical reconstruction | Primary closure | 17 (57) | 16 (33) | 23 (51) | 0.000 b*** |
| Local flap | 1 (3) | 2 (4) | 1 (2) | ||
| Fasciocutaneous free flap | 12 (40) | 12 (25) | 19 (42) | ||
| Bone graft/flap | 0 (0) | 18 (38) | 2 (4) | ||
| Water swallowing frequency | Normal | 28 (93) | 48 (100) | 43 (96) | 0.177 b |
| Impaired swallowing frequency | 2 (7) | 0 (0) | 2 (4) | ||
| Applesauce swallowing frequency | Normal | 23 (77) | 39 (81) | 43 (96) | 0.031 b* |
| Impaired swallowing frequency | 7 (23) | 9 (19) | 2 (4) | ||
| Tongue mobility | Reaches beyond the lower lip/mouth corner | 29 (97) | 42 (88) | 27 (60) | 0.000 b*** |
| Reaches the lower lip/mouth corner | 1 (3) | 6 (13) | 16 (36) | ||
| Cannot reach the lower lip/mouth corner | 0 (0) | 0 (0) | 2 (4) | ||
| Thermal sensory function of the tongue | Unimpaired | 29 (97) | 46 (96) | 43 (96) | 1.000 b |
| Unilateral impairment | 1 (3) | 2 (4) | 2 (4) | ||
| Bilateral impairment | 0 (0) | 0 (0) | 0 (0) | ||
| Tactile sensory function of the tongue | Unimpaired | 29 (97) | 44 (94) | 40 (89) | 0.116 b |
| Unilateral impairment | 0 (0) | 3 (6.4) | 5 (11.1) | ||
| Bilateral impairment | 1 (3) | 0 (0) | 0 (0) | ||
| Dental status | ED | 7 (23) | 13 (27) | 5 (11) | 0.000 b*** |
| FD | 7 (23) | 8 (17) | 13 (29) | ||
| FD&FDI | 0 (0) | 2 (4) | 4 (9) | ||
| FD&D | 4 (14) | 8 (17) | 3 (7) | ||
| FDI&FDI | 0 (0) | 0 (0) | 0 (0) | ||
| FDI&D | 1 (3) | 0 (0) | 0 (0) | ||
| D | 11 (37) | 17 (35) | 20 (44) | ||
| Continuous variables (mean, SD) | |||||
| Age | 68.6 (12.3) | 66.7 (12.7) | 61.4 (13.1) | 0.036 c* | |
| Water swallowing duration | 2.6 (1.5) | 2.9 (1.6) | 2.4 (2.2) | 0.343 c | |
| Applesauce swallowing duration | 3.6 (2.2) | 4.4 (3.02) | 3.1 (1.7) | 0.040 c* | |
| Maximum tongue force | 12.9 (6.3) | 15.9 (7.7) | 15.2 (7.5) | 0.651 c |
| Mixed Model | Main Effects | SE | Interactions with the Assessment Moment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | 0.859 | 0.231 | |||||||||||
| Before | SE | After | SE | 6 months | SE | 1 Year | SE | 5 Years | SE | ||||
| Assessment moment | Before | 0.520 | 0.325 | ||||||||||
| After | −0.507 | 0.201 | |||||||||||
| 6 Months | −0.294 | 0.203 | |||||||||||
| 1 Year | −0.114 | 0.222 | |||||||||||
| 5 Years | 0 | 0 | |||||||||||
| Age | 0.009 | 0.002 | |||||||||||
| Location | Maxilla | −0.049 | 0.155 | 0.220 | 0.166 | 0.417 | 0.174 | 0.537 | 0.234 | −0.121 | 0.183 | 0 | 0 |
| Mandible | 0.030 | 0.120 | 0.314 | 0.131 | 0.155 | 0.138 | 0.174 | 0.134 | −0.031 | 0.139 | 0 | 0 | |
| TFM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T classification | T1 | −0.134 | 0.150 | 0.259 | 0.159 | 0.023 | 0.167 | 0.219 | 0.176 | −0.002 | 0.180 | 0 | 0 |
| T2 | −0.166 | 0.152 | 0.243 | 0.160 | 0.222 | 0.170 | 0.130 | 0.172 | 0.099 | 0.180 | 0 | 0 | |
| T3 | −0.192 | 0.207 | 0.195 | 0.233 | 0.010 | 0.234 | 0.203 | 0.229 | 0.519 | 0.238 | 0 | 0 | |
| T4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Maximum tongue force | −0.009 | 0.003 | |||||||||||
| Tongue mobility | Normal mobility | −0.163 | 0.138 | −1.064 | 0.315 | 0.081 | 0.169 | −0.004 | 0.179 | 0.123 | 0.192 | 0 | 0 |
| Impaired mobility | −0.083 | 0.149 | −1.006 | 0.321 | 0.230 | 0.179 | −0.005 | 0.186 | −0.151 | 0.197 | 0 | 0 | |
| No mobility | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tactile sensory function | Bilateral impairment | 0.248 | 0.214 | 0.832 | 0.458 | −0.134 | 0.264 | −0.010 | 0.301 | −0.592 | 0.444 | 0 | 0 |
| of the tongue | Unimpaired | −0.134 | 0.120 | 0.070 | 0.194 | 0.159 | 0.140 | −0.127 | 0.143 | 0.129 | 0.146 | 0 | 0 |
| Unilateral impairment | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mixed Model | Main Effects | SE | |
|---|---|---|---|
| Intercept | 2.662 | 1.153 | |
| Assessment moment | Before | 0.930 | 0.464 |
| After | 0.926 | 0.458 | |
| 6 Months | 0.788 | 0.459 | |
| 1 Year | 0.306 | 0.452 | |
| 5 Years | 0 | 0 | |
| Age | −0.051 | 0.015 | |
| Treatment | Surgery | 0.245 | 0.358 |
| Surgery and radiotherapy | 0 | 0 | |
| Maximum tongue force | 0.066 | 0.024 | |
| Tactile sensory function of the tongue | Bilateral impairment | −1.030 | 0.759 |
| Unimpaired | 1.096 | 0.356 | |
| Unilateral impairment | 0 | 0 |
| Mixed Model | Main Effects | SE | Interactions with the Assessment Moment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | 1.014 | 0.200 | |||||||||||
| Before | SE | After | SE | 6 Months | SE | 1 Year | SE | 5 Years | SE | ||||
| Assessment moment | Before | −0.237 | 0.100 | ||||||||||
| After | −0.330 | 0.103 | |||||||||||
| 6 Months | −0.196 | 0.101 | |||||||||||
| 1 Year | −0.138 | 0.104 | |||||||||||
| 5 Years | 0 | 0 | |||||||||||
| Age | 0.014 | 0.002 | |||||||||||
| Gender | Male | −0.199 | 0.072 | ||||||||||
| Female | 0 | 0 | |||||||||||
| Alcohol consumption | ≤1 unit alcohol per day | −0.179 | 0.071 | ||||||||||
| >1 unit alcohol per day | 0 | 0 | |||||||||||
| Location | Maxilla | 0.159 | 0.156 | −0.188 | 0.172 | 0.420 | 0.183 | 0.134 | 0.179 | 0.037 | 0.189 | 0 | 0 |
| Mandible | −0.030 | 0.131 | 0.241 | 0.148 | 0.346 | 0.152 | 0.211 | 0.151 | 0.221 | 0.156 | 0 | 0 | |
| TFM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Maximum tongue force | −0.013 | 0.003 | |||||||||||
| Tactile sensory function | Bilateral impairment | 0.215 | 0.138 | ||||||||||
| of the tongue | Unimpaired | −0.219 | 0.060 | ||||||||||
| Unilateral impairment | 0 | 0 | |||||||||||
| Mixed Model | Main Effects | SE | Interactions with the Assessment Moment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | 1.806 | 1.345 | |||||||||||
| Before | SE | After | SE | 6 Months | SE | 1 Year | SE | 5 Years | SE | ||||
| Assessment moment | Before | 2.718 | 0.775 | ||||||||||
| After | 2.317 | 0.762 | |||||||||||
| 6 Months | 2.113 | 0.729 | |||||||||||
| 1 Year | 1.496 | 0.693 | |||||||||||
| 5 Years | 0 | 0 | |||||||||||
| Age | −0.060 | 0.018 | |||||||||||
| Treatment | Surgery | 2.784 | 0.886 | −3.220 | 1.074 | −3.012 | 1.074 | −2.579 | 1.050 | −2.625 | 1.028 | 0 | 0 |
| Surgery and radiotherapy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Maximum tongue force | 0.081 | 0.027 | |||||||||||
| Tactile sensory function | Bilateral impairment | −1.022 | 0.853 | ||||||||||
| of the tongue | Unimpaired | 1.158 | 0.403 | ||||||||||
| Unilateral impairment | 0 | 0 | |||||||||||
| Patients with Oral Cancer | WSD | WSF | ASD | ASF | |
|---|---|---|---|---|---|
| t0 | Mean (SD) | 2.68 (1.84) | 1.04 (0.24) | 3.75 (2.48) | 1.18 (0.46) |
| Median (IQR) | 1 (1–1) | 1 (1–1) | |||
| t1 | Mean (SD) | 3.22 (2.65) | 1.13 (0.41) | 5.14 (5.17) | 1.42 (0.97) |
| Median (IQR) | 1 (1–1) | 1 (1–1) | |||
| t2 | Mean (SD) | 2.94 (1.72) | 1.14 (0.38) | 4.48 (3.41) | 1.32 (0.75) |
| Median (IQR) | 1 (1–1) | 1 (1–1) | |||
| t3 | Mean (SD) | 3.08 (1.80) | 1.15 (0.47) | 4.86 (4.10) | 1.40 (0.79) |
| Median (IQR) | 1 (1–1) | 1 (1–1) | |||
| t4 | Mean (SD) | 2.99 (1.92) | 1.12 (0.32) | 4.37 (3.26) | 1.37 (0.67) |
| Median (IQR) | 1 (1–1) | 1 (1–1) | |||
| Healthy persons | Mean (SD) | 1.40 (0.64) | 1.00 (0.00) | 2.52 (1.07) | 1.17 (0.38) |
| Median (IQR) | 1 (1–1) | 1 (1–1) | |||
| t0 vs. healthy | p-value | 0.000 *** | 0.159 | 0.000 *** | 0.793 |
| t1 vs. healthy | p-value | 0.000 *** | 0.014 * | 0.000 *** | 0.123 |
| t2 vs. healthy | p-value | 0.000 *** | 0.004 ** | 0.000 *** | 0.348 |
| t3 vs. healthy | p-value | 0.000 *** | 0.010 ** | 0.000 *** | 0.099 |
| t4 vs. healthy | p-value | 0.000 *** | 0.007 ** | 0.000 *** | 0.095 |
3.2. Water Swallowing Duration
3.3. Water Swallowing Frequency
3.4. Applesauce Swallowing Duration
3.5. Applesauce Swallowing Frequency
4. Discussion
4.1. Clinical Implications
4.2. Strengths and Limitations
4.3. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Formulae for the WSD, WSF, ASD, and ASF
| Estimated WSD = 0.86 + 0.01Age + 0.03Mand − 0.05Max − 0.13pT1 − 0.16pT2 − 0.19pT3 − 0.01MTF − 0.16TM0 − 0.08TM1 − 0.13TSFt1 + 0.25TSFt3 + t0(0.52 + 0.31Mand + 0.22Max + 0.26pT1 + 0.24pT2 + 0.19pT3 − 1.06TM0 − 1.00TM1 + 0.07TSFt1 + 0.83TSFt3) + t1(−0.5 + 0.15Mand + 0.41Max + 0.02pT1 + 0.22pT2 + 0.01pT3 + 0.08TM0 + 0.23TM1 + 0.16TSFt1 − 0.13TSFt3) + t2(−0.29 + 0.23Mand + 0.54Max + 0.22pT1 + 0.13pT2 + 0.20pT3 − 0.004TM0 − 0.005TM1 − 0.12TSFt1 − 0.01TSFt3) + t3(−0.11 − 0.03Mand − 0.12Max − 0.002pT1 + 0.09pT2 + 0.52pT3 + 0.12TM0 − 0.15TM1 + 0.13TSFt1 − 0.59TSFt3) |
| Estimated WSF = 2.66 − 0.05Age + 0.93t0 + 0.92t1 + 0.78t2 + 0.30t3 + 0.24Surgery + 0.06MTF − 1.03TSFt1 + 1.09TSFt3 |
| Estimated ASD = 1.01 − 0.20Men + 0.01Age − 0.18No-alcohol − 0.3Mand + 0.16Max − 0.01MTF − 0.22TSFt1 + 0.21TSFt3 + t0(−0.24 + 0.24Mand − 0.19Max) + t1(−0.33 + 0.34Mand + 0.42Max) + t2(−0.19 + 0.21Mand + 0.13Max) + t3(−0.14 + 0.22Mand + 0.38Max) |
| Estimated ASF = 1.80 − 0.06Age + 2.78Surgery + 0.08MTF − 1.02TSFt1 + 1.15TSFt3 + t0(2.71 − 3.22Surgery) + t1(2.31 − 3.01Surgery) + t2(2.11 − 2.57Surgery) + t3(1.49 − 2.62Surgery) |
| ASD: Applesauce swallowing duration; ASF: Applesauce swallowing frequency; Mand: Mandible; Max: Maxilla; MTF: Maximum tongue force; No-Alcohol: ≤1 alcohol unit per day; pT1: Tumor stage 1; pT2: tumor stage 2; pT3: tumor stage 3; TSFt1: normal tactile sensory function of the tongue; TSFt3: bilateral impairment on the tactile sensory function of the tongue; TM0: normal mobility of the tongue; TM1: Impaired mobility of the tongue; WSD: Water swallowing duration; WSF: water swallowing frequency |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.; et al. SEER Cancer Statistics Review, 1975–2017, National Cancer Institute; Bethesda: Rockville, MD, USA, 2019. [Google Scholar]
- Maghami, E.; Ho, A.S. (Eds.) Multidisciplinary Care of the Head and Neck Cancer Patient; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-654121-8. [Google Scholar]
- Riaz, N.; Wolden, S.L.; Gelblum, D.Y.; Eric, J. HHS Public Access. Percutaneous 2016, 118, 6072–6078. [Google Scholar]
- Logemann, J.A. Evaluation and Treatment of Swallowing Problems. Brain Inj. Med. 2018, 26, 203–209. [Google Scholar] [CrossRef]
- Teismann, I.K.; Steinstraeter, O.; Stoeckigt, K.; Suntrup, S.; Wollbrink, A.; Pantev, C.; Dziewas, R. Functional Oropharyngeal Sensory Disruption Interferes with the Cortical Control of Swallowing. BMC Neurosci. 2007, 8, 62. [Google Scholar] [CrossRef]
- Namaki, S.; Tanaka, T.; Hara, Y.; Ohki, H.; Shinohara, M.; Yonhehara, Y. Videofluorographic Evaluation of Dysphagia before and after Modification of the Flap and Scar in Patients with Oral Cancer. J. Plast. Surg. Hand. Surg. 2011, 45, 136–142. [Google Scholar] [CrossRef]
- Ohkoshi, A.; Ogawa, T.; Nakanome, A.; Ishida, E.; Ishii, R.; Kato, K.; Katori, Y. Predictors of Chewing and Swallowing Disorders after Surgery for Locally Advanced Oral Cancer with Free Flap Reconstruction: A Prospective, Observational Study. Surg. Oncol. 2018, 27, 490–494. [Google Scholar] [CrossRef]
- Romesser, P.; Romanyshyn, J.; Schupak, K.; Setton, J.; Riaz, N.; Wolden, S.; Gelblum, D.; Sherman, E.; Kraus, D.; Lee, N. Percutaneous Endoscopic Gastrotomy in Oropharyngeal Cancer Patients Treated with Intensity-Modulated Radiotherapy with Concurrent Chemotherapy. Cancer 2012, 118, 6072–6078. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, S.Y.; Lui, L.T.; Wang, T.G.; Wang, T.C.; Hsiao, T.Y.; Li, Y.W.; Lien, I.N. Dysphagia in Patients with Nasopharyngeal Cancer after Radiation Therapy: A Videofluoroscopic Swallowing Study. Dysphagia 2003, 18, 135–143. [Google Scholar] [CrossRef]
- Caudell, J.J.; Schaner, P.E.; Meredith, R.F.; Locher, J.L.; Nabell, L.M.; Carroll, W.R.; Magnuson, J.S.; Spencer, S.A.; Bonner, J.A. Factors Associated with Long-Term Dysphagia after Definitive Radiotherapy for Locally Advanced Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 410–415. [Google Scholar] [CrossRef]
- Pauloski, B.R.; Rademaker, A.W.; Logemann, J.A.; McConnel, F.M.S.; Heiser, M.A.; Cardinale, S.; Lazarus, C.L.; Pelzer, H.; Stein, D.; Beery, Q. Surgical Variables Affecting Swallowing in Patients Treated for Oral/Oropharyngeal Cancer. Head Neck 2004, 26, 625–636. [Google Scholar] [CrossRef]
- Lazarus, C.; Logemann, J.; Pauloski, B.; Rademaker, A.; Helenowski, I.; Vonesh, E.; MacCracken, E.; Mittal, B.; Vokes, E.; Haraf, D. Effects of Radiotherapy with or without Chemotherapy on Tongue Strength and Swallowing in Patients with Oral Cancer. Head Neck 2007, 29, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Pauloski, B.R.; Rademaker, A.W.; Logemann, J.A.; Colangelo, L.A. Speech and Swallowing in Irradiated and Nonirradiated Postsurgical Oral Cancer Patients. Otolaryngol. Head Neck Surg. 1998, 118, 616–624. [Google Scholar] [PubMed]
- Deng, W.; Zhao, G.; Li, Z.; Yang, L.; Xiao, Y.; Zhang, S.; Guo, K.; Xie, C.; Liang, Y.; Liao, G. Recovery Pattern Analysis of Swallowing Function in Patients Undergoing Total Glossectomy and Hemiglossectomy. Oral Oncol. 2022, 132, 105981. [Google Scholar] [CrossRef] [PubMed]
- Vermaire, J.A.; Raaijmakers, C.P.J.; Monninkhof, E.M.; Leemans, C.R.; de Jong, R.J.B.; Takes, R.P.; Leeuw, I.M.V.; de Jansen, F.; Langendijk, J.A.; Terhaard, C.H.J.; et al. The Course of Swallowing Problems in the First 2 Years after Diagnosis of Head and Neck Cancer. Support. Care Cancer 2022, 30, 9527–9538. [Google Scholar] [CrossRef]
- Vermaire, J.A.; Raaijmakers, C.P.J.; Monninkhof, E.M.; Verdonck-de Leeuw, I.M.; Terhaard, C.H.J.; Speksnijder, C.M. Factors Associated with Swallowing Dysfunction in Patients with Head and Neck Cancer. Oral Dis. 2023, 29, 1937–1946. [Google Scholar] [CrossRef]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. International Classification of Diseases for Oncology (ICD-O), 3rd ed.; 1st Revision; World Health Organization: Geneva, Switzerland, 2013. [CrossRef]
- Snehal, G.; Patel, M.; Shah, J. TNM Staging of Cancers of the Head and Neck: Striving for Uniformity among Diversity. CA Cancer J. Clin. 2005, 55, 242–258. [Google Scholar]
- Logemann, J.A. Evaluation and Treatment of Swallowing Disorders, 2nd ed.; Pro Ed.: Austin, TX, USA, 1998. [Google Scholar]
- Speksnijder, C.M.; Van Der Bilt, A.; Van Der Glas, H.W.; Koole, R.; Merkx, M.A.W. Tongue Function in Patients Treated for Malignancies in Tongue and/or Floor of Mouth; A One Year Prospective Study. Int. J. Oral Maxillofac. Surg. 2011, 40, 1388–1394. [Google Scholar] [CrossRef]
- Beumer, J., Jr.; Curtis, T.A.; Marunick, M.T. Maxillofacial Rehabilitation; Prosthodontic and Surgical Considerations; Ishiyaku EuroAmerica: St. Louis, MI, USA; Tokyo, Japan, 1996. [Google Scholar]
- de Groot, R.J.; Merkx, M.A.W.; Hamann, M.N.S.; Brand, H.S.; de Haan, A.F.J.; Rosenberg, A.J.W.P.; Speksnijder, C.M. Tongue Function and Its Influence on Masticatory Performance in Patients Treated for Oral Cancer: A Five-Year Prospective Study. Support. Care Cancer 2020, 28, 1491–1501. [Google Scholar] [CrossRef]
- Dwivedi, R.C.; Strose, S.; Chisholm, E.J.; Georgalas, C.; Bisase, B.; Amen, F.; Kerawala, C.J.; Clarke, P.M.; Nutting, C.M.; Rhys-Evans, P.H.; et al. Evaluation of Swallowing by Sydney Swallow Questionnaire (SSQ) in Oral and Oropharyngeal Cancer Patients Treated with Primary Surgery. Dysphagia 2012, 27, 491–497. [Google Scholar] [CrossRef]
- Kletzien, H.; Cullins, M.J.; Connor, N.P. Age-Related Alterations in Swallowing Biomechanics. Exp. Gerontol. 2019, 118, 45–50. [Google Scholar] [CrossRef]
- Hughes, T.A.T.; Wiles, C.M. Clinical Measurement of Swallowing in Health and in Neurogenic Dysphagia. QJM 2012, 89, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Tulunay-Ugur, O.E. Dysphagia in the Older Patient. Otolaryngol. Clin. N. Am. 2018, 51, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.M.; Hildreth, A.; McColl, E.; Carding, P.N.; Hamilton, D.; Wilson, J.A. The Clinical Application of the 100 ML Water Swallow Test in Head and Neck Cancer. Oral Oncol. 2011, 47, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Wang, J.W.; Li, Y.M. Is Alcohol Consumption Associated with Gastroesophageal Reflux Disease? J. Zhejiang Univ. Sci. B 2010, 11, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Mellion, M.; Gilchrist, J.M.; De La Monte, S. Alcohol-Related Peripheral Neuropathy: Nutritional, Toxic, or Both? Muscle Nerve 2011, 43, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Speksnijder, C.M.; van der Glas, H.; van der Bilt, A.; van Es, R.J.J.; van der Rijt, E.; Koole, R. Oral Function After Oncological Intervention in the Oral Cavity: A Retrospective Study. J. Oral Maxillofac. Surg. 2010, 68, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Palmer, J. Anatomy and Physiology of Feeding and Swallowing: Normal and Abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef]
- Shin, Y.S.; Koh, Y.W.; Kim, S.-H.; Jeong, J.H.; Ahn, S.; Hong, H.J.; Choi, E.C. Radiotherapy Deteriorates Postoperative Functional Outcome after Partial Glossectomy with Free Flap Reconstruction. J. Oral Maxillofac. Surg. 2012, 70, 216–220. [Google Scholar] [CrossRef]
- Strojan, P.; Hutcheson, K.A.; Eisbruch, A.; Beitler, J.J.; Langendijk, J.A.; Lee, A.W.M.; Corry, J.; Mendenhall, W.M.; Smee, R.; Rinaldo, A.; et al. Treatment of Late Sequelae after Radiotherapy for Head and Neck Cancer. Cancer Treat. Rev. 2017, 59, 79–92. [Google Scholar] [CrossRef]
- Pradat, P.F.; Delanian, S. Late Radiation Injury to Peripheral Nerves. Handb. Clin. Neurol. 2013, 115, 743–758. [Google Scholar] [CrossRef]
- Miller, J.L.; Watkin, K.L. The Influence of Bolus Volume and Viscosity on Anterior Lingual Force during the Oral Stage of Swallowing. Dysphagia 1996, 11, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A.; Rademaker, A.W.; Mcconnel, F.M.S.; Stein, D.; Johnson, J.; Baker, T. Speech and Swallow Function after Tonsil/Base of Tongue Resection With Primary Closure. J. Speech Lang. Hear. Res. 1993, 36, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Kamstra, J.I.; Jager-Wittenaar, H.; Dijkstra, P.U.; Huisman, P.M.; Van Oort, R.P.; Van Der Laan, B.F.A.M.; Roodenburg, J.L.N. Oral Symptoms and Functional Outcome Related to Oral and Oropharyngeal Cancer. Support. Care Cancer 2011, 19, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A. Evaluation and Treatment of Swallowing Problems. In Brain Injury Medicine; Zasler, N.D., Katz, D.I., Zafonte, R.D., Arciniegas, D.B., Bullock, M.R., Kreutzer, J.S., Eds.; Springer Publishing Company: New York, NY, USA, 2012; pp. 1111–2012. ISBN 978-1-9362-8727-7. [Google Scholar]
- Riva, G.; Sapino, S.; Ravera, M.; Elia, G.; Pecorari, G. Long-Term Functional Outcomes and Quality of Life after Partial Glossectomy for T2 Squamous Cell Carcinomas. Braz. J. Otorhinolaryngol. 2022, 88, S33–S43. [Google Scholar] [CrossRef]
- Biglioli, F.; Liviero, F.; Frigerio, A.; Rezzonico, A.; Brusati, R. Function of the Sensate Free Forearm Flap after Partial Glossectomy. J. Cranio-Maxillofac. Surg. 2006, 34, 332–339. [Google Scholar] [CrossRef]
- Benfield, J.K.; Everton, L.F.; Bath, P.M.; England, T.J. Does Therapy with Biofeedback Improve Swallowing in Adults with Dysphagia? A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 551–561. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Komar, A.; Ringash, J.; Chan, C.; Davis, A.M.; Jones, J.; Martino, R.; McEwen, S. A Scoping Review of Rehabilitation Interventions for Survivors of Head and Neck Cancer. Disabil. Rehabil. 2019, 41, 2093–2107. [Google Scholar] [CrossRef]
- van der Berg, M.G.A.; Kalf, J.G.; Hendriks, J.C.M.; Takes, R.P.; van Herpen, C.M.L.; Wanten, G.J.A.; Drenth, J.P.H.; Kaanders, J.H.A.M.; Merkx, M.A.W. Normalcy of Food Intake in Patients with Head and Neck Cancer Supported by Combined Dietary Counseling and Swallowing Therapy: A Randomized Clinical Trial. Head Neck 2016, 38, E198–E206. [Google Scholar] [CrossRef]
- Perry, A.; Lee, S.H.; Cotton, S.; Kennedy, C. Therapeutic Exercises for Affecting Post-Treatment Swallowing in People Treated for Advanced-Stage Head and Neck Cancers. Cochrane Database Syst. Rev. 2016, 2016, CD011112. [Google Scholar] [CrossRef]
- Pedersen, A.; Wilson, J.; McColl, E.; Carding, P.; Patterson, J. Swallowing Outcome Measures in Head and Neck Cancer—How Do They Compare? Oral Oncol. 2016, 52, 104–108. [Google Scholar] [CrossRef]
- Wu, M.C.; Chang, Y.C.; Wang, T.G.; Lin, L.C. Evaluating Swallowing Dysfunction Using a 100-Ml Water Swallowing Test. Dysphagia 2004, 19, 43–47. [Google Scholar] [CrossRef]
- Clavé, P.; Arreola, V.; Romea, M.; Medina, L.; Palomera, E.; Serra-Prat, M. Accuracy of the Volume-Viscosity Swallow Test for Clinical Screening of Oropharyngeal Dysphagia and Aspiration. Clin. Nutr. 2008, 27, 806–815. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Wu, H.Y.; Yang, Y. A Systematic Review of Swallowing Training Measures for Postoperative Oral Cancer Patients. Dysphagia 2022, 37, 1839–1850. [Google Scholar] [CrossRef]
- Mathew, A.; Lockwood, M.B.; Steffen, A.; Tirkey, A.J.; Pavamani, S.P.; Patil, C.L.; Doorenbos, A.Z. Symptom Cluster Experiences of Patients Operated for Oral Cancer: A Mixed Methods Study. Semin. Oncol. Nurs. 2023, 39, 151407. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speksnijder, C.M.; Ortiz-Comino, L.; de Haan, A.F.J.; Fernández-Lao, C.; de Bree, R.; Merkx, M.A.W. Swallowing after Oral Oncological Treatment: A Five-Year Prospective Study. Cancers 2023, 15, 4371. https://doi.org/10.3390/cancers15174371
Speksnijder CM, Ortiz-Comino L, de Haan AFJ, Fernández-Lao C, de Bree R, Merkx MAW. Swallowing after Oral Oncological Treatment: A Five-Year Prospective Study. Cancers. 2023; 15(17):4371. https://doi.org/10.3390/cancers15174371
Chicago/Turabian StyleSpeksnijder, Caroline M., Lucía Ortiz-Comino, Anton F. J. de Haan, Carolina Fernández-Lao, Remco de Bree, and Matthias A. W. Merkx. 2023. "Swallowing after Oral Oncological Treatment: A Five-Year Prospective Study" Cancers 15, no. 17: 4371. https://doi.org/10.3390/cancers15174371
APA StyleSpeksnijder, C. M., Ortiz-Comino, L., de Haan, A. F. J., Fernández-Lao, C., de Bree, R., & Merkx, M. A. W. (2023). Swallowing after Oral Oncological Treatment: A Five-Year Prospective Study. Cancers, 15(17), 4371. https://doi.org/10.3390/cancers15174371









