Outcome of Second Primary Malignancies Developing in Multiple Myeloma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistics
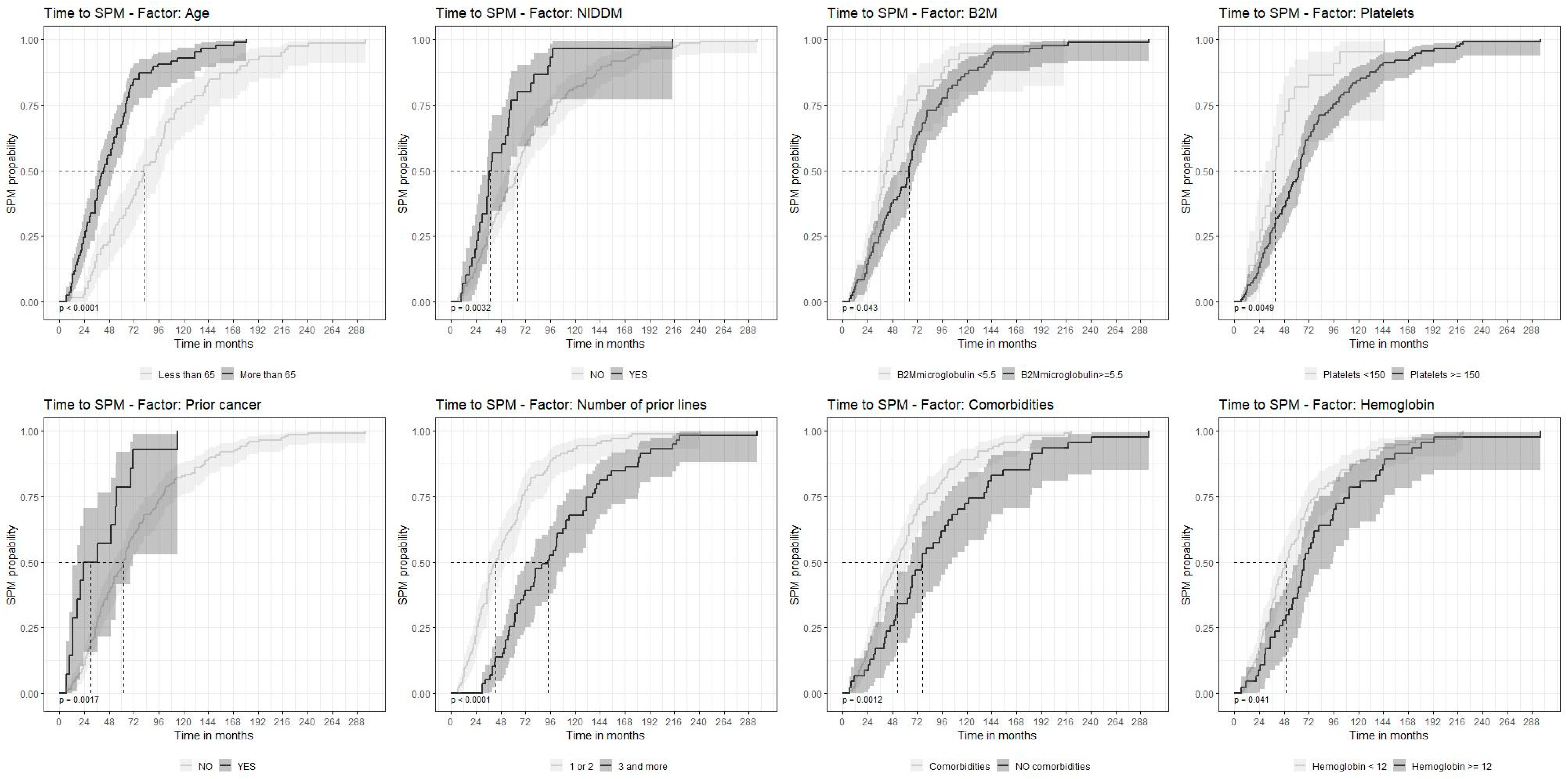
3. Results
3.1. Patient Characteristics
3.2. SPM Characteristics
3.3. SPM and MM Management following SPM Detection
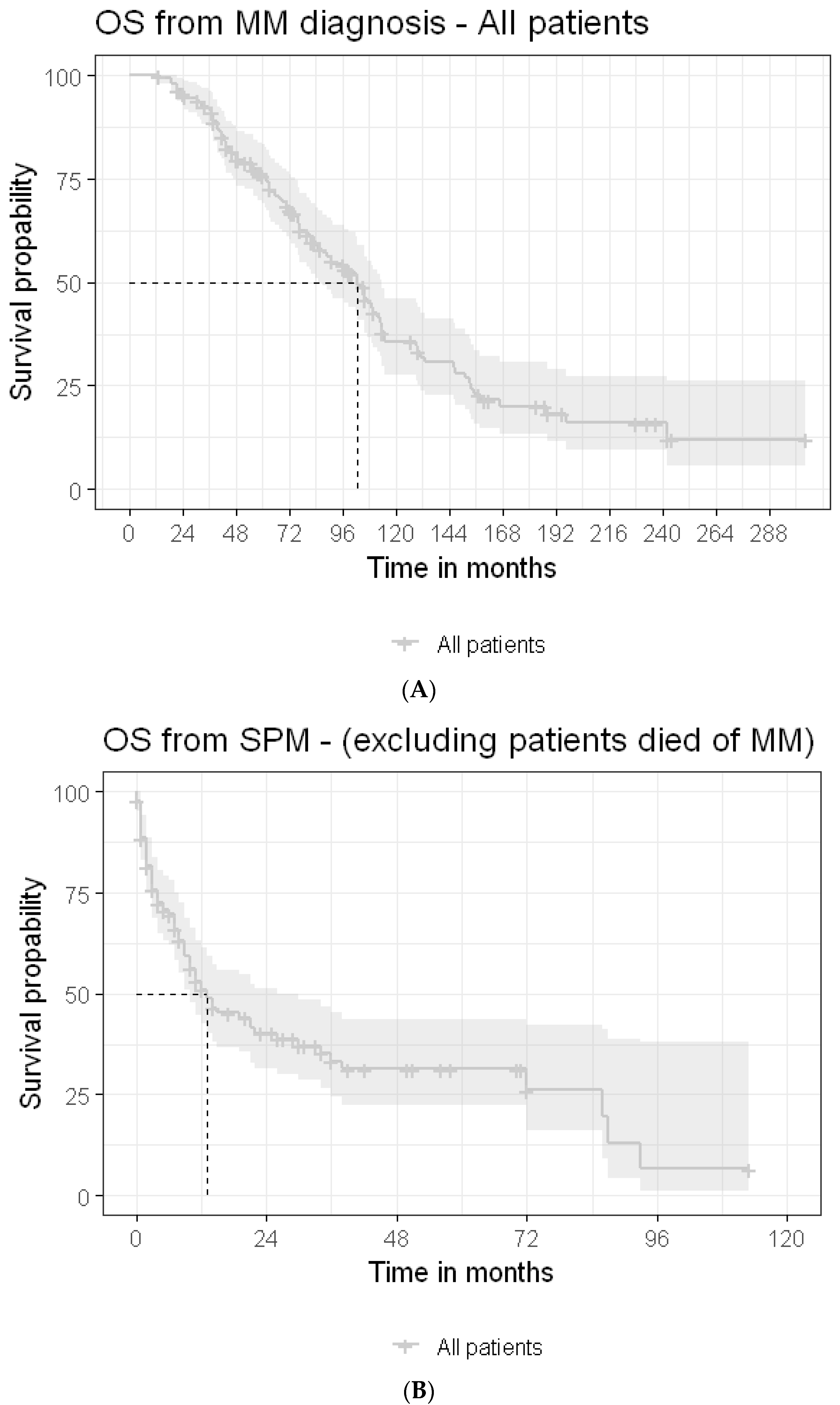
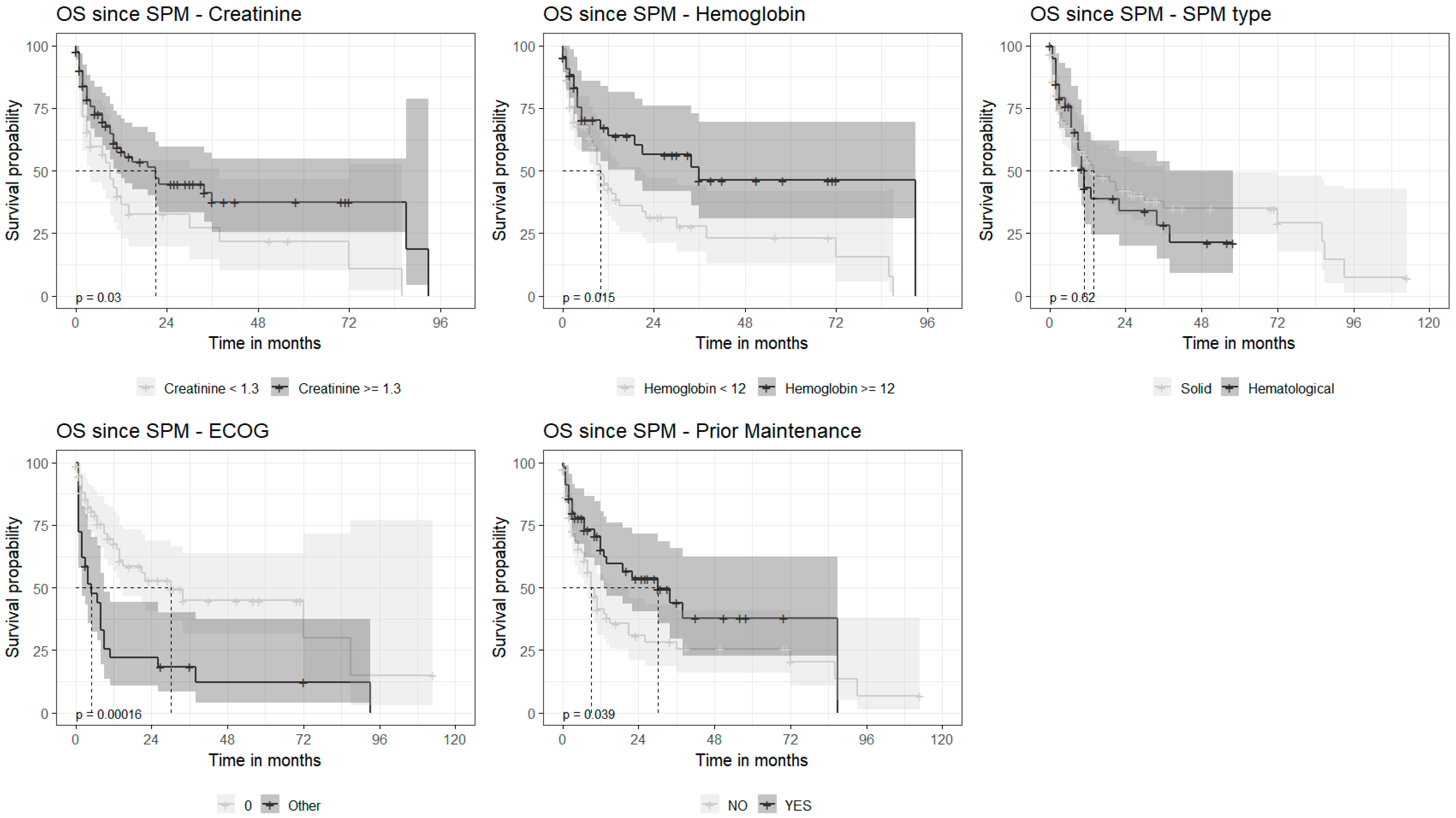
3.4. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brink, M.; Minnema, M.C.; Visser, O.; Levin, M.D.; Posthuma, E.F.M.W.; Broijl, A.; Sonneveld, P.; van der Klift, M.; Roeloffzen, W.W.H.; Westerman, M.; et al. Increased mortality risk in multiple-myeloma patients with subsequent malignancies: A population-based study in the Netherlands. Blood Cancer J. 2022, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, K.; Diamond, B.; Maura, F.; Hillengas, J.; Turesson, I.; Landgren, C.O.; Kazandjian, D. Second malignancies in multiple myeloma; emerging patterns and future directions. Best Pract. Res. Clin. Haematol. 2020, 33, 101144. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.Y.; Mei, M.; Sun, C.L.; Thomas, S.H.; Teh, J.B.; Kang, T.; Htut, M.; Somlo, G.; Sahebi, F.; Forman, S.J.; et al. Second primary malignancies after autologous hematopoietic cell transplantation for multiple myeloma. Biol. Blood Marrow Transpl. 2013, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Musto, P.; Anderson, K.C.; Attal, M.; Richardson, P.G.; Badros, A.; Hou, J.; Comenzo, R.; Du, J.; Durie, B.G.M.; Miguel, J.S.; et al. Second primary malignancies in multiple myeloma: An overview and IMWG consensus. Ann. Oncol. 2017, 28, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.S.; Brunson, A.; Tuscano, J.; Jonas, B.A.; Hoeg, R.; Wun, T.; Keegan, T.H.M. Effect of autologous hematopoietic stem cell transplant on the development of second primary malignancies in multiple myeloma patients. Blood Cancer J. 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Haserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Gulla, A.; Anderson, K.C. Multiple myeloma: The (r)evolution of current therapy and a glance into the future. Haematologica 2020, 105, 2358. [Google Scholar] [CrossRef]
- Finkelstein, D.M.; Horick, N.K.; Ramchandani, R.; Boyd, K.L.; Rana, H.Q.; Bychkovsky, B.L. Are rare cancer survivors at elevated risk of subsequent new cancers? BMC Cancer 2019, 19, 166. [Google Scholar] [CrossRef]
- Zheng, X.; Li, X.; Wang, M.; Shen, J.; Sisti, G.; He, Z.; Huang, J.; Li, Y.M.; Wu, A. Multidisciplinary Oncology Research Collaborative Group (MORCG). Second primary malignancies among cancer patients. Ann. Transl. Med. 2020, 8, 638. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, H.A.; Qiu, H.; Tang, C.H. Is the risk of second primary malignancy increased in multiple myeloma in the novel therapy era? A population-based, retrospective cohort study in Taiwan. Sci. Rep. 2020, 10, 14393. [Google Scholar] [CrossRef]
- Jonsdottir, G.; Lund, S.H.; Bjorkholm, M.; Turesson, I.; Wahlin, A.; Mailankody, S.; Blimark, C.; Hulcrantz, M.; Porwit, A.; Landgren, O.; et al. Survival in multiple myeloma patients who develop second malignancies: A population-based cohort study. Haematologica 2016, 101, e145–e148. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D.; Mo, C.C.; Landgren, O.; Richardson, P.G. The role of high-dose melphalan with autologous stem-cell transplant in multiple myeloma: Is it time for a paradigm shift? Br. J. Haematol. 2020, 191, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, G.; Björkholm, M.; Turesson, I.; Hultcrantz, M.; Diamond, B.; Porwit, A.; Landgren, O.; Kristinsson, S.Y. Cumulative exposure to melphalan chemotherapy and subsequent risk of developing acute myeloid leukemia and myelodysplastic syndromes in patients with multiple myeloma. Eur. J. Haematol. 2021, 107, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, D.; Bertoli, D.; Cattaneo, C.; Almici, C.; Re, A.; Beloti, A.; Borlenghi, E.; Lanzi, G.; Archetti, S.; Verardi, R.; et al. The role of clonal hematopoiesis as driver of therapy-related myeloid neoplasms after autologous stem cell transplantation. Ann. Hematol. 2022, 101, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Franz, G.; McClune, B.; Buadi, F.; Walsh, W.; White, F.; Przepiorka, D. Myelodysplasia after Autologous Stem Cell Transplantation for Multiple Myeloma. Blood 2006, 108, 5329. [Google Scholar] [CrossRef]
- Mouhieddine, T.H.; Sperling, A.S.; Redd, R.; Park, J.; Leventhal, M.; Gibson, C.J.; Manier, S.; Nassar, A.H.; Capelletti, M.; Huynh, D.; et al. Clonal hematopoiesis is associated with adverse outcomes in multiple myeloma patients undergoing transplant. Nat. Commun. 2020, 11, 2996. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Díaz-Tejedor, A.; Lorenzo-Mohamed, M.; Puig, N.; Garcia-Sanz Ramon Mateos, M.V.; Garayoa, M.; Paino, T. Immune system alterations in multiple myeloma: Molecular mechanisms and therapeutic strategies to reverse immunosuppression. Cancers 2021, 13, 1353. [Google Scholar] [CrossRef]
- Swan, D.; Lynch, K.; Gurney, M.; O’Dwyer, M. Current and emerging immunotherapeutic approaches to the treatment of multiple myeloma. Ther. Adv. Hematol. 2019, 10, 2040620719854171. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Incidence of haematological malignancy by sub-type: A report from the Haematological Malignancy Research Network. Br. J. Cancer 2011, 105, 1684–1692. [Google Scholar] [CrossRef]
- Wang, J.; Lv, C.; Zhou, M.; Xu, J.Y.; Chen, B.; Wan, Y. Second Primary Malignancy Risk in Multiple Myeloma from 1975 to 2018. Cancers 2022, 14, 4919. [Google Scholar] [CrossRef] [PubMed]
- Ragon, B.K.; Shah, M.V.; D’Souza, A.; Estrada-Merly, N.; Gowda, L.; George, G.; de Lima, M.; Hashmi, S.; Kharfan-Dabaja, M.A.; Majhail, N.S.; et al. Impact of Second Primary Malignancy Post-Autologous Transplantation on Outcomes of Multiple Myeloma: A CIBMTR Analysis. Blood Adv. 2023, 7, 2746–2757. [Google Scholar] [CrossRef]
- Palumbo, A.; Hajek, R.; Delforge, M.; Kropff, M.; Petrucci, M.T.; Catalano, J.; Gisslinger, H.; Jędrzejczak, W.W.; Zoledava, M.; Weisel, K.; et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N. Engl. J. Med. 2012, 366, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agrawal, A.; Agrawal, S.; Su, H.; Gollapudi, S. A paradox of immunodeficiency and inflammation in human aging: Lessons learned from apoptosis. Immun. Ageing 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Qu, S. The relationship between diabetes mellitus and cancers and its underlying mechanisms. Front. Endocrinol. 2022, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Sperling, A.S.; Guerra, V.A.; Kennedy, J.A.; Yan, Y.; Hsu, J.I.; Wang, F.; Nguyen, A.T.; Miller, P.G.; McConkey, M.E.; Quevedo Barrios, V.A.; et al. Lenalidomide promotes the development of TP53-mutated therapy-related myeloid neoplasms. Blood 2022, 140, 1753–1763. [Google Scholar] [CrossRef]
- Jones, J.R.; Cairns, D.; Menzies, T.; Pawlyn, C.; Davis, F.E.; Sigsworth, F.; Jenner, M.W.; Kaiser, M.F.; Mottram, M.; Drayson, M.T.; et al. Second Primary Malignancy Incidence in Patients Receiving Lenalidomide at Induction and Maintenance; Long-Term Follow up of 4358 Patients Enrolled to the Myeloma XI Trial. Blood 2022, 140 (Suppl. S1), 1823–1825. [Google Scholar] [CrossRef]
- Cooper, J.D.; Thornton, J.A.; Gibson, S.J.; Pham, K.; Sunderland, K.; deStefano, C.B. Survival of Patients with Multiple Myeloma Diagnosed with Second Primary Malignancies: An ASCO Cancerlinq Analysis. Blood 2022, 140 (Suppl. S1), 10039–10040. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Sexton, R.; Hoering, A.; Heuck, C.J.; Nair, B.; Waheed, S.; Al Sayed, J.; Chauhan, N.; Ahmed, N.; Atrash, S.; et al. Second malignancies in total therapy 2 and 3 for newly diagnosed multiple myeloma: Influence of thalidomide and lenalidomide during maintenance. Blood 2012, 120, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
| All SPMs n = 165 | Solid SPMs n = 113 | Hemato-l SPMs n = 52 | p-Value | ||
|---|---|---|---|---|---|
| Demographics and prior medical history | |||||
| Age at MM diagnosis in years, median (range) | 65 (57–70) | 66 (57–71) | 62.5 (56–68) | 0.053 | |
| Age at SPM in years, median (range) | 70 (64–75) | 71 (64–77) | 68 (64–72) | 0.090 | |
| Sex | Female | 62 (37.6%) | 47 (41.6%) | 15 (28.8%) | 0.124 |
| Male | 103 (62.4%) | 66 (58.4%) | 37 (71.2%) | ||
| Prior cancer | NO | 151 (91.5%) | 103 (91.2%) | 48 (92.3%) | 1.000 |
| YES | 14 (8.5%) | 10 (8.8%) | 4 (7.7%) | ||
| T2DM | NO | 135 (81.8%) | 88 (77.9%) | 47 (90.4%) | 0.081 |
| YES | 30 (18.2%) | 25 (22.1%) | 5 (9.6%) | ||
| MM-related parameters | |||||
| Albumin (g/dL) | <3.50 | 51 (30.9%) | 39 (34.5%) | 12 (23.1%) | 0.241 |
| ≥3.5 | 79 (47.9%) | 52 (46.0%) | 27 (51.9%) | ||
| Missing | 35 (21.2%) | 22 (19.5%) | 13 (25.0%) | ||
| B2M level (mg/L) | <5.5 | 85 (51.5%) | 61 (54.0%) | 24 (46.2%) | 1.0 |
| ≥5.5 | 39 (23.6%) | 28 (24.8%) | 11 (21.2%) | ||
| Missing | 41 (24.8%) | 24 (21.2%) | 17 (32.7%) | ||
| ISS | 1 | 40 (24.2%) | 26 (23.0%) | 14 (26.9%) | 0.546 |
| 2 | 42 (25.5%) | 32 (28.3%) | 10 (19.2%) | ||
| 3 | 49 (29.7%) | 35 (31.0%) | 14 (26.9%) | ||
| Missing | 34 (20.6%) | 20 (17.7%) | 14 (26.9%) | ||
| Creatinine (mg/dL) | <1.3 | 99 (60.0%) | 69 (61.1%) | 30 (57.7%) | 0.542 |
| ≥1.3 | 41 (24.8%) | 31 (27.4%) | 10 (19.2%) | ||
| Missing | 25 (15.2%) | 13 (11.5%) | 12 (23.1%) | ||
| Hemoglobin (gr/dL) | <12 | 95 (57.6%) | 67 (59.3%) | 28 (53.8%) | 0.695 |
| ≥12 | 47 (28.5%) | 35 (31.0%) | 12 (23.1%) | ||
| Missing | 23 (13.9%) | 11 (9.7%) | 12 (23.1%) | ||
| Platelets (109/L) | <150 | 22 (13.3%) | 15 (13.3%) | 7 (13.5%) | 0.798 |
| ≥150 | 114 (69.1%) | 82 (72.6%) | 32 (61.5%) | ||
| Missing | 29 (17.6%) | 16 (14.2%) | 13 (25.0%) | ||
| LDH level | Normal | 90 (54.5%) | 64 (56.6%) | 26 (50.0%) | 0.670 |
| Increased | 36 (21.8%) | 24 (21.2%) | 12 (23.1%) | ||
| Missing | 39 (23.6%) | 25 (22.1%) | 14 (26.9%) | ||
| Time from first therapy to SPM (months) | 60 (35–95) | 57 (30–94) | 63 (35.5–100.5) | 0.312 | |
| Prior IMiD | NO | 22 (13.3%) | 15 (13.3%) | 7 (13.5%) | 1.000 |
| YES | 143 (86.7%) | 98 (86.7%) | 45 (86.5%) | ||
| Prior PI | NO | 37 (22.4%) | 29 (25.7%) | 8 (15.4%) | 0.163 |
| YES | 128 (77.6%) | 84 (74.3%) | 44 (84.6%) | ||
| Prior chemotherapy (excluding AutoHCT) | NO | 47 (28.5%) | 35 (31.0%) | 12 (23.1%) | 0.355 |
| YES | 118 (71.5%) | 78 (69.0%) | 40 (76.9%) | ||
| No. of prior therapies median, (range) | 2 (1–7) | 2 (1–7) | 2 (1–7) | 0.624 | |
| No. of prior therapies ≤ 2 | 106 (64.2%) | 74 (65%) | 32 (62%) | 0.727 | |
| Prior AutoHCT | NO | 82 (49.7%) | 64 (56.6%) | 18 (34.6%) | 0.012 |
| YES | 83 (50.3%) | 49 (43.4%) | 34 (65.4%) | ||
| Prior maintenance * | NO | 101 (61.2%) | 72 (63.7%) | 29 (55.8%) | 0.391 |
| YES | 64 (38.8%) | 41 (36.3%) | 23 (44.2%) | ||
| MM treatment at the time of SPM detection | CHEMO | 4 (2.4%) | 2 (1.8%) | 2 (3.8%) | 0.639 |
| IMiD | 55 (33.3%) | 40 (35.4%) | 15 (28.8%) | ||
| IMiD-CHEMO | 5 (3.0%) | 5 (4.4%) | 0 (0.0%) | ||
| MOAB | 8 (4.8%) | 6 (5.3%) | 2 (3.8%) | ||
| Not specified | 15 (0.9%) | 12 (11%) | 3 (0.6%) | ||
| OTHER | 1 (0.6%) | 1 (0.9%) | 0 (0.0%) | ||
| PI | 7 (4.2%) | 5 (4.4%) | 2 (3.8%) | ||
| PI-IMiD | 5 (3.0%) | 4 (3.5%) | 1 (1.9%) | ||
| No anti-MM therapy | 65 (57.5%) | 38 (34%) | 27 (52%) |
| (A) | |||
|---|---|---|---|
| HR | CI95% | p | |
| Age ≥ 65 (years) | 2.53 | 1.83–3.51 | <0.001 |
| Sex (Females) | |||
| Males | 1.11 | 0.81–1.52 | 0.52 |
| Prior cancer | 2.36 | 1.35–4.11 | 0.003 |
| Concomitant T2DM | 1.82 | 1.21–2.72 | 0.004 |
| No concomitant comorbidities | |||
| 0 | 0.56 | 0.40–0.80 | 0.001 |
| 1 | 1.67 | 1.11–2.50 | 0.013 |
| 2 | 1.91 | 1.23–2.95 | 0.004 |
| 3 | 1.85 | 1.13–3.03 | 0.014 |
| Creatinine level | |||
| ≥1.3 (mg/dL) | 1.07 | 0.74–1.54 | 0.73 |
| Albumin level | |||
| ≥3.5 (g/dL) | 0.85 | 0.60–1.21 | 0.37 |
| B2-microglobulin | |||
| ≥5.5 (mg/L) | 1.48 | 1.01–2.18 | 0.044 |
| ISS (1) | |||
| 2 | 1.24 | 0.80–1.92 | 0.34 |
| 3 | 1.42 | 0.93–2.17 | 0.10 |
| Platelets > 150 (109/L) | 0.52 | 0.3–0.83 | 0.006 |
| Hemoglobin ≥ 12 (gr/dL) | 0.69 | 0.49–0.99 | 0.042 |
| Any prior chemotherapy for MM | 0.75 | 0.53–1.06 | 0.10 |
| Any prior IMiD | 0.93 | 0.59–1.46 | 0.74 |
| Any prior PI | 1.07 | 0.73–1.56 | 0.72 |
| Any maintenance therapy | 1.11 | 0.81–1.53 | 0.51 |
| Prior AutoHCT | 0.75 | 0.55–1.03 | 0.076 |
| Number of prior lines (≤2 vs. ≥3) | 0.41 | 0.29–0.57 | <0.001 |
| SPM type | |||
| Solid vs. Hematolo | 0.83 | 0.60–1.16 | 0.28 |
| ECOG PS 0 vs. ≥1 | 0.62 | 0.42–0.93 | 0.019 |
| (B) | |||
| HR | CI95% | p | |
| Age < 65 vs. ≥65 (years) | 2.87 | 0.25–0.59 | <0.001 |
| Prior cancer history | 0.97 | 0.47–1.99 | 0.927 |
| Number of prior lines <2 vs. ≥3 | 0.38 | 0.25–0.59 | <0.001 |
| PLT < 150 vs. >150 (109/L) | 0.58 | 0.35–0.94 | 0.026 |
| B2-microglobulin ≥ 5.5 (mg/L) | 1.14 | 0.73–1.78 | 0.575 |
| Concomitant T2DM | 1.56 | 0.98–2.49 | 0.059 |
| Hemoglobin < 12 vs. ≥12 (gr/dL) | 0.63 | 0.40–1.00 | 0.048 |
| Characteristic | 2—Change of Anti-MM Tx, n = 15 (%) | 1—Discontinuation of Tx, n = 52 (%) | 3—Continuation of Tx, N = 33 (%) | p-Value | |
|---|---|---|---|---|---|
| SPM Type, n (%) | Solid (75) | 10 (13.3) | 33 (44) | 32 (43) | <0.001 |
| Hemato (25) | 5 (20) | 19 (76) | 1 (4) | ||
| HR | CI95% | p | |
|---|---|---|---|
| Any maintenance therapy | 0.65 | 0.26–1.61 | 0.351 |
| Creatinine level < 1.3 | |||
| ≥1.3 | 0.74 | 0.40–136 | 0.334 |
| ECOG PS 0 | |||
| 1 | 2.62 | 1.16–5.91 | 0.020 |
| 2 | 5.16 | 1.76–15.08 | 0.003 |
| 3 | 6.38 | 2.73–14.90 | <0.001 |
| Hemoglobin < 12 | |||
| ≥12 | 0.38 | 0.18–0.81 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avivi, I.; Vesole, D.H.; Davila-Valls, J.; Usnarska-Zubkiewicz, L.; Olszewska-Szopa, M.; Milunovic, V.; Baumert, B.; Osękowska, B.; Kopińska, A.; Gentile, M.; et al. Outcome of Second Primary Malignancies Developing in Multiple Myeloma Patients. Cancers 2023, 15, 4359. https://doi.org/10.3390/cancers15174359
Avivi I, Vesole DH, Davila-Valls J, Usnarska-Zubkiewicz L, Olszewska-Szopa M, Milunovic V, Baumert B, Osękowska B, Kopińska A, Gentile M, et al. Outcome of Second Primary Malignancies Developing in Multiple Myeloma Patients. Cancers. 2023; 15(17):4359. https://doi.org/10.3390/cancers15174359
Chicago/Turabian StyleAvivi, Irit, David H. Vesole, Julio Davila-Valls, Lidia Usnarska-Zubkiewicz, Magdalena Olszewska-Szopa, Vibor Milunovic, Bartłomiej Baumert, Bogumiła Osękowska, Anna Kopińska, Massimo Gentile, and et al. 2023. "Outcome of Second Primary Malignancies Developing in Multiple Myeloma Patients" Cancers 15, no. 17: 4359. https://doi.org/10.3390/cancers15174359
APA StyleAvivi, I., Vesole, D. H., Davila-Valls, J., Usnarska-Zubkiewicz, L., Olszewska-Szopa, M., Milunovic, V., Baumert, B., Osękowska, B., Kopińska, A., Gentile, M., Puertas-Martinez, B., Robak, P., Crusoe, E., Rodriguez-Lobato, L. G., Gajewska, M., Varga, G., Delforge, M., Cohen, Y., Gozzetti, A., ... Jurczyszyn, A. (2023). Outcome of Second Primary Malignancies Developing in Multiple Myeloma Patients. Cancers, 15(17), 4359. https://doi.org/10.3390/cancers15174359













