Predictive Value of Modified Glasgow Prognostic Score and Persistent Inflammation among Patients with Non-Small Cell Lung Cancer Treated with Durvalumab Consolidation after Chemoradiotherapy: A Multicenter Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunological and Nutritional Markers
2.3. Response Evaluation and Outcome Assessment
2.4. Statistical Analysis
2.5. Software Tools
3. Results
3.1. Characteristics of Patients
3.2. Identification of the Most Significant Immunological and Nutritional Marker among Candidate Markers
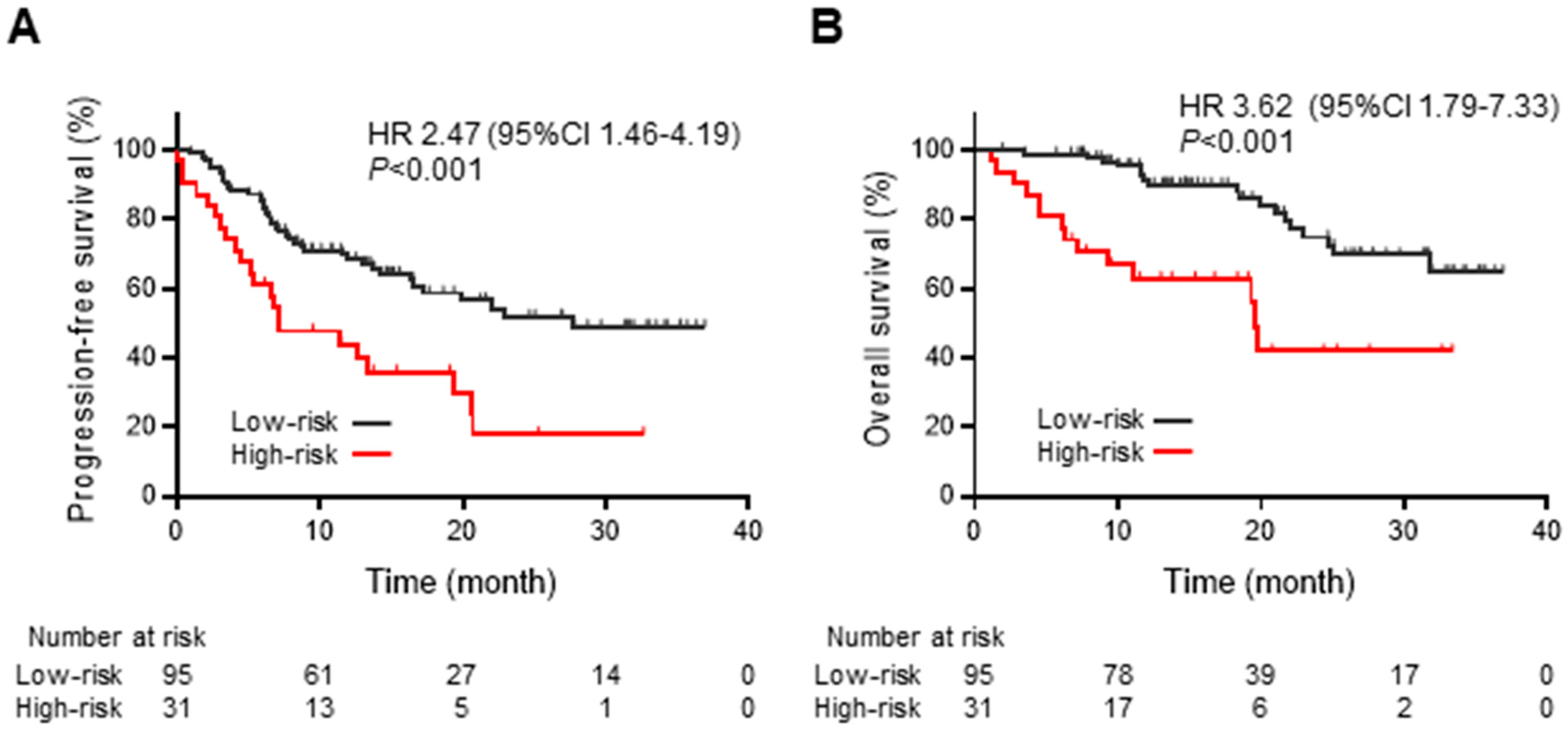
3.3. Systemic Inflammation-Based Prognostic Risk Classification in Patients Treated with Chemoradiotherapy Followed by Durvalumab Consolidation
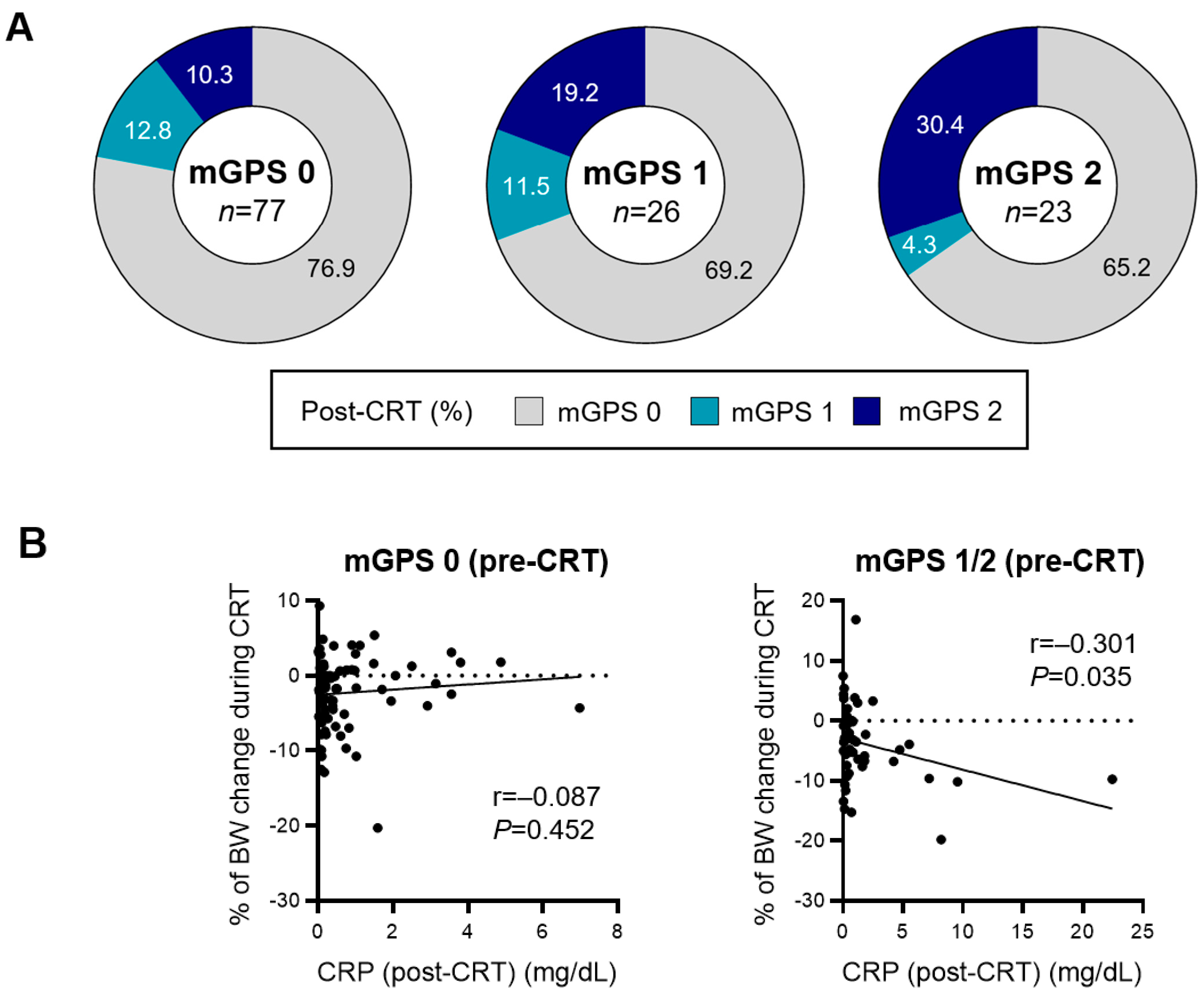
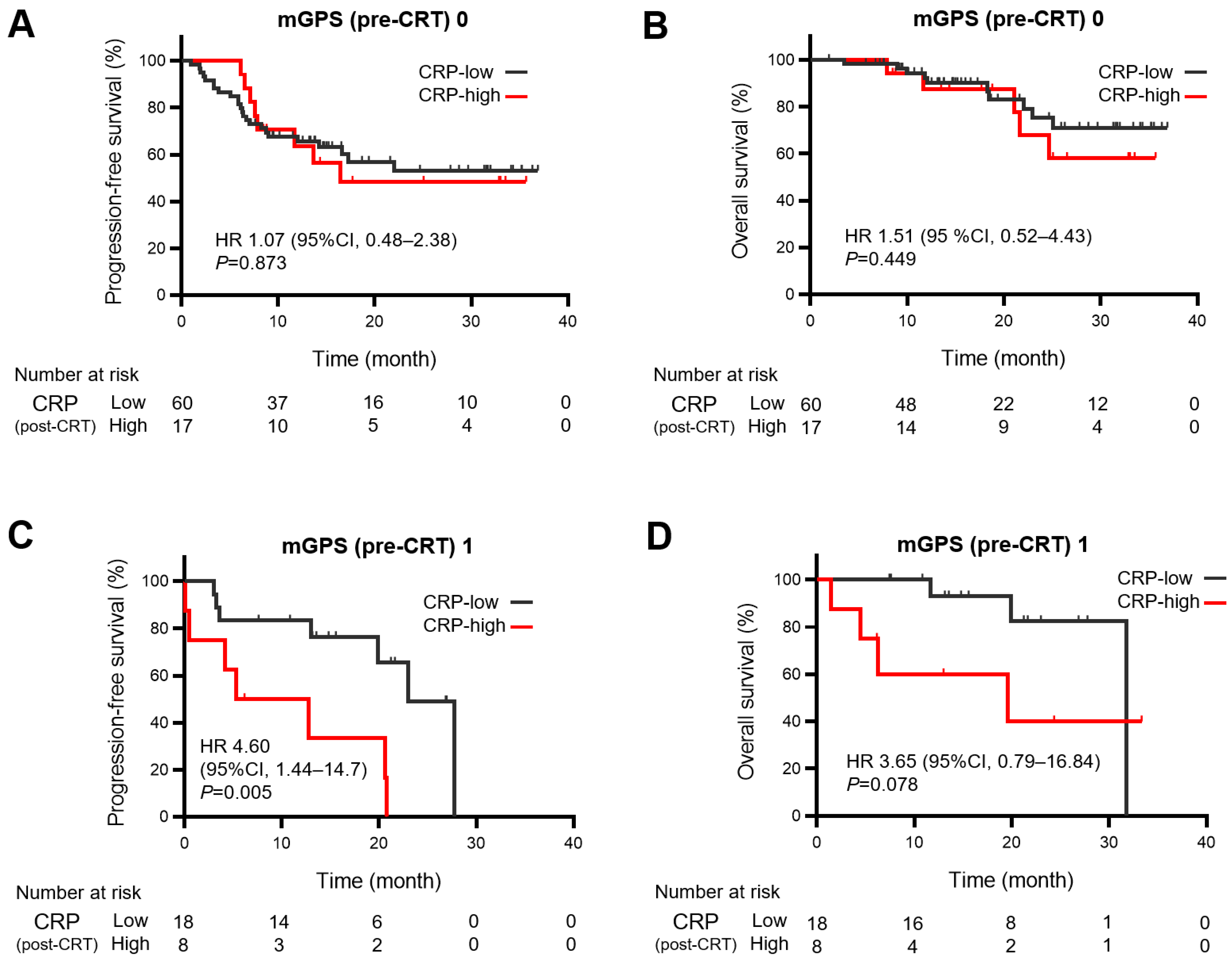
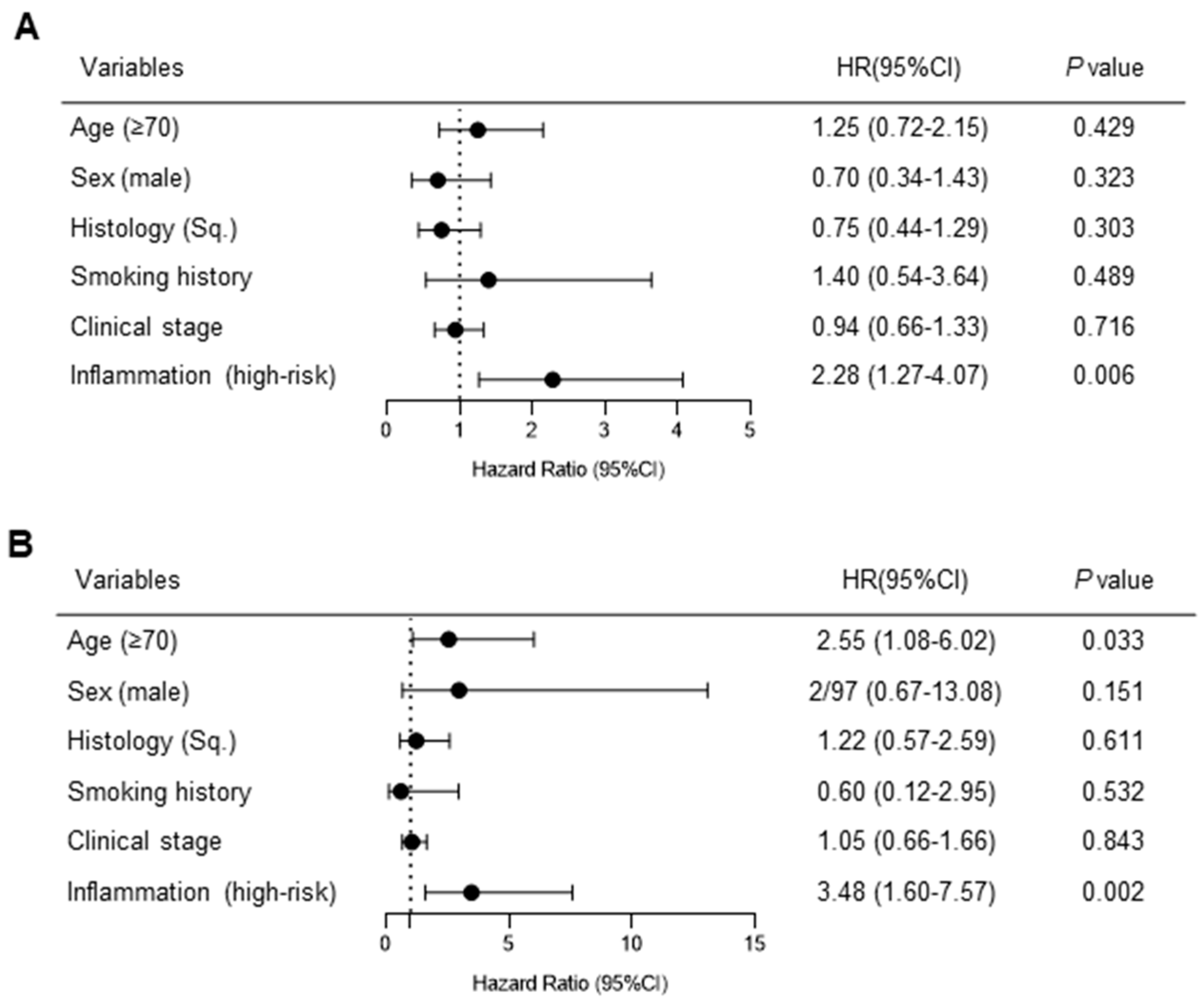
3.4. Relationship between Persistent Inflammation after Chemoradiotherapy and Survival Outcomes of Durvalumab Consolidation
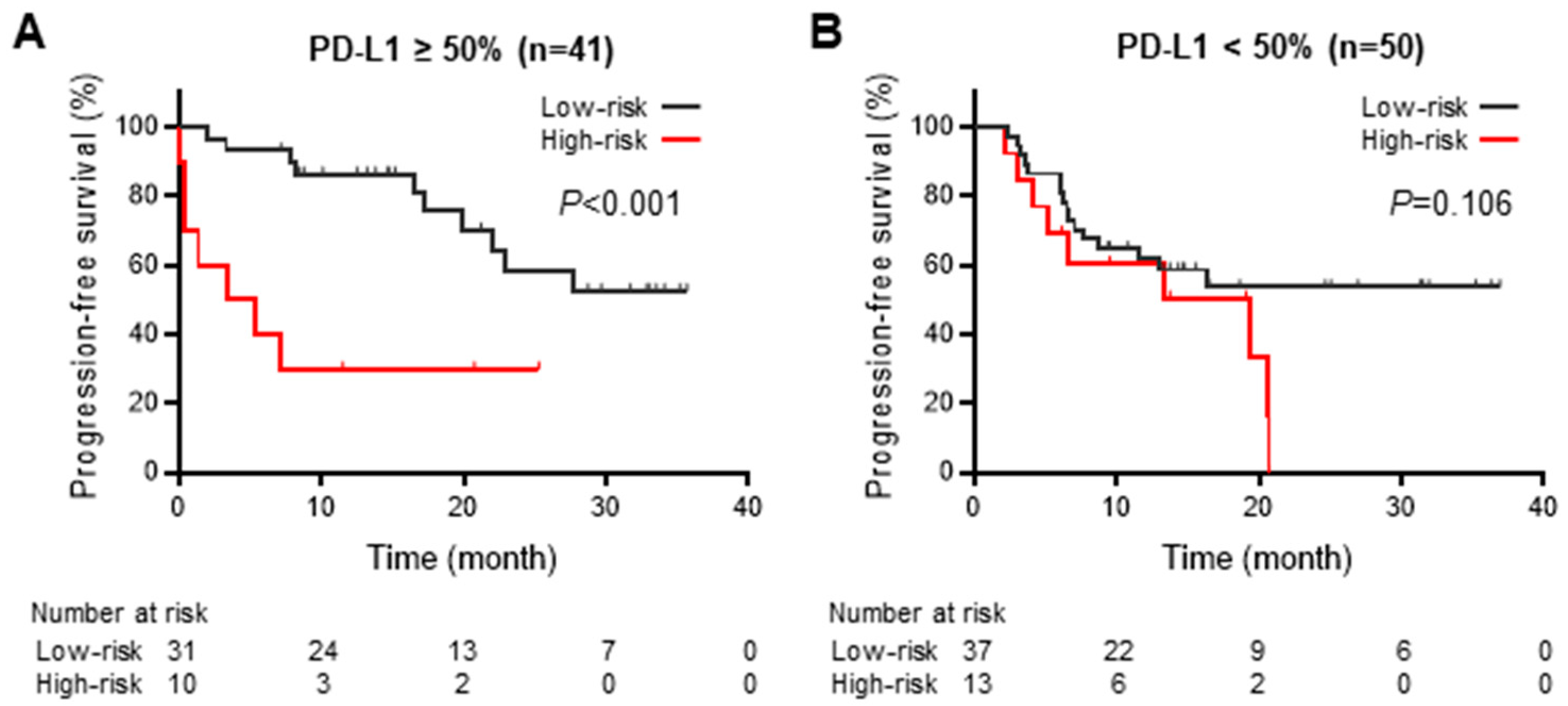
3.5. Impact of PD-L1 Expression on Progression-Free Survival during Durvalumab Consolidation
3.6. Treatment-Related Adverse Events during Durvalumab Consolidation after Chemoradiotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Lebow, E.S.; Shepherd, A.; Eichholz, J.E.; Offin, M.; Gelblum, D.Y.; Wu, A.J.; Simone, C.B.; Schoenfeld, A.J.; Jones, D.R.; Rimner, A.; et al. Analysis of Tumor Mutational Burden, Progression-Free Survival, and Local-Regional Control in Patents with Locally Advanced Non-Small Cell Lung Cancer Treated with Chemoradiation and Durvalumab. JAMA Netw. Open 2023, 6, e2249591. [Google Scholar] [CrossRef]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef]
- Carmi, Y.; Dotan, S.; Rider, P.; Kaplanov, I.; White, M.R.; Baron, R.; Abutbul, S.; Huszar, M.; Dinarello, C.A.; Apte, R.N.; et al. The Role of IL-1β in the Early Tumor Cell–Induced Angiogenic Response. J. Immunol. 2013, 190, 3500–3509. [Google Scholar] [CrossRef]
- Kitamura, H.; Ohno, Y.; Toyoshima, Y.; Ohtake, J.; Homma, S.; Kawamura, H.; Takahashi, N.; Taketomi, A. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017, 108, 1947–1952. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Kamp, C.O.D.; Langen, R.; Minnaard, R.; Kelders, M.; Snepvangers, F.; Hesselink, M.; Dingemans, A.; Schols, A. Pre-cachexia in patients with stages I–III non-small cell lung cancer: Systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer 2012, 76, 112–117. [Google Scholar] [CrossRef]
- Smith, I.J.; Aversa, Z.; Hasselgren, P.-O.; Pacelli, F.; Rosa, F.; Doglietto, G.B.; Bossola, M. CALPAIN activity is increased in skeletal muscle from gastric cancer patients with no or minimal weight loss. Muscle Nerve 2011, 43, 410–414. [Google Scholar] [CrossRef]
- Miyawaki, T.; Naito, T.; Kodama, A.; Nishioka, N.; Miyawaki, E.; Mamesaya, N.; Kawamura, T.; Kobayashi, H.; Omori, S.; Wakuda, K.; et al. Desensitizing Effect of Cancer Cachexia on Immune Checkpoint Inhibitors in Patients with Advanced NSCLC. JTO Clin. Res. Rep. 2020, 1, 100020. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Uchino, J.; Yokoi, T.; Kijima, T.; Goto, Y.; Nakao, A.; Hibino, M.; Takeda, T.; Yamaguchi, H.; Takumi, C.; et al. Impact of cancer cachexia on the therapeutic outcome of combined chemoimmunotherapy in patients with non-small cell lung cancer: A retrospective study. OncoImmunology 2021, 10, 1950411. [Google Scholar] [CrossRef] [PubMed]
- Bagley, S.J.; Kothari, S.; Aggarwal, C.; Bauml, J.M.; Alley, E.W.; Evans, T.L.; Kosteva, J.A.; Ciunci, C.A.; Gabriel, P.E.; Thompson, J.C.; et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017, 106, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Lee, M.; Hoen, D.; Weiss, K.; Kelly, D.W.; Adusumilli, P.S.; Paik, P.K.; Plitas, G.; Ladanyi, M.; Postow, M.A.; et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 2021, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Du, Y.; Huang, Z.; Xu, J.; Qiu, T.; Wang, J.; Wang, T.; Zhu, W.; Liu, P. Prognostic Value of PLR in Various Cancers: A Meta-Analysis. PLoS ONE 2014, 9, e101119. [Google Scholar] [CrossRef]
- Gu, X.; Sun, S.; Gao, X.-S.; Xiong, W.; Qin, S.; Qi, X.; Ma, M.; Li, X.; Zhou, D.; Wang, W.; et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: Evidence from 3430 patients. Sci. Rep. 2016, 6, 23893. [Google Scholar] [CrossRef]
- Frey, A.; Martin, D.; D’cruz, L.; Fokas, E.; Rödel, C.; Fleischmann, M. C-Reactive Protein to Albumin Ratio as Prognostic Marker in Locally Advanced Non-Small Cell Lung Cancer Treated with Chemoradiotherapy. Biomedicines 2022, 10, 598. [Google Scholar] [CrossRef]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Tanaka, K.; Fushiya, N.; Koike, K.; Nishino, H.; Matsushima, M. The C-Reactive Protein/Albumin Ratio, a Novel Inflammation-Based Prognostic Score, Predicts Outcomes in Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2015, 22, 803–810. [Google Scholar] [CrossRef]
- Mountzios, G.; Samantas, E.; Senghas, K.; Zervas, E.; Krisam, J.; Samitas, K.; Bozorgmehr, F.; Kuon, J.; Agelaki, S.; Baka, S.; et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open 2021, 6, 100254. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J.; et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients after Curative Resection for Hepatocellular Carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Tian, B.-W.; Yang, Y.-F.; Yang, C.-C.; Yan, L.-J.; Ding, Z.-N.; Liu, H.; Xue, J.-S.; Dong, Z.-R.; Chen, Z.-Q.; Hong, J.-G.; et al. Systemic immune–inflammation index predicts prognosis of cancer immunotherapy: Systemic review and meta-analysis. Immunotherapy 2022, 14, 1481–1496. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Mcmillan, D.C. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc. Nutr. Soc. 2008, 67, 257–262. [Google Scholar] [CrossRef]
- Mcmillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Chu, C.H.; Chiu, T.H.; Wang, C.C.; Chang, W.C.; Huang, A.C.; Liu, C.Y.; Wang, C.L.; Ko, H.W.; Chung, F.T.; Hsu, P.C.; et al. Consolidation treatment of durvalumab after chemoradiation in real-world patients with stage III unresectable non-small cell lung cancer. Thorac. Cancer 2020, 11, 1541–1549. [Google Scholar] [CrossRef]
- Araki, T.; Tateishi, K.; Komatsu, M.; Sonehara, K.; Wasamoto, S.; Koyama, S.; Yoshiike, F.; Hama, M.; Nishie, K.; Kondo, D.; et al. Predictive value of post-treatment C-reactive protein-to-albumin ratio in locally advanced non-small cell lung cancer patients receiving durvalumab after chemoradiotherapy. Thorac. Cancer 2022, 13, 2031–2040. [Google Scholar] [CrossRef]
- Silva, G.A.D.; Wiegert, E.V.M.; Calixto-Lima, L.; Oliveira, L.C. Clinical utility of the modified Glasgow Prognostic Score to classify cachexia in patients with advanced cancer in palliative care. Clin. Nutr. 2020, 39, 1587–1592. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Lim, M.; Deweese, T.L.; Drake, C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef]
- Choe, E.A.; Cha, Y.J.; Kim, J.H.; Pyo, K.H.; Hong, M.H.; Park, S.Y.; Shim, H.S.; Jung, I.; Lee, C.Y.; Cho, B.C.; et al. Dynamic changes in PD-L1 expression and CD8+ T cell infiltration in non-small cell lung cancer following chemoradiation therapy. Lung Cancer 2019, 136, 30–36. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; Meylan, M.; de Reyniès, A.; Sautès-Fridman, C.; Fridman, W.H. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front. Immunol. 2020, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F.; Montfort, A.; Marcheteau, E.; Imbert, C.; Gilhodes, J.; Filleron, T.; Rochaix, P.; Andrieu-Abadie, N.; Levade, T.; Meyer, N.; et al. TNFalpha blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 2017, 8, 2256. [Google Scholar] [CrossRef] [PubMed]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti–PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Flint, T.R.; Janowitz, T.; Connell, C.M.; Roberts, E.W.; Denton, A.E.; Coll, A.P.; Jodrell, D.I.; Fearon, D.T. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016, 24, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Xu, L.J.; Yang, L.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O.; Chen, Y. Radiation alters PD-L1/NKG2D ligand levels in lung cancer cells and leads to immune escape from NK cell cytotoxicity via IL-6-MEK/Erk signaling pathway. Oncotarget 2017, 8, 80506–80520. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Johnson, D.H.; Abdel-Wahab, N.; Foo, W.C.; Bentebibel, S.E.; Daher, M.; Haymaker, C.; Wani, K.; Saberian, C.; Ogata, D.; et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022, 40, 509–523. [Google Scholar] [CrossRef]
- Sealy, M.J.; Dechaphunkul, T.; van der Schans, C.P.; Krijnen, W.P.; Roodenburg, J.L.; Walker, J.; Jager-Wittenaar, H.; Baracos, V.E. Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clin. Nutr. 2020, 39, 501–509. [Google Scholar] [CrossRef]
- Karaca, F.; Afsar, C.U.; Gunaldi, M.; Erkurt, E.; Ercolak, V.; Sertdemir, Y.; Paydas, S. Alterations of ghrelin with weights and correlation among ghrelin, cytokine and survival in patients receiving chemoradiotherapy for gastrointestinal cancers. Int. J. Clin. Exp. Med. 2015, 8, 876–882. [Google Scholar]
- Hiura, Y.; Takiguchi, S.; Yamamoto, K.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Miyata, H.; Fujiwara, Y.; Mori, M.; et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: A prospective, randomized, placebo-controlled phase 2 study. Cancer 2012, 118, 4785–4794. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Uchino, J.; Yokoyama, T.; Naito, T.; Kondo, M.; Yamada, K.; Kitajima, H.; Yoshimori, K.; Sato, K.; Saito, H.; et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 2018, 124, 606–616. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n = 126 | |
|---|---|---|
| Age—median (IQR), year | 71.0 (64.3, 76.0) | |
| Sex-Male—n (%) | 98 (77.8) | |
| Smoking history—n (%) | Never smoked | 16 (12.7) |
| Ex- or current smoker | 110 (87.3) | |
| ECOG-PS—n (%) | 0 or 1 | 118 (93.7) |
| ≥2 | 8 (6.3) | |
| Histology—n (%) | Squamous cell carcinoma | 65 (51.6) |
| Adenocarcinoma | 57 (45.2) | |
| Others | 4 (3.2) | |
| Driver mutations—n (%) | Wild-type | 19 (15.1) |
| EGFR or ALK | 6 (4.8) | |
| Others | 2 (1.6) | |
| Unknown | 99 (78.6) | |
| PD-L1 expression—n (%) | ≥50% | 41 (32.5) |
| <50% | 50 (39.7) | |
| Unknown | 35 (27.8) | |
| Clinical stage—n (%) | ≤IIb | 11 (8.7) |
| IIIA | 48 (38.1) | |
| IIIB | 56 (44.4) | |
| IIIC | 11 (8.7) | |
| Chemotherapy—n (%) | Carboplatin/paclitaxel | 64 (50.8) |
| Carboplatin monotherapy | 22 (17.5) | |
| Cisplatin/S-1 | 16 (12.7) | |
| Cisplatin/docetaxel | 14 (11.1) | |
| Others | 10 (8.0) | |
| Best overall response to CRT—n (%) | PR/CR | 93 (73.8) |
| SD | 32 (25.4) | |
| NE | 1 (0.8) | |
| Interval of CRT and durvalumab -median (IQR), day | 15.5 (13.0, 29.8) | |
| PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre/Post CRT | C-Index | SE | HR (95% CI) | p-Value | C-Index | SE | HR (95% CI) | p-Value | |
| mGPS (0,1,2) | Pre | 0.572 | 0.035 | 1.45 (1.07–1.97) | 0.016 | 0.653 | 0.049 | 1.84 (1.22–2.78) | 0.004 |
| Post | 0.549 | 0.032 | 1.35 (0.99–1.83) | 0.056 | 0.615 | 0.052 | 1.47 (0.98–2.20) | 0.065 | |
| CAR (<0.32 vs. ≥0.32) | Pre | 0.557 | 0.033 | 1.67 (1.0–2.77) | 0.049 | 0.64 | 0.044 | 2.33 (1.16–4.68) | 0.018 |
| Post | 0.535 | 0.03 | 1.41 (0.81–2.45) | 0.22 | 0.61 | 0.047 | 2.11 (1.03–4.33) | 0.041 | |
| NLR (<5 vs. ≥5) | Pre | 0.566 | 0.03 | 1.92 (1.04–3.55) | 0.037 | 0.603 | 0.046 | 2.36 (1.09–5.11) | 0.029 |
| Post | 0.512 | 0.032 | 0.99 (0.58–1.69) | 0.962 | 0.543 | 0.047 | 1.46 (0.71–3.01) | 0.302 | |
| ALI (≥18 vs. <18) | Pre | 0.553 | 0.032 | 1.56 (0.91–2.67) | 0.103 | 0.604 | 0.047 | 2.43 (1.21–4.9) | 0.013 |
| Post | 0.489 | 0.034 | 1.02 (0.61–1.70) | 0.931 | 0.526 | 0.048 | 1.24 (0.61–2.49) | 0.556 | |
| PLR (<180 vs. ≥180) | Pre | 0.507 | 0.034 | 1.04 (0.63–1.72) | 0.879 | 0.532 | 0.048 | 1.12 (0.56–2.24) | 0.756 |
| Post | 0.54 | 0.028 | 0.64 (0.36–1.13) | 0.122 | 0.54 | 0.043 | 0.69 (0.32–1.49) | 0.34 | |
| SII (<750 vs. ≥750) | Pre | 0.539 | 0.034 | 1.37 (0.82–2.27) | 0.226 | 0.581 | 0.048 | 1.54 (0.76–3.12) | 0.232 |
| Post | 0.548 | 0.034 | 0.77 (0.46–1.27) | 0.304 | 0.506 | 0.049 | 0.99 (0.49–1.98) | 0.967 | |
| LIPI (0,1,2) | Pre | 0.547 | 0.034 | 1.26 (0.86–1.84) | 0.236 | 0.546 | 0.046 | 1.45 (0.85–2.47) | 0.173 |
| Post | 0.51 | 0.036 | 1.1 (0.73–1.67) | 0.655 | 0.562 | 0.052 | 1.5 (0.87–2.59) | 0.141 | |
| Low-Risk (n = 95) | High-Risk (n = 31) | p-Value | ||
|---|---|---|---|---|
| Age—median (IQR), y | 70.0 [44.0, 89.0] | 72.0 [36.0, 84.0] | 0.351 | |
| Sex—n (%) | Male | 75 (78.9) | 23 (74.2) | 0.622 |
| ECOG-PS—n (%) | 0 or 1 | 89 (93.7) | 29 (93.5) | 1 |
| ≥2 | 6 (6.3) | 2 (6.5) | ||
| Smoking history—n (%) | Yes | 83 (87.4) | 27 (87.1) | 1 |
| No | 12 (12.6) | 4 (12.9) | ||
| Histology—n (%) | Non-Sq. | 48 (50.5) | 17 (54.8) | 0.686 |
| Sq. | 47 (49.5) | 14 (45.2) | ||
| PD-L1 expression—n (%) | ≥50% | 31 (32.6) | 10 (32.3) | 0.293 |
| 1–49% | 24 (25.3) | 12 (38.7) | ||
| <1% | 13 (13.7) | 1 (3.2) | ||
| Unknown | 27 (28.4) | 8 (25.8) | ||
| Clinical Stage | ≤IIb | 11 (11.6) | 0 (0.0) | 0.031 |
| IIIA | 36 (37.9) | 12 (38.7) | ||
| IIIB | 43 (45.3) | 13 (41.9) | ||
| IIIC | 5 (5.3) | 6 (19.4) | ||
| % of change from pre-CRT BW—median (IQR) | −1.72 (−5.10, 0.76) | −4.86 (−8.24, −2.19) | 0.037 | |
| Best overall response to CRT—n (%) | CR/PR | 69 (72.6) | 24 (77.4) | 0.859 |
| SD | 25 (26.3) | 7 (22.6) | ||
| NE | 1 (1.1) | 0 (0) | ||
| Total (n = 126) | Low-Risk (n = 95) | High-Risk (n = 31) | ||||
|---|---|---|---|---|---|---|
| Adverse Events—n (%) | Any Grade | Grade 3–5 | Any Grade | Grade 3–5 | Any Grade | Grade 3–5 |
| Any AE | 101 (80) | 17 (13) | 76 (80) | 10 (10.5) | 25 (80.6) | 7 (22.6) |
| Pneumonitis | 82 (65) | 10 (8) | 62 (65.2) | 5 (5.3) | 20 (64.5) | 5 (16.1) |
| Hypothyroidism | 19 (15) | 5 (4) | 16 (16.8) | 4 (4.4) | 3 (9.6) | 1 (3.2) |
| Rash | 11 (9) | 0 | 9 (9.5) | 0 | 2 (6.4) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanimura, K.; Takeda, T.; Yoshimura, A.; Honda, R.; Goda, S.; Shiotsu, S.; Fukui, M.; Chihara, Y.; Uryu, K.; Takei, S.; et al. Predictive Value of Modified Glasgow Prognostic Score and Persistent Inflammation among Patients with Non-Small Cell Lung Cancer Treated with Durvalumab Consolidation after Chemoradiotherapy: A Multicenter Retrospective Study. Cancers 2023, 15, 4358. https://doi.org/10.3390/cancers15174358
Tanimura K, Takeda T, Yoshimura A, Honda R, Goda S, Shiotsu S, Fukui M, Chihara Y, Uryu K, Takei S, et al. Predictive Value of Modified Glasgow Prognostic Score and Persistent Inflammation among Patients with Non-Small Cell Lung Cancer Treated with Durvalumab Consolidation after Chemoradiotherapy: A Multicenter Retrospective Study. Cancers. 2023; 15(17):4358. https://doi.org/10.3390/cancers15174358
Chicago/Turabian StyleTanimura, Keiko, Takayuki Takeda, Akihiro Yoshimura, Ryoichi Honda, Shiho Goda, Shinsuke Shiotsu, Mototaka Fukui, Yusuke Chihara, Kiyoaki Uryu, Shota Takei, and et al. 2023. "Predictive Value of Modified Glasgow Prognostic Score and Persistent Inflammation among Patients with Non-Small Cell Lung Cancer Treated with Durvalumab Consolidation after Chemoradiotherapy: A Multicenter Retrospective Study" Cancers 15, no. 17: 4358. https://doi.org/10.3390/cancers15174358
APA StyleTanimura, K., Takeda, T., Yoshimura, A., Honda, R., Goda, S., Shiotsu, S., Fukui, M., Chihara, Y., Uryu, K., Takei, S., Katayama, Y., Hibino, M., Yamada, T., & Takayama, K. (2023). Predictive Value of Modified Glasgow Prognostic Score and Persistent Inflammation among Patients with Non-Small Cell Lung Cancer Treated with Durvalumab Consolidation after Chemoradiotherapy: A Multicenter Retrospective Study. Cancers, 15(17), 4358. https://doi.org/10.3390/cancers15174358






